Abstract
Background:
Diabetes is positively associated with various cancers, but its relationship with tumors of the esophagus/esophagogastric junction remains unclear.
Methods:
Data were harmonized across 13 studies in the International Barrett’s and Esophageal Adenocarcinoma Consortium, comprising 2309 esophageal adenocarcinoma (EA) cases, 1938 esophagogastric junction adenocarcinoma (EGJA) cases, 1728 Barrett’s esophagus (BE) cases, and 16,354 controls. Logistic regression was used to estimate study-specific odds ratios (ORs) and 95% CIs for self-reported diabetes in association with EA, EGJA, and BE. Adjusted ORs were then combined using random-effects meta-analysis.
Results:
Diabetes was associated with a 34% increased risk of EA (OR, 1.34; 95% CI, 1.00-1.80; I2 = 48.8% [where 0% indicates no heterogeneity, and larger values indicate increasing heterogeneity between studies]), 27% for EGJA (OR, 1.27; 95% CI, 1.05-1.55; I2 = 0.0%), and 30% for EA/EGJA combined (OR, 1.30; 95% CI, 1.06-1.58; I2 = 34.9%). Regurgitation symptoms modified the diabetes-EA/EGJA association (P for interaction = .04) with a 63% increased risk among participants with regurgitation (OR, 1.63; 95% CI, 1.19-2.22), but not among those without regurgitation (OR, 1.03; 95% CI, 0.74-1.43). No consistent association was found between diabetes and BE.
Conclusions:
Diabetes was associated with increased EA and EGJA risk, which was confined to individuals with regurgitation symptoms. Lack of an association between diabetes and BE suggests that diabetes may influence progression of BE to cancer.
Keywords: Barrett’s esophagus, diabetes, epidemiology, esophageal adenocarcinoma, meta-analysis
Condensed Abstract:
The positive association between diabetes and esophageal and esophagogastric junction adenocarcinomas, but not BE, suggests that diabetes may be acting later in the carcinogenesis pathway.
INTRODUCTION
Esophageal adenocarcinoma (EA) incidence has increased approximately 600% over the last 35 years and, until the recent time period, was one of the most rapidly increasing cancer types in the United States (US) and other Western countries.1–3 The incidence of the anatomically-linked esophagogastric junction adenocarcinoma (EGJA) has also increased but less rapidly than EA.4 EA and EGJA are often considered as similar clinical entities because both are cancers at or near the gastroesophageal junction, have similar 5-year survival rates, and have comparable survival according to tumor stage.5 The only known potential precursor of EA/EGJA is Barrett’s esophagus (BE), which is associated with 10–40-times increased risk of EA/EGJA.6–8 Among individuals over the age of 50 years with gastroesophageal reflux disease (GERD), an estimated 10% also have BE.9 In the last 30 years, BE diagnosis increased 200–300% – independent of the number of gastrointestinal endoscopies performed.10–12
Obesity has previously been associated with BE13 and EA/EGJA.14 Two hypotheses have been proposed for the association between obesity and these tumors: 1) mechanical effects on the integrity of the esophagogastric junction including both direct somatic pathways, whereby central adiposity promotes the development of GERD which may then promote BE development,15 and indirect pathways, whereby diabetes induces mechanophysiological changes in the esophagus which may then promote the development of tumors,16 or 2) systemic metabolic alterations due to obesity increasing the levels of various hormones, including insulin, which may promote the development of BE or the progression of BE to cancer.17
Type 2 diabetes, which accounts for 95% of diabetes diagnoses,18 is often characterized by hyperinsulinemia and has been associated with a higher risk of various cancers, including malignancies of the liver, pancreas, endometrium, colorectal, breast and bladder.19, 20 Diabetes has been increasing in prevalence, and 12% of adults in the US18, 19 and 9% worldwide21 have prevalent diabetes. However, the association between diabetes and EA/EGJA remains understudied,22 with several studies suggesting an increased risk23–28 and others showing no association.29–31 Additionally, few studies have assessed the association between diabetes and BE, with inconsistent results.31–35 Thus, we utilized studies from the International Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON, http://beacon.tlvnet.net/) to examine the associations of diabetes with risk of BE and EA/EGJA.
MATERIALS AND METHODS
Study Design and Population
This study draws on the resources of BEACON, which, as previously described,36 was formed to facilitate pooling of resources from epidemiologic studies focused on the Barrett’s esophagus/esophageal adenocarcinoma continuum. In brief, this consortium was formed in 2005 by an international group of investigators to provide an open epidemiologic research forum for all completed or ongoing population-based studies of EA or BE.36 Thusly, this consortium allows for pooled analysis of individual-level data and avoids reliance on only published data. The present study pools information from 13 BEACON studies that included assessment of diabetes: three cohort and six case-control studies examining EA or EGJA (Supplementary Table S1); and one cohort and five case-control studies examining BE (Supplementary Table S2). Two studies included examination of both BE and EA/EGJA.37, 38 Of the nine EA and EGJA studies, six were conducted in North America, two in Europe, and one in Australia. Of the six BE studies, three were conducted in North America, two in Europe, and one in Australia. Detailed descriptions of recruitment procedures can be found in study-specific publications.32, 37–48 Institutional review board or research ethics committee approval was obtained by each sponsoring institution.
Cases were categorized as EA, EGJA, or BE. We also combined EA and EGJA cases (EA/EGJA). Case eligibility was determined by the parent studies, based on endoscopy, pathology, and/or medical records. Histology and site determination were based on radiology, surgery, pathology, and/or endoscopy reports or linkage with a cancer registry.
For the cohort studies, a nested case-control approach was employed. A case to control ratio of 1:2 was used in the Netherlands Cohort Study (NLCS); 1:4 in the National Institutes of Health-AARP Diet and Health Study (NIH-AARP); and 1:8 in the Kaiser-Permanente Multiphasic Health Checkup Study (MHC). The EA/EGJA case-control studies recruited population-based controls, whereas the BE case-control studies recruited population-based and/or endoscopy controls.
The analytic study population was restricted to non-Hispanic whites because there were few cases from nonwhite ethnic groups (EA/EGJA: 60 black, 127 Hispanic, 77 other race or ethnic group; BE: 54 black, 23 Hispanic, 10 other race or ethnic group). Our pooled study population included 2309 EA cases, 1938 EGJA cases, 1728 BE cases, and 16,354 controls. Participants had a mean age of 60 years and were more likely to be men (73.5%).
Exposure
To provide comparable data from the parent studies, we harmonized responses from the nine EA/EGJA and six BE parent studies for diabetes and potential covariates. To assess diabetes, most used a variation of the question “Did a doctor ever tell you that you had diabetes?” Prior studies have shown that the agreement between self-reported diabetes compared to medical record review ranges from 83–98%.49–51 Additionally, the Newly Diagnosed Barrett’s Esophagus Study (NDB) also measured fasting glucose to determine undiagnosed diabetes, which resulted in 11 additional participants classified with diabetes (7.6% of total participants with diabetes in the NDB study).33 Only one study, Factors Influencing the Barrett’s/Adenocarcinoma Relationship Study (FINBAR),37 specifically asked about type 1 versus type 2 diabetes. For the studies with age at diabetes diagnosis available (five EA/EGJA studies and two BE studies), a diagnosis after age 30 was assumed to be type 2 diabetes, as most type 1 diabetes is diagnosed peri-puberty. Additionally, 95% of diabetes is type 2.18 Duration of type 2 diabetes in years was examined as a continuous variable for tests of linear trends and categorized as 0.5–3, >3–5, >5–10, and >10 years.23, 24 BEACON study responses for other covariates were harmonized and have been previously described for cigarette smoking,52, 53 alcohol consumption,54, 55 body mass index (BMI),13, 14 waist circumference,13, 14 non-steroidal anti-inflammatory drug use,56, 57 and reflux symptoms.58
Statistical Analyses
For our pooled study, we conducted a two-step analytic approach. First, we used multivariable unconditional (for case-control studies) or conditional (for matched case-control studies nested within cohorts) logistic regression to estimate study-specific odds ratios (ORs) and 95% confidence intervals (CIs) for the association between diabetes and EA, EGJA, combined EA/EGJA, or BE. Then, the study-specific estimates were pooled using meta-analytic techniques.59 Estimates generated with fixed-effects and random-effects models were similar. Given that the latter are more robust to study heterogeneity,60 we only present random-effects models. Between-study heterogeneity was assessed using a χ2 test based on the Q statistic and the I2 statistic (where 0% indicates no heterogeneity and larger values indicate increasing heterogeneity between studies).61 While investigating differences across studies includes quantification of heterogeneity, the most critical aspects are ensuring that the studies are comparable in clinical and methodologic aspects,61 as BEACON was designed. To demonstrate that there was no selection bias (i.e., “publication bias”), funnel plots were visually inspected for asymmetry and quantitatively assessed using Begg and Mazumdar’s rank correlation test62 and Egger’s linear regression test.63 To determine the influence of individual studies, we conducted a meta-influence analysis, whereby we excluded one study at a time and re-estimated the summary effect estimates.
