Iron is an essential element for life. While it plays fundamental roles in processes such as oxygen transport, DNA synthesis, and electron transport, it can also be toxic1. As such its homeostasis is tightly controlled. Disorders of iron metabolism are well recognized as a cause of disease in humans. Yet the role of iron in the context of atherosclerosis and coronary artery disease (CAD) remains uncertain, despite iron having been known to be present in atherosclerotic lesions for decades2. Sullivan proposed iron as a cardiovascular risk factor, suggesting modest increases in levels of stored iron, promote cardiovascular disease while relative iron deficiency might protect against it. The “iron hypothesis” was initially presented as an explanation for sex-based differences in cardiovascular disease with an increase in women after menopause3. The more basic idea was that free iron released from tissues might accelerate lipid peroxidation and inflammation through production of hydroxyl radicals via the Fenton reaction.
As our knowledge of the mechanisms involved in iron metabolism has improved, it appears that such an understanding might be overly simplistic. Movement of iron throughout the body is critical to maintaining cellular iron homeostasis. For the purposes of this discussion we will focus on the regulation of systemic and cellular iron. Macrophages are especially important in maintaining systemic iron levels4. Ferroportin (FPN) is the only known iron exporter and mediates exit of iron from macrophages into the circulation. This molecule is an extremely important mechanism for immediate control of available and circulating serum iron. The peptide hormone hepcidin is the key hormone regulating the expression of FPN. Hepcidin binds to FPN, inducing its internalization and degradation thus inhibiting cellular iron export from macrophages. The hepcidin-FPN axis is a major regulatory mechanism that maintains iron homeostasis and when dysregulated can cause disease5. In hereditary hemochromatosis, a disease of iron overload, either the expression or function of hepcidin is disturbed. In these situations, FPN is elevated because of low circulating hepcidin levels leading to increased gut iron absorption and pathologic deposition of iron in tissues.
One would expect states of iron overload such as this to lead to increased risk of atherosclerosis if Sullivan was correct. However, an autopsy study found the extent of CAD to be less in those with hemochromatosis than the general population while a clinical study of 9000 individuals found carriers of the hemochromatosis genotypes C282Y did not have an increased risk of CAD for ischemic heart disease or myocardial infarction6, 7. Furthermore, whether serum iron increases risk for CAD remains uncertain8–11. In ApoE−/− mice, dietary iron overload reduces rather than exacerbates the severity of atherosclerosis12. These and other data challenge the paradigm of iron as a pro-oxidant capable of accelerating CAD.
Likewise, data from experimental studies seems to contradict the iron hypothesis. Mice with genetic defects meant to mimic human hemochromatosis (i.e. genetic deficiency of the hemochromatosis gene HFE) show attenuated inflammatory responses with decreased macrophage tumor necrosis factor-alpha and Interleukin-6 after exposure to the toll-like receptor-4 (TLR-4) agonist lipopolysaccharide. Exposing wild type mouse macrophage to iron chelators reproduced these effects suggesting lowered intracellular iron within macrophages leads to impaired inflammatory TLR-4 signaling. Because hepcidin is a central molecule regulating macrophage intracellular iron levels, it would seem logical to target hepcidin to reduce atherosclerosis through its effects on lowering macrophage iron. Earlier work by our group examined the effect of inhibiting bone morphogenic protein (BMP) signaling to indirectly inhibit hepcidin. BMP signaling is involved in hepcidin gene transcription via SMAD 1/5/8 phosphorylation13. BMP inhibitors, such as LDN, potently inhibit hepcidin production by blocking BMP type-I receptors, ALK 2/3/6 preventing its downstream effects on SMAD14, 15. Effects of BMP inhibition using LDN inhibited macrophage inflammatory polarization and led to increased ATP binding cassette (ABC) transporters, decreased cytokine and reactive oxygen species (ROS) production and increased FPN production15, 16. Interestingly, LDN treatment inhibited atherosclerosis progression in transgenic ApoE knockout mice and increased serum iron suggesting its effect on plaque progression might be mediated via changes in macrophage function. Because LDN does not directly inhibit hepcidin but yields its effect by inhibiting upstream BMP signaling, other mechanisms for the decrease in atherosclerosis could not be ruled out.
