Abstract
Inflammation plays an important role in the pathogenesis of diabetic retinopathy. We have previously demonstrated the effect of cathepsin D (CD) on the mechanical disruption of retinal endothelial cell junctions and increased vasopermeability, as well as increased levels of CD in retinas of diabetic mice. Here, we have also examined the effect of CD on endothelial–pericyte interactions, as well as the effect of dipeptidyl peptidase-4 (DPP4) inhibitor on CD in endothelial–pericyte interactions in vitro and in vivo. Cocultured cells that were treated with pro-CD demonstrated a significant decrease in the expression of platelet-derived growth factor receptor-β, a tyrosine kinase receptor that is required for pericyte cell survival; N-cadherin, the key adherens junction protein between endothelium and pericytes; and increases in the vessel destabilizing agent, angiopoietin-2. The effect was reversed in cells that were treated with DPP4 inhibitor along with pro-CD. With pro-CD treatment, there was a significant increase in the phosphorylation of the downstream signaling protein, PKC-α, and Ca2+/calmodulin-dependent protein kinase II in endothelial cells and pericytes, which disrupts adherens junction structure and function, and this was significantly reduced with DPP4 inhibitor treatment. Increased CD levels, vasopermeability, and alteration in junctional-related proteins were observed in the retinas of diabetic rats, which were significantly changed with DPP4 inhibitor treatment. Thus, DPP4 inhibitors may be used as potential adjuvant therapeutic agents to treat increased vascular leakage observed in patients with diabetic macular edema.—Monickaraj, F., McGuire, P., Das, A. Cathepsin D plays a role in endothelial–pericyte interactions during alteration of the blood–retinal barrier in diabetic retinopathy.
Keywords: DPP4 inhibitor, endothelium, CI-MPR/IGF2R, CaMKII, PKC-α
Inflammation plays an important role in structural and molecular alterations of the blood–retinal barrier (BRB) in diabetic retinopathy and leakage from retinal blood vessels, which leads to diabetic macular edema (1–3). The exact molecular mechanism that underlies damage to retinal blood vessels is unknown. Subclinical, chronic inflammation and leukostasis results in the adhesion of leukocytes to the vascular endothelium via adhesion molecules that are present on the endothelium, and triggers the release of inflammatory cytokines, growth factors, and vascular permeability factors. We have previously shown monocyte trafficking to the retina in early diabetes in an animal model involving interaction of angiopoietin-2 (Ang2) and the chemokine, CCL2 (4, 5), which subsequently alters endothelial cell junctions, allowing for diapedesis of leukocytes into the retina and breakdown of the BRB.
It is well known that pericyte–endothelial interactions are necessary for the development and maintenance of a functional microcirculation in many different tissues (6). One of the first abnormalities in diabetes is pericyte dropout and subsequent capillary remodeling (7, 8). The degree of pericyte coverage of retinal capillaries is greater than that observed in other tissues and, thus, likely provides greater integrity to retinal microcirculation (9). We have previously shown that, in normal circumstances, retinal capillary pericytes help to maintain pericyte–endothelial contacts and the integrity of vascular barrier function via secretion of a lipid, sphingosine 1-phosphate (6).
Previous studies have demonstrated that cadherins are targeted by extracellular and cytoplasmic proteases, including metalloproteinases, elastase, and cathepsin G (10, 11), that could lead to the down-regulation of cell–cell adhesion. In an earlier study, we showed that the macrophage-derived factor, cathepsin D (CD), an aspartyl protease, increases endothelial cell permeability by disrupting the endothelial junctional barrier via increased Rho/ROCK-dependent cell contractility (12). Of importance, CD protein is increased in the retinas of diabetic mice and serum of patients with diabetic macular edema (12). Thus, CD may play an important role in the alteration of the BRB in diabetic retinopathy, and it can be used as a therapeutic target in the management of diabetic macular edema.
To further understand the involvement of CD in the loss of pericyte–endothelial junction integrity, we studied the effect of CD on endothelial–pericyte interactions. CD functions in this context by binding to the cation-independent mannose 6 phosphate receptor (CIMPR) (13). Similarly, dipeptidyl peptidase-4 (DPP4), which plays an important role in glucose metabolism, interacts with the same receptor via a mannose-6-phosphate (M6P) recognition moiety (14–16).
Therefore, we hypothesize that the exposure of endothelial cells and pericytes to CD impairs the ability of these cells to interact with each other via changes in PKC-α and Ca2+/calmodulin-dependent protein kinase II (CaMKII) downstream signaling, receptor expression, and junctional protein alterations. In the present study, we examined the effect of cathepsin D on endothelium–pericyte interactions and the effect of DPP4 inhibitors on this interaction and prevention of alteration of the BRB.
MATERIALS AND METHODS
Animal model of diabetes
Diabetes was induced in male Sprague-Dawley rats with an intraperitoneal injection of 50 mg/kg streptozotocin. Age-matched, nondiabetic control animals received injections of an equal volume of citrate buffer only. Animals with plasma glucose concentrations of >250 mg/dl were considered diabetic. Blood glucose levels and body weight were monitored regularly. The total study period was 2 mo. At the start of the second month of diabetes, one group of diabetic animals (n = 6) were treated with DPP4 inhibitor (100 μg/kg body weight; EMD Millipore, Billerica, MA, USA) every other day for 30 d (15 injections). All animal protocols were approved by the Animal Care and Use Committee at the New Mexico Veterans Affairs and University of New Mexico Health Sciences Center.
Cell culture
Human retinal microvascular endothelial cells (HRECs; ACBRI-181) and human retinal microvascular pericytes (HRPs; ACBRI-183) were obtained from Cell Systems (Kirkland, WA, USA). Green fluorescent protein (GFP)–positive HRP cells were obtained from Angio-Proteomie (Boston, MA, USA). Cells were used in all experiments between passages 5 and 8. Concentration of DPP4 inhibitor for in vitro conditions was 250 nM.
Immunofluorescence
HREC and HRP cells were characterized and confirmed by immunofluorescence using von Willebrand factor, an endothelial cell marker, Desmin (8, 17), and neural/glial antigen 2 (8, 18, 19) markers for pericytes. In brief, HREC and HRP cells were washed twice with 1× PBS and fixed with 3.7% paraformaldehyde for 10 min at room temperature, then permeabilized with 0.1% Triton X-100 for 5 min at room temperature, blocked with 10% normal goat serum, and incubated with primary von Willebrand factor Ab (1:100; Thermo Fisher Scientific, Waltham, MA, USA), Desmin (1:50), and neural/glial antigen 2 (1:50; both from Abcam, Cambridge, MA, USA), followed by fluorescently labeled secondary Abs (Thermo Fisher Scientific). Cells were washed twice with PBS-Tween, stained with Draq-5 nuclear stain, and coverslipped with Prolong Gold (both from Thermo Fisher Scientific). Slides were imaged by confocal microscopy (Leica TCS SP5; Leica Microsystems, Buffalo Grove, IL, USA; Supplemental Fig. 1).
