Abstract
Myofibroblasts are key contributors to pathological fibrotic conditions of several major organs. Fibroblast transdifferentiation into myofibroblasts requires both a mechanical signal and transforming growth factor beta (TGF-β) signaling. The cation channel transient receptor potential vanilloid 4 (TRPV4) is a critical mediator of myofibroblast transdifferentiation and in vivo fibrosis through its mechanosensitivity to extracellular matrix stiffness. Here, we show that TRPV4 promoted transdifferentiation of human and mouse lung myofibroblasts through interaction with phosphoinositide 3-kinase γ (PI3Kγ), forming nanomolar-affinity, intracellular TRPV4-PI3Kγ complexes. TGF-β induced the recruitment of TRPV4-PI3Kγ complexes to the plasma membrane and increased the activities of both TRPV4 and PI3Kγ. Using gain- and loss-of-function approaches, we show that both TRPV4 and PI3Kγ proteins were required for myofibroblast transdifferentiation as measured by increased production of α-smooth muscle actin and its incorporation into stress fibers, cytoskeletal changes, collagen I production, and contractile force. Expression of various mutant forms of the PI3Kγ catalytic subunit (p110γ) in cells lacking PI3Kγ revealed that only the non-catalytic, amino-terminal domain of p110γ was necessary and sufficient for TGF-β–induced TRPV4 plasma membrane recruitment and myofibroblast transdifferentiation. These data demonstrate that TGF-β stimulates a non-canonical scaffolding action of PI3Kγ, which recruits TRPV4-PI3Kγ complexes to the plasma membrane, thereby increasing myofibroblast transdifferentiation. Given that both TRPV4 and PI3Kγ have pleiotropic actions, targeting the interaction between them could provide a specific therapeutic approach for inhibiting myofibroblast transdifferentiation.
Introduction
Fibroproliferative diseases most prominently affect the heart, vasculature, kidney, liver, and lungs, and collectively account for over 45% of the overall mortality in the United States (1–4). Myofibroblasts play a major role in fibroproliferative diseases by secreting extracellular matrix proteins and pro-fibrotic cytokines, and through their contractile function (5, 6). The mechanisms that drive myofibroblast generation from fibroblasts and their persistence remain an area of active investigation (7). The two main signals required for myofibroblast generation are mechanical signaling and active transforming growth factor–β (TGF-β) (8, 9).
The process by which a cell transduces extracellular mechanical stimuli into intracellular chemical signals is known as mechanotransduction (10, 11). Mechanotransduced signals affect many vital cell functions, including cell fate, proliferation, migration, apoptosis, and survival (10, 12, 13). Emerging work demonstrates that cells utilize integrins and stretch-sensitive plasma membrane ion channels to transduce mechanical signals that are then integrated with signals from soluble ligands through growth factor or G protein–coupled receptors (GPCRs) (10, 14). However, the specifics of the mechanical signal, the sensing capabilities, the precise receptors, the phenotypic cell responses, and the intracellular pathways involved are highly context-dependent and poorly understood.
Transient receptor potential vanilloid 4 (TRPV4) is a ubiquitous mechanosensitive cation channel that functions in the plasma membrane. TRPV4 is activated by a wide range of chemical [for example, 4α-phorbol-12,13-didecanoate (4α-PDD) and arachidonic acid metabolites] and physiological (such as hypotonicity, cell swelling, and heat) stimuli (15). Previous work from our lab revealed that TRPV4 action drives the TGF-β–induced transdifferentiation of fibroblasts into myofibroblasts that underlies pulmonary fibrosis in vivo and is dysregulated in idiopathic pulmonary fibrosis (IPF) in humans (16). We further showed that TRPV4 drives myofibroblast transdifferentiation, in part through promoting extracellular calcium (Ca2+) influx in a mechanosensitive manner, over a physiological range of matrix stiffness (16). Furthermore, this effect occurred through crosstalk with SMAD-independent, non-canonical TGF-β signaling (16). TRPV4 has large intracellular amino- and carboxy-terminal regions that have been shown to interact with several intracellular signaling pathways (15). Here, we aimed to identify the intracellular molecules with which TRPV4 interacts to drive myofibroblast transdifferentiation and thereby in vivo organ fibrosis. We found that TGF-β–driven, TRPV4-dependent transdifferentiation of human and mouse lung fibroblasts required the non-catalytic, amino-terminal domain of phosphoinositide 3-kinase γ (PI3Kγ), in order to form TRPV4-PI3Kγ complexes. Upon TGF-β stimulation, TRPV4 and PI3Kγ were mutually required for one another’s accumulation at the plasma membrane and for lung fibroblasts to transdifferentiate. Targeting the interaction between TRPV4 and PI3Kγ may disrupt fibrogenic processes that contribute to organ fibrosis in vivo.
Results
The mechanosensitive ion channel TRPV4 mediates TGF-β–induced PI3Kγ activity
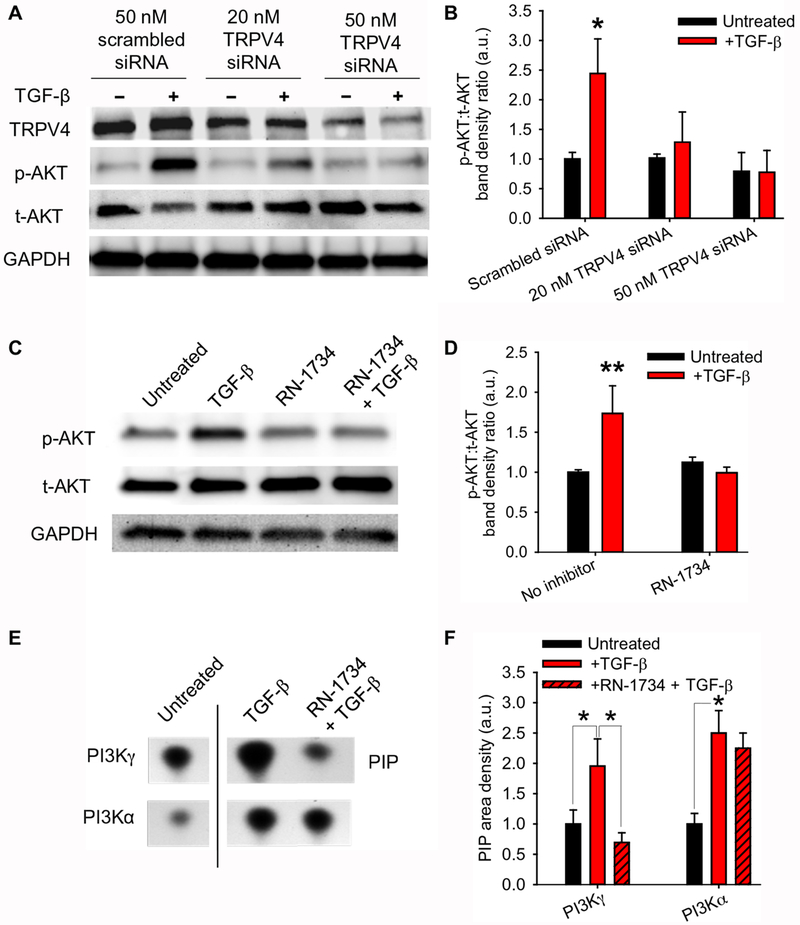
We previously showed that TRPV4 activity is essential for TGF-β–driven myofibroblast transdifferentiation in a manner that depends on matrix stiffness but does not depend on the mediators of canonical TGF-β signaling SMAD2 and SMAD3 (SMAD2/3) (16). Because there is evidence indicating that activation of the phosphoinositide 3-kinase (PI3K) pathway is sensitive to matrix stiffness (17), we examined whether PI3K pathway activation occurred downstream of TRPV4 activation. Knocking down TRPV4 in human lung fibroblasts (HLFs; specifically, 19Lu cells, plated on plastic) with small interfering RNAs (siRNAs) or treating the cells with the TRPV4-specific antagonist RN-1734 significantly blocked activation of the kinase AKT, a PI3K downstream effector, upon TGF-β stimulation (Fig. 1, A–D). Whereas TGF-β increased the activity of both PI3Kα and PI3Kγ isoforms at the plasma membrane, only PI3Kγ exhibited increased lipid kinase activity that depended on TRPV4 (Fig. 1, E–F). These data indicate that TGF-β–induced activation of PI3Kγ depends on TRPV4 (Fig. 1, E–F).