In this issue of ATVB Malhotra et al. resolve some of the remaining issues regarding the role of hepcidin in atherogenesis using gene knock-out mouse model. Mice with combined deficiency of hepcidin (Hamp −/−) and the LDL receptor (LDLR−/−) demonstrated decreased atherosclerosis and a reduced macrophage pro-inflammatory phenotype in a 21 week high fat diet model17. Because hepcidin deficiency is associated with both increased serum iron and decreased macrophage iron, they also explored whether increasing serum iron might be the mechanism behind the anti-atherogenic effects rather than decreased macrophage iron. Hamp+/+/ Ldlr−/− mice were treated with iron dextran so as to produce a 2-fold increase in serum iron. However, increased serum iron did not decrease atherosclerosis. These data suggest that reduced pro-inflammatory phenotype in macrophages could be the key contributing to the vascular benefits of hepcidin inhibition17.
However, whether inhibiting hepcidin truly would reduce atherosclerosis is still uncertain especially in the context of human atherosclerosis. Different populations of macrophages have been detected within an atherosclerotic plaques18. A key mechanism of plaque progression is thought to be intraplaque hemorrhage (IPH) resulting from leakage of red cells from thin-walled microvessels which develop within advanced atherosclerosis plaques19. Iron metabolism is a key factor in the development of a special subtype of macrophage we have term M(Hb) which is found in these areas. While foam cell macrophages maintain higher intracellular iron levels, M(Hb), characterized by high CD163 hemoglobin:haptoglobin (HH) scavenger receptor expression, have reduced free intracellular iron and ROS levels likely due to increased export of free iron outside the cell via FPN. However other aspects of these cells may be pro-atherogenic. We recently showed that in human atherosclerotic lesions, M(Hb) macrophages were associated with plaque progression, microvascularity, and a high level of HIF-1α and VEGF-A expression20. A relationship between iron and the proangiogenic transcription factor HIF1α can be found through its interactions with the prolyl hydroxylase proteins (PHDs). Under conditions of normoxia or hyperoxia, HIF1α becomes hydroxylated by PHDs on proline residues 402 and 564, within its oxygen-dependent domain. This allows it to be recognized by the von Hippel-Lindau tumor-suppressor protein (pVHL), which targets it for ubiquitin-mediated degradation. While hypoxia is a well-known activator of HIF1α, indirectly controls the activity of HIF1α, because it is an essential cofactor for the activity of PHDs. Our work showed that within M(Hb) cells, activation of HIF1α via inhibition of iron-dependent PHDs promoted VEGF-mediated increases in intraplaque angiogenesis, vascular permeability, inflammatory cell recruitment, and plaque progression.
This work initially appears to contradict the findings of Malhotra et al. regarding genetic depletion of hepcidin. How then can we resolve this? An important unanswered question, of course, is whether hepcidin lowering increases the appearance of the proatherosclerotic M(Hb) cells. It also remains possible that hepcidin may have different roles in atherogenesis depending upon the stage of atherosclerosis. In advancing plaques inhibition of hepcidin may have a beneficial effect by affecting macrophage pro-inflammatory activity while in areas of IPH its effect may actually be detrimental. Such nuanced understanding would not have been seen in the mouse model used by Malhotra et al since in mice 21 weeks of high fat diet feeding does not result in IPH.
In conclusion, the results of the elegant work by Malhotra et al. advance our knowledge of the role of hepcidin and iron in atherosclerosis. However, more work needs to be done to understand completely the relationship between iron metabolism and atherosclerosis.
