Aortic dissection (AD) is defined as tearing of the aortic wall, a disease that results in high mortality due to aortic rupture and malperfusion.1 Unfortunately, the current available techniques cannot predict the onset of AD. Therefore, understanding the pathophysiology and mechanisms of AD is in a dire medical need. In this issue of Arteriosclerosis, Thrombosis, and Vascular Biology, an elegant publication by Nishida et al. reported that high salt intake augmented AD through an interleukin-17 (IL-17)-dependent mechanism (Figure 1).2
Figure 1. Schematic summary of the findings reported by Nishida et al.2.
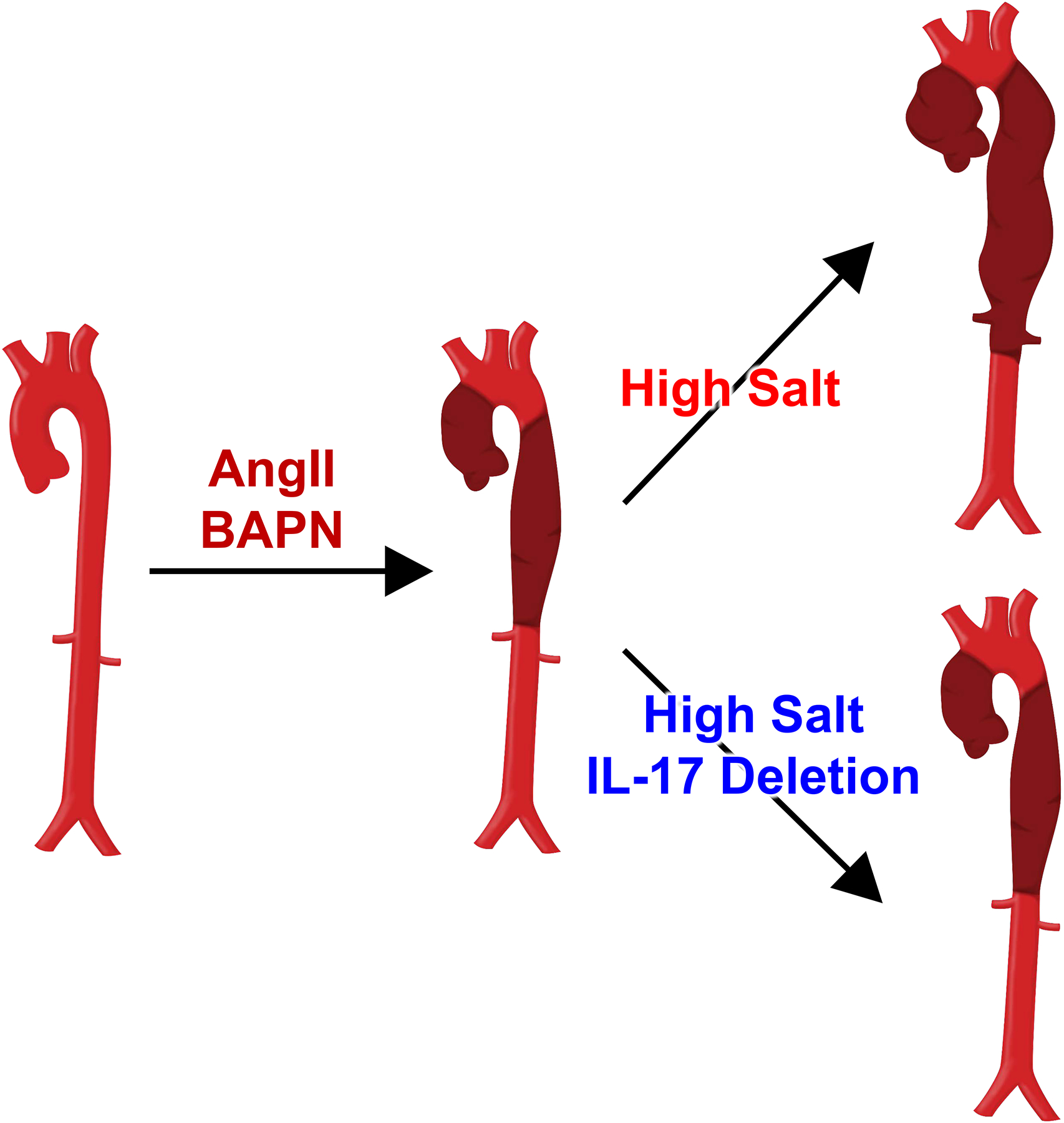
Administration of angiotensin II (AngII) and β-aminopropionitrile (BAPN) leads to focal aortic dissection in thoracic and abdominal aortic regions in mice with normal salt intake. High salt intake leads to more extensive pathological changes in mice administered AngII and BAPN. Whole body interleukin-17 deletion diminishes high salt-induced augmentation of aortic dissection.