Fig. 1. TRPV4 selectively mediates TGF-β–driven PI3Kγ activity.
(A) Representative Western blot showing TRPV4, phosphorylated AKT [p-AKT (Ser473)], total AKT (t-AKT), and GAPDH (loading control) in lysates of human lung fibroblast (HLF) treated with TRPV4 or scrambled siRNA ±TGF-β as indicated. (B) Quantification of p-AKT (Ser473) relative to total AKT as in (A) by densitometry. *P<0.05 difference between untreated and +TGF-β conditions. Band densities were measured with respect to the untreated scrambled siRNA condition. (C) Representative Western blot showing p-AKT (Ser473) and total AKT (t-AKT) in lysates from HLFs treated with the TRPV4 antagonist RN-1734 ± TGF-β as indicated. (D) Quantification of p-AKT (Ser473) relative to total AKT as in (C). **P<0.01 difference between untreated and +TGF-β conditions. Band densities were measured with respect to the untreated condition. (E) Lipid kinase activity of PI3Kγ and PI3Kα immunoprecipitated from plasma membrane fractions of HLFs treated with the TRPV4 antagonist RN-1734 ±TGF-β as indicated. PI3Kγ and PI3Kα were assayed for their capacity to generate PIP by thin layer chromatography. The line between the untreated and +TGF-β conditions indicates non-contiguous lanes from the same blot. (F) Quantification of PIP densities as in (E). *P<0.05 as indicated. PIP densities were measured with respect to the untreated condition for each PI3K isoform. All graphs show means ± SEM from N = 3 independent experiments (*P≤0.05, **P≤0.01, using ANOVA followed by Student-Newman-Keuls multiple comparisons test).
TRPV4 binds directly to PI3Kγ, and TRPV4-PI3Kγ complexes are recruited to the plasma membrane upon TGF-β stimulation
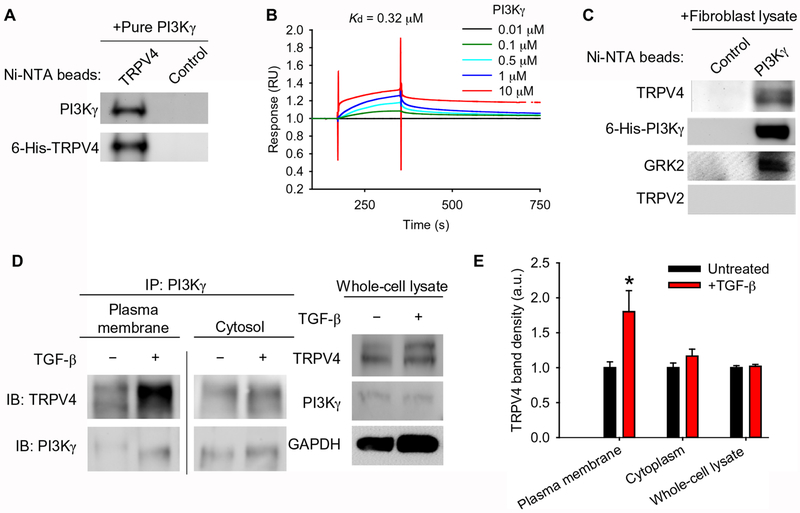
We next determined whether TRPV4 and PI3Kγ directly interact. TRPV4 bound to PI3Kγ with nanomolar affinity in several independent techniques and assay methods using His-tagged forms of the human proteins. First, purified His-tagged TRPV4 coupled to nickel nitrilotriacetic acid (Ni-NTA) beads bound to purified PI3Kγ in an in vitro solution binding assay (Fig. 2A). Second, purified PI3Kγ bound to immobilized, purified TRPV4 in a concentration-dependent manner with nanomolar affinity (Kd = 320 nM) by surface plasmon resonance (SPR) assay (Fig. 2B). Third, purified PI3Kγ coupled to Ni-NTA beads directly interacted with endogenous TRPV4 in HLF whole cell lysates in an in vitro pulldown assay (Fig. 2C) (18). In order to determine the location of the TRPV4-PI3Kγ complex in cells, the plasma membrane and cytosolic fractions of human lung fibroblast lysates were analyzed for TRPV4-PI3Kγ complexes by co-immunoprecipitation. Co-immunoprecipitation revealed that TRPV4-PI3Kγ complexes were present in both the plasma membrane and cytosolic fractions in human lung fibroblasts under basal conditions. Treating the cells with TGF-β significantly increased the amount of TRPV4-PI3Kγ complexes at the plasma membrane but had no effect on the amount in the cytoplasm (Fig. 2, D–E). The observed effect was not simply due to changes in overall TRPV4 or PI3Kγ cellular protein abundance upon TGF-β treatment (Fig. 2, D–E). Taken together, these data demonstrate a nanomolar affinity, direct interaction between TRPV4 and PI3Kγ that localizes to the plasma membrane upon TGF-β treatment.
Fig. 2. TRPV4 and PI3Kγ interact directly and translocate to the plasma membrane in response to TGF-β.