Potential confounders64 included age (at diagnosis for cases and interview for controls), sex, education (<high school, high school graduate/vocational school, college graduate for BE studies; study-specific education categories for EA and EGJA studies), cigarette smoking (ever, never; current, former, never; duration in years; and pack-years), alcohol consumption (drinks per day), BMI (kg/m2), waist circumference (cm), non-steroidal anti-inflammatory drug use (ever/never), and fruit and vegetable consumption (servings per day). Age, sex, and BMI were included a priori as confounders. For the other potential confounders, if the log OR changed by ≥10% due to variable elimination in any parent study, the variable was considered a confounder and retained in all models.64 Final models included age (continuous), sex, smoking status (ever, never), and BMI (continuous).
We considered effect measure modification by sex, BMI (<25/≥25 kg/m2), smoking (ever/never), and age (<60/≥60 years). Additionally, report of heartburn (retrosternal burning), regurgitation, and any reflux symptoms (i.e., heartburn or regurgitation) were assessed as potential modifiers (ever/never). Heartburn symptoms were defined as burning or aching pain behind the breastbone/sternum, and regurgitation symptoms were defined as a sour taste in the mouth resulting from regurgitation of acid, bile or other stomach contents. In the NLCS, reflux symptoms were defined as any report of heartburn, hiatal hernia, esophagitis, gastritis, or an esophageal or stomach/duodenal condition treated with antacids or H2 antagonists. For analyses of effect measure modification, we used a pooled dataset of individual-level data with additional adjustment for parent study, instead of the two-step meta-analytic approach. Departures from the multiplicative null were evaluated through stratification and by using likelihood ratio tests from nested logistic regression models.64 Departures from the additive null were approximated by calculating the relative excess risk due to interaction (RERIOR = OR11 – OR10 – OR01 + 1; null hypothesis = 0), synergy index (SOR = (OR11 – 1)/((OR10 – 1) + (OR01 – 1)); null hypothesis = 1), and the attributable proportion due to interaction, which assumes a causal relationship (APOR = RERIOR/OR11; null hypothesis = 0).65, 66
Sensitivity Analysis
As not all studies assessed heartburn and regurgitation symptoms, we conducted additional effect measure modification models that were restricted to studies that ascertained both of these exposures. For EA/EGJA, this included the Australian Cancer Study (ACS), FINBAR, and the Los Angeles County Multi-ethnic Case-control Study (LAS). For BE, this included FINBAR, NDB, the Study of Digestive Health (SDH), and the Epidemiologic Case-Control Study of Barrett’s Esophagus (UNC). We also examined heartburn and regurgitation symptoms categorized as “none”, “weekly”, and “≥weekly”, the latter of which we term “recurrent” symptoms. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and Stata version 14.0 (StataCorp LP, College Station, TX).
RESULTS
Descriptive characteristics of cases and controls are shown in Table 1. Both EA and EGJA cases were more likely than the population-based controls to be smokers (79.2 and 79.7 vs. 63.6%) and had a slightly higher BMI (28.0 and 27.4 vs. 26.4 kg/m2). In the six studies with symptoms available, EA and EGJA cases were more likely to have reported reflux symptoms than controls (58.1 and 58.8 vs. 45.2%). In the BE studies, mean BMI was comparable between the BE cases and population-based controls (27.8 vs. 27.4 kg/m2, respectively) but lower than the endoscopy-based controls (29.1 kg/m2). However, BE cases had a higher proportion of smokers (67.7%) compared with both control groups (60.7 and 61.5%, respectively). Prevalence of reflux symptoms was similar between BE cases and endoscopy controls (56.3 vs. 66.5%), but lower in population-based controls (24.6%).
Table 1.
Descriptive characteristics of the pooled study population, Barrett’s and Esophageal Adenocarcinoma Consortium.
| Barrett’s Esophagus Population |
Adenocarcinoma Population |
|||||
|---|---|---|---|---|---|---|
| Study | BE Cases (n=1,728) | Controls |
Adenocarcinoma Cases |
Controls (n=11,841) | ||
| Population-based (n=2,830) | GERD/Endoscopy (n=1,683) | EA (n=2,309) | EGJA (n=1,938) | |||
|
|
||||||
| Age, years (SD) | 60.5 (9.0) | 60.5 (7.9) | 57.5 (11.8) | 62.3 (8.8) | 62.0 (8.7) | 60.1 (9.4) |
| Sex, n (%) | ||||||
| Male | 1,256 (72.7) | 2,073 (73.2) | 1,231 (73.1) | 2,059 (89.2) | 1,666 (86.0) | 8,127 (68.6) |
| Female | 472 (27.3) | 757 (26.8) | 452 (26.9) | 250 (10.8) | 272 (14.0) | 3,713 (31.4) |
| Missing | 0 | 0 | 0 | 0 | 0 | 1 |
| Cigarette Smoking, n (%) | ||||||
| Never | 547 (32.3) | 1,103 (39.3) | 624 (38.5) | 471 (20.8) | 383 (20.3) | 4,187 (36.4) |
| Ever | 1,147 (67.7) | 1,700 (60.7) | 995 (61.5) | 1,792 (79.2) | 1,505 (79.7) | 7,324 (63.6) |
| Missing | 34 | 27 | 64 | 46 | 50 | 330 |
| Body Mass Index, kg/m2 (SD) | 27.8 (4.8) | 27.4 (5.1) | 29.1 (5.8) | 28.0 (4.9) | 27.4 (4.7) | 26.4 (4.4) |
| Missing, n | 41 | 60 | 31 | 52 | 47 | 298 |
The associations between diabetes and risk of EA/EGJA are shown in Table 2 and Figure 1. In multivariable analyses, a diabetes diagnosis was associated with a 34% increased risk of EA (OR=1.34, 95% CI: 1.00–1.80; I2=48.8%), a 27% increased risk of EGJA (OR=1.27, 95% CI: 1.05–1.55; I2=0.0%), and a 30% increased risk of EA/EGJA (OR=1.30, 95% CI: 1.06–1.58; I2=34.9%). Results were robust in the meta-influence analysis, dropping one study at a time (Supplementary Figure S1). Selection bias of studies included in BEACON was unlikely as assessed examination of the funnel plots and by the Begg and Mazumdar’s (p>0.05) and Egger’s tests (p>0.05) (Supplementary Figure S3). When the analysis was restricted to individuals with self-reported or suspected type 2 diabetes (i.e., classifying individuals with a self-report of diabetes after age 30 years as type 2), compared to individuals without a diabetes diagnosis, diabetes was associated with a non-significant 17% increased risk of EA, EGJA, and EA/EGJA (Table 2). There was no trend between duration of type 2 diabetes and EA (ptrend=0.3), EGJA (ptrend=0.5), or EA/EGJA (ptrend=0.3).
Table 2.
