Abstract
Perceived life stress (PLS) and cognitive restraint are associated with increased comfort food intake under stress and lead to weight gain and obesity, but the mechanisms by which they do so remain unclear. Stress and negative affect (NA) are associated with increased reward-driven comfort food intake as a means to ‘feel better’, particularly for individuals with higher PLS and cognitive restraint. Thus, we propose that PLS and cognitive restraint increase stress-eating by strengthening the relationship between stress-induced NA and comfort food intake. Upon comfort eating, individuals with higher PLS show greater reductions in the negative consequences of stress (e.g. NA). The rewarding effects of this ‘emotional relief’ may promote future stress-induced comfort eating, but this has yet to be examined. Thus, we investigate the pathways by which PLS or cognitive restraint increase snack intake under stress by proposing that 1) stress-induced NA is a stronger predictor of increased snack intake for women with greater PLS and cognitive restraint, and 2) greater PLS will be associated with greater reductions in NA upon snacking under stress (i.e. emotional relief). Forty-three healthy women were given snacks (chips, golden oreos, and M&Ms) to eat after a Trier Social Stress Test or rest period on separate days in counterbalanced order. Following linear regression analyses, we determined that stress-induced NA predicted more snack intake for women with higher PLS, and that higher PLS was associated with heightened emotional relief upon snacking under stress. Future studies are needed to directly assess whether greater emotional relief following stress-eating reinforces the learned association between stress-induced NA and intake, and ultimately explains greater stress-eating and obesity in women with higher PLS. This work may lead clinicians to focus on NA in the treatment of obesity-and stress-related illnesses for women with higher PLS.
1. Introduction
Individuals either increase or decrease the amount of food consumed when exposed to chronic and acute stressors [1]. It is unknown what factors are responsible for this dichotomy. One possibility lies in inconsistent methods of measuring stress responses and food intake, and whether the measurements are made via self-report or during objective observation in the laboratory [15,47]. Approximately one-third of adults self-report overeating due to stress (hyperphagia); yet another one-third self-report skipping meals or undereating when stressed (hypophagia; [2]). The majority of laboratory studies report increases in food intake when exposed to acute mental stress; however, some studies report reduced intake [1,9,48,49]. Despite being a critical barrier in advancing the field [15], a lack of consistent and precise measurement cannot fully explain the variance in stress-eating.
Another explanation comes from individual differences models, which state that heterogeneity in psychological, environmental, and physiological vulnerability factors contribute to variance in eating behavior under stress [23,25,38]. For instance, individual differences in negative affect (NA), cortisol, and hunger reactions to stress have been associated with variance in palatable food intake, via interactions with neural networks involved in reward, emotion, and cognition (for review, see [47]; also [1,6,14,53,57,61]). NA enhances reward-driven ‘wanting’ for comfort food as a means to reduce the deleterious effects of stress [18,40], and cortisol increases dopamine release from brain reward centers, promoting the motivational drive for comfort food intake under stress [16]. Hunger is often self-reported as a reason for eating unhealthy snacks [7] and hunger-eating (i.e. eating because of hunger) increases with perceived stress [41], possibly due to stress-induced increases in ghrelin [45]. These factors do not uniformly increase the consumption of palatable foods under stress, as other studies report that negative mood, cortisol, and hunger are unrelated to stress-eating, or are associated with decreased intake [3,17,21,34,44]. These inconsistencies in how stress-induced NA, cortisol, and hunger influence food intake may be explained by a failure to investigate potential moderating factors; in particular, perceived life stress (PLS) and cognitive restraint (i.e. intentional restriction of caloric intake to lose or maintain weight; [27]). They could also be associated with the definition of stress utilized by the investigator or the type of stressful situation administered [15]. In this report, the approach will center on the possibility that moderating factors of PLS and cognitive restraint may be at work.
2. PLS and cognitive restraint may increase stress-eating by moderating the hyperphagic effects of NA, cortisol, and hunger
PLS and cognitive restraint are associated with increased stress-induced comfort food intake and ultimately weight gain and obesity [13,33,35,38,53,56,58,60]. We propose that this is because PLS and cognitive restraint enhance the salience of NA as a trigger for stress-eating. Individuals with high chronic and perceived life stress have greater baseline and stress-induced NA [31,54], and show a relationship between stress-induced NA and consuming a larger percentage of portioned snack food [30]. Similarly, individuals with high cognitive restraint show increased food intake in response to negative emotion compared to those with low cognitive restraint [6,17].
For women with greater PLS and cognitive restraint, stronger hyperphagic effects of stress-induced NA may coincide with weakened hyperphagic effects of stress-induced cortisol and hunger. A recent study from our group found that stress-induced cortisol and hunger were not associated with snacking under stress in women with high PLS [30]. Similarly, individuals with high cognitive restraint tend to be less responsive to internal hunger cues and physiological stress than individuals with low restraint [26,43]. Thus, preliminary evidence suggests that psychological factors such as NA may drive stress-induced eating for women with higher PLS and cognitive restraint, rather than physiological and homeostatic factors such as cortisol and hunger. To our knowledge, no study to date has directly assessed how PLS and cognitive restraint moderate these factors to increase stress-eating. Therefore, we propose that PLS and cognitive restraint increase snacking under stress by enhancing the hyperphagic effects of stress-induced NA. Secondarily, we propose that PLS and cognitive restraint reduce the hyperphagic effects of stress-induced hunger and cortisol. Gaining a better understanding of the mechanisms by which PLS and cognitive restraint increase snack intake under stress may inform the treatment of stressor obesity-related illnesses. If our hypothesis is supported, such treatment for individuals with high PLS or cognitive restraint may focus on negative emotions rather than physiological or homeostatic factors as the primary drivers of stress-eating and obesity.