(A) The binding of purified PI3Kγ to purified 6-His-TRPV4 coupled to Ni-NTA beads was assessed by immunoblotting with an antibody specific for PI3Kγ. The blots were stripped and reprobed with an antibody for TRPV4. Control indicates beads alone (no protein bound to beads), N=3 independent experiments. (B) The direct interaction between immobilized purified 6-His-TRPV4 and purified 6-His-PI3Kγ was measured by surface plasmon resonance. N=3 independent experiments. (C) The binding of purified 6-His-PI3Kγ coupled to Ni-NTA beads to endogenous TRPV4 from human lung fibroblast (HLF) lysates was assessed by Western blotting. Blots were stripped and reprobed with antibodies specific for PI3Kγ, GRK2 (positive control), and TRPV2 (negative control). Control indicates beads alone (no protein bound to beads), N=3 independent experiments. (D) PI3Kγ immunoprecipitates (IP) from plasma membrane and cytosolic fractions of HLFs treated with TGF-β as indicated were immunoblotted for PI3Kγ and TRPV4. The line between the plasma membrane and cytosolic fractions indicates non-contiguous lanes from the same blot. Total whole cell lysate of cells treated ±TGF-β (input) was immunoblotted for TRPV4 and PI3Kγ. GAPDH is a loading control. (E) Quantification of TRPV4 in plasma membrane and cytosolic fractions normalized to TRPV4 in whole cell lysate as in (D). Data represent means ± SEM. N=3 independent experiments. *P≤0.05 between untreated and TGF-β conditions, using ANOVA followed by Student-Newman-Keuls multiple comparisons test.
TRPV4 and PI3Kγ are mutually required for translocation of TRPV4-PI3Kγ complexes to the plasma membrane
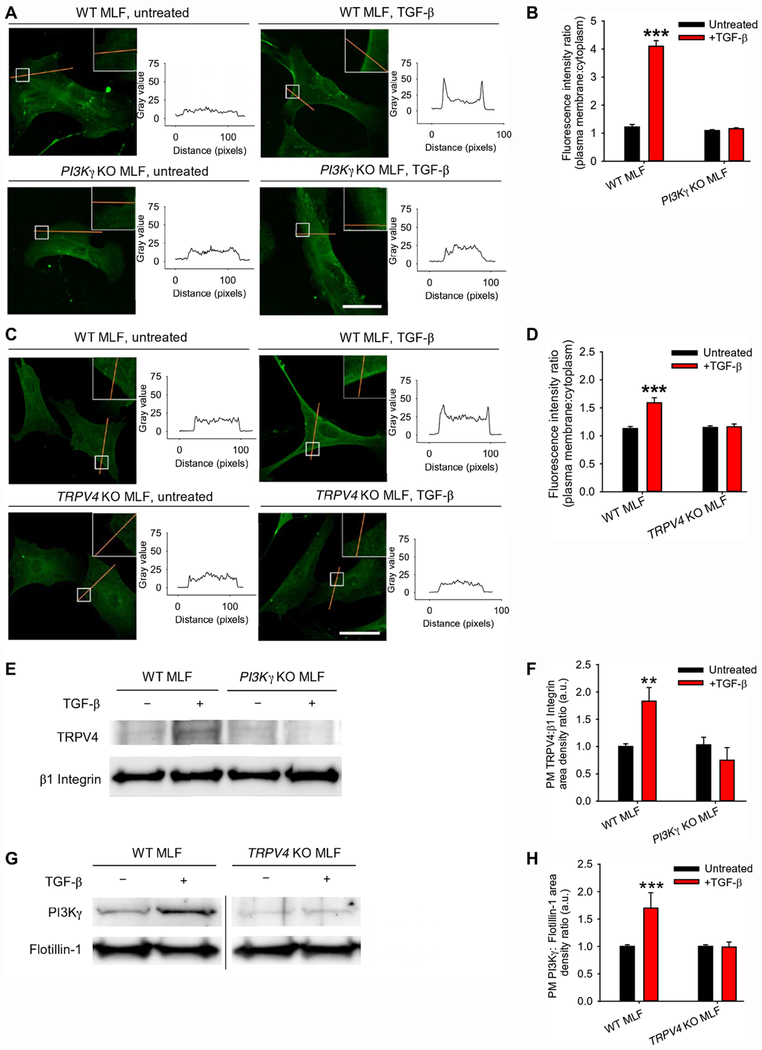
In order to better understand the molecular requirements for PI3Kγ and TRPV4 translocation to the plasma membrane, we utilized loss-of-function experiments to determine the individual roles of PI3Kγ and TRPV4 in the observed TGF-β–driven membrane translocation of the TRPV4-PI3Kγ complex. Under basal conditions, TRPV4 was present in the cytosol and plasma membrane of lung fibroblasts isolated from PI3Kγ knockout mice, as shown by immunofluorescence (Fig. 3, A–B). Similarly, PI3Kγ was found in the cytosol and plasma membrane of lung fibroblasts from TRPV4 knockout mice (Fig. 3, C–D). Treating the cells with TGF-β induced the translocation of TRPV4-PI3Kγ complexes to the plasma membrane, based on the increase of both TRPV4 and PI3Kγ in the plasma membrane, as shown by immunofluorescence (Fig. 3, A–D), by Western blot of plasma membrane TRPV4 from a surface biotinylation assay (Fig. 3, E–F), or by Western blot of PI3Kγ in plasma membrane fractions (Fig. 3, G–H). TGF-β–induced translocation of TRPV4 was lost in cells lacking PI3Kγ, and TGF-β–induced translocation of PI3Kγ was lost in cells lacking TRPV4, indicating that the proteins were mutually required for one another’s translocation to the plasma membrane (Fig. 3, A–H). Taken together, these data suggest that pre-formed cytosolic TRPV4-PI3Kγ complexes translocate to the plasma membrane upon TGF-β treatment.
Fig. 3. TRPV4 and PI3Kγ are mutually required for translocation to the plasma membrane.
(A) Representative confocal images showing TRPV4 in wild-type (WT) and PI3Kγ knockout (KO) murine lung fibroblasts (MLFs) ±TGF-β as indicated and the corresponding plot profiles of the TRPV4 immunofluorescence. Orange lines indicate regions where plot profiles were obtained. White boxes indicate areas shown in higher magnification in insets. (B) Quantification of plasma membrane:cytoplasm fluorescence for experiments in (A). N = 3 independent experiments with at least 30 cells per condition. ***P<0.005 compared with untreated WT MLF or with PI3Kγ KO MLF ± TGF-β. (C) Representative confocal images showing PI3Kγ in WT and TRPV4 KO MLFs ±TGF-β and the corresponding plot profiles of the PI3Kγ immunofluorescence. (D) Quantification of results in (C). N = 3 independent experiments with at least 30 cells per condition. ***P<0.005 compared with untreated WT MLF or with TRPV4 KO MLF ±TGF-β. (E) Representative immunoblots for TRPV4 and β1 integrin (loading control) from a surface biotinylation assay in WT and PI3Kγ KO MLF ±TGF-β. (F) Quantification of results from (E). N=3 independent experiments. **P<0.01 compared with untreated WT MLF or with PI3Kγ KO MLF ± TGF-β. (G) Representative immunoblots for PI3Kγ and flotillin-1 (loading control) from the plasma membrane fractions of WT and TRPV4 KO MLF ± TGF-β. The line between WT and TRPV4 KO MLF conditions indicates non-contiguous lanes from the same blot. (H) Quantification of results from (G). N=3 independent experiments. ***P<0.005 compared with untreated WT MLF or with TRPV4 KO ±TGF-β. All graphs show means ± SEM from N = 3 independent experiments. **P≤0.01, ***P≤0.005, using ANOVA followed by Student-Newman-Keuls multiple comparisons test. Scale bars, 50 μm.