Adjusteda odds ratios (ORs) and 95% confidence intervals (CIs) for associations between diabetes and esophageal and esophagogastric junction adenocarcinomas.
| Esophageal Adenocarcinoma |
|||||
|---|---|---|---|---|---|
| Case N |
Control N |
OR (95% CI) |
I2 (%) |
Pheterogeneity |
|
| Diabetes | |||||
| No | 1,942 | 7,577 | Referent | ||
| Yes | 238 | 554 | 1.34 (1.00–1.80) | 48.8 | 0.05 |
| Type 2 Diabetes b | |||||
| No | 928 | 5,444 | Referent | ||
| Yes | 147 | 544 | 1.17 (0.90–1.51) | 0.0 | 0.8 |
| Duration of type 2 diabetes | |||||
| 0.5-3 years | 38 | 174 | 1.01 (0.67–1.52) | 0.0 | 0.8 |
| >3-5 years | 14 | 39 | 1.31 (0.67–2.56) | 0.0 | 0.7 |
| >5-10 years | 47 | 148 | 1.33 (0.89–1.97) | 0.0 | 0.9 |
| >10 years | 45 | 183 | 1.22 (0.78–1.90) | 19.6 | 0.3 |
|
| |||||
| Esophagogastric Junction Adenocarcinomac | |||||
|
|
|||||
| Diabetes | |||||
| No | 1,622 | 6,584 | Referent | ||
| Yes | 184 | 463 | 1.27 (1.05–1.55) | 0.0 | 0.6 |
| Type 2 Diabetes b | |||||
| No | 972 | 4,918 | Referent | ||
| Yes | 123 | 459 | 1.17 (0.91–1.51) | 0.0 | 0.6 |
| Duration of type 2 diabetes | |||||
| 0.5-3 years | 35 | 138 | 1.29 (0.84–1.99) | 0.0 | 0.7 |
| >3-5 years | 11 | 39 | 1.12 (0.45–2.74) | 27.2 | 0.2 |
| >5-10 years | 32 | 119 | 1.31 (0.68–2.54) | 43.7 | 0.1 |
| >10 years | 42 | 163 | 1.12 (0.66–1.92) | 40.7 | 0.1 |
|
| |||||
| All Esophageal and Esophagogastric Junction Adenocarcinomas | |||||
|
|
|||||
| Diabetes | |||||
| No | 3,564 | 10,498 | Referent | ||
| Yes | 422 | 735 | 1.30 (1.06–1.58) | 34.9 | 0.1 |
| Type 2 Diabetes b | |||||
| No | 1,900 | 6,699 | Referent | ||
| Yes | 270 | 734 | 1.17 (0.96–1.42) | 0.0 | 0.7 |
| Duration of type 2 diabetes | |||||
| 0.5-3 years | 73 | 250 | 1.12 (0.82–1.54) | 0.0 | 0.6 |
| >3-5 years | 25 | 39 | 1.08 (0.60–1.93) | 4.2 | 0.4 |
| >5-10 years | 79 | 202 | 1.37 (1.00–1.88) | 0.0 | 0.7 |
| >10 years | 87 | 243 | 1.09 (0.67–1.76) | 56.0 | 0.04 |
Adjusted for age (continuous), sex, smoking (never, ever), and body mass index (continuous).
Available for ACS, BKW, FINBAR, LAS, LEO, and a subset of NIH-AARP.
PMCC does not include EGJA.
Figure 1.
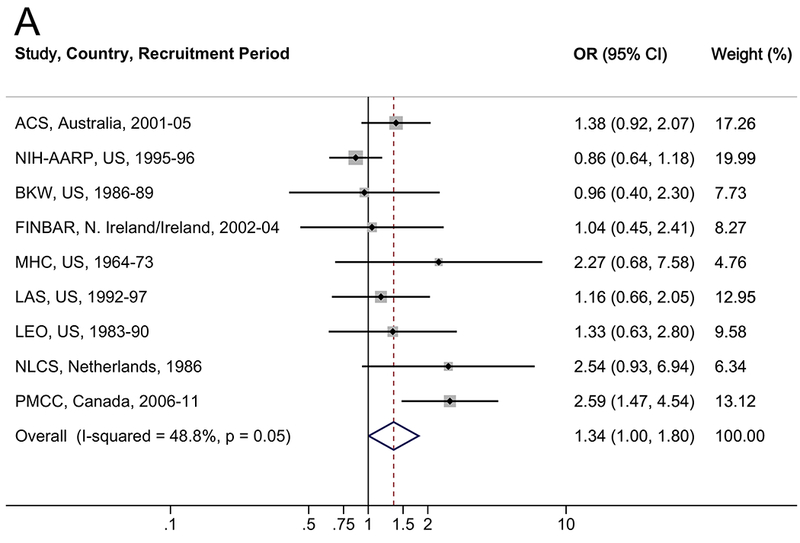
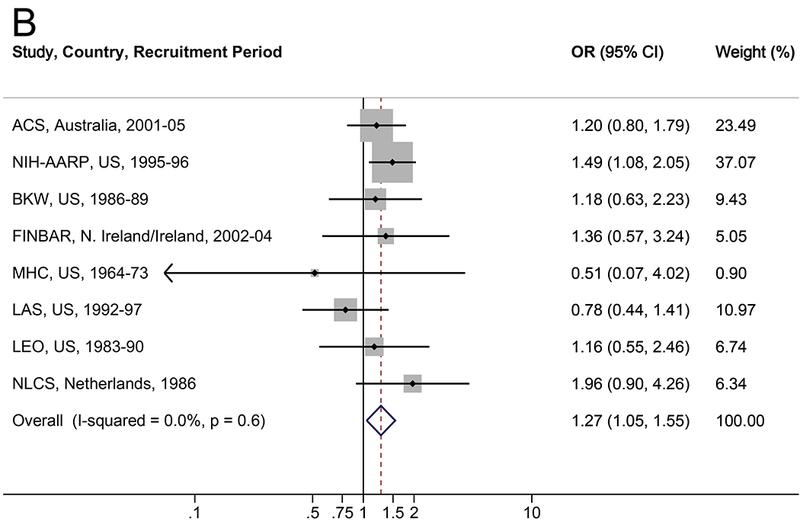
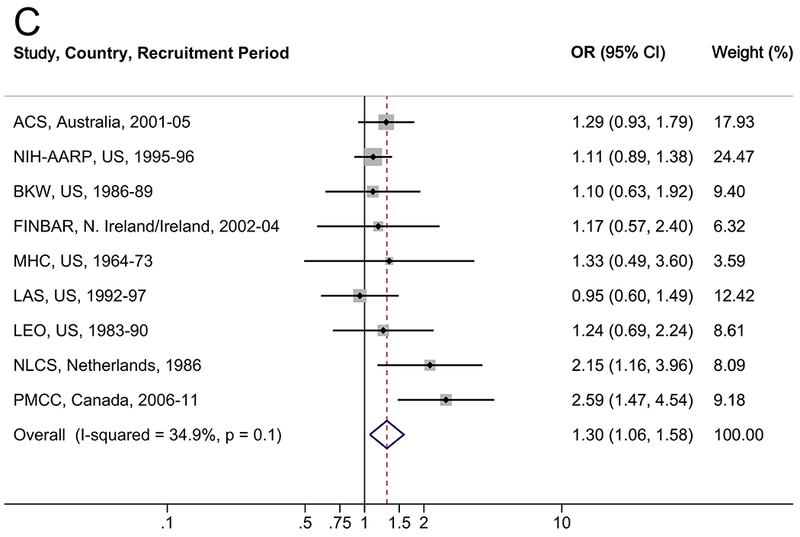
Forrest plot of the relationship between diabetes and (A) esophageal adenocarcinoma, (B) esophagogastric junction adenocarcinoma, and (C) all esophageal and esophagogastric junction adenocarcinomas, by random effects model adjusted for age, sex, smoking (never, ever), and body mass index (continuous).
As shown in Table 3, the association between diabetes and risk of EA/EGJA was modified by regurgitation symptoms (pinteraction=0.04). Diabetes was associated with a 63% increased risk of EA/EGJA among individuals with regurgitation symptoms (OR=1.63, 95% CI: 1.19–2.22), but there was no association between diabetes and EA/EGJA among individuals without regurgitation symptoms (OR=1.03, 95% CI: 0.74–1.43). When cross-classified, the group with diabetes but without regurgitation symptoms was not at an increased risk of EA/EGJA (OR=1.03, 95% CI: 0.74–1.43), the group with regurgitation but without diabetes was at a 2-times increased risk of EA/EGJA (OR=2.10, 95% CI: 1.81–2.45), and the group with both regurgitation and diabetes was at a 3.4-times increased risk of EA/EGJA (OR=3.42, 95% CI: 2.49–4.69), each compared with the referent group of individuals without diabetes or regurgitation (data not tabulated). Regurgitation and diabetes provided a relative excess risk due to interaction (RERI) of 1.29 (95% CI: 0.22–2.36, p=0.02), a synergy index of 2.14 (95% CI: 1.23–3.72, p=0.007), and an attributable proportion due to interaction of 0.38 (95% CI: 0.16–0.59, p=0.001) in relation to the risk of EA/EGJA. Results were similar when we restricted the analyses to only those studies with information on both symptoms of heartburn and regurgitation (Supplemental Table S3). When we examined regurgitation and heartburn symptoms categorized as “none”, “<weekly” (non-recurrent), and “≥weekly” (recurrent), there was also evidence of effect modification in relation to EA/EGJA (pinteraction=0.009, Supplemental Table S4), with the increased risk observed among individuals reporting non-recurrent (<weekly) regurgitation symptoms (OR=1.77, 95% CI: 1.17–2.70) and no associations among individuals reporting recurrent (≥weekly) regurgitation symptoms (OR=1.10, 95% CI: 0.64–1.90) or reporting no symptoms (OR=0.84, 95% CI: 0.57–1.24). There was little evidence of effect modification of the diabetes-adenocarcinoma association by BMI, smoking, and sex (Table 3).