3. Greater reductions in negative emotions following stress-eating may enhance the salience of NA as a trigger of stress-eating
Consumption of palatable foods induces feelings of comfort (i.e. comfort eating) and provides short-term relief from negative mood and feelings of stress ([9,20,37,52,59,63]). Stress-induced comfort food intake is driven and maintained in part by negative reinforcement, as it reduces the discomforts associated with stress via multiple brain networks [16]. Eating palatable foods under stress activates dopamine release in brain reward pathways, resulting in feelings of pleasure, and also dampens hypothalamic pituitary adrenal (HPA) axis and behavioral stress reactivity within the central stress response network [16,19,32,39]. Dampening of physiological and behavioral stress responses may explain ‘feeling better’ immediately after stress-eating comfort foods, and may promote the use of palatable food as a form of self-medication [9–11]. Stress-eating comfort foods may be more reinforcing for individuals with higher chronic or perceived life stress. PLS may strengthen the wanting and pleasure associated with comfort food intake, as well as memories of emotional relief upon comfort eating [16]. In a prior study from our group, we reported that snacking under stress reduced NA for women with high PLS to a greater extent than for women with low PLS [30]. Our prior study did not, however, control for greater stress-induced NA in women with high PLS, and thus greater emotional relief upon snacking may have been due to higher initial levels of NA.
Because stress-eating comfort foods tends to provide relief from the negative effects of stress, particularly for those with PLS, we propose that PLS predicts greater reductions in stress-induced NA upon snacking. Although not directly assessed in the current study, greater reductions in NA upon stress-eating for women with greater PLS may reinforce the relationship between stress-induced NA and comfort food intake. Ultimately, this hypothetical conditioning effect may increase the ability of stress-induced NA to trigger snacking in women with greater PLS, and potentially explain the heightened prevalence of comfort food intake, emotional eating, and obesity in this population [24,46,51].
In the current study, we attempt to explain how PLS and cognitive restraint increase snack intake under stress. We predict that 1) for women with greater PLS and cognitive restraint, stress-induced NA will be a stronger predictor of increased snack intake under stress, but stress-induced cortisol and hunger will be weaker predictors. This is because 2) PLS will predict greater reductions in NA upon snacking under stress, the rewarding effects of which may promote future comfort intake in response to stress-induced NA.
4. Methods
4.1. Participants
Female undergraduates (n = 43) between the ages of 18 and 22 responded to an advertisement for research investigating stress, and participants were told that the study was investigating stress physiology in college females. Given that women report eating greater amounts of food in response to stress than men, and because the relationship between stress and obesity is greater in women than men [55], we specifically recruited women for our study.
Participants were excluded from study participation if they were currently in treatment for eating or weight problems, were regular smokers, currently taking blood pressure, stimulant, or psychoactive medications, or self-reported current or prior cardiovascular disease, diabetes, or hypertension. The research was approved by the college’s Institutional Review Board, and all participants received partial course credit for their time.
4.2. Procedure
Women responding to our advertisements completed preliminary screening questions aimed at assessing PLS and the exclusionary criteria described above, as well as measures closely tied to PLS: depressive symptoms, uncontrolled eating, emotional eating, cognitive restraint, and body mass index [5,12,64]. In order to collect data from a parent study not reported here, participants also completed a computerized task in which they were asked to create digital projections of the food portions they would typically eat and would eat in response to a recalled memory from a stressful life event. If inclusionary criteria were met, participants were invited to schedule both of their laboratory sessions (rest and stress days).
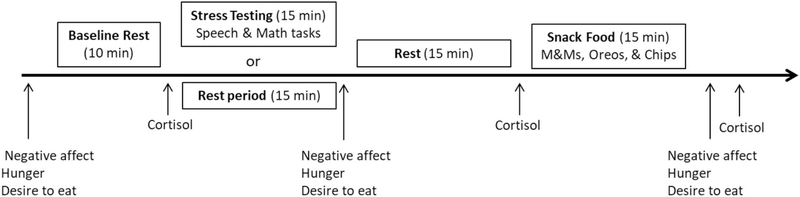
Each laboratory testing session began between the hours of 4:00 pm and 5:30 pm. The order of rest and stress laboratory sessions was counterbalanced and the average time between them was 6.5 days (standard deviation = 2 days). The rest day was identical to the stress day, with the exception that the stress testing was replaced with a rest period of the same length (Fig. 1). On the day of testing, participants refrained from exercising strenuously, waking from sleep less than 2 h prior to the testing session, drinking more than a single caffeinated beverage in the morning, eating or drinking (except water) 2 h prior to the study, consuming any alcohol 12 h prior to the study, or taking any antihistamines, psychotropic medications, and neural stimulants. The experimenter confirmed that participants had followed all testing-day requirements. Participants were also asked to arrive “not too hungry, but not too full” and to “make sure to eat some food at 2 hours before the study visit to avoid excess hunger.”
Fig. 1.
Laboratory protocol for stress or rest day.
4.3. Laboratory protocol
4.3.1. Baseline rest
First, the researcher placed a blood pressure cuff on the non-dominant arm of the participant. Next, participants completed questionnaires assessing their subjective well-being: stress intensity, positive and negative affect, hunger, and desire to eat (see below). Participants then rested quietly for 10 min, during which time cardiovascular activity was assessed every 5 min. Cardiovascular measures were comprised of systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR). HPA-axis activity was assessed immediately following baseline rest via salivary cortisol (see below).
4.3.2. The Trier Social Stress Test (TSST)
The TSST [29] is a stress test that reliably induces large and consistent cardiovascular responses. During pre-task instructions (5 min), the researcher informed the participants that they would be giving a speech that will be audio- and video-recorded for later analysis and would be followed by a serial subtraction task. Participants were then introduced to the selection committee composed of three research assistants wearing white laboratory coats who would be evaluating their speech. The researcher then asked participants to imagine that they were applying for their ideal job and to take 5 min to prepare their speech describing why they would be the ideal candidate for the position. Immediately following the preparation period, the selection committee returned to the testing room and asked the participants to deliver their speech. If the participant finished before 5 min, the committee responded in a standardized way by asking the participant to continue. If necessary, the committee asked prepared questions to ensure that participants spoke for the entire period. Finally, the researcher asked the participants to perform mental math for 5 min by serially subtracting 7 from 2000 aloud as quickly and accurately as possible. Their progress was monitored, and when an error was made, the experimenter told the participants to start over from the beginning. During rest day, participants rested quietly while listening to classical music and were given the option to read any of 3–4 magazines about the city of Memphis. Cardiovascular and HPA-axis activity were assessed throughout the TSST and during rest (see below). Immediately following stress and rest, participants completed assessments measuring stress intensity, positive and negative affect, hunger, and desire to eat (see below). For the purposes of a parent study, participants also completed a computerized task in which they were asked to create food portions they wanted to eat at the present moment. Researchers then asked participants to sit and rest quietly. During this time, both the 35 min post-stress or post-rest induction measures of salivary cortisol were taken.