The PI3Kγ isoform is required for TRPV4 ion channel function
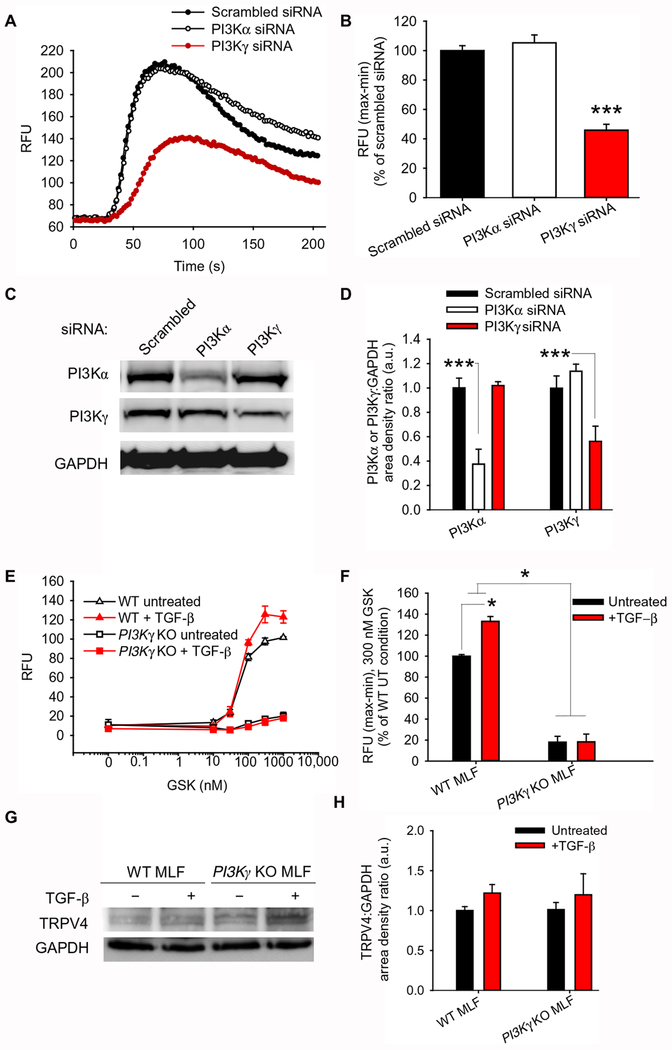
To determine the functional relevance of TRPV4-PI3Kγ complexes at the plasma membrane, we examined whether PI3Kγ was required for TRPV4 ion channel function in lung fibroblasts. Knocking down PI3Kγ by siRNA in HLFs resulted in a loss of TRPV4 function, as measured by TRPV4 agonist–induced Ca2+ influx (Fig. 4, A–D). Similarly, TRPV4 function was almost completely lost in fibroblasts from PI3Kγ knockout mice, indicating that PI3Kγ is required for TRPV4 function in lung fibroblasts (Fig. 4, E–F). In contrast, knocking down the PI3Kα isoform had no effect on TRPV4 function (Fig. 4, A–D). This demonstrates that only the PI3Kγ isoform functionally interacts with TRPV4. As a control, immunoblotting analysis of whole cell lysates confirmed that the absence of TRPV4 function seen in PI3Kγ knockout fibroblasts was not simply due to changes in TRPV4 abundance (Fig. 4, G–H). Taken together, these data demonstrate the selective importance of PI3Kγ to TRPV4 ion channel function.
Fig. 4. PI3Kγ is required for TRPV4 ion channel function.
(A) Representative plots showing the effects of scrambled, PI3Kα, and PI3Kγ siRNA on Ca2+ influx induced by the TRPV4 agonist GSK1016790A (GSK) in human lung fibroblasts. Ca2+ influx was measured in relative fluorescence units (RFU) using Calcium 5 dye on intact human lung fibroblast (19Lu) monolayers treated with scrambled, PI3Kα, or PI3Kγ siRNA. (B) Quantification of experiments in (A). ***P<0.005 compared with PI3Kα siRNA or scrambled siRNA. (C) Representative immunoblots showing PI3Kα and PI3Kγ in 19Lu cells treated with scrambled, PI3Kα, or PI3Kγ siRNA. GAPDH is a loading control. (D) Quantification of PI3Kα and PI3Kγ band density relative to GAPDH in (C). ***P<0.005 as indicated. (E) Representative plot showing GSK1016790A-induced Ca2+ influx in WT and PI3Kγ KO mouse lung fibroblasts (MLFs) treated ±TGF-β as indicated. (F) Quantification of RFU in 300 nM GSK1016790A conditions in (E). *P<0.05 as indicated. (G) Representative immunoblot showing TRPV4 in WT and PI3Kγ KO MLFs treated ±TGF-β. (H) Quantification of TRPV4 band density relative to GAPDH in (G). All graphs show means ± SEM from N = 3 independent experiments (*P≤0.05, ***P≤0.005, using ANOVA followed by Student-Newman-Keuls multiple comparisons test).
Both TRPV4 and PI3Kγ are necessary for lung fibroblast responses to stiff substrates
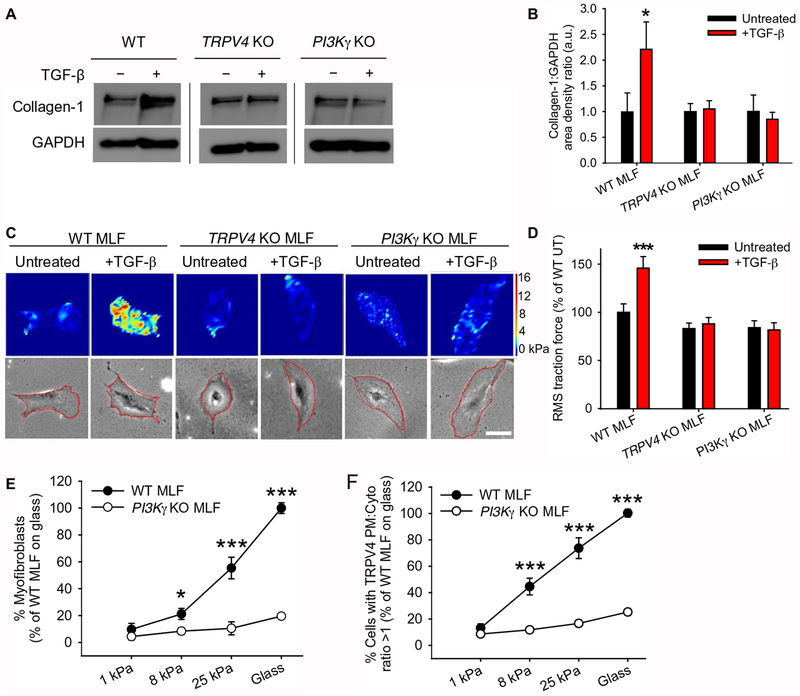
In order to determine the role of TRPV4-PI3Kγ complexes in the myofibroblast phenotype, we measured collagen production, contractility, myofibroblast transdifferentiation, and TRPV4 translocation in response to pathophysiological matrix stiffness in a loss-of-function context. TGF-β stimulation promoted collagen I production (as determined by Western blotting) and contractile activity (as determined by traction force microscopy), in lung fibroblasts from wild-type mice but not in lung fibroblasts from TRPV4 or PI3Kγ knockout mice (Fig. 5, A–D), indicating a requirement for both TRPV4 and PI3Kγ in these processes.
