Abstract
Background.
Routine alcohol screening scores are increasingly available in electronic health records (EHRs). Changes in such scores could be useful for monitoring response to brief intervention or treatment of alcohol use disorder. However, it is unclear whether changes in clinically-documented AUDIT-C alcohol screening scores reflect true changes in drinking. This study evaluated associations between changes in EHR AUDIT-C scores and changes in high density lipoprotein cholesterol (HDL), a laboratory test that reflects average alcohol consumption.
Methods.
National Veterans Affairs EHR data (2004–2007) were used to identify patients screened with the AUDIT-C (0 to 12 points) on two occasions at least a year apart, who had HDL measured in the year after each screen. First differencing linear regression estimated associations between changes in AUDIT-C score (−12 to 12 points; modeled categorically to allow for non-linearity) and subsequent changes in HDL (mg/dL), adjusting for baseline HDL. Additional analyses evaluated whether associations between changes in AUDIT-C and changes in HDL were modified by baseline AUDIT-C.
Results.
Among 316,712 patients, increases—but not decreases—in AUDIT-C scores were associated with commensurate changes in HDL. However, a significant interaction was observed with baseline AUDIT-C score (p<0.00001), which revealed that decreases in AUDIT-C scores were also associated with commensurate decreases in HDL (p-values<0.05) except in the 1.5% of patients with the highest baseline AUDIT-C scores (10–12).
Conclusions.
Findings suggest that changes in EHR AUDIT-C scores reflect changes in drinking. These results support the use of clinically-documented alcohol screening scores for monitoring patients’ alcohol use over time.
Keywords: alcohol drinking, screening, monitoring, AUDIT-C
1.0. Introduction
Over a decade ago, experts recommended that high quality care for unhealthy alcohol use requires monitoring changes in alcohol use over time (Institute of Medicine (US) Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders 2006). With increasing implementation of routine screening for unhealthy alcohol use, the need for simple and effective methods of monitoring alcohol use has only become more pressing (Bradley and Kivlahan 2014; National Council for Behavioral Health 2018). Such monitoring would be appropriate for all patients identified to have unhealthy alcohol use, and particularly important for assessing response to interventions, whether counseling, behavioral therapy, or medication.
Some have suggested that scaled screening questionnaires for unhealthy alcohol use may be useful for monitoring changes in drinking over time (National Council for Behavioral Health 2018), much the same way scores on the PHQ-9 screen for depression can also be used to monitor changes in depression severity (Kroenke and Spitzer 2002; Unutzer et al. 2006). Brief alcohol screening questionnaires, such as the 3-item Alcohol Use Disorders Identification Test – Consumption (AUDIT-C) questionnaire, have been implemented in routine clinical care across diverse settings through the use of electronic health records (EHRs) (Johnson, Woychek, et al. 2013; Rose et al. 2008; Ornstein et al. 2013; Bradley et al. 2006; Bobb et al. 2017). Because AUDIT-C scores are strongly associated with average alcohol consumption based on confidential in-depth interviews (Rubinsky et al. 2013), they may be useful for monitoring changes in drinking over time. However, it is unknown whether within-person changes in AUDIT-C scores documented in EHRs as part of routine care reflect changes in alcohol consumption. Specifically, clinically-documented AUDIT-C scores can be biased (Bradley et al. 2011; Hawkins et al. 2007; Davis, Thake, and Vilhena 2010; Williams et al. 2015; McGinnis et al. 2016) and changes in scores could reflect measurement error due to inconsistent administration of the questionnaire by clinicians (e.g., skipping and altering items) and/or patient social desirability bias (Williams et al. 2015).
The primary aim of this study was to determine whether changes in AUDIT-C scores documented as part of routine medical care are associated with commensurate changes in a laboratory test known to reflect average alcohol consumption—high density lipoprotein cholesterol (HDL) (Brien et al. 2011). HDL is commonly measured in routine primary care (Helfand and Carson 2008) and has been shown to increase in response to increased drinking in numerous experimental interventional studies (Brien et al. 2011). Prior studies have demonstrated a strong association between cross-sectional EHR AUDIT-C scores and HDL (Berger et al. 2013) and between changes in EHR AUDIT-C score groups and subsequent HDL (Bradley et al. 2016), but no study has evaluated whether within-person changes in AUDIT-C scores reflect changes in drinking. The present study examines the within-person association between changes in EHR AUDIT-C scores and changes in HDL using analytic methods that implicitly control for unobserved confounding by time-invariant factors. Additionally, to understand whether EHR AUDIT-C scores could be used for monitoring within-person changes in drinking equally across subgroups of patients, this study planned a priori to evaluate whether the association between changes in AUDIT-C score and changes in HDL was modified by baseline AUDIT-C groups, as well as demographic or clinical factors known to be associated with alcohol use.