4.3.3. Snack food
Forty minutes following the initiation of stress or rest, participants were given a tray containing three plastic containers with a pourable lid, each filled with a different snack food. Three bowls were provided in which to pour each of the three snack foods, so participants could serve themselves. Each plastic container was the same size and filled to the top with either M&Ms. (935 g, 19.5 servings, 4684 kcal), mini golden Oreos (380 g, 13 servings, 1834 kcal), or potato chips (110 g, 4 servings, 629 kcal). Participants were told that the purpose of this part of the study was to determine the effect of liking or disliking certain foods on salivary function. Participants were asked to sample each snack food and then rate it on the taste dimensions of salty, sweet, and crunchy, as well as how much they liked the snack. Participants were then left alone for 15 min to portion, consume, and rate the snacks, and were free to move about the private testing room.
4.3.4. Post-snack
Following the snack period, participants again completed assessments measuring stress intensity, positive and negative affect, hunger, and desire to eat (see below). During the second laboratory testing session, height (cm) and weight (kg) were then assessed to calculate BMI (kg/m2) using a Seca 769 digital column scale. Waist circumference, a measure of central obesity, was also measured at the midway point between the lowest ribs and the iliac crest with an anthropometric tape measure by trained research assistants.
4.4. Physiological measures
The Oscar 2 oscillometric ambulatory blood pressure monitor (SunTech Medical Instruments, Inc., Raleigh, NC) with an appropriately sized arm cuff provided automated measurement of systolic blood pressure (SBP), diastolic blood pressure (DBP) and HR during the testing session. All measurements took place while participants were in a comfortable seated position. Blood pressure and heart rate measures were taken at minutes 0, 5, and 10 of baseline, minutes 0, 2, and 4 of both the speech and serial subtraction periods during stress day, and minutes 0, 5, and 10 of quiet rest during the rest day. The cardiovascular measures taken at minute 10 of baseline constituted the baseline value for each participant. For cardiovascular measures during stress, the average value for each participant during each task constituted the speech and math stress values.
Saliva was collected in 1.5 mL Eppendorf tubes at the end of the baseline rest period, and 35 and 70 min following stress or rest induction. Participants passively drooled into the tube for a maximum of 2 min per sample. Saliva samples were frozen within 30 min of collection at −20 °C until assayed. On the day of testing, all samples were centrifuged at 3000 rpm for 15 min to remove mucins and were analyzed in duplicate by enzyme immunoassay (Salimetrics, State College, PA). The mean intra-assay coefficient of variation was 8.03% and the inter-assay coefficient was 9.88%.
4.5. Psychological measures – preliminary screening
4.5.1. Perceived life stress (PLS)
Levels of perceived psychological stress were measured using the Perceived Stress Scale (PSS-10; [8]). The PSS measures “the degree to which situations in one’s life are appraised as stressful” ([8], p. 387). The ten items on the PSS assess how unpredictable, uncontrollable, and overloaded respondents view their lives, and directly inquire about levels of experienced stress in the past 3 months with answer choices ranging from 0 (Never) to 4 (Very Often). A sample item is: “How often have you felt nervous and stressed?” Scores range from 0 to 40 and higher scores indicate greater PLS.
4.5.2. Depressive symptoms
Depressive symptoms were assessed using the Beck Depression Inventory (BDI; [4]). The BDI comprehensively assesses self-reported dysphoric symptoms, including affective, cognitive, somatic, overt behavioral, and interpersonal symptoms of depression. Each forced-choice question has a set of at least four possible answer choices, with increasing severity of depressive symptoms from 0 to 3.
4.5.3. Subjective eating measures
The Three Factor Eating Questionnaire (TFEQ-R18; [28]), which is a revised and shortened version of the original 51-item TFEQ. [50] assessed uncontrolled eating (eating more than usual due to a loss of control over food intake accompanied by subjective feelings of hunger; range 3–12), emotional eating (inability to resist emotional cues; range 9–36), and restrained eating (conscious restriction of food intake in order to control body weight or to promote weight loss; range 6–24). Greater scores indicate greater eating-related psychopathology.
4.6. Subjective psychological measures-baseline and post-stress/rest
4.6.1. Stress intensity
Current level of stress intensity was measured on a computerized sliding scale from 0 (None) to 100 (Strongest experienced).
4.6.2. Positive and negative affect
Affect was quantified with the Positive and Negative Affect Schedule (PANAS), a 20-item multiple-choice survey validated in a university population [62]. For each word describing a different feeling or emotion felt at the present moment (e.g. distressed, hostile, nervous), participants had a choice of ratings from 1 (Very Slightly or Not At All) to 5 (Extremely). The positive subscale consisted of 10 words and a possible range from 10 to 50, with higher scores indicating more positive affect. The negative subscale consisted of 10 words and a possible range from 10 to 50, with higher scores indicating more NA.
4.6.3. Drive to eat
Current hunger and desire to eat were measured on separate visual analog scales from 0 (None) to 10 (Most imaginable).
4.7. Data analyses
Hypothesis #1.
PLS and cognitive restraint increase stress-eating by enhancing the hyperphagic effects of NA, and by reducing the hyperphagic effects of cortisol and hunger.
In order to test our first hypothesis, we performed a hierarchical linear regression analysis to predict snack intake under stress based on stress-induced changes in hunger, cortisol, and NA, as well as PLS, cognitive restraint, and snack intake at rest (step 1). In step 2 of the analysis, we combined PLS and cognitive restraint with NA, hunger, cortisol (i.e. PLS × NA, PLS × hunger, PLS × cortisol, cognitive restraint × NA, cognitive restraint × hunger, cognitive restraint × cortisol) and added these interaction one by one to determine whether each interaction added a significant amount of variance to the model predicting snack intake under stress.
Hypothesis #2.
PLS will predict greater reductions in NA upon snacking under stress.