Fig. 5. Both TRPV4 and PI3Kγ are necessary for profibrotic responses of myofibroblasts.
(A) Representative immunoblots showing collagen-1 in WT, TRPV4 KO, and PI3Kγ KO mouse lung fibroblasts (MLFs) treated ±TGF-β. Black lines indicate non-contiguous lanes from the same blot. GAPDH is a loading control. (B) Quantification of collagen-1:GAPDH band density ratios (means ± SEM) from (A). N=3 independent experiments. *P<0.05 compared with untreated WT MLF, TRPV4 KO MLF ±TGF-β, or PI3Kγ KO MLF ±TGF-β. (C) Representative traction force intensity images and corresponding phase images with the region of interest outlined in red for WT, TRPV4 KO, and PI3Kγ KO MLFs treated ±TGF-β. Red and orange, areas of high contraction; blue, areas of low contraction. Scale bar, 50 μm. (D) Quantification of root mean square (RMS) traction force (means ± SEM) results from (C). N=3 independent experiments with at least 30 cells per condition. ***P<0.005 compared with untreated WT MLF, TRPV4 KO MLF ±TGF-β, or PI3Kγ KO MLF ±TGF-β. (E) Quantification of WT and PI3Kγ KO MLFs that differentiated into myofibroblasts when plated on polyacrylamide gels of varying stiffness and treated with TGF-β, as measured by α-smooth muscle actin (α-SMA) in stress fibers. Data shown as % of WT MLF on glass. N=3 independent experiments with at least 30 cells per condition *P<0.05 between WT and PI3Kγ KO MLF at 8 kPa, ***P<0.005 between WT and PI3Kγ KO MLF at 25kPa and glass. (F) Quantification of WT and PI3Kγ KO MLFs plated on polyacrylamide gels of varying stiffness ad treated with TGF-β that showed a TRPV4 plasma membrane:cytoplasm ratio >1 as measured by TRPV4 immunofluorescence. Data shown as % of WT MLF on glass. N=3 independent experiments with at least 30 cells per condition. ***P<0.005 between WT and PI3Kγ KO MLF at 8 kPa, 25 kPa, and glass. All graphs show means ± SEM from N = 3 independent experiments (*P≤0.05, ***P≤0.005, using ANOVA followed by Student-Newman-Keuls multiple comparisons test).
We have previously shown that TRPV4 promotes myofibroblast transdifferentiation in response to mechanical stimuli – specifically, that induced by culturing lung fibroblasts on substrates with the stiffness of fibrotic lungs (16). Consistent with our previous findings, wild-type mouse lung fibroblasts maintained the fibroblast cell identity on substrates with the stiffness of normal lungs (1 kPa) but transdifferentiated into myofibroblasts, as assayed by the presence of α-smooth muscle actin (α-SMA) in stress fibers, when they were cultured on substrates with the stiffness of fibrotic lungs (25 kPa) (Fig. 5E). The myofibroblast transdifferentiation response did not occur in lung fibroblasts from TRPV4 (Fig. 5C and (16)) or PI3Kγ knockout mice (Fig. 5E), indicating that PI3Kγ and likely the plasma membrane translocation of TRPV4 were required for the response to alterations in matrix stiffness over the pathophysiological range (Fig. 5, E–F). These results highlight the biological importance of TRPV4-PI3Kγ complex translocation in the myofibroblast phenotype and thereby in the development of organ fibrosis.
Both TRPV4 and PI3Kγ are required for myofibroblast transdifferentiation
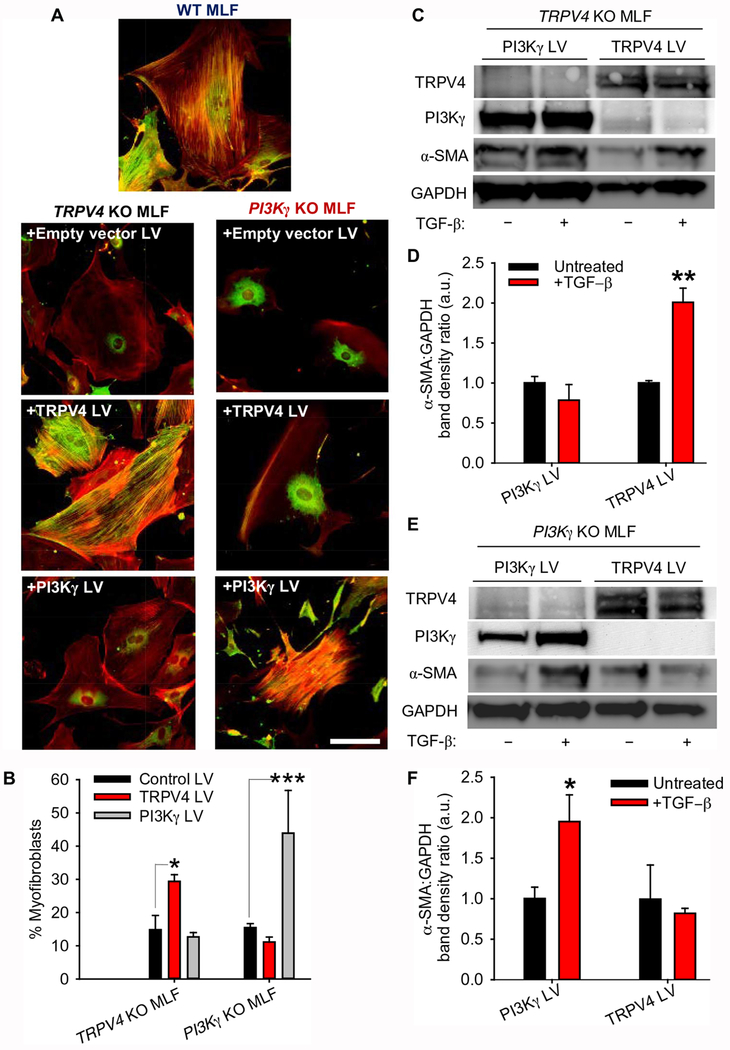
To determine the physiological effects of TRPV4-PI3Kγ complexes, we studied the effect of gain- and loss-of-function of TRPV4 and PI3Kγ on myofibroblast transdifferentiation. Whereas Lentivirus-mediated expression of TRPV4 stimulated transdifferentiation of lung fibroblasts from TRPV4 knockout mice into myofibroblasts, transgenic expression of PI3Kγ did not (Fig. 6, A–F). Myofibroblast transdifferentiation was defined by incorporation of α-SMA into actin stress fibers (Fig. 6, A and B) and an increase in the production of α-SMA (Fig. 6, C–F). Conversely, transgenic expression of PI3Kγ stimulated transdifferentiation of lung fibroblasts from PI3Kγ knockout mice into myofibroblasts, transgenic expression of TRPV4 did not (Fig. 6, A–F). That myofibroblast transdifferentiation only occurred in fibroblasts that produced both TRPV4 and PI3Kγ extends our previous findings (16) by demonstrating that both TRPV4 and PI3Kγ are required for myofibroblast transdifferentiation. Taken together, these data clearly demonstrate a requirement for both TRPV4 and PI3Kγ to drive myofibroblast transdifferentiation.