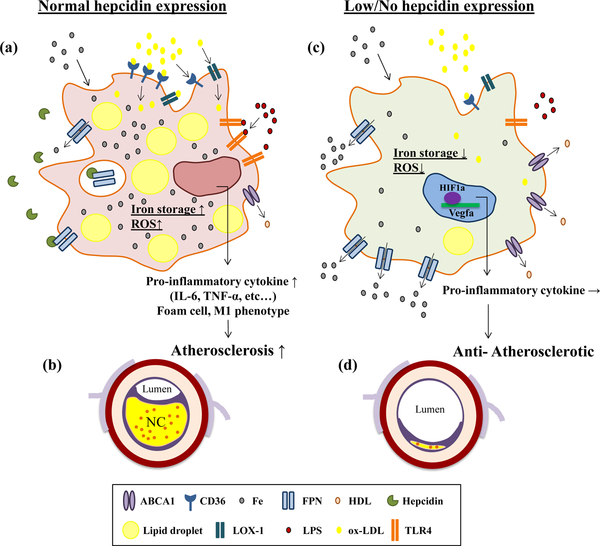
Figure 1. Effect of Hepcidin on Macrophages Phenotype and Function in the Setting of Atherosclerosis.
(a): Hepcidin inhibits iron release from macrophage via down-regulation of iron transporter ferroportin (FPN) and enhance intra-cellular iron storage. Elevated intra-cellular iron accumulation results in incorporation increased oxidized low-density lipoprotein cholesterol (ox-LDL) retention via transporter such as CD36 and LDL recepter-1 (LOX-1), and increased inflammatory signaling (LPS) via toll-like recepter-4 (TLR4), decreasing cholesterol efflux, and intra-cellular reactive oxygen species (ROS) generation. Overall these cells exhibit a phenotype consistent with pro-inflammatory foamy macrophage phenotype. In this condition, macrophages contribute to atherosclerosis progression (b).
(c): In the low/no hepcidin environment, intra-cellular iron is actively exported out of the macrophage via FPN. Lowering intracellular iron within the macrophage suppresses LDL uptake and increases its export via ABC transporters, lowers TLR4-dependent inflammatory signaling and ROS production. These effects are thought to be anti-atherogenic (d). At the same time low iron level enhance hypoxia inducible factor-1 alpha (HIF-1α) nuclear translocation, promoting vascular endothelial target gene expression, endothelial permeability, and inflammatory signaling. The latter effect may be pro-atherogenic especially in the setting of intraplaque hemorrhage.
Abbreviations: ABCA1, ATP-binding cassette sub-family A member 1; CD, cluster differentiation; Fe, iron; FPN, ferroportin; HIF1α, Hypoxia inducible factor-1 alpha; HDL, high-density lipoprotein; LOX-1, low-density lipoprotein recepter-1; LPS, lipopolysaccharide; NC, necrotic core; ox-LDL, oxidized low-density lipoprotein cholesterol; ROS, reactive oxygen species; TLR4, tall-like receptor; TNF-α, tumor necrosis factor-alpha; VEGF-α, vascular endothelial grows factor-alpha.
Acknowledgments
Source of Funding
This work was supported by CVPath Institute, Leducq Foundation Transatlantic Networks of Excellence Grant (18CVD02) to PlaqOmics Research Network (A.V.F.), and Research Rotation Program of the Medical Faculty of RWTH Aachen University (A.C.).
Disclosure
CVPath Institute has received institutional research support from 480 Biomedical, Abbott Vascular, ART, BioSensors International, Biotronik, Boston Scientific, Celonova, Claret Medical, Cook Medical, Cordis, Edwards Lifescience, Medtronic, MicroPort, MicroVention, Celonova, OrbusNeich, ReCore, SINO Medical Technology, Spectranetics, Surmodics, Terumo Corporation, W.L. Gore and Xeltis. A.V. Finn has sponsored research agreements with Boston Scientific and Medtronic CardioVascular and is an advisory board member to Medtronic CardioVascular. C.C.Hong has licensed the BMP inhibitor technology to La Jolla Pharmaceutical Co.
Footnotes
Editorial on ATVB paper “Hepcidin deficiency protects against Atherosclerosis” by Malhotra et al. 2018 Dec 27, :ATVBAHA118312215.