Electrical cell-substrate impedance sensing
Monolayer permeability was determined by using an electrical cell substrate impedance sensing system from Applied Biophysics (Troy, NY, USA). HRECs (1 × 105) were plated into fibronectin-coated multiwell chambers (8W10E+; Applied Biophysics) and grown for 16 h until maximum resistance was attained (∼1200 Ω). Cells were treated as described in Results, and changes in resistance were monitored for up to 18 h. Resistance values for multiple wells were normalized to identical starting resistance values and averaged and presented as normalized resistance over time.
Western blot
Cells/tissues were lysed using RIPA buffer, sonicated, and incubated on ice for 10 min. Lysates were collected by centrifugation at 12,000 rpm for 10 min at 4°C. Protein concentration of each sample was estimated by using the Bradford assay method. Protein samples were separated in precast TGX gels (Bio-Rad, Carlsbad, CA, USA), transferred to blotting membranes, and probed with the specific primary Abs, N-Cad (1:500), platelet-derived growth factor receptor-β (PDGFRβ; 1:2500), cerebral cavernous malformation 3 (CCM3; 1:500), albumin (1:500; Abcam), PKCα (1:1000), phospho-PKCα (1:1000), CaMKII (1:500), and phospho-CaMKII (1:500; Thermo Fisher Scientific), followed by incubation with fluorescently labeled secondary Ab (Li-Cor Biosciences, Lincoln, NE, USA). Images were collected on the Li-Cor Odyssey. Protein levels were normalized to β-tubulin.
RT-PCR
Total RNA was isolated from treated cells (Direct-zol RNA MiniPrep; Zymo Research, Irvine, CA, USA), converted to cDNA (RT2 First Strand Kit; Qiagen, Germantown, MD, USA), and analyzed by using specific probes of the TaqMan assay (Thermo Fisher Scientific) using the 7500 ABI Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). Relative levels of mRNA were determined by using the Ct method with normalization to 18s mRNA.
Endothelial–pericyte binding assay
HREC and HRP binding assay was performed using GFP-positive HRP cells and normal HRECs. Cells were treated with 25 μg pro-CD (Abcam) with or without DPP4. In brief, HRECs and GFP-HRPs were treated separately for 16 h, followed by the addition of GFP-HRPs to the top of a preformed HREC monolayer. Cells were incubated for 24 h and subsequently washed with room temperature PBS to remove unbound cells. Before fluorescence reading, cells were imaged by using the Evos fluorescence cell imaging system (Life Technologies, Carlsbad, CA, USA). Cells were lysed, and the fluorescence intensity was measured by using a spectrofluorimeter at 395-nm excitation and 509-nm emission wavelengths.
ELISA-based receptor-ligand binding assay
An ELISA-based receptor-ligand binding assay was performed by coating 96-well plates with recombinant IGF2 receptor (IGF2R) protein (R&D Systems, Minneapolis, MN, USA) that was dissolved in carbonate coating buffer at 4°C overnight. Plates were rinsed and blocked with 5% bovine serum albumin for 2 h at room temperature. Ligands and inhibitors were added in respective wells, incubated, washed, and incubated with respective primary Abs. Wells were rinsed and probed with horseradish peroxidase–conjugated secondary Ab. 3,3′,5,5′-Tetramethylbenzidine substrate was added and incubated for 30 min at room temperature. The reaction was stopped by adding stopping solution. Plates were immediately read at 450 nm.
ELISA
CD levels were measured in rat retinas by ELISA (Elabscience, Bethesda, MD, USA) according to the manufacturer’s protocol.
Statistical methods
For all quantitative experiments, statistical analyses were performed with an unpaired Student’s t test or a 1-way ANOVA (Prism; GraphPad Software, La Jolla, CA, USA). Differences indicated by ANOVA were compared with the Newman-Keuls test. Values of P <0.05 were considered significant.
RESULTS
Pro-CD decreases endothelial permeability and pericyte PDGFRβ and N-Cad expression
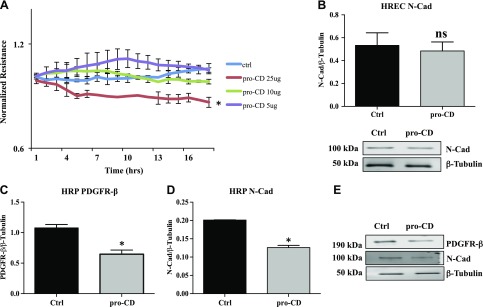
Electrical cell substrate impedance sensing was used to determine the effect of pro-CD on the permeability of HREC. Recombinant pro-CD at a concentration of 25 μg induced a significant reduction in resistance compared with cells that were grown with normal growth medium (P = 0.02). Pro-CD at lower concentrations (5 and 10 μg) did not demonstrate significant differences compared with cells that were grown with normal growth medium (Fig. 1A). On the basis of this observation, we used 25 μg as our treatment concentration in all subsequent experiments.
Figure 1.
Reduction in endothelial permeability and pericyte PDGFRβ and N-Cad expression with pro-CD. A) Normalized resistance of HREC monolayers that were treated with recombinant pro-CD. A significant decrease is observed in the resistance of cells treated with 25 μg of recombinant pro-CD compared with HREC cells that were treated with normal growth medium (ctrl) or recombinant pro-CD of 5 and 10 μg concentrations. *P = 0.02. B) N-Cad levels in pro-CD–treated HRECs as measured by Western blot. No significant difference was observed compared with cells that were grown in normal growth medium (ctrl). Representative Western blot images of N-Cad and β-tubulin of HRECs are shown at the bottom of the graph. C) PDGFRβ levels in pro-CD–treated HRPs measured by Western blot were significantly reduced compared with cells that were grown in normal growth medium.*P = 0.02. D) N-Cad levels in pro-CD–treated HRPs were significantly reduced compared with cells that were grown in normal growth medium (ctrl). *P = 0.004. E) Representative Western blot images of PDGFRβ, N-Cad, and β-tubulin of HRPs. ns, not significant. Data are presented as means ± sd.
For the proper function of the BRB, the stability of endothelial–pericyte junctions depends on the appropriate expression of PDGFRβ and N-Cad in HRECs and HRPs. PDGFRβ is not expressed in endothelial cells in culture, and the expression of N-Cad was not altered in HRECs that were exposed to pro-CD compared with control cells (Fig. 1B). In the case of pericytes (HRPs), the expression of PDGFRβ was significantly reduced with pro-CD treatment compared with cells that were grown in normal growth medium (P = 0.02; Fig. 1C). Similarly, the protein expression of N-Cad in pericytes that were treated with pro-CD demonstrated a significant reduction compared with untreated cells (P = 0.004; Fig. 1D). Representative Western blot images of PDGFRβ, N-Cad, and β-tubulin are shown.