Fig. 6. Both PI3Kγ and TRPV4 are required for myofibroblast transdifferentiation.
(A) Representative fluorescence images showing α-SMA (green) and F-actin (red) in TRPV4 KO and PI3Kγ KO mouse lung fibroblasts (MLFs) treated with empty vector (control) lentivirus (LV), TRPV4 LV, or PI3Kγ LV, then stimulated with TGF-β. Non-transfected WT MLF were used as a positive control. Scale bar, 100 μm. (B) Quantification of cells with α-SMA–positive stress fibers in (A). Data are presented as % myofibroblasts (means ± SEM). N = 3 independent experiments with at least 30 cells per condition. *P<0.05 and ***P<0.005 as indicated. (C) Representative Western blot showing TRPV4, PI3Kγ, and α-SMA in TRPV4 KO MLFs transfected with PI3Kγ LV or TRPV4 LV then stimulated with TGF-β. (D) Quantification α-SMA:GAPDH band density ratios (means ± SEM) in (C). N=3 independent experiments. **P<0.01 difference between untreated and +TGF-β conditions with TRPV4 LV. (E) Representative Western blot showing TRPV4, PI3Kγ, and α-SMA in PI3Kγ KO MLFs transfected with PI3Kγ LV or TRPV4 LV then stimulated with TGF-β. GAPDH is a loading control. (F) Quantification α-SMA:GAPDH band density ratios (means ± SEM) in (E). N=3 independent experiments. *P<0.05 difference between untreated and +TGF-β conditions with PI3Kγ LV. All graphs show means ± SEM from N = 3 independent experiments (*P≤0.05, **P≤0.01, ***P≤0.005, using ANOVA followed by Student-Newman-Keuls multiple comparisons test).
The non-catalyic, amino-terminal domain of PI3Kγ is necessary and sufficient for TRPV4 translocation and myofibroblast transdifferentiation
Class I PI3Ks are composed of a regulatory subunit (p85) and a catalytic subunit (p110). The catalytic activity of p110γ resides in the C-terminal portion of the protein; the N-terminal domain has no catalytic activity and has a unique sequence and tertiary structure compared to the corresponding regions of the other class I PI3K catalytic subunits (19). To more clearly define the functional domain of PI3Kγ that was involved in its interaction with TRPV4, we tested full-length, wild-type PI3Kγ (p110γ), a PI3Kγ (p110γ) mutant including only the non-catalytic N-terminal domain (AA 1-142), or a PI3Kγ (p110γ) mutant with deletions of the N-term domain and the ATP binding site (N-del, AA Δ1-142, AA Δ948-981) for binding to and activation TRPV4 (Fig. 7A). In vitro, purified PI3Kγ bound endogenous TRPV4 from lysates of human lung fibroblasts through its non-catalytic N-terminal domain (Fig. 7B). The purified N-terminal domain of PI3Kγ also bound with nanomolar affinity to chip-immobilized purified TRPV4 in a concentration-dependent manner (Kd = 280 nM), as measured by SPR (Fig. 7C). Lentivirus-mediated expression of the N-terminal domain of PI3Kγ in lung fibroblasts from PI3Kγ knockout mice promoted the translocation of endogenous TRPV4 to the plasma membrane, as measured by immunofluorescence and immunoblotting of plasma membrane fractions (Fig. 7, D–F). Furthermore, the N-terminal domain of PI3Kγ restored myofibroblast transdifferentiation comparably to full-length, wild-type PI3Kγ (Fig. 7, G–H). In contrast, the PI3Kγ fragment lacking the N-terminal domain did not bind TRPV4 in human lung fibroblast lysates or induce TRPV4 translocation or rescue myofibroblast transdifferentiation in PI3Kγ-deficient murine lung fibroblasts (Fig. 7, B–H). Thus, the N-terminal domain of PI3Kγ mediates the interaction between PI3Kγ with TRPV4. In conclusion, our findings are consistent with the scaffolding function of PI3Kγ being a critical, non-redundant function of PI3Kγ that is not redundant with other class I PI3Ks, such as PI3Kα, and drives TGF-β–induced myofibroblast transdifferentiation through translocation of TRPV4-PI3Kγ complexes to the plasma membrane.
Fig. 7. The non-catalytic, amino-terminal domain of PI3Kγ is necessary and sufficient for TRPV4 translocation and myofibroblast transdifferentiation.
(A) Domain structure of WT and mutant PI3Kγ constructs consisting of only the non-catalytic N-terminal domain (N-term) or lacking both the N-terminal domain and the ATP binding site in the catalytic domain (N-del). (B) Immunoblotting of His-tagged N-del or N-term forms of PI3Kγ coupled to Ni-NTA beads incubated with lysates of human lung fibroblasts (HLFs). Blots were probed with an antibody recognizing TRPV4, then stripped and reprobed for PI3Kγ. N=3 independent experiments. (C) Direct interaction between immobilized purified 6-His-TRPV4 and purified 6-His-N-term-PI3Kγ was measured by surface plasmon resonance. N=3 independent experiments. (D) Representative fluorescence images showing TRPV4 in PI3Kγ KO murine lung fibroblasts (MLFs) transfected with N-del or N-term PI3Kγ lentivirus (LV) and treated with TGF-β and the corresponding plot profiles of TRPV4 immunofluorescence. White arrows indicate TRPV4 at the plasma membrane; orange lines indicate regions where plot profiles were obtained; white boxes indicate higher magnification insets. Scale bar, 50 μm. (E) Quantification of results from (D). N = 3 independent experiments with at least 30 cells per condition. ***P<0.005 compared with PI3Kγ KO MLF + N-del PI3Kγ LV by Student’s t-test. (F) Western blotting of plasma membrane fractions of PI3Kγ KO MLFs transfected with lentivirus encoding WT PI3Kγ, N-term PI3Kγ, or N-del PI3Kγ, then treated with TGF-β as indicated. Blots were probed for TRPV4, PI3Kγ, and flotillin-1 (loading control). N=3 independent experiments. (G) Representative fluorescence images showing α-SMA in PI3Kγ KO MLFs transfected with control LV (empty vector), WT PI3Kγ LV, N-term PI3Kγ LV or N-del PI3Kγ LV, then stimulated with TGF-β. White boxes indicate areas enlarged in insets. Scale bar, 100 μm. (H) Quantification of results from (G). N = 3 independent experiments with at least 30 cells per condition. ***P<0.005 untreated vs +TGF-β conditions by ANOVA followed by Student-Newman-Keuls multiple comparisons test. All graphs show means ± SEM from N = 3 independent experiments.