Table 3.
Adjusteda odds ratios (ORs) and 95% confidence intervals (CIs) for interaction between diabetes and other risk factors and esophageal and esophagogastric junction adenocarcinomas.
| Esophageal Adenocarcinoma |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Diabetes |
Diabetes |
||||||||
| Case N |
Control N |
OR (95% CI) |
Case N |
Control N |
OR (95% CI) |
Pinteraction |
|||
| Reflux Symptoms b | |||||||||
| No | 374 | 3,202 | Referent | 40 | 157 | 1.51 (1.01–2.25) | |||
| Yes | 625 | 2,082 | Referent | 99 | 130 | 1.56 (1.14–2.12) | 0.9 | ||
| Regurgitation c | |||||||||
| No | 313 | 1,409 | Referent | 47 | 118 | 1.26 (0.84–1.88) | |||
| Yes | 429 | 1,186 | Referent | 76 | 84 | 1.67 (1.16–2.41) | 0.3 | ||
| Heartburn b | |||||||||
| No | 438 | 3,553 | Referent | 54 | 183 | 1.61 (1.13–2.29) | |||
| Yes | 554 | 1,717 | Referent | 83 | 104 | 1.51 (1.07–2.12) | 0.8 | ||
| Sex | |||||||||
| Male | 1,730 | 5,323 | Referent | 215 | 427 | 1.17 (0.97–1.41) | |||
| Female | 212 | 2,254 | Referent | 23 | 127 | 1.44 (0.89–2.34) | 0.4 | ||
| BMI | |||||||||
| <25 kg/m2 | 549 | 33,237 | Referent | 37 | 124 | 1.39 (0.93–2.07) | |||
| ≥25 kg/m2 | 1,393 | 4,341 | Referent | 201 | 430 | 1.16 (0.96–1.41) | 0.4 | ||
| Smoking | |||||||||
| Never | 404 | 2,780 | Referent | 49 | 176 | 1.36 (0.95–1.94) | |||
| Ever | 1,538 | 4,798 | Referent | 189 | 378 | 1.15 (0.95–1.41) | 0.4 | ||
| Age | |||||||||
| <60 years | 668 | 3,333 | Referent | 53 | 151 | 1.31 (0.93–1.87) | |||
| ≥60 years | 1,274 | 4,245 | Referent | 185 | 403 | 1.16 (0.95–1.41) | 0.5 | ||
|
| |||||||||
| Esophagogastric Junction Adenocarcinomad | |||||||||
|
|
|||||||||
| Reflux Symptoms b | |||||||||
| No | 392 | 2,985 | Referent | 26 | 139 | 0.99 (0.63–1.56) | |||
| Yes | 469 | 2,047 | Referent | 56 | 127 | 1.32 (0.93–1.86) | 0.3 | ||
| Regurgitation c | |||||||||
| No | 249 | 1,169 | Referent | 22 | 98 | 0.73 (0.44–1.20) | |||
| Yes | 338 | 1,174 | Referent | 45 | 83 | 1.47 (0.99–2.20) | 0.03 | ||
| Heartburn b | |||||||||
| No | 493 | 3,345 | Referent | 40 | 165 | 1.12 (0.77–1.63) | |||
| Yes | 362 | 1,685 | Referent | 42 | 101 | 1.28 (0.86–1.90) | 0.6 | ||
| Sex | |||||||||
| Male | 1,383 | 4,857 | Referent | 165 | 378 | 1.32 (1.08–1.62) | |||
| Female | 239 | 1,978 | Referent | 19 | 106 | 1.21 (0.72–2.03) | 0.8 | ||
| BMI | |||||||||
| <25 kg/m2 | 561 | 2,914 | Referent | 29 | 127 | 0.98 (0.64–1.49) | |||
| ≥25 kg/m2 | 1,061 | 3,922 | Referent | 155 | 357 | 1.41 (1.14–1.74) | 0.1 | ||
| Smoking | |||||||||
| Never | 333 | 2,541 | Referent | 31 | 159 | 1.26 (0.84–1.91) | |||
| Ever | 1,289 | 4,295 | Referent | 153 | 325 | 1.32 (1.07–1.63) | 0.9 | ||
| Age | |||||||||
| <60 years | 574 | 2,969 | Referent | 45 | 135 | 1.31 (0.91–1.89) | |||
| ≥60 years | 1,048 | 3,867 | Referent | 139 | 349 | 1.30 (1.05–1.62) | 1.0 | ||
|
| |||||||||
| All Esophageal and Esophagogastric Junction Adenocarcinoma | |||||||||
|
|
|||||||||
| Reflux Symptoms b | |||||||||
| No | 766 | 3,202 | Referent | 66 | 157 | 1.24 (0.91–1.72) | |||
| Yes | 1,094 | 2,082 | Referent | 155 | 130 | 1.49 (1.15–1.94) | 0.4 | ||
| Regurgitation c | |||||||||
| No | 562 | 1,409 | Referent | 69 | 118 | 1.03 (0.74–1.43) | |||
| Yes | 767 | 1,186 | Referent | 121 | 84 | 1.63 (1.19–2.22) | 0.04 | ||
| Heartburn b | |||||||||
| No | 931 | 3,553 | Referent | 94 | 183 | 1.35 (1.02–1.78) | |||
| Yes | 916 | 1,717 | Referent | 125 | 104 | 1.46 (1.09–1.95) | 0.7 | ||
| Sex | |||||||||
| Male | 3,128 | 7,135 | Referent | 380 | 552 | 1.25 (1.08–1.44) | |||
| Female | 452 | 3,321 | Referent | 42 | 182 | 1.31 (0.91–1.88) | 0.8 | ||
| BMI | |||||||||
| <25 kg/m2 | 1,110 | 4,445 | Referent | 66 | 174 | 1.17 (0.86–1.58) | |||
| ≥25 kg/m2 | 2,470 | 6,012 | Referent | 356 | 560 | 1.28 (1.10–1.49) | 0.6 | ||
| Smoking | |||||||||
| Never | 740 | 3,818 | Referent | 80 | 240 | 1.34 (1.01–1.77) | |||
| Ever | 2,840 | 6,639 | Referent | 342 | 494 | 1.23 (1.05–1.43) | 0.6 | ||
| Age | |||||||||
| <60 years | 925 | 2,966 | Referent | 71 | 134 | 1.32 (1.02–1.72) | |||
| ≥60 years | 1,650 | 3,859 | Referent | 257 | 349 | 1.23 (1.05–1.43) | 0.6 | ||
Adjusted for age (continuous), sex, smoking (never, ever), and body mass index (continuous).
Available for ACS, FINBAR, MHC, LAS, NLCS, and PMCC.
Available for ACS, FINBAR, LAS, and PMCC.
PMCC does not include EGJA.
The association between diabetes and BE is shown in Table 4 and Figure 2. There was no consistent association between diabetes and BE when compared to population-based controls (OR=0.87, 95% CI: 0.67–1.13; I2=18.3%) or endoscopy controls (OR=0.93, 95% CI: 0.71–1.20; I2=0.0%). Results were robust in the meta-influence analysis, dropping one study at a time (Supplementary Figure S2). Selection bias of studies included in BEACON was unlikely as assessed examination of the funnel plots and by the Begg and Mazumdar’s (p>0.05) and Egger’s tests (p>0.05) (Supplementary Figure S4). Similarly, when results were restricted to individuals with self-reported or suspected type 2 diabetes, there was no association between diabetes and BE (Table 4). No trend was found between duration of type 2 diabetes and BE compared to population-based (ptrend=0.7) or endoscopy controls (ptrend=0.8). There was no evidence of effect measure modification between diabetes and reflux symptoms, sex, BMI, smoking, or age in relation to BE (Table 5 and Supplemental Table S5).