Expression of PDGFRβ, N-Cad, and Ang2 in the coculture setup (HRECs + HRP) in response to pro-CD and DPP4 inhibitor
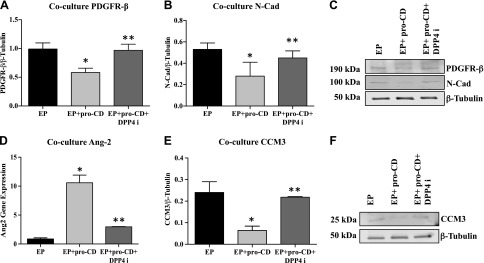
HREC and HRP cocultures were established to more closely mimic the physiologic condition. Cells were treated with pro-CD and the DPP4 inhibitor. Protein expression of PDGFRβ and N-Cad was significantly reduced in cells that were treated with pro-CD compared with untreated cells (P = 0.05 and 0.03). Addition of the DPPP4 inhibitor rescued the expression of PDGFRβ and N-Cad in these cells (Fig. 2A, B). Representative Western blot images of PDGFRβ, N-Cad, and β-tubulin are shown in Fig. 2C.
Figure 2.
DPP4 inhibitor increases the expression of PDGFRβ, N-Cad, CCM3, and decreases the expression of Ang2 in a coculture of the endothelial–pericyte (EP) model. A) Protein expression as measured by Western blot of PDGFRβ levels in EP treated with pro-CD (EP + pro-CD) is significantly decreased compared with EP grown in normal growth medium. *P = 0.05. DPP4 inhibitor (DPP4i)–treated cells (EP + pro-CD + DPP4i) significantly increased PDGFRβ expression compared with EP + pro-CD. **P = 0.05. B) N-Cad expression in EP + pro-CD was significantly decreased compared with EP grown in normal growth medium. *P = 0.03. DPP4i–treated cells (EP + pro-CD + DPP4i) significantly increased N-Cad expression compared with EP + pro-CD. **P = 0.05. C) Representative Western blot images of PDGFRβ, N-Cad, and β-tubulin of EP. D) Ang2 gene expression levels as measured by RT-PCR in EP + pro-CD was significantly higher compared with EP alone. *P = 0.009. Levels were significantly reduced in EP + pro-CD + DPP4i compared with EP + pro-CD. **P = 0.01. E) CCM3 protein levels by Western blot shows a significantly reduced expression in EP + pro-CD compared with EP. *P = 0.02. Levels were significantly increased in EP + pro-CD + DPP4i compared with EP + pro-CD. **P = 0.01. F) Representative Western blot images of CCM3 and β-tubulin of EP. Data are presented as means ± sd.
We next examined the levels of Ang2 and CCM3. Cells that were treated with pro-CD demonstrated significantly increased levels of Ang2 compared with untreated cells (P = 0.009). Levels of Ang2 were significantly reduced when cocultured cells were treated with the DPP4 inhibitor (P = 0.01; Fig. 2D) in addition to pro-CD. In contrast, the protein level of CCM3 was significantly reduced in pro-CD–treated cells compared with untreated cells (P = 0.02), and DPPP4 inhibitor treatment resulted in significantly increased levels of CCM3 (P = 0.01; Fig. 2E). Representative Western blot images of CCM3 and β-tubulin are shown in Fig. 2F.
Endothelial–pericyte binding and ligand-receptor interactions are affected by pro-CD and the DDP4 inhibitor
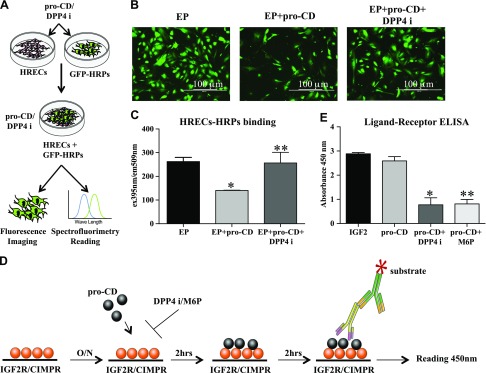
We next examined the effect of pro-CD and DPP4 inhibitor on endothelial–pericyte interactions that help to maintain BRB function. Cells that were treated with pro-CD had significantly less binding of HRPs to HRECs, as evidenced by a significant decrease in fluorescence intensity compared with untreated cells (P = 0.01). DPP4 inhibitor treatment significantly improved binding between the cells, despite the presence of pro-CD (P = 0.05; Fig. 3C).
Figure 3.
Endothelial and pericyte binding and receptor-ligand binding. A) Schematic flow chart of the endothelial–pericyte (EP) binding experiment. B) Representative fluorescence images of GFP-HRPs of EP, EP + pro-CD, and EP + pro-CD + DPP4 inhibitor (DPP4i). Original magnification, ×4. Scale bars, 100 μm. C) Fluorescence intensity as measured by spectrofluorimetry is significantly reduced in EP + pro-CD compared with EP. Intensity is significantly improved in EP + pro-CD + DPP4i cells compared with EP + pro-CD. *P = 0.01, **P = 0.05. D) A pictorial representation of the ELISA procedure of the ligand-receptor binding assay. E) Absorbance of ligand binding as measured by spectrophotometer. Absorbance of IGF2 (positive control) is similar with pro-CD. Absorbance in the wells with pro-CD + DPP4i and M6P is significantly reduced compared with pro-CD alone. Data are presented as means ± sd. *P = 0.0001, **P = 0.0001.
We have previously shown that the addition of M6P with CD stabilizes endothelial resistance (12). In the current study, we have shown the direct interaction of the ligand and the receptor using an ELISA-based method (Fig. 3D). The level of pro-CD binding to IGF2R in an in vitro system is similar to that observed with the positive control, IGF2. These levels were significantly reduced when DPP4 inhibitor (P = 0.0001) or M6P (P = 0.0001) were added to the wells with pro-CD (Fig. 3E).