Many studies have reported that high salt intake is associated with cardiovascular diseases including hypertension and aortic aneurysms.3–8 Although hypertension is a risk factor for AD,9 the impact of high salt intake on AD formation has not been investigated. The study by Nishida et al. revealed adverse effects of high salt intake on AD induced by subcutaneous co-infusion of angiotensin II (AngII) and β-aminopropionitrile monofumarate (BAPN).2 The authors performed detailed characterization on histological features of medial disruption and aortic rupture. They subsequently demonstrated that increased salt intake led to augmentation of the aortic pathology (Figure 1).
In the study of Nishida et al,2 salt (1% wt/vol NaCl) was provided in drinking water. In humans, high salt intake is due to excessive salt in diet, in contrast to the drinking water that was the delivery mode in this study. A recent human study demonstrated that high salt intake (12 g/day) in food increased urinary cortisol and cortisone excretions, and changed catabolic hormone profile.10 In contrast, a diet containing high salt (4% wt/wt NaCl) fed to mice with ad libitum access to water did not alter urinary glucocorticoid concentrations or catabolic state.11 However, combination of a 4% wt/wt NaCl diet and isotonic saline (0.9% wt/vol) in drinking water increased urinary glucocorticoid excretion and recapitulated the catabolic hormone profile change observed in the human study.10, 11 Thus, in mouse studies, the route of salt administration is critical for elucidating effects of high salt intake. Salt administered in drinking water, as reported by Nishida et al.,2 can mimic the human response to high salt loading.
AD exhibits disruption of the intima and medial layers of the aorta with intramural hematoma that forms a false lumen.9 These features were also detected in mice infused with AngII and BAPN.12, 13 In the study by Nishida et al.2, AD was described as an intramural hematoma with greater diameters (> 1.5-fold) than the external diameter at the distal site of the corresponding aortic region (used as the reference diameter). This criterion to define AD formation was based on the authors’ previous report that progression of medial disruption was associated with formation of intramural hematoma and increased aortic diameter.14 On the basis of these definitions, the authors found that high salt administration exacerbated AD induced by co-infusion of AngII and BAPN. In addition to the incidence, the authors used length of lesions with aortic wall destruction to represent AD severity. End-organ ischemia due to dissection-related obstruction of aortic branch vessels is a deleterious consequence of AD and is associated with higher mortality.9 Since longer AD lesions have higher risk for complicating perfusion to branch vessels, lesion length is an optimal parameter to determine AD severity. However, it is unclear whether short lesions would be associated with lower incidence of aortic rupture. Therefore, multiple parameters may need to be considered in order to predict aortic rupture.
To investigate mechanisms by which high salt intake exacerbated AD, the authors2 explored IL-17 signaling pathway. IL-17 is a cytokine secreted from helper T lymphocytes (Th17) that was increased in AD tissues,15, 16 and high salt intake increased Th17 lymphocytes and inflammation.15 These findings support potential links among high salt intake, IL-17, and AD formation. Indeed, whole body deletion of IL-17 reduced high salt-accelerated AD incidence and severity, whereas deletion of IL-17 had no effects on AD in mice with normal salt intake (mice were given only normal drinking water).2 This study therefore provides a mechanistic insight into linking high salt intake and AD augmentation through IL-17 signaling pathway (Figure 1).2 Salt intake regulates the renin-angiotensin-aldosterone system.17 There has been consistent evidence that this hormonal system contributes to aortopathies.18 Therefore, it would be also interesting to determine the regulation of the renin-angiotensin-aldosterone system in high salt induced augmentation of AD.
A frequent approach for exploring mechanisms is to compare the abundance of components between normal and diseased tissues. However, this approach does not discriminate whether the differences are a consequence of the pathology or the cause. To overcome this common shortcoming, Nishida et al.2 harvested aortic samples at early phase (3 days of infusion) prior to AD development. A spectrum of changes were noted between mice given normal versus high salt, including alterations of IL-17 receptor and TGF-β signaling, collagen deposition, and aortic force displacement. Since these parameters were altered prior to detecting overt pathologies, they may represent causative mechanisms. AD is initiated by disruption of the intima that consequently tears medial layers.9 Therefore, aortic pathologies, including aortic dilatation and intramural hematoma, are consequences of this initial disruption.9 It is important to define the entry site of blood in AD. Nishida et al.2 performed detailed histological anaysis from the intact proximal region throughout the diseased region, providing insights into the complex AD pathology.