In order to test our second hypothesis, we performed a hierarchical linear regression analysis to predict the change in NA following snacking under stress based on PLS. We wanted to test whether PLS explained significant variance in the reduction in NA after stress-eating above and beyond that explained by other factors associated with post-ingestive reductions in NA, and we did so by including these factors in step 1 of the model: snack intake under stress and rest, as well as stress-induced changes in NA, desire to eat, and cortisol. In step 2, we added PLS in order to determine whether it added a significant amount of variance explained to the model.
In order to determine whether our predictions pertained to snack intake in general, or if the effects were specific to eating under stress, we performed the same two linear regression analyses with rest day variables. We then sought to determine whether the post-ingestive alleviation of negative affect was greater under stress versus rest by performing a repeated measures ANCOVA, with stress day and rest day as the repeated factor, and with change in NA from baseline on rest and stress days as covariates.
We examined the data for outliers on both stress and rest days and eliminated extreme scores (i.e. greater than the third quartile plus 1.5 times the interquartile range) from two participants who reported extreme decreases in their hunger ratings from baseline to stress, one participant who reported extremely high baseline NA ratings on the stress day, and two participants who showed extremely large increases in cortisol from baseline to stress. Results reflect removal of these outliers.
5. Results
Hypothesis #1.
PLS and cognitive restraint increase stress-eating by enhancing the hyperphagic effects of NA, and by reducing the hyperphagic effects of cortisol and hunger.
In step 1 of the linear regression analysis, snack intake at rest was the only significant predictor of snack intake under stress, Beta = 0.87, F(6,35) =16.1, p =.000, R2 = 0.77 (Table 1; Figs. 2 and 3). In step 2, the interaction of PLS and NA explained additional variance in snack intake under stress F(7, 35) =16.3, p =.000, R2 change = 0.03, with PLS enhancing the effect of stress-induced NA on increased snack intake. That is to say, higher stress-induced NA predicted greater snack intake for women with higher PLS. No other interactions resulted in additional variance explained (Ps > 0.05).
Table 1.
Hierarchical linear regression analysis predicting snack intake under stress.
| Variable | Step 1 | Step 2 | ||||
|---|---|---|---|---|---|---|
| B | SE B | β | B | SE B | β | |
| Stress-induced change in negative affect (NA) | −0.68 | 1.2 | −0.06 | −7.6 | 3.3 | −0.67* |
| Stress-induced change in cortisol | 0.11 | 0.09 | 0.12 | 0.12 | 0.09 | 0.13 |
| Stress-induced change in hunger | 4.0 | 4.0 | 0.10 | 4.6 | 3.8 | 0.11 |
| Perceived Life Stress (PLS) | −0.19 | 0.87 | −0.02 | −1.8 | 1.1 | −0.20 |
| Cognitive restraint | −0.71 | 1.2 | −0.05 | −0.92 | 1.1 | −0.07 |
| Rest day snack intake | 0.79 | 0.09 | 0.87** | 0.77 | 0.09 | 0.84** |
| PLS×Stress-induced change in NA | 0.40 | 0.18 | 0.72* | |||
| R2 | 0.77 | 0.80 | ||||
| F for change in R2 | 16.1** | 4.9* | ||||
p <.05
p <.01.
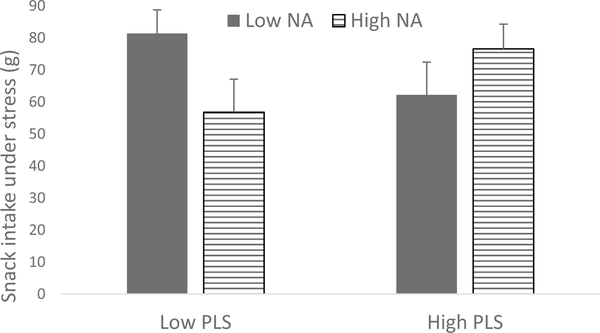
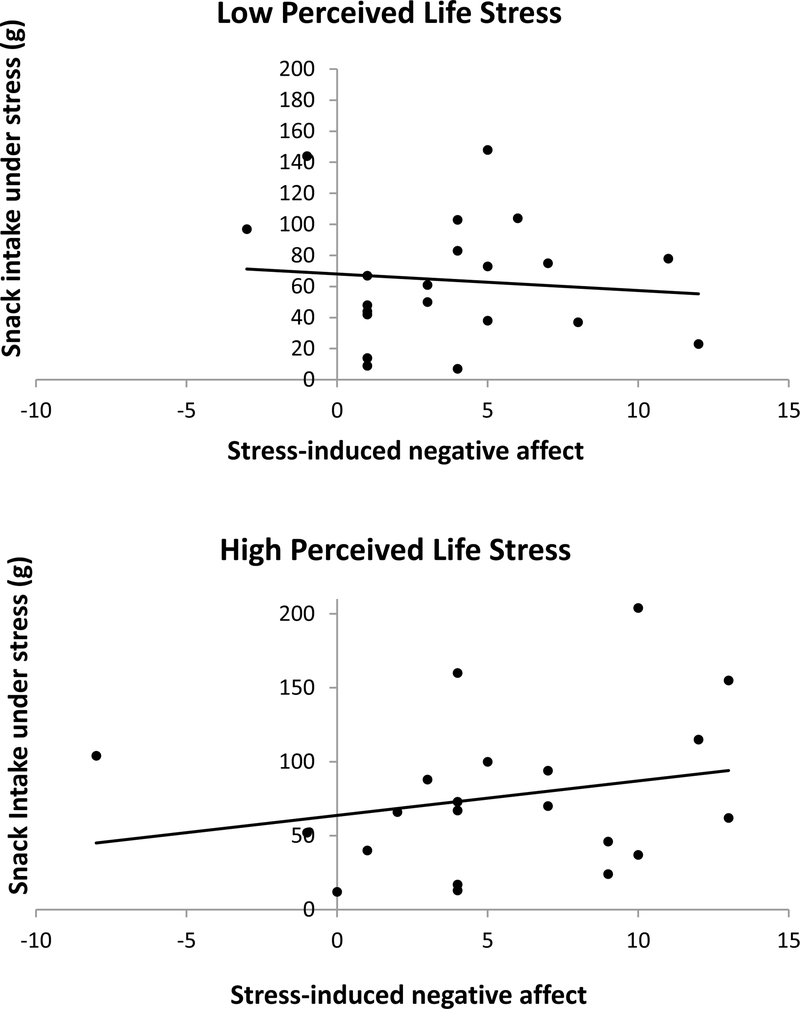
Fig. 2.