Table 4.
Adjusteda odds ratios (ORs) and 95% confidence intervals (CIs) for the association between diabetes and Barrett’s esophagus, compared to population-based and endoscopy controls, respectively.
| Population-based Controls |
|||||
|---|---|---|---|---|---|
| BE Case N |
Control N |
OR (95% CI) |
I2 (%) |
Pheterogeneity |
|
| Diabetes | |||||
| No | 1,325 | 2,438 | Referent | ||
| Yes | 158 | 301 | 0.87 (0.67–1.13) | 18.3 | 0.3 |
| Type 2 Diabetes b | |||||
| No | 709 | 913 | Referent | ||
| Yes | 116 | 144 | 1.00 (0.64–1.56) | 49.3 | 0.1 |
| Duration of type 2 diabetes | |||||
| 0.5-3 years | 35 | 27 | 1.74 (0.73–4.15) | 48.3 | 0.1 |
| >3-5 years | 19 | 25 | 0.85 (0.46–1.60) | 0.0 | 0.5 |
| >5-10 years | 25 | 31 | 0.98 (0.44–2.20) | 42.4 | 0.2 |
| >10 years | 21 | 42 | 0.58 (0.33–1.01) | 0.0 | 0.6 |
|
| |||||
| Endoscopy Controls | |||||
|
|
|||||
| Diabetes | |||||
| No | 816 | 1,195 | Referent | ||
| Yes | 130 | 268 | 0.93 (0.71–1.20) | 0.0 | 1.0 |
| Type 2 Diabetes b | |||||
| No | 709 | 1006 | Referent | ||
| Yes | 116 | 239 | 0.97 (0.73–1.28) | 0.0 | 1.0 |
| Duration of type 2 diabetes | |||||
| 0.5-3 years | 35 | 55 | 1.11 (0.52–2.36) | 48.3 | 0.1 |
| >3-5 years | 19 | 34 | 1.05 (0.55–2.00) | 0.0 | 0.5 |
| >5-10 years | 25 | 58 | 0.77 (0.45–1.31) | 0.0 | 0.5 |
| >10 years | 21 | 44 | 0.90 (0.51–1.57) | 0.0 | 0.5 |
Adjusted for age (continuous), sex, smoking (never, ever), and body mass index (continuous).
Available for FINBAR, SDH, and VAT.
Figure 2.
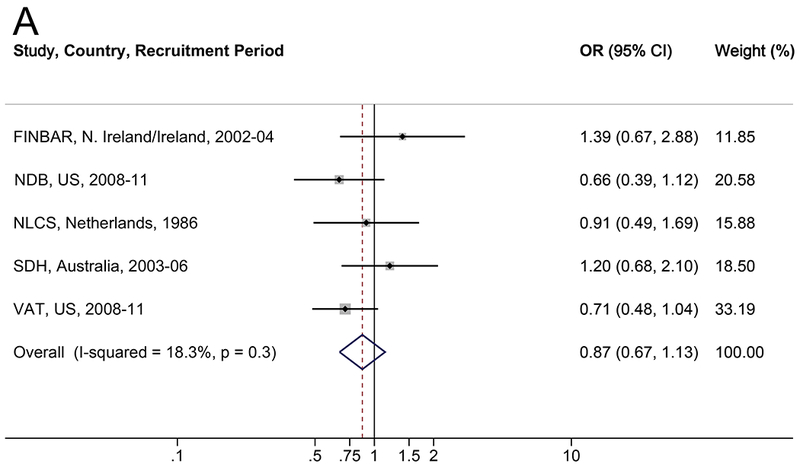
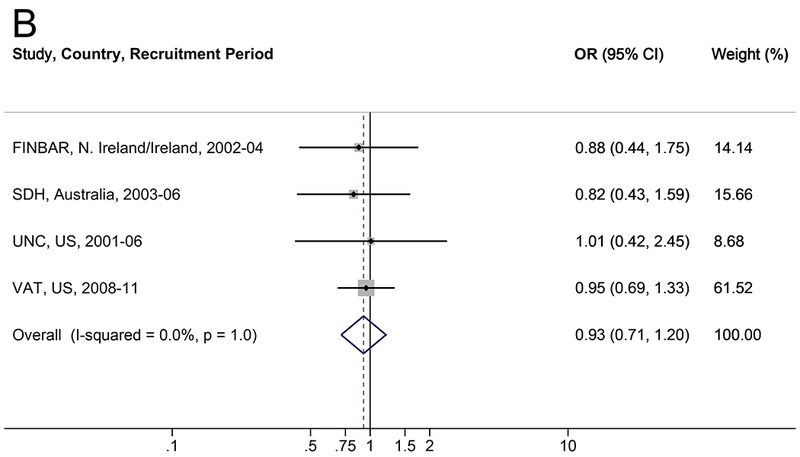
Forrest plot of the relationship between diabetes and Barret155t’s esophagus, versus (A) population controls and (B) GERD/endoscopy controls, by random effects model adjusted for age (continuous), sex, smoking (never, ever), and body mass index (continuous).
Table 5.
Adjusteda odds ratios (ORs) and 95% confidence intervals (CIs) for interaction between diabetes and other risk factors and Barrett’s esophagus, compared to population-based and endoscopy controls, respectively.
| Population-based Controls |
|||||||
|---|---|---|---|---|---|---|---|
| Non-Diabetes |
Diabetes |
||||||
| BE Case N |
Control N |
OR (95% CI) |
BE Case N |
Control N |
OR (95% CI) |
Pinteraction |
|
| Reflux Symptoms | |||||||
| No | 622 | 1,844 | Referent | 60 | 215 | 0.91 (0.66–1.27) | |
| Yes | 703 | 594 | Referent | 98 | 86 | 0.73 (0.52–1.02) | 0.3 |
| Regurgitation b | |||||||
| No | 201 | 925 | Referent | 27 | 134 | 1.00 (0.63–1.59) | |
| Yes | 434 | 342 | Referent | 40 | 31 | 0.98 (0.59–1.64) | 1.0 |
| Heartburn | |||||||
| No | 708 | 1,962 | Referent | 62 | 227 | 0.85 (0.62–1.18) | |
| Yes | 617 | 476 | Referent | 96 | 74 | 0.77 (0.54–1.10) | 0.9 |
| Sex | |||||||
| Male | 945 | 1,730 | Referent | 140 | 272 | 0.79 (0.62–1.02) | |
| Female | 380 | 708 | Referent | 18 | 29 | 1.01 (0.55–1.86) | 0.5 |
| BMI | |||||||
| <25 kg/m2 | 446 | 955 | Referent | 21 | 33 | 1.21 (0.68–2.15) | |
| ≥25 kg/m2 | 879 | 1,483 | Referent | 137 | 268 | 0.78 (0.61–1.00) | 0.2 |
| Smoking | |||||||
| Never | 425 | 981 | Referent | 42 | 101 | 0.87 (0.59–1.30) | |
| Ever | 900 | 1,457 | Referent | 116 | 200 | 0.80 (0.61–1.05) | 0.7 |
| Age | |||||||
| <60 years | 523 | 1,088 | Referent | 41 | 86 | 0.93 (0.61–1.41) | |
| ≥60 years | 802 | 1,350 | Referent | 117 | 215 | 0.78 (0.60–1.02) | 0.5 |
|
| |||||||
| Endoscopy Controls | |||||||
|
|
|||||||
| Non-Diabetes |
Diabetes |
||||||
| BE Case N |
Control N |
OR (95% CI) |
BE Case N |
Control N |
OR (95% CI) |
Pinteraction |
|
| Reflux Symptoms | |||||||
| No | 149 | 383 | Referent | 35 | 108 | 1.05 (0.67–1.65) | |
| Yes | 662 | 802 | Referent | 95 | 160 | 0.87 (0.64–1.17) | 0.5 |
| Regurgitation c | |||||||
| No | 170 | 264 | Referent | 14 | 14 | 1.14 (0.52–2.49) | |
| Yes | 429 | 359 | Referent | 39 | 38 | 0.76 (0.46–1.25) | 0.9 |
| Heartburn | |||||||
| No | 191 | 429 | Referent | 36 | 111 | 1.01 (0.65–1.56) | |
| Yes | 603 | 737 | Referent | 93 | 156 | 0.87 (0.64–1.18) | 1.0 |
| Sex | |||||||
| Male | 633 | 842 | Referent | 114 | 233 | 0.92 (0.70–1.21) | |
| Female | 183 | 353 | Referent | 16 | 35 | 0.98 (0.51–1.90) | 0.8 |
| BMI | |||||||
| <25 kg/m2 | 225 | 305 | Referent | 17 | 23 | 1.03 (0.52–2.06) | |
| ≥25 kg/m2 | 591 | 890 | Referent | 113 | 245 | 0.91 (0.69–1.19) | 0.7 |
| Smoking | |||||||
| Never | 264 | 489 | Referent | 35 | 77 | 1.11 (0.70–1.76) | |
| Ever | 552 | 706 | Referent | 95 | 191 | 0.85 (0.64–1.15) | 0.3 |
| Age | |||||||
| <60 years | 366 | 655 | Referent | 36 | 77 | 1.17 (0.74–1.84) | |
| ≥60 years | 450 | 540 | Referent | 94 | 191 | 0.84 (0.62–1.13) | 0.2 |
Adjusted for age (continuous), sex, smoking (never, ever), body mass index (continuous), and parent study.