Discussion
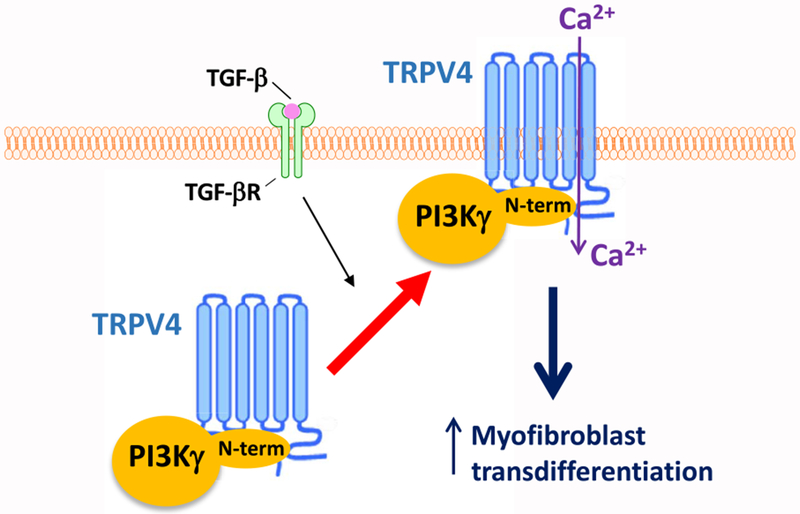
Our prior work revealed that the mechanosensitive, Ca2+-permeable ion channel TRPV4 plays a key role in myofibroblast transdifferentiation and in vivo pulmonary fibrosis (16). The current study was undertaken to define the intracellular signaling pathway by which TRPV4 mediates these effects. The results presented here reveal that TRPV4 interacts with the non-catalytic, N-terminal domain of PI3Kγ to form TRPV4-PI3Kγ complexes that translocate to the plasma membrane, thereby mediating myofibroblast transdifferentiation (Fig. 8). Although previous reports classically show PI3Kγ activation by GPCRs, data presented herein show that an ion channel (TRPV4) was required for PI3Kγ activity in lung fibroblasts. Conversely, PI3Kγ was required for the activity of TRPV4. TRPV4 binding to PI3Kγ was demonstrable with a Kd in the nanomolar range in vitro and by TRPV4-PI3Kγ complex formation in fibroblasts. Upon TGF-β stimulation, TRPV4 and PI3Kγ were mutually required for one another’s plasma membrane translocation, and thereby their Ca2+ influx and lipid kinase functional activities, respectively. The requirement for both TRPV4 and PI3Kγ to induce myofibroblast transdifferentiation upon TGF-β stimulation was demonstrated using gain-of-function approaches in either TRPV4- or PI3Kγ-deficient cells. TRPV4 binding to the N-terminal domain of PI3Kγ was demonstrated both in vitro and in human lung fibroblasts and shown to be sufficient for TGF-β–induced TRPV4 plasma membrane translocation and myofibroblast transdifferentiation. Taken together, this work demonstrates that the plasma membrane translocation of TRPV4-PI3Kγ complexes is required for myofibroblast transdifferentiation.
Fig. 8. Schematic working model showing TGF-β–stimulated activation of TRPV4-PI3Kγ complexes in myofibroblast transdifferentiation.
Our data suggest that TRPV4 binds to the non-catalytic, N-terminal domain of PI3Kγ, forming a TRPV4-PI3Kγ complex. Upon TGF-β stimulation, the N-terminus of PI3Kγ acts as a scaffold, recruiting TRPV4-PI3Kγ complexes from the cytoplasm to the plasma membrane. This translocation leads to an increase in TRPV4-mediated Ca2+ influx, which promotes myofibroblast transdifferentiation.
Myofibroblasts are key pro-fibrotic cell types through their capacity to secrete extracellular matrix proteins and pro-fibrotic cytokines, and by their contractile nature (20, 21). We have previously shown that TRPV4 is necessary for the cytoskeletal remodeling and increased production of α-SMA and collagen-1 in response to TGF-β (16). We now extend these previous data to additional biologically relevant, profibrotic, TRPV4-mediated effects. Data presented herein reveal that both collagen I production as well as contractility are increased in a TRPV4- and PI3Kγ-dependent manner in response to TGF-β treatment. The role of TRPV4 in cell-matrix interactions may be even more generalizable, because work from others has shown that TRPV4 mediates collagen fiber alignment and focal adhesion tension in mesenchymal stem cells (22). In addition, our previous work revealed that TRPV4 is required for the substrate stiffness–dependent increase in myofibroblast transdifferentiation (16). We show that PI3Kγ was necessary for the mechanosensitive responses of myofibroblast transdifferentiation and TRPV4 plasma membrane translocation upon TGF-β treatment. We previosuly showed that TRPV4 is required for the generation of myofibroblasts in vivo as well as experimental pulmonary fibrosis, and is dysregulated in human IPF (16). Because myofibroblasts are the critical fibrogenic cells in many organs (lung, heart, skin, kidney and liver), the translocation of TRPV4-PI3Kγ complexes may drive fibrosis in many disease contexts.
It has been reported that various stimuli, including hormones, cytokines and sheer stress, can induce translocation TRP-family channels to the plasma membrane, in a cell type–, stimulus-, and context-specific manner (23–25). There is some precedent for TRPV4 forming complexes with other proteins that alter its membrane trafficking (15). Both PACSIN3 (protein kinase C and casein kinase substrate in neurons protein 3) and OS-9 (OS9 endoplasmic reticulum lectin) have been shown to bind TRPV4’s N-terminal domain and thereby mediate TRPV4 endocytosis or release from the ER membrane, respectively (26, 27). TRPV4 phosphorylation at Ser824 promotes an interaction between TRPV4 and the store-operated ion channel STIM1 (stromal interaction molecule 1), thereby increasing TRPV4 abundance at the plasma membrane (28). In addition, evidence supports a role for glycosylation of TRPV4 in regulating its maturation and trafficking (29, 30). This study reveals that TRPV4 functional activity was induced at the plasma membrane in response to TGF-β in a manner that depended on PI3Kγ’s non-canonical scaffolding function. The specific membrane trafficking events involved in our observed TGF-β–mediated increase in plasma membrane TRPV4-PI3Kγ complexes is an area of active investigation.