Visual depiction of the perceived life stress (PLS) × stress-induced negative affect (NA) interaction predicting snack intake under stress, using 1 standard deviation above and below the mean. Higher stress-induced NA predicted greater snack intake for women with higher PLS, F(7, 35) =16.3, p =.000, R2 change = 0.03.
Fig. 3.
Visual depiction of the perceived life stress × stress-induced negative affect interaction predicting snack intake under stress, using median split.
Snack intake under stress was the only significant predictor of snack intake at rest, F(7, 38) = 10.20, p =.000, R2 = 0.697.
Hypothesis #2.
PLS predicts greater reductions in NA upon snacking under stress.
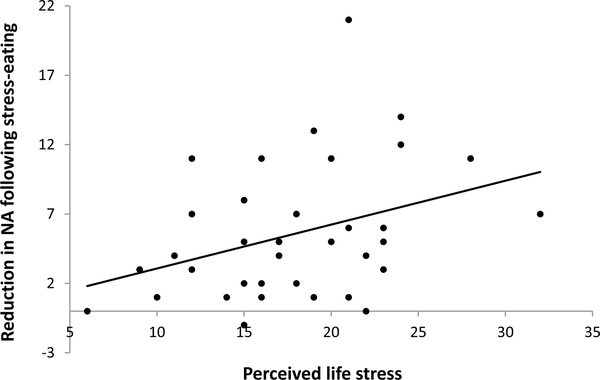
In step 1 of the linear regression analysis, the rise in NA following stress was the only significant predictor of the reduction in NA following snack intake under stress, Beta = 0.553, p =.000, F(5, 37) = 11.618, p =.000, R2 = 0.645. In step 2, PLS added significant variance explained to the model, Beta = 0.244, p =.032, F(6, 37) = 11.74, p =.000, R2 change = 0.05, as PLS predicted greater reductions in NA following snack intake under stress (Table 2; Fig. 4). There were no significant predictors of post-ingestive changes in NA on rest day, F(5, 38) = 0.838, p =.532, R2 = 0.113.
Table 2.
Linear regression analysis predicting decreases in negative affect upon snacking under stress.
| Variable | Step 1 | Step 2 | ||||
|---|---|---|---|---|---|---|
| B | SEB | β | B | SEB | β | |
| Stress-induced change in negative affect | 0.64 | 0.14 | 0.55** | 0.54 | 0.14 | 0.47** |
| Stress-induced change in cortisol | −0.01 | 0.01 | −0.05 | 0.00 | 0.01 | 0.00 |
| Stress-induced change in desire to eat | −0.60 | 0.36 | −0.22 | −0.60 | 0.34 | −0.22 |
| Rest day snack intake | 0.03 | 0.02 | 0.33 | 0.03 | 0.02 | 0.32 |
| Stress day snack intake | 0.01 | 0.02 | 0.09 | 0.01 | 0.02 | 0.13 |
| Perceived Life Stress | 0.22 | 1.0 | 0.24* | |||
| R2 | 0.645 | 0.694 | ||||
| F for change in R2 | 11.62** | 5.04* | ||||
p <.05
p <.01.
Fig. 4.
Relationship between perceived life stress and reduction in negative affect (NA) upon snacking under stress (i.e. emotional relief).
In the entire sample, we found greater reductions in NA following snacking on stress day compared to rest day, controlling for pre-snack NA, F(1, 37) = 5.98, p =.019. Post-hoc analyses revealed significant decreases in NA following snacking under stress F(1, 42) = 59.8, p =.000, but no change in NA following snacking at rest F(1, 40) = 0.005, p =.95.
5.1. Physiological stress measures
The speech task induced significant increases in SBP, F (1,41) = 208.71, p =.001, DBP, F(1,41) = 272.11, p =.001, and HR, F (1,41) = 109.35, p =.001. The math task also induced significant increases in SBP, F(1,41) = 185.62, p =.001, DBP, F(1,41) = 214.95, p =.001, and HR, F(1,41) = 54.18, p =.001. However, the TSST did not cause any overall changes in cortisol, F(1,37) = 1.1, p =.30. On rest day, cardiovascular measures did not change significantly over time (Ps > 0.05), but cortisol decreased from baseline through the rest period, F(1, 40) = 6.37, p =.016.
5.2. Subjective measures
The stress task induced significant increases from baseline rest in subjective stress, F(1, 41) = 36.89, p =.001, and NA, F(1, 41) = 42.99, p =.001, but no changes in hunger or desire to eat (Ps > 0.05). The rest period was associated with decreases from baseline rest in subjective stress ratings, F(1, 41) = 24.48, p =.001, and NA, F (1,41) = 13.61, p=.001, and with increases in hunger, F(1, 41) = 12.78, p =.001, and desire to eat, F(1, 41) = 5.83, p =.02.
There was no difference in snack intake between rest and stress days, F(1, 41) = 0.138, p =.71 (see Table 3).
Table 3.
Mean ( ± SD) demographic factors and variables of interest from our sample of undergraduate women (n = 43).
| Age | 19.5 ( ±1.3) |
| Body mass index | 23.4 ( ±5.8) |
| Participants self-reporting minority race (%) | 9 (23.7%) |
| Perceived life stress | 18.1 ( ±5.6) |
| Cognitive restraint | 13.6 ( ±3.8) |
| Stress-induced increase in negative affect | 19.5 ( ±6.2) |
| Stress-induced increase in cortisol (mg/dL) | 0.4 ( ±2.3) |
| Stress-induced increase in hunger | 0.20 ( ±1.1) |
| Rest day snack intake (g) | 78.4 ( ±57.4) |
| Stress day snack intake (g) | 76.5 ( ±54.7) |
| Decrease in negative affect after snacking under stress | 5.6 ( ±4.8) |
6. Discussion
In the current study, we attempted to explain how PLS and cognitive restraint increase snack intake under stress. In line with our first prediction, we found that greater PLS enhanced the hyperphagic effects of stress-induced NA. That is to say, stress-induced NA predicted greater snack intake for women with higher PLS. Cognitive restraint, however, was not a significant predictor in our linear regression model. These results highlight NA as a primary driver of stress-eating in women with higher PLS and build upon previous findings that stress-induced NA, but not cortisol and hunger, was associated with more snack intake under stress for women with high PLS [30]. For this group of women, the psychological and emotional components of eating may be more potent triggers for snacking under stress than physiological and homeostatic factors. These findings are clinically relevant, as individuals with high chronic or perceived life stress show more NA, depression, and emotional eating than individuals with low chronic or perceived life stress [30,31,51,54].