2.0. Methods
2.1. Study Sample and Data Sources
This longitudinal cohort study included patients from 24 VA medical centers in the Northern and Western U.S. (VA Networks 18–22) who had (1) documented alcohol screening with the AUDIT-C on two occasions at least a year apart (1/1/04 – 12/31/07) and (2) HDL laboratory results within a year after each screen. Alcohol screening scores, HDL levels, diagnoses, addictions treatment utilization, and demographic characteristics documented in the EHR were obtained from the VA Informatics and Computing Infrastructure (VINCI), which includes a national warehouse of VA clinical and administrative data. VA Medicare claims were used to supplement information on demographic characteristics and diagnoses. Approval of this study and waivers of informed consent and Health Insurance Portability and Accountability Act (HIPAA) authorization were granted by the VA Puget Sound Health Care System Institutional Review Board (IRB).
2.2. Measures
2.2.1. AUDIT-C Alcohol Screening Scores
The VA has required annual alcohol screening with the AUDIT-C since 2003 and screened over 90% of primary care outpatients during the study period (Bradley et al. 2006). The AUDIT-C is a validated screen for past-year unhealthy alcohol use scored 0–12 points (Johnson, Lee, et al. 2013; Bradley et al. 2007), with a score of 0 indicating no past-year alcohol use and increasing scores indicative of greater consumption (Rubinsky et al. 2013; Berger et al. 2013). Patients’ first AUDIT-C during the study period is referred to as their baseline AUDIT-C, and their first subsequent AUDIT-C at least 12 months later is referred to as their follow-up AUDIT-C. Change in AUDIT-C was computed as the difference in scores from the patient’s baseline AUDIT-C to follow-up AUDIT-C (range: −12 to 12 points; 0 indicates no change, positive values indicate an increase in AUDIT-C score, and negative values indicate a decrease in AUDIT-C score).
2.2.2. HDL Cholesterol
HDL was selected as the outcome for this study because it is commonly measured in primary care as part of routine cardiovascular risk assessment (U.S. Preventive Services Task Force et al. 2016), it reflects average daily alcohol consumption and changes rapidly in response to experimentally manipulated changes in drinking (Rimm et al. 1999; Brien et al. 2011), and is therefore amenable to studying within-person change. Experimental trials of alcohol administration have shown that HDL increases with consumption in a dose-response manner: drinking 1–2, 2–4 or ≥4 drinks per day for a period of at least two weeks was associated with increases in HDL of 2.8, 3.9, and 5.4 milligrams (mg) of cholesterol per deciliter (dL) of blood, respectively (Brien et al. 2011). Because HDL is typically ordered for reasons unrelated to drinking, it is available on a sample of medical outpatients relatively unbiased by level of alcohol use.
The first HDL measurement on the day of, or in the 365 days following, the patient’s baseline and follow-up AUDIT-Cs were used as the baseline HDL and follow-up HDL, respectively. When there were more than one HDL recorded on the same day, the mean was used. Change in HDL was computed as the difference in mg/dL from the patient’s baseline HDL to their follow-up HDL (such that 0 indicates no change, positive values indicate an increase in HDL, and negative values indicate a decrease in HDL).
2.2.3. Other Measures
Demographic characteristics included gender (male, female), race/ethnicity (white, non-white), age at the time of baseline AUDIT-C (21–29, 30–44, 55–64, 65+ years), and marital status (married, divorced/separated/widowed, never married) at the time of each AUDIT-C. VA service coverage, based on multiple factors including military service history, service-connected disability rating and income level, was determined at the time of each AUDIT-C and categorized into no copay (indicating higher service-connected medical need and/or lower socioeconomic status), partial copay or full copay. Clinical measures included 1) past-year smoking (yes, no) determined at the time of each AUDIT-C using EHR text data elements (McGinnis et al. 2011), 2) medical comorbidity measured using the Deyo-Charlson comorbidity index computed based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes in the year prior to each AUDIT-C and dichotomized at a threshold of ≥3 points (Deyo, Cherkin, and Ciol 1992), and 3) any recognized substance use disorder (yes, no) measured based on any documented VA addictions treatment or any ICD-9-CM diagnosis indicating a substance use disorder or an alcohol-related medical condition in the year prior to the baseline AUDIT-C. Time from AUDIT-C to HDL was calculated based on the number of days between each AUDIT-C and the subsequent HDL; as well as a single indicator of whether both intervals (days from baseline AUDIT-C to baseline HDL and days from follow-up AUDIT-C to follow-up HDL) were ≤ 90 days.