The study by Nishida et al.2 provides solid evidence that high salt intake plays a critical role in augmenting AD formation through IL-17 signaling pathway. We look forward to future studies that examine the effect of salt restriction, as a potential therapeutic strategy, on AD formation and mechanisms beyond IL-17 and its signaling pathway.
Sources of Funding
The authors’ research work is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers R01 HL133723, HL139748, and HL142973. The authors’ research project on thoracic aortic aneurysm is also supported by the American Heart Association SFRN in Vascular Disease (18SFRN33960001). HS is supported by an American Heart Association postdoctoral fellowship (18POST33990468). The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
None.
Conflicts of Interest: None
References
- 1.Nienaber CA, Clough RE. Management of acute aortic dissection. The Lancet. 2015;385:800–811 [DOI] [PubMed] [Google Scholar]
- 2.Nishida N, Aoki H, Ohno-Urabe S, Nishihara M, Furusho A, Hirakata S, Hayashi M, Ito S, Yamada H, Hirata Y, Yasukawa H, Imaizumi T, Tanaka H, Y F. High salt intake worsens aortic dissection in mice: Involvement of IL-17a-dependent extracellular matrix metabolism. Arterioscler Thromb Vasc Biol. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ. 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J, Global Burden of Diseases N, Chronic Diseases Expert G. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634 [DOI] [PubMed] [Google Scholar]
- 5.Mente A, O’Donnell M, Rangarajan S, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: A pooled analysis of data from four studies. The Lancet. 2016;388:465–475 [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Xie Z, Daugherty A, Cassis LA, Pearson KJ, Gong MC, Guo Z. Mineralocorticoid receptor agonists induce mouse aortic aneurysm formation and rupture in the presence of high salt. Arterioscler Thromb Vasc Biol. 2013;33:1568–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golledge J, Hankey GJ, Yeap BB, Almeida OP, Flicker L, Norman PE. Reported high salt intake is associated with increased prevalence of abdominal aortic aneurysm and larger aortic diameter in older men. PLoS One. 2014;9:e102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishijo N, Sugiyama F, Kimoto K, Taniguchi K, Murakami K, Suzuki S, Fukamizu A, Yagami K. Salt-sensitive aortic aneurysm and rupture in hypertensive transgenic mice that overproduce angiotensin II. Lab Invest. 1998;78:1059–1066 [PubMed] [Google Scholar]
- 9.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27–e129 [DOI] [PubMed] [Google Scholar]
- 10.Rakova N, Kitada K, Lerchl K, et al. Increased salt consumption induces body water conservation and decreases fluid intake. J Clin Invest. 2017;127:1932–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitada K, Daub S, Zhang Y, et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Invest. 2017;127:1944–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanematsu Y, Kanematsu M, Kurihara C, Tsou TL, Nuki Y, Liang EI, Makino H, Hashimoto T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension. 2010;55:1267–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren W, Liu Y, Wang X, Jia L, Piao C, Lan F, Du J. Beta-aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice. Sci Rep. 2016;6:28149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logghe G, Trachet B, Aslanidou L, Villaneuva-Perez P, De Backer J, Stergiopulos N, Stampanoni M, Aoki H, Segers P. Propagation-based phase-contrast synchrotron imaging of aortic dissection in mice: From individual elastic lamella to 3d analysis. Sci Rep. 2018;8:2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic Th17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju X, Ijaz T, Sun H, Ray S, Lejeune W, Lee C, Recinos A 3rd, Guo DC, Milewicz DM, Tilton RG, Brasier AR. Interleukin-6-signal transducer and activator of transcription-3 signaling mediates aortic dissections induced by angiotensin II via the t-helper lymphocyte 17-interleukin 17 axis in C57BL/6 mice. Arterioscler Thromb Vasc Biol. 2013;33:1612–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu H, Wu C, Howatt DA, Balakrishnan A, Charnigo RJ Jr., Cassis LA, Daugherty A. Differential effects of dietary sodium intake on blood pressure and atherosclerosis in hypercholesterolemic mice. J Nutr Biochem. 2013;24:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu CH, Mohammadmoradi S, Chen JZ, Sawada H, Daugherty A, Lu HS. Renin-angiotensin system and cardiovascular functions. Arterioscler Thromb Vasc Biol. 2018;38:e108–e116 [DOI] [PMC free article] [PubMed] [Google Scholar]