Our finding that NA is a stronger trigger for stress-eating in women with higher PLS may be explained by greater reductions in NA after stress-eating (i.e. emotional relief). The aversive state reduction hypothesis posits that individuals eat comfort food when stressed in order to gain relief from negative consequences of the stress response [42]. Eating comfort food in response to stress activates dopamine release in the mesolimbic reward pathway, leading to feelings of pleasure and reduced activity in the central stress response network [16]. Dallman and colleagues [9] suggest that when stress leads to comfort food intake, the association between feeling stressed and subsequent stress relief upon eating palatable foods is strengthened. This learned association likely promotes future stress-eating as a form of self-medication [9–11], and may be a result of experience-dependent synaptic plasticity in the basal ganglia, the home of habit [16,22].
Chronic and perceived life stress may amplify the rewarding effects of eating palatable foods under stress, and thus enhance this learned response. Chronic stress in animals and humans is associated with heightened glucocorticoid release, leading to an increased drive for comfort food intake, and subsequent relief from the deleterious physiological and affective consequences of chronic stress [11,39,52]. For instance, women with high PLS report heightened emotional eating coupled with greater reductions in HPA axis responses to stress [51]. Greater emotional relief after stress-eating for women with more PLS may act as negative reinforcement, which strengthens the learned association between stress and eating palatable foods [9–11]. This hypothetical conditioning effect would enhance the hyperphagic effects of stress-induced NA and help to explain greater rates of obesity in women with chronic or perceived life stress [1,10,24]. Further studies are needed to directly test this proposed model of negative reinforcement learning.
An important methodological advancement of the current study was the use of continuous measures of PLS and cognitive restraint, as opposed to dichotomous categorization. Median split is a common statistical practice used to compare high and low groups, yet this dichotomization can lead to misleading results [36]. The use of continuous measures in our regression analyses prevented us from reporting inflated differences between arbitrary groups. A further strength of the current study was the use of a two-step model in our linear regression analyses, which enabled us to determine the unique impact of our main predictors on the outcome variables, above and beyond that of other factors related to stress-eating. Additionally, we employed a within-subjects methodological design to assess eating behavior on both rest and stress days, thereby allowing us to determine stress-specific effects. For instance, we were able to discern that snacking under stress induced greater reductions in NA than snacking at rest, controlling for the rise in NA due to stress. Thus, it is not snacking in general, but snacking in response to stress specifically, that is more strongly associated with ‘feeling better’. Finally, it is important to note that our analyses were only significant on the stress visit, and thus acute stress may be necessary to trigger the hyperphagic and reinforcing effects associated with NA in women with greater PLS.
Limitations of the current study include limited diversity in our sample, particularly the lack of participants who are obese or overweight. Chronic stress and obesity are highly comorbid [10] and interact to disrupt the HPA axis and metabolic feedback pathways that affect food consumption [54]. Our normal weight participants and limited BMI range precluded us from assessing how individual differences in PLS and cognitive restraint interact with BMI to increase snacking under stress, or whether our findings would be relevant in an obese or overweight sample. Furthermore, although our sample of undergraduate women had comparable PLS scores to those of caregivers of chronically ill children [51], the type of life stress experienced is likely to differ. We were not able to assess the nature of the stress and whether our results would replicate in community-dwelling participants whose life stress may differ qualitatively from that of undergraduate women.
Overall, we found that PLS enhanced the hyperphagic effects of stress-induced NA, which may be due to greater reductions in stress-induced NA upon snacking. Greater emotional relief upon stress-eating may strengthen the association between stress-induced NA and snacking for women with PLS. This enhanced conditioning effect may drive future episodes of stress-eating in response to NA, and may help to explain greater rates of obesity in those with high chronic or perceived life stress, but this needs to be tested in future studies with a more diverse sample. Further investigation in this area has the potential to inform clinical strategies for individuals with chronic or perceived life stress who are in treatment for obesity-related issues. Specifically, clinicians may encourage coping strategies that selectively target emotion regulation under stress for women in this population.
Footnotes
Declarations of interest
None.