2.3. Analyses
Initial analyses describe demographic and clinical characteristics overall and across groups of patients defined by their change in AUDIT-C score. Differences in patient characteristics across groups were tested based on Pearson chi-squared statistics for categorical variables and non-parametric Kruskal-Wallis tests for continuous variables.
Main analyses estimated the change in HDL associated with each possible change in AUDIT-C score (−12 to 12) based on a linear regression model that adjusted for baseline HDL using the best fitting fractional polynomial (powers −2 and 3) and estimated robust standard errors using the sandwich estimator to account for correlated data among patients from the same medical center. By design, such “change-in-change” or first-differencing analyses implicitly control for all patient-specific factors, whether measured or unmeasured, that do not vary over the study period (e.g., clinical and/or social factors that may be associated with propensity to change drinking and/or HDL) because they are present on both sides of the equation and subtract out in these differencing models (Liker, Augustyniak, and Duncan 1985). To allow for a non-linear association between change in AUDIT-C score and change in HDL, each possible change in AUDIT-C score was modeled as a separate categorical variable, with 0 points (no change in AUDIT-C) as the referent. A linear trend was evaluated based on a linear contrast test.
To understand whether the association between change in AUDIT-C and change in HDL held across subgroups of patients, additional analyses evaluated interactions between AUDIT-C change and baseline AUDIT-C score as well as demographic and clinical characteristics hypothesized a priori to potentially modify the association. Specifically, we repeated main analyses adding baseline AUDIT-C score and the interaction between baseline AUDIT-C score and change in AUDIT-C at follow-up screening. Baseline AUDIT-C scores (0, 1–4, 5–9, 10–12) and changes in AUDIT-C scores (± 0, 1, 2, 3–4, 5–9, 10–12 points) were grouped to facilitate interpretability and increase precision of the estimates. Average change in HDL was estimated across categories of change in AUDIT-C score for each baseline AUDIT-C group based on recycled prediction methods that hold the covariate distribution constant (Basu and Rathouz 2005). Wald tests were used to evaluate an overall interaction and to compare the average change in HDL associated with each category of change in AUDIT-C versus no change in AUDIT-C (the referent). We similarly evaluated interactions between the above AUDIT-C change groups and gender, age, and any recognized substance use disorder.
Sensitivity analyses, planned a priori, evaluated the association between change in AUDIT-C score and change in HDL in the subset of patients who had HDLs within the 90 days following each AUDIT-C, a timeframe that reduces the likelihood patients may have changed their drinking after an AUDIT-C. We also assessed whether the inclusion of covariates measured at the time of each AUDIT-C, that could possibly (but were unlikely to) vary with time, meaningfully changed results; these covariates included marital status, VA service coverage, past-year smoking status, and Deyo-Charlson comorbidity.
3.0. Results
Overall 549,563 patients had a documented pair of AUDIT-Cs at least a year apart during the study period. Of those, 316,712 (58%) had HDLs documented in the year after each AUDIT-C and were included in the study. Most eligible patients were male (96%), White (67%), 65 years and older (60%), and married (56%) (Table 1). Over half (51%) of eligible veterans reported no past-year alcohol use (AUDIT-C score 0) on their baseline AUDIT-C, while another 40% had low-level drinking (scores 1–4), and only 7.3% and 1.5% had AUDIT-C scores 5–9 and 10–12, respectively.
Table 1.