References
- 1.Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017;168:344–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Haehling S, Jankowska EA, Van Veldhuisen DJ, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nature Reviews Cardiology. 2015;12:659. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet (London, England). 1981;1:1293–1294 [DOI] [PubMed] [Google Scholar]
- 4.Soares MP, Hamza I. Macrophages and iron metabolism. Immunity. 2016;44:492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao N, Zhang AS, Enns CA. Iron regulation by hepcidin. The Journal of clinical investigation. 2013;123:2337–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller M, Hutchins GM. Hemochromatosis, multiorgan hemosiderosis, and coronary artery disease. Jama. 1994;272:231–233 [PubMed] [Google Scholar]
- 7.Ellervik C, Tybjaerg-Hansen A, Grande P, Appleyard M, Nordestgaard BG. Hereditary hemochromatosis and risk of ischemic heart disease: A prospective study and a case-control study. Circulation. 2005;112:185–193 [DOI] [PubMed] [Google Scholar]
- 8.Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern finnish men. Circulation. 1992;86:803–811 [DOI] [PubMed] [Google Scholar]
- 9.Sung KC, Kang SM, Cho EJ, Park JB, Wild SH, Byrne CD. Ferritin is independently associated with the presence of coronary artery calcium in 12,033 men. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2525–2530 [DOI] [PubMed] [Google Scholar]
- 10.Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: Prospective results from the bruneck study. Circulation. 1997;96:3300–3307 [DOI] [PubMed] [Google Scholar]
- 11.Gill D, Del Greco MF, Walker AP, Srai SKS, Laffan MA, Minelli C. The effect of iron status on risk of coronary artery disease: A mendelian randomization study-brief report. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:1788–1792 [DOI] [PubMed] [Google Scholar]
- 12.Kirk EA, Heinecke JW, LeBoeuf RC. Iron overload diminishes atherosclerosis in apoe-deficient mice. The Journal of clinical investigation. 2001;107:1545–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits bmp signals required for embryogenesis and iron metabolism. Nature chemical biology. 2008;4:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boergermann JH, Kopf J, Yu PB, Knaus P. Dorsomorphin and ldn-193189 inhibit bmp-mediated smad, p38 and akt signalling in c2c12 cells. The international journal of biochemistry & cell biology. 2010;42:1802–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saeed O, Otsuka F, Polavarapu R, Karmali V, Weiss D, Davis T, Rostad B, Pachura K, Adams L, Elliott J, Taylor WR, Narula J, Kolodgie F, Virmani R, Hong CC, Finn AV. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habib A, Polavarapu R, Karmali V, Guo L, Van Dam R, Cheng Q, Akahori H, Saeed O, Nakano M, Pachura K, Hong CC, Shin E, Kolodgie F, Virmani R, Finn AV. Hepcidin-ferroportin axis controls toll-like receptor 4 dependent macrophage inflammatory responses in human atherosclerotic plaques. Atherosclerosis. 2015;241:692–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhotra R, Wunderer F, Barnes HJ, Bagchi A, Buswell MD, O’Rourke CD, Slocum CL, Ledsky CD, Peneyra KM, Sigurslid H, Corman B, Johansson KB, Rhee DK, Bloch KD, Bloch DB. Hepcidin deficiency protects against atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2018: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butcher MJ, Galkina EV. Phenotypic and functional heterogeneity of macrophages and dendritic cell subsets in the healthy and atherosclerosis-prone aorta. Frontiers in physiology. 2012;3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:2054–2061 [DOI] [PubMed] [Google Scholar]
- 20.Guo L, Akahori H, Harari E, Smith SL, Polavarapu R, Karmali V, Otsuka F, Gannon RL, Braumann RE, Dickinson MH, Gupta A, Jenkins AL, Lipinski MJ, Kim J, Chhour P, de Vries PS, Jinnouchi H, Kutys R, Mori H, Kutyna MD, Torii S, Sakamoto A, Choi CU, Cheng Q, Grove ML, Sawan MA, Zhang Y, Cao Y, Kolodgie FD, Cormode DP, Arking DE, Boerwinkle E, Morrison AC, Erdmann J, Sotoodehnia N, Virmani R, Finn AV. Cd163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. The Journal of clinical investigation. 2018;128:1106–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]