Available for FINBAR, NDB, and SDH.
Available for FINBAR, SDH, and UNC.
DISCUSSION
In this pooled analysis of 13 studies from BEACON, diabetes was associated with a 27–34% increased risk of EA/EGJA but not BE. In the studies of EA/EGJA, we also report a synergistic interaction between diabetes and regurgitation symptoms. Compared to those without diabetes or regurgitation, individuals with diabetes and regurgitation had a 3.4-times increased risk of EA/EGJA. Finally, we did not find that a longer duration of type 2 diabetes was associated with an increased risk of EA/EGJA or BE. The increased risk of EA/EGJA, but not BE, associated with diabetes suggests that diabetes may be acting later in the carcinogenesis pathway.
The current study is the largest study of EA or EGJA reported to date with information on diabetes status of study participants. One prior meta-analysis examined the association between diabetes and esophageal cancer (all histologic types), but only three of the studies included in the meta-analysis were specific for EA.22 The other studies included were either esophageal squamous cell carcinoma, the other primary type of esophageal cancer which has a different etiology,67 or a mixture of histological subtypes. A number of previous studies have suggested that diabetes increases the risk of EA,23–28 while others showed no association.29–31 Of the three prior studies showing no association between diabetes and EA, one is included in the current pooled analysis.30 Another was conducted in the UK Clinical Practice Research Datalink, which is a large electronic medical record database.31 It is unclear why our results would be discrepant, but a further study included herein from Northern Ireland and Ireland (FINBAR) also found little to no association between diabetes and EA, suggesting potential geographic heterogeneity. The third and final previously published study that reported no association between diabetes and EA/EGJA included fewer cases (n=311) than we report herein (n=1,249).29 Moreover, it was conducted in a population of US veterans with GERD which provided an unusually high prevalence of diabetes (36% in EA/EGJA cases, 32% in controls) compared with our study (12% in EA/EGJA cases, 6% in controls), as well as other factors including male sex (90%),68 overweight or obesity (78%),69 and ever smoking (68%),70 which reduces the generalizability of its findings.
In the current study, we report a synergistic interaction between diabetes and symptomatic regurgitation but not overall reflux symptoms (defined as heartburn or regurgitation symptoms). Three prior studies that assessed this interaction reported that reflux did not modify the diabetes-EA/EGJA association.23, 28, 31 However, two of these studies defined GERD as coded in medical records and were not able to stratify by heartburn or regurgitation symptoms.28, 31
Few studies have assessed the association between diabetes and BE, and the results have been inconsistent.31–35 Two of the previous studies, which are included in the current analysis, found little to no association between diabetes and BE.32, 33 Similarly, two studies conducted within the UK Clinical Practice Research Datalink offer little evidence for any association between diabetes and BE.31, 34 However, after further adjustment for BMI, smoking, and GERD, Iyer et al. reported a 50% increased risk of BE associated with diabetes.34 Another study using SEER-Medicare data reported diabetes was associated with an increased risk of BE when compared with population controls, but not endoscopy negative controls.35 In the current study, we report no association between diabetes and BE regardless of the control group. If diabetes conferred no increased risk of progression from BE to adenocarcinoma, then the diabetes-EA/EGJA association would be approximately equal to that observed for diabetes-BE. However, as we observed an association between diabetes and EA/EGJA, but not BE, this suggests that diabetes may influence the risk of progression of BE to cancer. The synergistic interaction observed between diabetes and regurgitation symptoms also indicates that diabetes may be promoting progression from reflux or BE to EA/EGJA.
Our pooled study is the first to indicate that the association between diabetes and EA/EGJA may be strongest among those with regurgitation symptoms. Reasons for diabetes and regurgitation synergistically increasing risk of EA/EGJA are not completely understood. However, putative mechanisms of diabetes-induced mechanophysiological changes may explain our observation,16 including impaired lower esophageal sphincter relaxation71, 72 and dysfunction of the esophageal body (e.g., dysfunctional peristalsis) which may result in decreased motility,73, 74 antral spasms and gastroparesis75 providing more opportunities for reflux. Diabetes and hyperglycemia may also be associated with gastroparesis via diabetic (vagal) neuropathy,76 which is thought to result from modification of proteins by advanced glycation end-products leading to development of atrophy and degeneration of nerve fibers.16
The interaction between diabetes and regurgitation but not heartburn symptoms may be explained by diabetic neuropathy; GERD patients with diabetes report less frequent acid regurgitation symptoms than GERD patients without diabetes,77, 78 while prior research indicates little to no decline in frequency of heartburn symptoms in GERD patients with diabetes.77, 78 Symptomatic regurgitation compared to heartburn is associated with a higher proximal extent of the liquid reflux component,79 which has been utilized as a proxy measure of increased contact time between the esophageal mucosa and gastric refluxate.80 Assuming asymptomatic regurgitation is also more severe than heartburn, individuals with diabetes may be experiencing regurgitation symptoms less frequently due to diabetic neuropathy, but they may still be exposed to high levels of gastric refluxate if they are experiencing asymptomatic regurgitation. When we stratified on frequency of reflux symptoms, we found that the association between diabetes and EA/EGJA was primarily confined to individuals with non-recurrent regurgitation symptoms, suggesting that they may be experiencing asymptomatic reflux. These putative mechanisms aptly explain why we may observe a synergistic interaction between diabetes and regurgitation in relation to EA/EGJA without an independent association between diabetes and cancer risk.
This study has several limitations. First, few BEACON studies assessed age at diabetes diagnosis or differentiated between type 1 and type 2 diabetes. Thus, to improve our assessment of type 2 diabetes, we restricted our analysis to diabetes diagnosed after age 30 years as a proxy for type 2 diabetes. Therefore, some degree of misclassification is likely, as type 1 diabetes can be diagnosed at any age. However, type 1 diabetes is most commonly diagnosed under age 20 and only accounts for 5% of all diabetes diagnoses;18 thus, misclassification of exposure is likely to be minimal. In addition, the little information on diabetes medication usage collected in the parent BEACON studies was of insufficient detail to examine. For example, one questionnaire asked participants if they had diabetes treated with insulin injections or if they had diabetes treated with tablets and/or diet.40, 41 Thus, it was not possible to accurately discern whether individuals were treated with tablets (and if so, what type), dietary changes, or did not receive treatment for diagnosed diabetes. Moreover, different anti-diabetic drugs are heterogeneous with respect to their cancer effects, and hence detailed information would be needed to assess effects on EA/EGJA. We also did not have information on degree of diabetes control, such as hemoglobin A1c. The majority of participants in BEACON were males of European descent, and therefore the results may not be generalizable to women or non-Europeans. That said, the overwhelming majority of individuals that develop EA and EGJA are white males;81 thus, these findings are still likely relevant to those at highest risk of developing these cancers. Finally, the current study included populations with both high and low prevalence of diabetes, in the controls this ranged from 2.7% in Kaiser-Permanente Multiphasic Health Checkup Study (MHC)43 to 39.8% in the Houston Barrett’s Esophagus Study (VAT).32 However, we did not observe substantial between-study heterogeneity in the risk estimates, and results were robust in the meta-influence analysis. Additionally, this study included case-control and cohort studies. The diabetes diagnoses case-control studies may be subject to differential misclassification of exposure, whereby cases could be diagnosed incidentally when undergoing testing for a cancer diagnosis and controls would not be diagnosed. However, stratifying the results by case-control versus cohort studies resulted in nearly identical estimates for junctional adenocarcinomas (data not shown).