Characteristics of the Study Sample at the time of Baseline Alcohol Screening
| N | (Column %) | |
|---|---|---|
| Total | 316,712 | (100%) |
| Female | 13,582 | (4.3%) |
| Age* | ||
| 21–29 | 928 | (0.3%) |
| 30–44 | 9,474 | (3.1%) |
| 45–64 | 117,830 | (37%) |
| 65+ | 188,480 | (60%) |
| Race* | ||
| Non-white | 49,316 | (16%) |
| White | 212,353 | (67%) |
| Marital Status* | ||
| Single/Never Married | 40,305 | (13%) |
| Divorced/Separated/Widowed | 97,538 | (31%) |
| Married | 178,501 | (56%) |
| VA Service Coverage | ||
| No Copay (Full Coverage) | 59,390 | (19%) |
| Partial Copay | 64,592 | (20%) |
| Full Copay | 192,730 | (61%) |
| Deyo Comorbidity > 3 | 39,002 | (12%) |
| Past-year Smoker | 85,592 | (27%) |
| Baseline AUDIT-C | ||
| 0 points | 162,574 | (51%) |
| 1–4 points | 126,550 | (40%) |
| 5–9 points | 22,983 | (7.3%) |
| 10–12 points | 4,605 | (1.5%) |
| Days between: | Median | (Interquartile range) |
| Baseline and Follow-up AUDIT-C | 489 | (407, 658) |
| Baseline AUDIT-C and HDL | 47 | (0, 170) |
| Follow-up AUDIT-C and HDL | 67 | (0, 181) |
Total may not sum to 100% due to missing values
Most (83%) patients with non-drinking (AUDIT-C score 0) at baseline had no change in AUDIT-C score at follow-up, and most (72%) with low-level drinking (AUDIT-C score 1–4) at baseline changed their AUDIT-C score 0–1 points (Appendix A1). Changes in AUDIT-C score were more common among men vs. women, younger vs. older age groups, unmarried vs. married patients, patients reporting past-year smoking vs. not, patients with vs. without VA service copays, and patients with less vs. more comorbidity.
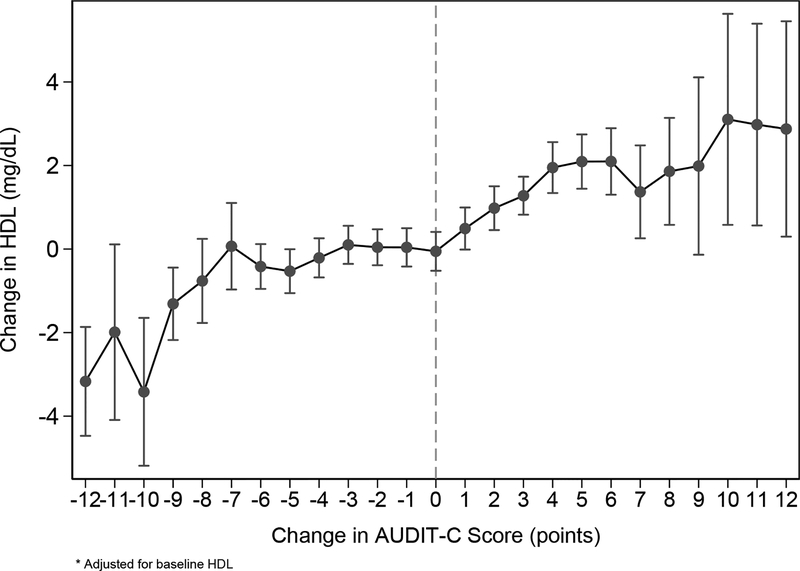
Main analyses based on first-differenced regression adjusting for baseline HDL revealed an overall dose-response association between change in AUDIT-C score and change in HDL, with a significant linear trend (p<0.0005). Among patients with no change in AUDIT-C score, HDL decreased by 0.05 mg/dL on average. Compared to no change in AUDIT-C score, increases in AUDIT-C score of every magnitude were associated with statistically significant increases in HDL (p-values<0.05), whereas significant decreases in HDL were observed only if AUDIT-C scores decreased by at least 9 points. Figure 1 depicts the estimated change in HDL associated with each possible change in AUDIT-C at follow-up screening (Appendix B1). Increases in AUDIT-C score of 1, 2, 3 and 4 points were associated with progressively larger increases in HDL: 0.5, 1.0, 1.3 and 2.0 mg/dL, respectively. Mean change in HDL stayed relatively stable across changes in AUDIT-C scores of 5–9 points (~2.0 mg/dL) but increased to approximately 3.0 mg/dL for those with changes in AUDIT-C scores of 10 or more points. A decrease in AUDIT-C score of 9 points was associated with a decrease in HDL of 1.3 mg/dL, whereas decreases in AUDIT-C score of 10–12 points were associated with decreases in HDL up to 3.4 mg/dL.
Figure 1.