Induction of PKC-α/CaMKII signaling by pro-CD, and inhibition by DPP4 inhibitor
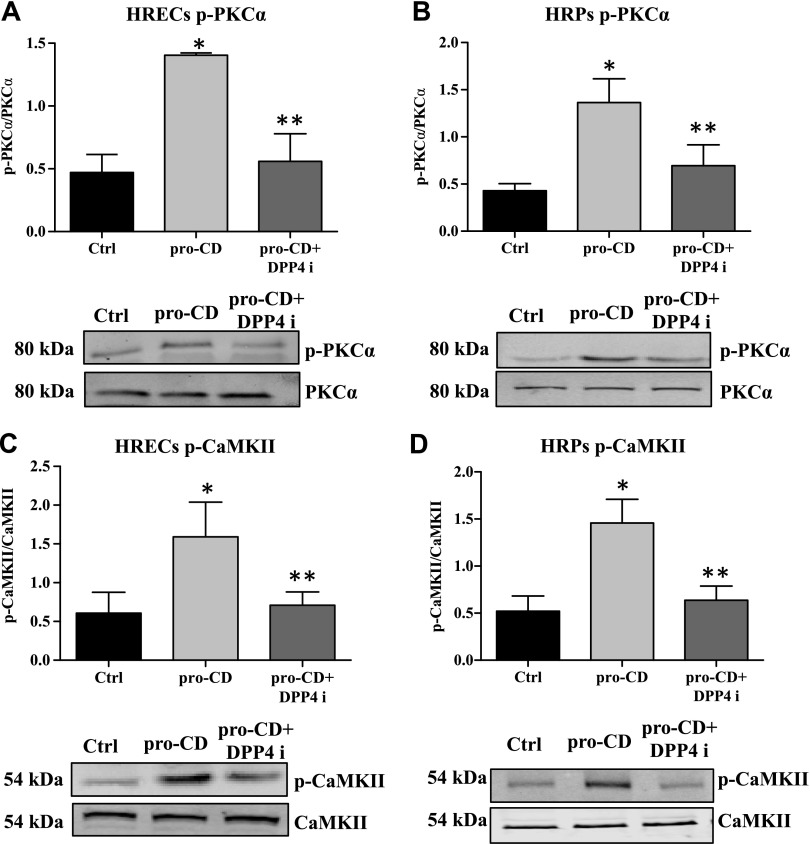
To determine the downstream signaling mechanisms of pro-CD that are involved in the alteration of PDGFRβ and N-Cad, we examined the phosphorylation status of PKC-α and CaMKII in HRECs and HRPs. The phosphorylation status of PKC-α was significantly increased in pro-CD–treated HRECs (P = 0.003) and HRPs (P = 0.007), and phosphorylation levels were significantly reduced by the addition of DPP4 inhibitor in both cell types (HRECs: P = 0.01; and HRP: P = 0.05; Fig. 4A, B). Phosphorylation of CaMKII was also significantly increased with pro-CD treatment in both HRECs (P = 0.03) and HRPs (P = 0.03), and this was significantly reduced by the addition of DPP4 inhibitor (HRECs: P = 0.03; and HRP: P = 0.05; Fig. 4C, D).
Figure 4.
Dephosphorylation of PKC-α/CaMKII by DPP4 inhibitor (DPP4i). Phosphorylation status of PKC-α/CaMKII in HRECs and HRPs as measured by Western blot. A) The phosphorylation level of PKC-α is significantly higher in pro-CD–treated HRECs compared with untreated cells. Pro-CD + DPP4i–treated cells have a significantly reduced phosphorylation form of PKC-α compared with pro-CD alone. *P = 0.003, **P = 0.01. B) In HRPs, the level of phosphor-PKC-α is significantly higher in pro-CD–treated cells compared with untreated cells. DPP4i treatment significantly reduced the level of phospho-PKC-α compared with pro-CD alone. *P = 0.007, **P = 0.05. C) The phosphorylation level of CaMKII is significantly higher in pro-CD–treated HRECs compared with untreated cells. Pro-CD + DPP4i cells have significantly reduced phospho-CaMKII compared with pro-CD alone. *P = 0.03, **P = 0.03. D) In HRPs, the level of phospho-CaMKII is significantly higher in pro-CD–treated cells compared with untreated cells. *P = 0.03. DPP4i treatment significantly reduced the level of phospho-CaMKII compared with pro-CD alone. Representative Western blots of total and phosphorylation forms of the proteins are placed at the bottom of the respective graphs. Data are presented as means ± sd. **P = 0.05.
CD levels and vasopermeability in the retina were reduced by DDP4 inhibitor treatment in diabetic rats
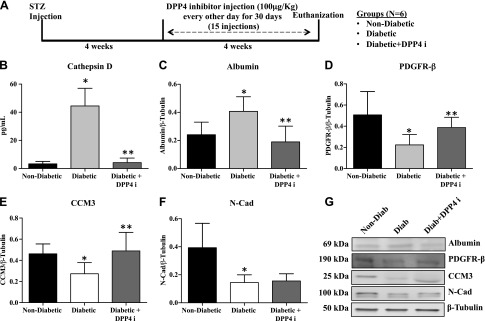
In vivo experiments were performed to determine whether DDP4 inhibitor has the potential to reduce the production of CD in diabetic rats as well as to assess its effect on retinal vascular permeability. A schematic representation of the study design is shown in Fig. 5A. Retinas of diabetic rats demonstrated significantly increased levels of CD compared with those from nondiabetic rats (P = 0.0001). Treatment of diabetic animals with DPP4 inhibitor resulted in significantly decreased levels of CD (P = 0.0003) as measured by ELISA (Fig. 5B). Retinal vascular permeability, as measured by albumin levels, was significantly increased compared with nondiabetic animals (P = 0.02). Treatment with DPP4 inhibitor led to significantly decreased levels of albumin (P = 0.01; Fig. 5C). Retinas from diabetic animals also demonstrated a significant decrease in the expressions of PDGFRβ (P = 0.01) and CCM3 (P = 0.02) compared with nondiabetic animals. Levels of these proteins were reversed with drug treatment (Fig. 5D, E). In the case of the adherens junction protein, N-Cad, we observed a significant decrease in diabetic retinas compared with nondiabetic retinas (P = 0.009); however, DPP4 inhibitor did not induce any significant change in the level of N-Cad (Fig. 5F). Representative Western blot images of albumin, PDGFRβ, CCM3, N-Cad, and β-tubulin of the animal groups are shown in Fig. 5G.
Figure 5.
DPP4 inhibitor (DPP4i) treatment reduces the increased permeability of retinal vasculature in diabetic rats. All protein levels were measured in the retinas of nondiabetic, diabetic rats, and diabetic rats that were treated with DPP4i. A) In vivo study design, timeline, and groups. B) CD levels as measured by ELISA showed a significant increase in retinas of diabetic rats compared with nondiabetic rats. DPP4i–treated diabetic rats had significantly less CD compared with diabetic rats. **P = 0.0003. C) Albumin levels as measured by Western blot to assess retinal vascular permeability demonstrated a significant increase in diabetic rats compared with nondiabetic rats. Drug treatment significantly reduced the albumin levels compared with untreated diabetic animals. *P = 0.02, **P = 0.01. D) PDGFRβ levels were significantly reduced in diabetic rats compared with nondiabetic rats. PDGFRβ levels were significantly increased in DPP4i–treated rats compared with untreated diabetic rats. *P = 0.01, **P = 0.05. E) CCM3 level is significantly reduced in the diabetic rats compared with the nondiabetic rats (*P = 0.02). CCM3 levels were significantly improved with drug treatment. **P = 0.05. F) The protein level of N-Cad was significantly reduced in diabetic animals compared with nondiabetic animals. There was no significant change in N-Cad expression in drug-treated animals compared with diabetic animals. *P = 0.009. G) Representative Western blot images of respective proteins. Data are presented as means ± sd (n = 6 in each group).