References
- [1].Adam TC, Epel ES, Stress, eating and the reward system, Physiol. Behav. 91 (4) (2007) 449–458, 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- [2].American Psychological Association, Stress in America™ Generation Z, (2018), p. 11. [Google Scholar]
- [3].Appelhans BM, Pagoto SL, Peters EN, Spring BJ, HPA axis response to stress predicts short-term snack intake in obese women, Appetite 54 (1) (2010) 217–220, 10.1016/j.appet.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beck AT, Beamesderfer A, Assessment of depression: the depression inventory, Mod. Probl. Pharmacopsychiatry 7 (1974) 151–169. [DOI] [PubMed] [Google Scholar]
- [5].Bekhbat M, Neigh GN, Sex differences in the neuro-immune consequences of stress: focus on depression and anxiety, Brain Behav. Immun. (2017), 10.1016/j.bbi.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cardi V, Leppanen J, Treasure J, The effects of negative and positive mood induction on eating behaviour: a meta-analysis of laboratory studies in the healthy population and eating and weight disorders, Neurosci. Biobehav. Rev. 57 (2015) 299–309, 10.1016/j.neubiorev.2015.08.011. [DOI] [PubMed] [Google Scholar]
- [7].Cleobury L, Tapper K, Reasons for eating ‘unhealthy’ snacks in overweight and obese males and females, J. Hum. Nutr. Diet. 27 (4) (2014) 333–341, 10.1111/jhn.12169. [DOI] [PubMed] [Google Scholar]
- [8].Cohen S, Kamarck T, Mermelstein R, A global measure of perceived stress, J. Health Soc. Behav. 24 (4) (1983) 385–396. [PubMed] [Google Scholar]
- [9].Dallman MF, Stress-induced obesity and the emotional nervous system, Trends Endocrinol. Metab. 21 (3) (2010) 159–165, 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gomez F, Houshyar H, ... Manalo S, Chronic stress and obesity: A new view of “comfort food.”, Proc. Natl. Acad. Sci. U. S. A. 100 (20) (2003) 11696–11701, 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dallman MF, Pecoraro NC, la Fleur SE, Chronic stress and comfort foods: self-medication and abdominal obesity, Brain Behav. Immun. 19 (4) (2005) 275–280, 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- [12].de Wit LM, Fokkema M, van Straten A, Lamers F, Cuijpers P, Penninx BWJH, Depressive and anxiety disorders and the association with obesity, physical, and social activities, Depress. Anxiety 27 (11) (2010) 1057–1065, 10.1002/da.20738. [DOI] [PubMed] [Google Scholar]
- [13].Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R, Are stress eaters at risk for the metabolic syndrome? Ann. N. Y. Acad. Sci. 1032 (1) (2004) 208–210, 10.1196/annals.1314.022. [DOI] [PubMed] [Google Scholar]
- [14].Epel E, Lapidus R, McEwen B, Brownell K, Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior, Psychoneuroendocrinology 26 (1) (2001) 37–49, 10.1016/S03064530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- [15].Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, Mendes WB, More than a feeling: a unified view of stress measurement for population science, Front. Neuroendocrinol. 49 (2018) 146–169, 10.1016/j.yfrne.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Epel ES, Tomiyama AJ, Dallman MF, Stress and reward: neural networks, eating, and obesity, Food and addiction: A comprehensive handbook, 2012, pp. 266–272, , 10.1093/med:psych/9780199738168.003.0040. [DOI] [Google Scholar]
- [17].Evers C, Dingemans A, Junghans AF, Boevé A, Feeling bad or feeling good, does emotion affect your consumption of food? A meta-analysis of the experimental evidence, Neurosci. Biobehav. Rev. 92 (2018) 195–208, 10.1016/j.neubiorev.2018.05.028. [DOI] [PubMed] [Google Scholar]
- [18].Fay SH, Finlayson G, Negative affect-induced food intake in non-dieting women is reward driven and associated with restrained-disinhibited eating subtype, Appetite 56 (3) (2011) 682–688, 10.1016/j.appet.2011.02.004. [DOI] [PubMed] [Google Scholar]
- [19].Finch LE, Tomiyama AJ, Stress-Induced Eating Dampens Physiological and Behavioral Stress Responses, (2014), 10.1016/B978-0-12-4078697.00018-0. [DOI] [Google Scholar]
- [20].Finch Laura E., A.J. Tomiyama, Comfort eating, psychological stress, and depressive symptoms in young adult women, Appetite 95 (2015) 239–244, 10.1016/j.appet.2015.07.017. [DOI] [PubMed] [Google Scholar]
- [21].Geliebter A, Gibson CD, Hernandez DB, Atalayer D, Kwon A, Lee MI, ... Gluck ME, Plasma cortisol levels in response to a cold pressor test did not predict appetite or ad libitum test meal intake in obese women, Appetite 59 (3) (2012) 956–959, 10.1016/j.appet.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Graybiel AM, Habits, rituals, and the evaluative brain, Annu. Rev. Neurosci. 31 (2008) 359–387, 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- [23].Greeno CG, Wing RR, Stress-induced eating, Psychol. Bull. 115 (3) (1994) 444–464. [DOI] [PubMed] [Google Scholar]
- [24].Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, ... Epel E, What is eating you? Stress and the drive to eat, Appetite 58 (2) (2012) 717–721, 10.1016/j.appet.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Habhab S, Sheldon JP, Loeb RC, The relationship between stress, dietary restraint, and food preferences in women, Appetite 52 (2) (2009) 437–444, 10.1016/j.appet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- [26].Heatherton TF, Polivy J, Herman CP, Restraint and internal responsiveness: effects of placebo manipulations of hunger state on eating, J. Abnorm. Psychol. 98 (1) (1989) 89–92. [DOI] [PubMed] [Google Scholar]
- [27].Herman CP, Mack D, Restrained and unrestrained eating1, J. Pers. 43 (4) (1975) 647–660, 10.1111/j.1467-6494.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- [28].Karlsson J, Persson LO, Sjöström L, Sullivan M, Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study, Int. J. Obes. Relat. Metab. Disord. 24 (12) (2000) 1715–1725. [DOI] [PubMed] [Google Scholar]
- [29].Kirschbaum C, Pirke KM, Hellhammer DH, The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting, Neuropsychobiology 28 (1–2) (1993) 76–81, 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- [30].Klatzkin RR, Baldassaro A, Hayden E, The impact of chronic stress on the predictors of acute stress-induced eating in women, Appetite 123 (2018) 343–351, 10.1016/j.appet.2018.01.007. [DOI] [PubMed] [Google Scholar]
- [31].Klatzkin RR, Baldassaro A, Rashid S, Physiological responses to acute stress and the drive to eat: the impact of perceived life stress, Appetite 133 (2019) 393–399, 10.1016/j.appet.2018.11.019. [DOI] [PubMed] [Google Scholar]