Change in AUDIT-C score and Change in HDL
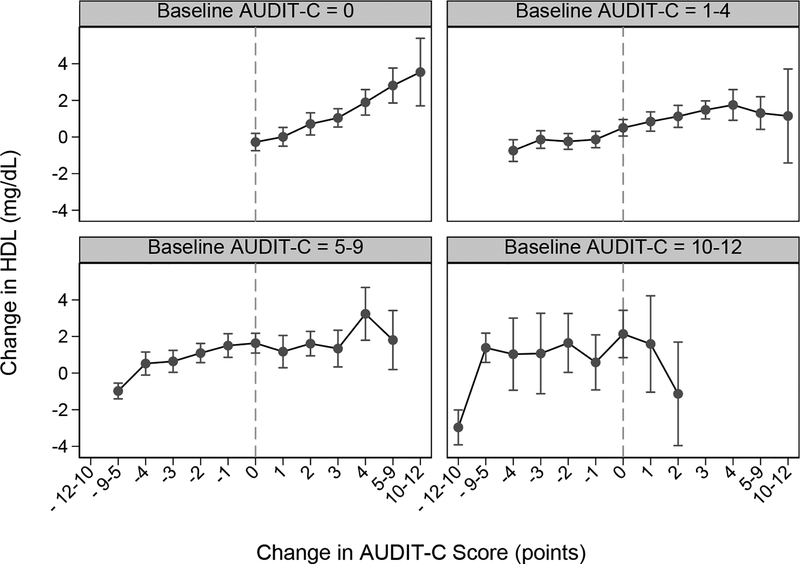
In analyses assessing differences in the association across subgroups, baseline AUDIT-C statistically significantly modified the association between changes in AUDIT-C and changes in HDL (Figure 2). Increases in AUDIT-C score were most strongly associated with increases in HDL in the large group of patients who reported no past-year drinking (AUDIT-C 0) at baseline. Decreases in AUDIT-C score of every magnitude were associated with significant decreases in HDL except in the small group (1.5%) of patients with the highest baseline AUDIT-C scores (AUDIT-C 10–12). Age and the presence of a substance use disorder diagnosis statistically significantly modified the association between changes in AUDIT-C and changes in HDL (interaction p-values<0.00001), but gender did not (p=0.06). Nonetheless, in stratified analyses, associations appeared similar across subgroups (Appendices C–D1).
Figure 2.
Change in AUDIT-C score and Change in HDL, by Baseline AUDIT-C Score Group
The median number of days between AUDIT-C screening and HDL was 54 (IQR 0, 171) at baseline and 68 (IQR 0, 180) at follow-up (Table 1). Analyses restricted to the subset of patients with HDL measured within 90 days following both AUDIT-Cs (n=115,481; 36%) revealed a stronger association between change in AUDIT-C and change in HDL, with extreme changes in AUDIT-C score in either direction associated with larger changes in HDL compared to overall findings (Appendix E1). As expected, adjusting for covariates at the time of each AUDIT-C did not appreciably change results (not shown).
4.0. Discussion
In this large study of over 300,000 outpatients, changes in AUDIT-C scores were associated with changes in HDL, a laboratory test known to reflect recent average alcohol consumption. Moreover, the association was stronger when the AUDIT-C and HDL were obtained closer together temporally, as would be expected if there was a causal association. Findings suggest that changes in AUDIT-C scores reflect changes in drinking and support the use of clinically-documented alcohol screening scores for monitoring patients’ alcohol use over time.
Although AUDIT-C scores are strongly associated with average alcohol consumption based on confidential interviews (Rubinsky et al. 2013), few studies have evaluated implications of changes in clinically-documented AUDIT-C scores over time, which may be subject to quality issues related to non-standard verbal administration of the questionnaire and/or patient response bias (Bradley et al. 2011; Hawkins et al. 2007; Davis, Thake, and Vilhena 2010; Williams et al. 2015; McGinnis et al. 2016). One study, restricted to patients with HIV, evaluated the association between within-person changes in clinically-documented AUDIT-C scores and changes in HIV outcomes and found that those with relatively stable drinking had the greatest improvements in HIV severity (Williams et al. 2018). Another study included HDL as one of three outcomes, and evaluated the association between changes in alcohol risk group based on routine AUDIT-C screening and subsequent HDL measured at a single timepoint (Bradley et al. 2016). In line with the present study, that study found that compared to patients who had no change in their alcohol risk group at follow-up screening, those who increased to a higher alcohol risk group had higher HDL and those who decreased to a lower alcohol risk group had a lower HDL on average.