DISCUSSION
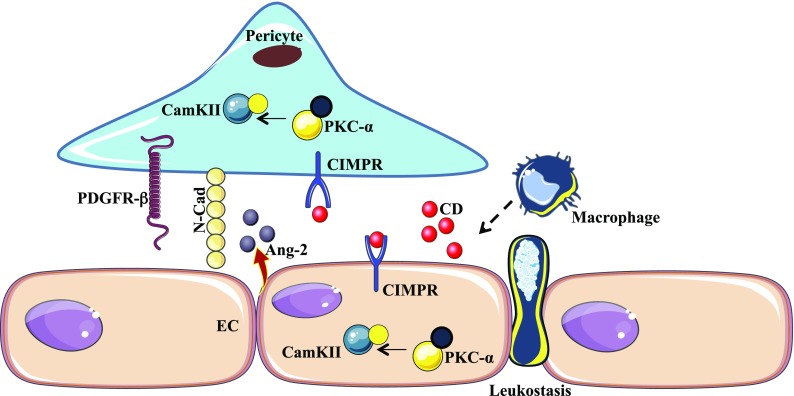
In our previous study, we reported the effect of the macrophage-derived factor, CD, on the mediation of increased endothelial cell permeability in the retinas of diabetic mice (12). Here, we have demonstrated the effect of pro-CD on the endothelial–pericyte interaction via the modulation of PDGFRβ, N-Cad, and Ang2. Cocultured cells that were treated with pro-CD showed a significant decrease in the expression of PDGFRβ ανδ N-Cad, and an increase in Ang2. Addition of DPP4 resulted in significantly decreased CD levels, decreased vasopermeability, and an alteration in junctional-related proteins in the retinas of diabetic rats. A conceptual model that summarizes the effect of CD in endothelial–pericyte interactions is shown in Fig. 6.
Figure 6.
Schematic diagram highlighting the cause and effect of CD in altered endothelial–pericyte interactions. Increased monocyte trafficking and the subsequent differentiation into macrophages leads to the secretion of CD. CD then binds to CIMPR on the endothelial and pericyte cell surface, activating the downstream signaling of PKC-α/CaMKII, which destabilizes endothelial–pericyte interactions. This leads to increased endothelial permeability and alteration of the BRB.
Leukocytes produce reactive oxygen species and inflammatory cytokines, which leads to increased vascular permeability (2, 20). Studies from our group have reported increased leukostasis and monocyte trafficking to the retinas of diabetic mice, as well as the effect of activated monocyte conditioned media on endothelial cells in vitro (5). The role of pro-CD in the alteration of endothelial–pericyte interactions has been demonstrated in the present study by the alteration of cell–cell interactions via changes in the expression of PDGFRβ, N-Cad, and the destabilizing agent, Ang2. Corroborating these results, Takashima et al. (21) have reported on the proteolytic effect of CD on PDGFRβ in hepatic myofibroblasts. There is an inverse correlation between Ang2 and PDGFRβ in the maintenance of stable vasculature and a reduction in retinal leakiness (22). Ang2, which is mainly produced by activated endothelial cells, antagonizes Ang1 and destabilizes blood vessels by promoting pericyte detachment and initiating endothelial cell sprouting (23–25). Tie2 and its ligand, Ang2, has been shown to increase concomitantly with protease activate receptor 2 during developmental retinal neovascularization (26). In our study, pro-CD seems to play a part in altering the junctional properties of endothelial cells and pericytes by deregulating the levels of Ang2 and PDGFRβ expression via CIMPR.
A recent study by Zhou et al. (27) has shown the deficiency of CCMs, particularly CCM3, in endothelial cells, which augments exocytosis and the secretion of Ang2, further leading to destabilized endothelial cell junctions, enlarged lumen formation, and endothelial cell–pericyte dissociation. Our in vitro and in vivo studies have demonstrated a decrease in CCM3 in response to pro-CD and in streptozotocin (STZ)-induced diabetic animals, which also show a significant increase in the CD levels. Additional molecular and in vivo studies are required to determine if there are any related interactions between CCM3 and Ang2 that link PDGFRβ to the alteration of the BRB.
Proteases have been shown to degrade cadherin proteins, which leads to vascular remodeling and permeability (12, 28). N-Cad is an essential junctional protein that helps to maintain the endothelial–pericyte interaction (29, 30). In the current study, we have demonstrated that the expression of N-Cad was significantly decreased in pro-CD–treated cells and diabetic rat retinas. The in vitro binding assay also demonstrated a decrease in endothelial–pericyte interaction with pro-CD. The cytoplasmic domain of cadherin interacts with β-catenin, a structural adaptor that links to the cytoskeleton for cell–cell adhesion (31). Reiss et al. (32) have reported that the regulation of proteases in the cleavage of N-Cad leads to the loss of β-catenin. On the basis of this evidence, we proposed that reduced N-Cad expression may be mediated by pro-CD, leading to the degradation of N-Cad and its associated cytoskeletal modifications. We have previously demonstrated that endothelial cells that are treated with CD display activation of the Rho/ROCK pathway, endothelial cell contractility, and vascular permeability (12, 33).
In the present study, we report the possible downstream signaling mechanisms of pro-CD that lead to the alteration of endothelial–pericyte interactions. A study by Mathieu et al. (13) reported that CD interacts with the surface of breast cancer cells via an M6P-dependent mechanism. CD transport is mediated by CIMPR (33), referred to as IGF2R. Both CD and IGF2 can bind to CIMPR via 2 M6P binding sites (34–37). We have previously shown that excess addition of M6P with CD prevents vascular permeability in endothelial cells. The present study helps to further confirm the interaction of CD with CIMPR.
The downstream effects of pro-CD may be a result of the activation of PKC-α/CaMKII, as treatment of cells with pro-CD increases the phosphorylation of PKC-α/CaMKII. A previous study in cardiomyoblasts demonstrated the phosphorylation of PKC-α/CaMKII in IGF2R/CIMPR in response to treatment with IGF2, leading to hypertrophy (38). CaMKII also has been shown to contribute to the iNOS-related death of pericytes in diabetic retinas (39).
The rationale for using DPP4 inhibitor in our study is evident because of the fact that both CD and DPP4 bind to CIMPR/IGF2R in the M6P domain (40, 41). DPP4 inhibitors are oral hypoglycemic drugs that are currently used to treat patients with type 2 diabetes. The DPP4 inhibitor could reverse the effects of CD both in the cell culture model and in the diabetic animal model. Studies have demonstrated the effect of DDP4 inhibitor in the protection of diabetic retinas in animal models (42–45). The efficacy of the DDP4 inhibitor has been studied in other diabetic complications, such as diabetic nephropathy (46–50), neuropathy (51, 52), and foot ulcers (53). DPP4 inhibitors have been shown to have antioxidant, anti-inflammatory, and antifibrotic properties (54). The present study is possibly the first to reveal a novel mechanism of the DPP4 inhibitor in relation to CD in the alteration of the BRB.