The large sample size of our pooled study improved the precision of our effect estimates and allowed us to investigate potential effect modification by reflux symptoms, sex, BMI, smoking, and age. However, the number of cases for the stratified analyses was still relatively small. Additionally, utilizing BEACON resources, we were able to examine the precursor lesion of BE and invasive cancer, allowing us to speculate about the possible timing of when diabetes may have an effect in the BE-adenocarcinoma continuum. Finally, our study utilized the pooled meta-analytic approach, which yields less biased effect estimates because we were able to use individual-level, uniform definitions for the exposure and the covariates across all parent studies (in contrast to the standard meta-analytic approach, which must rely on published group information only).
In summary, our large pooled study provides evidence that diabetes is associated with an increased risk of EA/EGJA, but not BE, suggesting that diabetes may influence the risk of progression of BE to cancer. The increased risk for EA/EGJA was primarily confined to individuals with regurgitation symptoms, which acted synergistically with diabetes. Future studies should evaluate whether the synergistic association between diabetes and regurgitation symptoms persists in individuals with well-controlled diabetes and elucidate mechanisms that underlie these observations.
Supplementary Material
Funding and Acknowledgements:
This work was supported by National Institutes of Health (NIH) Intramural Research Program, National Cancer Institute; NIH grants (K23DK079291, R01CA001833, R01CA116845, K23DK059311, R01DK063616, K08DK002697, R01CA059636, R01CA030022, R37CA041530, U01CA057949, R01CA109193, U01CA199336, U54CA163059); Ireland-Northern Ireland Co-operation Research Project grant sponsored by Research & Development Office (Belfast, Northern Ireland) and Health Research Board (Dublin, Ireland) and by Ulster Cancer Foundation (Belfast, Northern Ireland); The Netherlands Cancer Foundation and the Dutch Digestive Disease Foundation; Queensland Cancer Fund and the National Health and Medical Research Council (NHMRC) of Australia (Program No 199600); Kaiser Permanente Community Benefit Grant; California Tobacco Related Research Program (3RT-0122); and U.S. Department of Veteran’s Affairs Clinical Science Research and Development (I01-CX000899). We thank Michael Spriggs from Information Management Services, Inc., for data preparation.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62: 118–128. [DOI] [PubMed] [Google Scholar]
- 2.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97: 142–146. [DOI] [PubMed] [Google Scholar]
- 3.Islami F, DeSantis CE, Jemal A. Incidence Trends of Esophageal and Gastric Cancer Subtypes by Race, Ethnicity, and Age in the United States, 1997–2014. Clin Gastroenterol Hepatol. 2019;17: 429–439. [DOI] [PubMed] [Google Scholar]
- 4.Devesa SS, Blot WJ, Fraumeni JF, Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83: 2049–2053. [PubMed] [Google Scholar]
- 5.Wijnhoven BP, Siersema PD, Hop WC, van Dekken H, Tilanus HW. Adenocarcinomas of the distal oesophagus and gastric cardia are one clinical entity. Rotterdam Oesophageal Tumour Study Group. Br J Surg. 1999;86: 529–535. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127: 310–330. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. Jama. 2002;287: 1972–1981. [DOI] [PubMed] [Google Scholar]
- 8.Cook MB, Coburn SB, Lam JR, Taylor PR, Schneider JL, Corley DA. Cancer incidence and mortality risks in a large US Barrett’s oesophagus cohort. Gut. 2018;67: 418–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieberman DA, Oehlke M, Helfand M. Risk factors for Barrett’s esophagus in community-based practice. GORGE consortium. Gastroenterology Outcomes Research Group in Endoscopy. Am J Gastroenterol. 1997;92: 1293–1297. [PubMed] [Google Scholar]
- 10.van Soest EM, Dieleman JP, Siersema PD, Sturkenboom MC, Kuipers EJ. Increasing incidence of Barrett’s oesophagus in the general population. Gut. 2005;54: 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prach AT, MacDonald TA, Hopwood DA, Johnston DA. Increasing incidence of Barrett’s oesophagus: education, enthusiasm, or epidemiology? Lancet. 1997;350: 933. [DOI] [PubMed] [Google Scholar]
- 12.Coleman HG, Bhat S, Murray LJ, McManus D, Gavin AT, Johnston BT. Increasing incidence of Barrett’s oesophagus: a population-based study. Eur J Epidemiol. 2011;26: 739–745. [DOI] [PubMed] [Google Scholar]
- 13.Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s oesophagus: a pooled analysis from the international BEACON consortium. Gut. 2013;62: 1684–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol. 2012;41: 1706–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riddell RH. The genesis of Barrett esophagus: has a histologic transition from gastroesophageal reflux disease-damaged epithelium to columnar metaplasia ever been seen in humans? Arch Pathol Lab Med. 2005;129: 164–169. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Gregersen H. Diabetes-induced mechanophysiological changes in the esophagus. Ann N Y Acad Sci. 2016;1380: 139–154. [DOI] [PubMed] [Google Scholar]
- 17.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4: 579–591. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. [Google Scholar]
- 19.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33: 1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350: g7607. [DOI] [PubMed] [Google Scholar]
- 21.Roglic G, World Health Organization. Global report on diabetes. Geneva, Switzerland: World Health Organization, 2016. [Google Scholar]
- 22.Huang W, Ren H, Ben Q, Cai Q, Zhu W, Li Z. Risk of esophageal cancer in diabetes mellitus: a meta-analysis of observational studies. Cancer Causes Control. 2012;23: 263–272. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Bernstein L, Tseng CC, Wu AH. Diabetes and risk of esophageal and gastric adenocarcinomas. Int J Cancer. 2012;131: 1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neale RE, Doecke JD, Pandeya N, et al. Does type 2 diabetes influence the risk of oesophageal adenocarcinoma? Br J Cancer. 2009;100: 795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng KK, Sharp L, McKinney PA, et al. A case-control study of oesophageal adenocarcinoma in women: a preventable disease. Br J Cancer. 2000;83: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reavis KM, Morris CD, Gopal DV, Hunter JG, Jobe BA. Laryngopharyngeal reflux symptoms better predict the presence of esophageal adenocarcinoma than typical gastroesophageal reflux symptoms. Ann Surg. 2004;239: 849–856; discussion 856–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agrawal S, Patel P, Agrawal A, Makhijani N, Markert R, Deidrich W. Metformin use and the risk of esophageal cancer in Barrett esophagus. South Med J. 2014;107: 774–779. [DOI] [PubMed] [Google Scholar]
- 28.Drahos J, Ricker W, Pfeiffer RM, Cook MB. Metabolic syndrome and risk of esophageal adenocarcinoma in elderly patients in the United States: An analysis of SEER-Medicare data. Cancer. 2017;123: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubenstein JH, Davis J, Marrero JA, Inadomi JM. Relationship between diabetes mellitus and adenocarcinoma of the oesophagus and gastric cardia. Aliment Pharmacol Ther. 2005;22: 267–271. [DOI] [PubMed] [Google Scholar]