Measurement-based care is increasingly recommended to improve the quality of care for patients with unhealthy substance use in primary care as well as specialty treatment settings (Institute of Medicine (US) Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders 2006; National Council for Behavioral Health 2018; Marsden et al. 2019). Moreover, systematic measurement of substance use is a core dimension of such measurement-based care (Marsden et al. 2019), and AUDIT-C scores have been recommended as a practical tool for monitoring alcohol use over time in those with unhealthy alcohol use (National Council for Behavioral Health 2018). Our study findings therefore have key implications for clinical care and research. This study suggests that such an approach would be valid for monitoring whether patients decrease drinking after brief interventions or treatment for AUD in most patients. Specifically, findings suggest that clinically-documented AUDIT-C scores reflect “real” decreases in drinking, with the possible exception of the relatively few patients with the highest AUDIT-C scores (10–12). Results also suggest that AUDIT-Cs can be useful for monitoring changes in drinking among patients who report no alcohol use, most of whom have stable (non-)drinking over time. In the minority of patients with nondrinking who increase alcohol use—perhaps after relapse of an AUD—this study suggests that AUDIT-C scores are an effective indicator of increased consumption. Changes in AUDIT-C scores could also be used as an outcome for comparative effectiveness research (Williams et al. 2010; Williams et al. 2014; Williams et al. 2017) or future pragmatic trials comparing interventions aiming to decrease drinking in patients with unhealthy alcohol use. Study findings also suggest a potential role for HDL in alcohol intervention research, as an objective measure of changes in drinking over time.
This study has important limitations. First, HDL levels tend to be affected by other changeable behaviors such as diet, exercise, conditions such as diabetes and hypothyroidism, and medication use. Second, HDL changes rapidly with changes in alcohol consumption and is thus a shorter-term marker of alcohol use than the AUDIT-C score, which asks about drinking over the past year. Third, the utility of HDL at high levels of alcohol consumption is unclear; studies of the effect of experimentally manipulated alcohol consumption on HDL have evaluated levels of consumption below those associated with AUDIT-C scores 10–12 (Brien et al. 2011; Rubinsky et al. 2013). Finally, the study sample was drawn from patients in VA outpatient care and included relatively few women, racial/ethnic minorities, younger patients, and patients with the highest AUDIT-C scores. Findings warrant replication and further exploration, including in healthcare systems with higher representation of these groups. Further, if used for routine alcohol screening, other instruments (e.g., the full 10-item AUDIT) may similarly serve as practical tools for monitoring changes in drinking and could be evaluated in future studies. At the same time, the study has unique strengths. We used data from a national sample of outpatients screened with the AUDIT-C without recruitment bias, an objective reference standard (HDL) that reflects average alcohol consumption (avoiding biases associated with self-report), and a novel analytic approach to account for differences in all observed and unobserved time-invariant patient characteristics.
5.0. Conclusion
This study is the first to evaluate the utility of within-person changes in AUDIT-C scores as an indicator of changes in alcohol use based on an objective laboratory test that changes with changes in recent consumption. Findings provide empirical support for use of the AUDIT-C to monitor drinking and treatment outcomes in clinical care and research. Clinicians and researchers need practical measures of alcohol consumption and findings suggest the AUDIT-C as a candidate tool.
Supplementary Material
Appendix A. Characteristics of the study sample by change in AUDIT-C score at follow-up screening
Appendix B. Difference in change in HDL associated with each possible change in AUDIT-C, compared to no change
Appendix C. Graphical plot of the association between change in AUDIT-C score and change in HDL, by age group
Appendix D. Graphical plot of the association between change in AUDIT-C score and change in HDL, by any substance use disorder
Appendix E. Graphical plot of the association between change in AUDIT-C score and change in HDL among patients with HDL in the 90 days following each AUDIT-C
Highlights.
Changes in clinically-documented AUDIT-C scores reflect commensurate changes in HDL, a laboratory test known to reflect recent average alcohol consumption.
The association between changes in AUDIT-C scores and changes in HDL was stronger when the AUDIT-C and HDL were obtained closer together temporally, as would be expected if there was a causal association.
Findings suggest that changes in AUDIT-C scores reflect changes in drinking and support the use of clinically-documented AUDIT-C scores for monitoring patients’ alcohol use over time.
Acknowledgements:
David Au for his support of this study and Eric Goemer and Vicky Kim for assisting with manuscript submission.