The STZ-induced diabetic model used in our study represents type 1 diabetes only; however, the lesions observed in this model—capillary basement membrane thickening, endothelial cell damage, pericyte loss, ganglion cell loss, acellular capillaries, and BRB or increased vascular leakage—are similar to those observed in type 2 diabetes models (db/db mice, ZDF rats, GK rats, and OLETF rats) (55, 56). Because of the similarity of the lesions in two types of diabetes models, we chose to use only one animal model (type 1) in our study. Moreover, the clinical features of human diabetic retinopathy (DR)—microaneurysms, retinal hemorrhages, hard exudates, retinal edema, cotton-wool spots, and retinal neovascularization—are similar in both type 1 and 2 diabetes, although the incidence of any DR or sight-threatening DR is higher in type 1 diabetes than in type 2 diabetes (57, 58). In addition, the incidence of diabetic macular edema has been reported to be similar in both type 1 and 2 diabetes (57).
In summary, increased CD levels in diabetic retinas lead to decreases of N-Cad and PDGFRβ, thereby resulting in the alteration of endothelial–pericyte interactions and alterations of the BRB. Treatment with the drug, DPP4 inhibitor, can significantly diminish the increased retinal vascular permeability. Thus, DPP4 inhibitor may be used as a potential adjuvant therapeutic strategy for the treatment of increased retinal vascular leakage as seen in patients with diabetic macular edema.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Eye Institute Grant R01-EY022327, and Veterans Affairs (VA) Merit Review Award IO 1BX001801. The authors thank Alison Wagner and Susan Baca (New Mexico VA Animal Facility) for their help in the animal study, and Tamara Howard (New Mexico Health Sciences Centre, University of New Mexico) for her technical assistance in confocal microscopy and immunofluorescence. The authors declare no conflicts of interest.
Glossary
- Ang2
angiopoietin-2
- BRB
blood–retinal barrier
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CCM
cerebral cavernous malformation
- CD
cathepsin D
- CIMPR
cation-independent mannose-6-phosphate receptor
- DPP4
dipeptidyl peptidase-4
- DR
diabetic retinopathy
- GFP
green fluorescent protein
- HREC
human retinal microvascular endothelial cell
- HRP
human retinal pericyte
- IGF2R
IGF2 receptor
- M6P
mannose-6-phosphate
- N-Cad
N-cadherin
- PDGFRβ
platelet-derived growth factor receptor-β
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
F. Monickaraj, P. McGuire, and A. Das designed research and analyzed data; F. Monickaraj performed research; F. Monickaraj wrote the paper; and P. McGuire and A. Das edited the paper.
REFERENCES
- 1.El-Asrar A. M. (2012) Role of inflammation in the pathogenesis of diabetic retinopathy. Middle East Afr. J. Ophthalmol. 19, 70–74 10.4103/0974-9233.92118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang J., Kern T. S. (2011) Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 30, 343–358 10.1016/j.preteyeres.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamis A. P. (2002) Is diabetic retinopathy an inflammatory disease? Br. J. Ophthalmol. 86, 363–365 10.1136/bjo.86.4.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangasamy S., Srinivasan R., Maestas J., McGuire P. G., Das A. (2011) A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 52, 3784–3791 10.1167/iovs.10-6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rangasamy S., McGuire P. G., Franco Nitta C., Monickaraj F., Oruganti S. R., Das A. (2014) Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One 9, e108508 10.1371/journal.pone.0108508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuire P. G., Rangasamy S., Maestas J., Das A. (2011) Pericyte-derived sphingosine 1-phosphate induces the expression of adhesion proteins and modulates the retinal endothelial cell barrier. Arterioscler. Thromb. Vasc. Biol. 31, e107–e115 10.1161/ATVBAHA.111.235408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltramo E., Porta M. (2013) Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr. Med. Chem. 20, 3218–3225 10.2174/09298673113209990022 [DOI] [PubMed] [Google Scholar]
- 8.Armulik A., Abramsson A., Betsholtz C. (2005) Endothelial/pericyte interactions. Circ. Res. 97, 512–523 10.1161/01.RES.0000182903.16652.d7 [DOI] [PubMed] [Google Scholar]
- 9.Frank R. N., Turczyn T. J., Das A. (1990) Pericyte coverage of retinal and cerebral capillaries. Invest. Ophthalmol. Vis. Sci. 31, 999–1007 [PubMed] [Google Scholar]
- 10.Dejana E., Orsenigo F., Lampugnani M. G. (2008) The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 121, 2115–2122 10.1242/jcs.017897 [DOI] [PubMed] [Google Scholar]
- 11.Dreymueller D., Pruessmeyer J., Groth E., Ludwig A. (2012) The role of ADAM-mediated shedding in vascular biology. Eur. J. Cell Biol. 91, 472–485 10.1016/j.ejcb.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 12.Monickaraj F., McGuire P. G., Nitta C. F., Ghosh K., Das A. (2016) Cathepsin D: an Mϕ-derived factor mediating increased endothelial cell permeability with implications for alteration of the blood-retinal barrier in diabetic retinopathy. FASEB J. 30, 1670–1682 10.1096/fj.15-279802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathieu M., Rochefort H., Barenton B., Prebois C., Vignon F. (1990) Interactions of cathepsin-D and insulin-like growth factor-II (IGF-II) on the IGF-II/mannose-6-phosphate receptor in human breast cancer cells and possible consequences on mitogenic activity of IGF-II. Mol. Endocrinol. 4, 1327–1335 10.1210/mend-4-9-1327 [DOI] [PubMed] [Google Scholar]
- 14.Ikushima H., Munakata Y., Ishii T., Iwata S., Terashima M., Tanaka H., Schlossman S. F., Morimoto C. (2000) Internalization of CD26 by mannose 6-phosphate/insulin-like growth factor II receptor contributes to T cell activation. Proc. Natl. Acad. Sci. USA 97, 8439–8444 10.1073/pnas.97.15.8439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikushima H., Munakata Y., Iwata S., Ohnuma K., Kobayashi S., Dang N. H., Morimoto C. (2002) Soluble CD26/dipeptidyl peptidase IV enhances transendothelial migration via its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cell. Immunol. 215, 106–110 10.1016/S0008-8749(02)00010-2 [DOI] [PubMed] [Google Scholar]