- [32].La Fleur SE, Houshyar H, Roy M, Dallman MF, Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint, Endocrinology 146 (5) (2005) 2193–2199, 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- [33].Lattimore P, Caswell N, Differential effects of active and passive stress on food intake in restrained and unrestrained eaters, Appetite 42 (2) (2004) 167–173, 10.1016/j.appet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- [34].Lemmens SG, Rutters F, Born JM, Westerterp-Plantenga MS, Stress augments food ‘wanting’ and energy intake in visceral overweight subjects in the absence of hunger, Physiol. Behav. 103 (2) (2011) 157–163, 10.1016/j.physbeh.2011.01.009. [DOI] [PubMed] [Google Scholar]
- [35].Lowe MR, Kral TVE, Stress-induced eating in restrained eaters may not be caused by stress or restraint, Appetite 46 (1) (2006) 16–21, 10.1016/j.appet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- [36].MacCallum RC, Zhang S, Preacher KJ, Rucker DD, On the practice of dichotomization of quantitative variables, Psychol. Methods 7 (1) (2002) 19–40. [DOI] [PubMed] [Google Scholar]
- [37].Macht M, Mueller J, Immediate effects of chocolate on experimentally induced mood states, Appetite 49 (3) (2007) 667–674, 10.1016/j.appet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- [38].O’Connor DB, Jones F, Conner M, McMillan B, Ferguson E, Effects of daily hassles and eating style on eating behavior, Health Psychol. 27 (1 Suppl) (2008) S20–S31, 10.1037/0278-6133.27.1.S20. [DOI] [PubMed] [Google Scholar]
- [39].Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF, Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress, Endocrinology 145 (8) (2004) 3754–3762, 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- [40].Pool E, Brosch T, Delplanque S, Sander D, Stress increases cue-triggered “wanting” for sweet reward in humans, J. Exp. Psychol. Anim. Learn. Cogn. 41 (2) (2015) 128–136, 10.1037/xan0000052. [DOI] [PubMed] [Google Scholar]
- [41].Reichenberger J, Kuppens P, Liedlgruber M, Wilhelm FH, Tiefengrabner M, Ginzinger S, Blechert J, No haste, more taste: an EMA study of the effects of stress, negative and positive emotions on eating behavior, Biol. Psychol. 131 (2018) 54–62, 10.1016/j.biopsycho.2016.09.002. [DOI] [PubMed] [Google Scholar]
- [42].Robbins TW, Fray PJ, Stress-induced eating: fact, fiction or misunderstanding? Appetite 1 (2) (1980) 103–133, 10.1016/S0195-6663(80)80015-8. [DOI] [Google Scholar]
- [43].Rutledge T, Linden W, To eat or not to eat: affective and physiological mechanisms in the stress–eating relationship, J. Behav. Med. 21 (3) (1998) 221–240, 10.1023/A:1018784015771. [DOI] [PubMed] [Google Scholar]
- [44].Rutters F, Nieuwenhuizen AG, Lemmens SGT, Born JM, Westerterp-Plantenga MS, Acute stress-related changes in eating in the absence of hunger, Obesity (Silver Spring) 17 (1) (2009) 72–77, 10.1038/oby.2008.493. [DOI] [PubMed] [Google Scholar]
- [45].Schellekens H, Finger BC, Dinan TG, Cryan JF, Ghrelin signalling and obesity: at the interface of stress, mood and food reward, Pharmacol. Ther. 135 (3) (2012) 316–326, 10.1016/j.pharmthera.2012.06.004. [DOI] [PubMed] [Google Scholar]
- [46].Scott KA, Melhorn SJ, Sakai RR, Effects of chronic social stress on obesity, Curr. Obes. Rep. 1 (1) (2012) 16–25, 10.1007/s13679-011-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sinha R, Role of addiction and stress neurobiology on food intake and obesity, Biol. Psychol. 131 (2018) 5–13, 10.1016/j.biopsycho.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sinha R, Jastreboff AM, Stress as a common risk factor for obesity and addiction, Biol. Psychiatry 73 (9) (2013) 827–835, 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stone AA, Brownell KD, The stress-eating paradox: multiple daily measurements in adult males and females, Psychol. Health 9 (6) (1994) 425–436, 10.1080/08870449408407469. [DOI] [Google Scholar]
- [50].Stunkard AJ, Messick S, The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger, J. Psychosom. Res. 29 (1) (1985) 71–83. [DOI] [PubMed] [Google Scholar]
- [51].Tomiyama AJ, Dallman MF, Epel ES, Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women, Psychoneuroendocrinology 36 (10) (2011) 1513–1519, 10.1016/j.psyneuen.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tomiyama AJ, Finch LE, Cummings JR, Did that brownie do its job? Stress, eating, and the biobehavioral effects of comfort food, Emerging Trends in the Social and Behavioral Sciences, 2015, pp. 1–15, , 10.1002/9781118900772.etrds0324. [DOI] [Google Scholar]
- [53].Torres SJ, Nowson CA, Relationship between stress, eating behavior, and obesity, Nutrition 23 (11−12) (2007) 887–894, 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- [54].Tryon MS, DeCant R, Laugero KD, Having your cake and eating it too: a habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness, Physiol. Behav. 114–115 (2013) 32–37, 10.1016/j.physbeh.2013.02.018. [DOI] [PubMed] [Google Scholar]
- [55].Udo T, Grilo CM, McKee SA, Gender differences in the impact of stressful life events on changes in body mass index, Prev. Med. 69 (2014) 49–53, 10.1016/j.ypmed.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].van Strien T, Causes of emotional eating and matched treatment of obesity, Curr. Diab. Rep. 18 (6) (2018), 10.1007/s11892-018-1000-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].van Strien T, Ouwens MA, Engel C, de Weerth C, Hunger, inhibitory control and distress-induced emotional eating, Appetite 79 (2014) 124–133, 10.1016/j.appet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- [58].Wallis DJ, Hetherington MM, Stress and eating: the effects of ego-threat and cognitive demand on food intake in restrained and emotional eaters, Appetite 43 (1) (2004) 39–46, 10.1016/j.appet.2004.02.001. [DOI] [PubMed] [Google Scholar]
- [59].Wansink B, Cheney MM, Chan N, Exploring comfort food preferences across age and gender, Physiol. Behav. 79 (4–5) (2003) 739–747. [DOI] [PubMed] [Google Scholar]
- [60].Wardle J, Steptoe A, Oliver G, Lipsey Z, Stress, dietary restraint and food intake, J. Psychosom. Res. 48 (2) (2000) 195–202. [DOI] [PubMed] [Google Scholar]
- [61].Warne JP, Shaping the stress response: interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity, Mol. Cell. Endocrinol. 300 (1–2) (2009) 137–146, 10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- [62].Watson D, Clark LA, Tellegen A, Development and validation of brief measures of positive and negative affect: the PANAS scales, J. Pers. Soc. Psychol. 54 (6) (1988) 1063–1070. [DOI] [PubMed] [Google Scholar]
- [63].Wouters S, Jacobs N, Duif M, Lechner L, Thewissen V, Negative affective stress reactivity: the dampening effect of snacking, Stress. Health 34 (2) (2018) 286–295, 10.1002/smi.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Yau YHC, Potenza MN, Stress and eating behaviors, Minerva Endocrinol. 38 (3) (2013) 255–267. [PMC free article] [PubMed] [Google Scholar]