Role of funding source
This work was supported by the Department of Veterans Affairs (VA), Veterans Health Administration, Office of Research and Development, Health Services Research and Development [grant number IIR 08-314]. Support for VA/CMS data for this project was provided by the VA Information Resource Center. Dr. Williams is supported by a Career Development Award from VA HSR&D [CDA 12-276]. Dr. Bradley is supported by a mid-career mentoring award from the National Institute on Alcohol Abuse and Alcoholism [K24 AA022128]. Funding sources had no involvement in study design; collection, analysis and interpretation of data; in the writing of the report; or the decision to submit the article for publication.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org/[…] and by entering doi:[…]
Conflict of Interest: No conflict declared
References
- Basu A, and Rathouz PJ. 2005. ‘Estimating marginal and incremental effects on health outcomes using flexible link and variance function models’, Biostatistics, 6: 93–109. [DOI] [PubMed] [Google Scholar]
- Berger D, Williams EC, Bryson CL, Rubinsky AD, and Bradley KA. 2013. ‘Alcohol questionnaires and HDL: screening scores as scaled markers of alcohol consumption’, Alcohol, 47: 439–45. [DOI] [PubMed] [Google Scholar]
- Bobb JF, Lee AK, Lapham GT, Oliver M, Ludman E, Achtmeyer C, Parrish R, Caldeiro RM, Lozano P, Richards JE, and Bradley KA. 2017. ‘Evaluation of a Pilot Implementation to Integrate Alcohol-Related Care within Primary Care’, Int J Environ Res Public Health, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, and Kivlahan DR. 2007. ‘AUDIT-C as a brief screen for alcohol misuse in primary care’, Alcoholism, Clinical and Experimental Research, 31: 1208–17. [DOI] [PubMed] [Google Scholar]
- Bradley KA, and Kivlahan DR. 2014. ‘Bringing patient-centered care to patients with alcohol use disorders’, JAMA, 311: 1861–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Lapham GT, Hawkins EJ, Achtmeyer CE, Williams EC, Thomas RM, and Kivlahan DR. 2011. ‘Quality concerns with routine alcohol screening in VA clinical settings’, J Gen Intern Med, 26: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Rubinsky AD, Lapham GT, Berger D, Bryson C, Achtmeyer C, Hawkins EJ, Chavez LJ, Williams EC, and Kivlahan DR. 2016. ‘Predictive validity of clinical AUDIT-C alcohol screening scores and changes in scores for three objective alcohol-related outcomes in a Veterans Affairs population’, Addiction, 111: 1975–84. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, and Kivlahan DR. 2006. ‘Implementation of evidence-based alcohol screening in the Veterans Health Administration’, American Journal of Managed Care, 12: 597–606. [PubMed] [Google Scholar]
- Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, and Ghali WA. 2011. ‘Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies’, British Medical Journal, 342: d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CG, Thake J, and Vilhena N. 2010. ‘Social desirability biases in self-reported alcohol consumption and harms’, Addict Behav, 35: 302–11. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, and Ciol MA. 1992. ‘Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases’, Journal of Clinical Epidemiology, 45: 613–9. [DOI] [PubMed] [Google Scholar]
- Hawkins EJ, Kivlahan DR, Williams EC, Wright SM, Craig T, and Bradley KA. 2007. ‘Examining quality issues in alcohol misuse screening’, Substance Abuse, 28: 53–65. [DOI] [PubMed] [Google Scholar]
- Helfand M, and Carson S. 2008. Screening for Lipid Disorders in Adults: Selective Update of 2001 US Preventive Services Task Force Review (Rockville (MD)). [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders,. 2006. Improving the Quality of Health Care for Mental and Substance-Use Conditions: Quality Chasm Series (National Academies Press: Washington, DC: ). [PubMed] [Google Scholar]
- Johnson JA, Lee A, Vinson D, and Seale JP. 2013. ‘Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study’, Alcohol Clin Exp Res, 37 Suppl 1: E253–9. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Woychek A, Vaughan D, and Seale JP. 2013. ‘Screening for at-risk alcohol use and drug use in an emergency department: integration of screening questions into electronic triage forms achieves high screening rates’, Ann Emerg Med, 62: 262–6. [DOI] [PubMed] [Google Scholar]
- Kroenke K, and Spitzer RL. 2002. ‘The PHQ-9: a new depression diagnostic and severity measure’, Psychiatric Annals, 32: 509–15. [Google Scholar]
- Liker JK, Augustyniak S, and Duncan GJ. 1985. ‘Panel data and models of change: a comparison of first difference and conventional two-wave models’, Social Science Research, 14: 80–101. [Google Scholar]