- 16.Ghosh P., Dahms N. M., Kornfeld S. (2003) Mannose 6-phosphate receptors: new twists in the tale. Nat. Rev. Mol. Cell Biol. 4, 202–212 10.1038/nrm1050 [DOI] [PubMed] [Google Scholar]
- 17.Armulik A., Genové G., Betsholtz C. (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 18.Bergers G., Song S. (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro-oncol. 7, 452–464 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozerdem U., Grako K. A., Dahlin-Huppe K., Monosov E., Stallcup W. B. (2001) NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. 222, 218–227 10.1002/dvdy.1200 [DOI] [PubMed] [Google Scholar]
- 20.Zhang C., Wang H., Nie J., Wang F. (2014) Protective factors in diabetic retinopathy: focus on blood-retinal barrier. Discov. Med. 18, 105–112 [PubMed] [Google Scholar]
- 21.Takashima T., Kawada N., Maeda N., Okuyama H., Uyama N., Seki S., Arakawa T. (2002) Pepstatin A attenuates the inhibitory effect of N-acetyl-L-cysteine on proliferation of hepatic myofibroblasts (stellate cells). Eur. J. Pharmacol. 451, 265–270 [DOI] [PubMed] [Google Scholar]
- 22.Keskin D., Kim J., Cooke V. G., Wu C. C., Sugimoto H., Gu C., De Palma M., Kalluri R., LeBleu V. S. (2015) Targeting vascular pericytes in hypoxic tumors increases lung metastasis via angiopoietin-2. Cell Reports 10, 1066–1081 10.1016/j.celrep.2015.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Augustin H. G., Koh G. Y., Thurston G., Alitalo K. (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10, 165–177 10.1038/nrm2639 [DOI] [PubMed] [Google Scholar]
- 24.Scharpfenecker M., Fiedler U., Reiss Y., Augustin H. G. (2005) The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J. Cell Sci. 118, 771–780 10.1242/jcs.01653 [DOI] [PubMed] [Google Scholar]
- 25.Thomas M., Augustin H. G. (2009) The role of the Angiopoietins in vascular morphogenesis. Angiogenesis 12, 125–137 10.1007/s10456-009-9147-3 [DOI] [PubMed] [Google Scholar]
- 26.Zhu T., Sennlaub F., Beauchamp M. H., Fan L., Joyal J. S., Checchin D., Nim S., Lachapelle P., Sirinyan M., Hou X., Bossolasco M., Rivard G. E., Heveker N., Chemtob S. (2006) Proangiogenic effects of protease-activated receptor 2 are tumor necrosis factor-alpha and consecutively Tie2 dependent. Arterioscler. Thromb. Vasc. Biol. 26, 744–750 10.1161/01.ATV.0000205591.88522.d4 [DOI] [PubMed] [Google Scholar]
- 27.Jenny Zhou H., Qin L., Zhang H., Tang W., Ji W., He Y., Liang X., Wang Z., Yuan Q., Vortmeyer A., Toomre D., Fuh G., Yan M., Kluger M. S., Wu D., Min W. (2016) Endothelial exocytosis of angiopoietin-2 resulting from CCM3 deficiency contributes to cerebral cavernous malformation. Nat. Med. 22, 1033–1042 10.1038/nm.4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navaratna D., McGuire P. G., Menicucci G., Das A. (2007) Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes 56, 2380–2387 10.2337/db06-1694 [DOI] [PubMed] [Google Scholar]
- 29.Gerhardt H., Betsholtz C. (2003) Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 314, 15–23 10.1007/s00441-003-0745-x [DOI] [PubMed] [Google Scholar]
- 30.Gerhardt H., Wolburg H., Redies C. (2000) N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev. Dyn. 218, 472–479 [DOI] [PubMed] [Google Scholar]
- 31.Okabe T., Nakamura T., Nishimura Y. N., Kohu K., Ohwada S., Morishita Y., Akiyama T. (2003) RICS, a novel GTPase-activating protein for Cdc42 and Rac1, is involved in the beta-catenin-N-cadherin and N-methyl-D-aspartate receptor signaling. J. Biol. Chem. 278, 9920–9927 10.1074/jbc.M208872200 [DOI] [PubMed] [Google Scholar]
- 32.Reiss K., Maretzky T., Ludwig A., Tousseyn T., de Strooper B., Hartmann D., Saftig P. (2005) ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 24, 742–752 10.1038/sj.emboj.7600548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiba Y., Kametaka S., Waguri S., Presley J. F., Randazzo P. A. (2013) ArfGAP3 regulates the transport of cation-independent mannose 6-phosphate receptor in the post-Golgi compartment. Curr. Biol. 23, 1945–1951 10.1016/j.cub.2013.07.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hancock M. K., Haskins D. J., Sun G., Dahms N. M. (2002) Identification of residues essential for carbohydrate recognition by the insulin-like growth factor II/mannose 6-phosphate receptor. J. Biol. Chem. 277, 11255–11264 10.1074/jbc.M109855200 [DOI] [PubMed] [Google Scholar]
- 35.Marron-Terada P. G., Hancock M. K., Haskins D. J., Dahms N. M. (2000) Recognition of Dictyostelium discoideum lysosomal enzymes is conferred by the amino-terminal carbohydrate binding site of the insulin-like growth factor II/mannose 6-phosphate receptor. Biochemistry 39, 2243–2253 10.1021/bi992226o [DOI] [PubMed] [Google Scholar]
- 36.Schmidt B., Kiecke-Siemsen C., Waheed A., Braulke T., von Figura K. (1995) Localization of the insulin-like growth factor II binding site to amino acids 1508-1566 in repeat 11 of the mannose 6-phosphate/insulin-like growth factor II receptor. J. Biol. Chem. 270, 14975–14982 10.1074/jbc.270.25.14975 [DOI] [PubMed] [Google Scholar]
- 37.Garmroudi F., Devi G., Slentz D. H., Schaffer B. S., MacDonald R. G. (1996) Truncated forms of the insulin-like growth factor II (IGF-II)/mannose 6-phosphate receptor encompassing the IGF-II binding site: characterization of a point mutation that abolishes IGF-II binding. Mol. Endocrinol. 10, 642–651 [DOI] [PubMed] [Google Scholar]
- 38.Chu C. H., Tzang B. S., Chen L. M., Kuo C. H., Cheng Y. C., Chen L. Y., Tsai F. J., Tsai C. H., Kuo W. W., Huang C. Y. (2008) IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via Galphaq interaction and protein kinase C-alpha/CaMKII activation in H9c2 cardiomyoblast cells. J. Endocrinol. 197, 381–390 10.1677/JOE-07-0619 [DOI] [PubMed] [Google Scholar]