- 30.Lin SW, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. Prospective study of self-reported diabetes and risk of upper gastrointestinal cancers. Cancer Epidemiol Biomarkers Prev. 2011;20: 954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drahos J, Li L, Jick SS, Cook MB. Metabolic syndrome in relation to Barrett’s esophagus and esophageal adenocarcinoma: Results from a large population-based case-control study in the Clinical Practice Research Datalink. Cancer Epidemiol. 2016;42: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thrift AP, Hilal J, El-Serag HB. Metabolic syndrome and the risk of Barrett’s oesophagus in white males. Aliment Pharmacol Ther. 2015;41: 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubenstein JH, Morgenstern H, McConell D, et al. Associations of diabetes mellitus, insulin, leptin, and ghrelin with gastroesophageal reflux and Barrett’s esophagus. Gastroenterology. 2013;145: 1237–1244 e1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iyer PG, Borah BJ, Heien HC, Das A, Cooper GS, Chak A. Association of Barrett’s esophagus with type II Diabetes Mellitus: results from a large population-based case-control study. Clin Gastroenterol Hepatol. 2013;11: 1108–1114 e1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drahos J, Ricker W, Parsons R, Pfeiffer RM, Warren JL, Cook MB. Metabolic syndrome increases risk of Barrett esophagus in the absence of gastroesophageal reflux: an analysis of SEER-Medicare Data. J Clin Gastroenterol. 2015;49: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cronin-Fenton DP, Murray LJ, Whiteman DC, et al. Reproductive and sex hormonal factors and oesophageal and gastric junction adenocarcinoma: a pooled analysis. Eur J Cancer. 2010;46: 2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson LA, Johnston BT, Watson RG, et al. Nonsteroidal anti-inflammatory drugs and the esophageal inflammation-metaplasia-adenocarcinoma sequence. Cancer Res. 2006;66: 4975–4982. [DOI] [PubMed] [Google Scholar]
- 38.van den Brandt PA, Goldbohm RA, van ‘t Veer P, Volovics A, Hermus RJ, Sturmans F. A large-scale prospective cohort study on diet and cancer in The Netherlands. J Clin Epidemiol. 1990;43: 285–295. [DOI] [PubMed] [Google Scholar]
- 39.Rubenstein JH, Inadomi JM, Scheiman J, et al. Association between Helicobacter pylori and Barrett’s esophagus, erosive esophagitis, and gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol. 2014;12: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith KJ, O’Brien SM, Green AC, Webb PM, Whiteman DC, Study of Digestive H. Current and past smoking significantly increase risk for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2009;7: 840–848. [DOI] [PubMed] [Google Scholar]
- 41.Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57: 173–180. [DOI] [PubMed] [Google Scholar]
- 42.Brown LM, Silverman DT, Pottern LM, et al. Adenocarcinoma of the esophagus and esophagogastric junction in white men in the United States: alcohol, tobacco, and socioeconomic factors. Cancer Causes Control. 1994;5: 333–340. [DOI] [PubMed] [Google Scholar]
- 43.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States). Cancer Causes Control. 2001;12: 721–732. [DOI] [PubMed] [Google Scholar]
- 45.Vaughan TL, Davis S, Kristal A, Thomas DB. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 1995;4: 85–92. [PubMed] [Google Scholar]
- 46.O’Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut. 2012;61: 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spreafico A, Coate L, Zhai R, et al. Early adulthood body mass index, cumulative smoking, and esophageal adenocarcinoma survival. Cancer Epidemiol. 2017;47: 28–34. [DOI] [PubMed] [Google Scholar]
- 48.Shaheen NJ, Green B, Medapalli RK, et al. The perception of cancer risk in patients with prevalent Barrett’s esophagus enrolled in an endoscopic surveillance program. Gastroenterology. 2005;129: 429–436. [DOI] [PubMed] [Google Scholar]
- 49.Campbell PT, Deka A, Jacobs EJ, et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology. 2010;139: 1138–1146. [DOI] [PubMed] [Google Scholar]
- 50.Jackson JM, DeFor TA, Crain AL, et al. Validity of diabetes self-reports in the Women’s Health Initiative. Menopause. 2014;21: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colditz GA, Willett WC, Stampfer MJ, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 1990;132: 501–513. [DOI] [PubMed] [Google Scholar]
- 52.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst. 2010;102: 1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook MB, Shaheen NJ, Anderson LA, et al. Cigarette smoking increases risk of Barrett’s esophagus: an analysis of the Barrett’s and Esophageal Adenocarcinoma Consortium. Gastroenterology. 2012;142: 744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freedman ND, Murray LJ, Kamangar F, et al. Alcohol intake and risk of oesophageal adenocarcinoma: a pooled analysis from the BEACON Consortium. Gut. 2011;60: 1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thrift AP, Cook MB, Vaughan TL, et al. Alcohol and the risk of Barrett’s esophagus: a pooled analysis from the International BEACON Consortium. Am J Gastroenterol. 2014;109: 1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012;142: 442–452 e445; quiz e422–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thrift AP, Anderson LA, Murray LJ, et al. Nonsteroidal Anti-Inflammatory Drug Use is Not Associated With Reduced Risk of Barrett’s Esophagus. Am J Gastroenterol. 2016;111: 1528–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cook MB, Corley DA, Murray LJ, et al. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: a pooled analysis from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON). PLoS One. 2014;9: e103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith-Warner SA, Spiegelman D, Ritz J, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163: 1053–1064. [DOI] [PubMed] [Google Scholar]
- 60.Poole C, Greenland S. Random-effects meta-analyses are not always conservative. Am J Epidemiol. 1999;150: 469–475. [DOI] [PubMed] [Google Scholar]
- 61.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50: 1088–1101. [PubMed] [Google Scholar]
- 63.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 65.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3: 452–456. [DOI] [PubMed] [Google Scholar]
- 66.VanderWeele TJ, Knol MJ. A Tutorial on Interaction. Epidemiol. Methods. 2014;3: 33–72. [Google Scholar]
- 67.Blot WJ, Tarone RE. Esophageal Cancer. In: Thun MJ, Linet MS, Cerhan JR, Haiman C, Schottenfeld D, editors. Schottenfeld and Fraumeni Cancer Epidemiology and Prevention. New York, NY: Oxford University Press, 2018:p. 579–592. [Google Scholar]
- 68.National Center for Veterans Analysis and Statistics. VA Utilization Profile FY 2016, 2017. [Google Scholar]
- 69.The Management of Overweight and Obesity Working Group. VA/DoD Clinical Practice Guideline for Screening and Management of Overweight and Obesity Guideline Summary, 2014. [Google Scholar]
- 70.Office of the Assistant Deputy Under Secretary for Health for Policy and Planning. 2011 Survey of Veteran Enrollees’ Health and Reliance Upon VA, 2011. [Google Scholar]
- 71.Zhang Q, Horowitz M, Rigda R, Rayner C, Worynski A, Holloway RH. Effect of hyperglycemia on triggering of transient lower esophageal sphincter relaxations. Am J Physiol Gastrointest Liver Physiol. 2004;286: G797–803. [DOI] [PubMed] [Google Scholar]
- 72.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24: 371–381. [DOI] [PubMed] [Google Scholar]
- 73.Kinekawa F, Kubo F, Matsuda K, et al. Relationship between esophageal dysfunction and neuropathy in diabetic patients. Am J Gastroenterol. 2001;96: 2026–2032. [DOI] [PubMed] [Google Scholar]
- 74.Kahrilas PJ, Dodds WJ, Hogan WJ. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology. 1988;94: 73–80. [DOI] [PubMed] [Google Scholar]
- 75.Samsom M, Akkermans LM, Jebbink RJ, van Isselt H, vanBerge-Henegouwen GP, Smout AJ. Gastrointestinal motor mechanisms in hyperglycaemia induced delayed gastric emptying in type I diabetes mellitus. Gut. 1997;40: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feldman M, Corbett DB, Ramsey EJ, Walsh JH, Richardson CT. Abnormal gastric function in longstanding, insulin-dependent diabetic patients. Gastroenterology. 1979;77: 12–17. [PubMed] [Google Scholar]
- 77.Sakitani K, Suzuki N, Ihara S, et al. Decline in perception of acid regurgitation symptoms from gastroesophageal reflux disease in diabetes mellitus patients. PLoS One. 2018;13: e0194466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Promberger R, Lenglinger J, Riedl O, et al. Gastro-oesophageal reflux disease in type 2 diabetics: symptom load and pathophysiologic aspects - a retro-pro study. BMC Gastroenterol. 2013;13: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bredenoord AJ, Weusten BL, Curvers WL, Timmer R, Smout AJ. Determinants of perception of heartburn and regurgitation. Gut. 2006;55: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sifrim D Relevance of volume and proximal extent of reflux in gastro-oesophageal reflux disease. Gut. 2005;54: 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Falk GW. Risk factors for esophageal cancer development. Surg Oncol Clin N Am. 2009;18: 469–485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