- Marsden J, Tai B, Ali R, Hu L, Rush AJ, and Volkow N. 2019. ‘Measurement-based care using DSM-5 for opioid use disorder: Can we make opioid medication treatment more effective?’, Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, Brown ST, Freiberg MS, Gibert CL, Goetz MB, Kim JW, Pisani MA, Rimland D, Rodriguez-Barradas MC, Sico JJ, Tindle HA, and Crothers K. 2011. ‘Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source’, Nicotine Tob Res, 13: 1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis KA, Tate JP, Williams EC, Skanderson M, Bryant KJ, Gordon AJ, Kraemer KL, Maisto SA, Crystal S, Fiellin DA, and Justice AC. 2016. ‘Comparison of AUDIT-C collected via electronic medical record and self-administered research survey in HIV infected and uninfected patients’, Drug and Alcohol Dependence, 168: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Council for Behavioral Health. 2018. “Implementing Care for Alcohol & Other Drug Use in Medical Setting: An Extension of SBIRT.” In. Washington DC: National Council for Behavioral Health. [Google Scholar]
- Ornstein SM, Miller PM, Wessell AM, Jenkins RG, Nemeth LS, and Nietert PJ. 2013. ‘Integration and sustainability of alcohol screening, brief intervention, and pharmacotherapy in primary care settings’, Journal of Studies on Alcohol and Drugs, 74: 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm EB, Williams P, Fosher K, Criqui M, and Stampfer MJ. 1999. ‘Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors’, British Medical Journal, 319: 1523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose HL, Miller PM, Nemeth LS, Jenkins RG, Nietert PJ, Wessell AM, and Ornstein S. 2008. ‘Alcohol screening and brief counseling in a primary care hypertensive population: a quality improvement intervention’, Addiction, 103: 1271–80. [DOI] [PubMed] [Google Scholar]
- Rubinsky AD, Dawson DA, Williams EC, Kivlahan DR, and Bradley KA. 2013. ‘AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers’, Alcohol Clin Exp Res, 37: 1380–90. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr., Garcia FA, Gillman MW, Kemper AR, Krist AH, Kurth AE, Landefeld CS, LeFevre ML, Mangione CM, Phillips WR, Owens DK, Phipps MG, and Pignone MP. 2016. ‘Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement’, The Journal of the American Medical Association, 316: 1997–2007. [DOI] [PubMed] [Google Scholar]
- Unutzer J, Schoenbaum M, Druss BG, and Katon WJ. 2006. ‘Transforming mental health care at the interface with general medicine: report for the presidents commission’, Psychiatr Serv, 57: 37–47. [DOI] [PubMed] [Google Scholar]
- Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, Ludman EJ, Berger D, and Bradley KA. 2015. ‘Factors Underlying Quality Problems with Alcohol Screening Prompted by a Clinical Reminder in Primary Care: A Multi-site Qualitative Study’, Journal of General Internal Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Lapham G, Achtmeyer CE, Volpp B, Kivlahan DR, and Bradley KA. 2010. ‘Use of an electronic clinical reminder for brief alcohol counseling is associated with resolution of unhealthy alcohol use at follow-up screening’, J Gen Intern Med, 25 Suppl 1: 11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Lapham GT, Bobb JF, Rubinsky AD, Catz SL, Shortreed SM, Bensley KM, and Bradley KA. 2017. ‘Documented brief intervention not associated with resolution of unhealthy alcohol use one year later among VA patients living with HIV’, J Subst Abuse Treat, 78: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, McGinnis KA, Bobb JF, Rubinsky AD, Lapham GT, Skanderson M, Catz SL, Bensley KM, Richards JE, Bryant KJ, Edelman EJ, Satre DD, Marshall BDL, Kraemer KL, Blosnich JR, Crystal S, Gordon AJ, Fiellin DA, Justice AC, and Bradley KA. 2018. ‘Changes in alcohol use associated with changes in HIV disease severity over time: A national longitudinal study in the Veterans Aging Cohort’, Drug and Alcohol Dependence, 189: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Rubinsky AD, Chavez LJ, Lapham GT, Rittmueller SE, Achtmeyer CE, and Bradley KA. 2014. ‘An early evaluation of implementation of brief intervention for unhealthy alcohol use in the US Veterans Health Administration’, Addiction, 109: 1472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Characteristics of the study sample by change in AUDIT-C score at follow-up screening
Appendix B. Difference in change in HDL associated with each possible change in AUDIT-C, compared to no change
Appendix C. Graphical plot of the association between change in AUDIT-C score and change in HDL, by age group
Appendix D. Graphical plot of the association between change in AUDIT-C score and change in HDL, by any substance use disorder
Appendix E. Graphical plot of the association between change in AUDIT-C score and change in HDL among patients with HDL in the 90 days following each AUDIT-C