- 39.Kim Y. H., Kim Y. S., Park S. Y., Park C. H., Choi W. S., Cho G. J. (2011) CaMKII regulates pericyte loss in the retina of early diabetic mouse. Mol. Cells 31, 289–293 10.1007/s10059-011-0038-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong M. G., Panchapakesan U., Qi W., Silva D. G., Chen X. M., Pollock C. A. (2011) Cation-independent mannose 6-phosphate receptor inhibitor (PXS25) inhibits fibrosis in human proximal tubular cells by inhibiting conversion of latent to active TGF-beta1. Am. J. Physiol. Renal Physiol. 301, F84–F93 10.1152/ajprenal.00287.2010 [DOI] [PubMed] [Google Scholar]
- 41.Panchapakesan U., Mather A., Pollock C. (2013) Role of GLP-1 and DPP-4 in diabetic nephropathy and cardiovascular disease. Clin. Sci. (Lond.) 124, 17–26 10.1042/CS20120167 [DOI] [PubMed] [Google Scholar]
- 42.Dietrich N., Kolibabka M., Busch S., Bugert P., Kaiser U., Lin J., Fleming T., Morcos M., Klein T., Schlotterer A., Hammes H. P. (2016) The DPP4 inhibitor linagliptin protects from experimental diabetic retinopathy. PLoS One 11, e0167853 10.1371/journal.pone.0167853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonçalves A., Leal E., Paiva A., Teixeira Lemos E., Teixeira F., Ribeiro C. F., Reis F., Ambrósio A. F., Fernandes R. (2012) Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood-retinal barrier in a type 2 diabetes animal model. Diabetes Obes. Metab. 14, 454–463 10.1111/j.1463-1326.2011.01548.x [DOI] [PubMed] [Google Scholar]
- 44.Gonçalves A., Marques C., Leal E., Ribeiro C. F., Reis F., Ambrósio A. F., Fernandes R. (2014) Dipeptidyl peptidase-IV inhibition prevents blood-retinal barrier breakdown, inflammation and neuronal cell death in the retina of type 1 diabetic rats. Biochim. Biophys. Acta 1842, 1454–1463 10.1016/j.bbadis.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 45.Maeda S., Yamagishi S., Matsui T., Nakashima S., Ojima A., Maeda S., Nishino Y., Ishibashi Y., Yoshida Y., Yamakawa R. (2013) Beneficial effects of vildagliptin on retinal injury in obese type 2 diabetic rats. Ophthalmic Res. 50, 221–226 10.1159/000354116 [DOI] [PubMed] [Google Scholar]
- 46.Kirino Y., Sato Y., Kamimoto T., Kawazoe K., Minakuchi K., Nakahori Y. (2009) Interrelationship of dipeptidyl peptidase IV (DPP4) with the development of diabetes, dyslipidaemia and nephropathy: a streptozotocin-induced model using wild-type and DPP4-deficient rats. J. Endocrinol. 200, 53–61 10.1677/JOE-08-0424 [DOI] [PubMed] [Google Scholar]
- 47.Mega C., de Lemos E. T., Vala H., Fernandes R., Oliveira J., Mascarenhas-Melo F., Teixeira F., Reis F. (2011) Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). Exp. Diabetes Res. 2011, 162092 10.1155/2011/162092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marques C., Mega C., Gonçalves A., Rodrigues-Santos P., Teixeira-Lemos E., Teixeira F., Fontes-Ribeiro C., Reis F., Fernandes R. (2014) Sitagliptin prevents inflammation and apoptotic cell death in the kidney of type 2 diabetic animals. Mediators Inflamm. 2014, 538737 10.1155/2014/538737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaghasiya J., Sheth N., Bhalodia Y., Manek R. (2011) Sitagliptin protects renal ischemia reperfusion induced renal damage in diabetes. Regul. Pept. 166, 48–54 10.1016/j.regpep.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 50.Nakashima T., Kiba T., Ogawa Y., Hosokawa A., Shintani H., Okada Y., Taniguchi T., Shigeta M., Kozawa K. (2012) A case of paclitaxel-induced peripheral neuropathy successfully treated with pregabalin [in Japanese]. Gan To Kagaku Ryoho 39, 1443–1445 [PubMed] [Google Scholar]
- 51.Bianchi R., Cervellini I., Porretta-Serapiglia C., Oggioni N., Burkey B., Ghezzi P., Cavaletti G., Lauria G. (2012) Beneficial effects of PKF275-055, a novel, selective, orally bioavailable, long-acting dipeptidyl peptidase IV inhibitor in streptozotocin-induced diabetic peripheral neuropathy. J. Pharmacol. Exp. Ther. 340, 64–72 10.1124/jpet.111.181529 [DOI] [PubMed] [Google Scholar]
- 52.Sharma A. K., Sharma A., Kumari R., Kishore K., Sharma D., Srinivasan B. P., Sharma A., Singh S. K., Gaur S., Jatav V. S., Sharma P., Srivastava V., Joshi S., Joshi M., Dhakad P. K., Kanawat D. S., Mishra A., Sharma A., Singh D., Singh R. P., Chawda H. S., Singh R., Raikwar S. K., Kurmi M. K., Khatri P., Agarwal A., Munajjam A. (2012) Sitagliptin, sitagliptin and metformin, or sitagliptin and amitriptyline attenuate streptozotocin-nicotinamide induced diabetic neuropathy in rats. J. Biomed. Res. 26, 200–210 10.7555/JBR.26.20110054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schürmann C., Linke A., Engelmann-Pilger K., Steinmetz C., Mark M., Pfeilschifter J., Klein T., Frank S. (2012) The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J. Pharmacol. Exp. Ther. 342, 71–80 10.1124/jpet.111.191098 [DOI] [PubMed] [Google Scholar]
- 54.Panchapakesan U., Pollock C. (2015) The role of dipeptidyl peptidase-4 inhibitors in diabetic kidney disease. Front. Immunol. 6, 443 10.3389/fimmu.2015.00443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olivares A. M., Althoff K., Chen G. F., Wu S., Morrisson M. A., DeAngelis M. M., Haider N. (2017) Animal models of diabetic retinopathy. Curr. Diab. Rep. 17, 93 10.1007/s11892-017-0913-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson R., Barathi V. A., Chaurasia S. S., Wong T. Y., Kern T. S. (2012) Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Dis. Model. Mech. 5, 444–456 10.1242/dmm.009597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romero-Aroca P., Navarro-Gil R., Valls-Mateu A., Sagarra-Alamo R., Moreno-Ribas A., Soler N. (2017) Differences in incidence of diabetic retinopathy between type 1 and 2 diabetes mellitus: a nine-year follow-up study. Br. J. Ophthalmol. 101, 1346–1351 10.1136/bjophthalmol-2016-310063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Looker H. C., Nyangoma S. O., Cromie D. T., Olson J. A., Leese G. P., Black M. W., Doig J., Lee N., Lindsay R. S., McKnight J. A., Morris A. D., Pearson D. W., Philip S., Wild S. H., Colhoun H. M., Scottish Diabetes Research Network Epidemiology Group ; Scottish Diabetic Retinopathy Collaborative . (2014) Rates of referable eye disease in the Scottish National Diabetic Retinopathy screening programme. Br. J. Ophthalmol. 98, 790–795 10.1136/bjophthalmol-2013-303948 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.