Myofibroblast transdifferentiation requires extensive remodeling of the cytoskeleton, the consequences of which are a highly organized stress fiber network, large focal adhesions, and induction of myosin activity, resulting in increases in intracellular tension (31–33). The binding of TRPV4 to various cytoskeletal elements has been shown to regulate its subcellular location in a manner that depends on the phosphorylation state of its C-terminal cytoplasmic tail region (34–36). Furthermore, the C-terminal region of TRPV4, which has a class II PDZ binding motif (Asp-Ala-Pro-Leu), has been shown to bind to PDZ domain–containing proteins, such as NHERF-4 (Na+/H+ exchanger regulatory factor 4), that anchor cell surface receptors to the cytoskeleton (37, 38). Evidence supports the notion that the regulation of TRPV4 activity through its interaction with the cytoskeleton is both bidirectional and critical to myofibroblast transdifferentiation (34). For example, the effect of TRPV4 on myofibroblast transdifferentiation is intimately linked to cytoskeletal remodeling, because TGF-β–induced actin polymerization depends on TRPV4, and the newly polymerized actin releases and enables the nuclear translocation of myocardin-related transcription factor A (MRTF-A, also known as MKL-1), a key transcriptional factor that drives myofibroblast transdifferentiation (16).
PI3Ks participate in myofibroblast transdifferentiation; however, the mechanism of their action has not yet been elucidated (39–44). Although class 1A PI3Ks (PI3Kα, -β, and -δ) are downstream of tyrosine kinase receptors, the class 1B isoform PI3Kγ plays a critical role in coupling signals downstream of activated GPCRs (39, 45). Classically, Gβγ subunits recruit PI3Kγ to the GPCR complex, wherein its kinase lipid function generates membrane PtdIns 3,4,5 P3 (PIP3) from phosphatidylinositol 4,5-bis-phosphate (PtdIns 4,5 P2 ;PIP2), resulting in activation of downstream signaling, including Akt (46, 47). Our findings demonstrate a unique mechanism of PI3Kγ recruitment to the plasma membrane through formation of an intracellular complex with TRPV4. Analysis of the crystal structure of PI3Kγ reveals a horseshoe-shaped assembly of the enzyme with domains that have the propensity to interact with many proteins in a regulatory manner, including the p101 adaptor subunit, the small G-protein Ras, GPCR kinase 2 (GRK2, also known as βARK1), phosphodiesterase (PDE), and protein phosphatase 2A (PP2A) (48–51). For example, interaction of PI3Kγ with GRK2 results in the effective recruitment of the PI3Kγ-GRK2 complex to the plasma membrane, independently of the Gβγ subunits of the G proteins (52). Alternatively, the interaction of PI3Kγ with the small GTPase Ras involves reciprocal regulation, wherein Ras is thought to activate PI3Kγ and PI3Kγ increases Ras activity (53). Such reciprocal regulation is observed in our current study, wherein TRPV4 and PI3Kγ were required for one other’s recruitment to the plasma membrane following TGF-β stimulation.
Whereas both the α and γ isoforms of PI3K were activated in response to TGF-β, only PI3Kγ was activated in a TRPV4-dependent manner and required for TRPV4 channel activity. Our observation that TRPV4 was required for PI3Kγ recruitment to the membrane in response to TGF-β supports a non-canonical, Gβγ-independent mechanism for the membrane recruitment of TRPV4-PI3Kγ complexes. We provide strong data for a critical scaffolding role for the non-catalytic, N-term domain of PI3Kγ and its interaction with TRPV4 in mediating myofibroblast transdifferentiation. The N-terminus of PI3Kγ contains an amino acid sequence that is not homologous to that of the other class I PI3Ks (19). Although we found that the non-catalytic, N-terminal PI3Kγ domain was required for the formation and translocation of TRPV4-PI3Kγ complexes and for myofibroblast transdifferentiation, our data do not exclude the possibility that PI3Kα and PI3Kγ also play additional, potentially redundant, roles that require their enzymatic activities in fibroblast activation (54, 55). Further, because the N-terminus of PI3Kγ binds to protein kinase A (PKA), thereby promoting the degradation of cyclic adenosine monophosphate (cAMP), and cAMP has been implicated in myofibroblast transdifferentiation, the relevance of PKA binding to PI3Kγ in fibroblast activation will be studied in future work (50, 56–59). After a more complete analysis of other binding partners of the TRPV4-PI3Kγ complex, we aim to find a specific inhibitor for use both in vitro and in vivo so that we can more accurately determine the specific role of the PI3Kγ-TRPV4 complexes in myofibroblast transdifferentiation and in an in vivo model of pulmonary fibrosis.
Although we have uncovered a role for PI3Kγ in translocating and sensitizing TRPV4, the precise membrane-targeting mechanism is an area for future investigation. We also show the reciprocal finding that TRPV4 can selectively translocate and activate PI3Kγ. Thus, our studies have revealed a unique TRPV4-PI3Kγ reciprocal regulatory mechanism following activation by TGF-β. This work has important therapeutic implications. Given that both TRPV4 and PI3Kγ have pleiotropic actions—both beneficial and detrimental—we hypothesize that specific targeting of the membrane translocation of TRPV4-PI3Kγ complexes, or disruption of complex formation, which involves the N-terminal domain of PI3Kγ, could provide a specific therapeutic approach for inhibiting myofibroblast transdifferentiation. Doing so may lead to treatment options for a broad array of fibrotic disorders.
Materials and Methods
Antibodies and reagents
Antibodies specific for the following proteins were used: α-SMA (1:5000) and collagen-1 (1:1000, Sigma Aldrich); TRPV4 (1:500, Alomone Labs); p-AKT (Ser473) (1:1000) and total AKT (1:1000, Cell Signaling Technology); PI3Kα (1:250), PI3Kγ (1:250), and GRK2 (1:500, Santa Cruz); GAPDH (1:10,000, Fitzgerald); β1 integrin (1:1000) and flotillin-1 (1:1000, BD Biosciences); TRPV2 (1:500, Boster); and 6-His (1:1000, Roche). Purified TGF-β1 was purchased from R&D Systems and used at 2 ng/ml in all experiments. The TRPV4 agonist GSK1016790A as well as the TRPV4 antagonist (RN-1734, 50 μM) were purchased from Sigma-Aldrich. Alexa Fluor-phalloidin, ProLong Gold Antifade Reagent, and Alexa-Fluor conjugated secondary antibodies were obtained from Invitrogen. Ni-NTA beads were purchased from Qiagen. Polyacrylamide gels of varying stiffness and the polyacrylamide substrates (shear moduli 25 kPa) embedded with 0.2 μm yellow/green fluorospheres used for traction force microscopy were purchased from Matrigen. All other chemicals were purchased from Sigma-Aldrich and Fisher Scientific.
Cell Culture
Adult normal human lung fibroblasts (19Lu, catalog # CCl-210) were purchased from American Type Culture Collection (ATCC). Fibroblasts were maintained and propagated in Modified Eagle’s Medium (MEM) supplemented with 10% fetal bovine serum (FBS), and all experiments were performed on early passages of cells. Primary murine lung fibroblasts were derived from 7-10 week old PI3Kγ knock-out (KO), TRPV4 KO, or wild type (WT) C57BL/6 mice and propagated as described previously (16, 51, 60–62). All animal protocols were performed according to guidelines approved by Cleveland Clinic Institutional Review Board.
Western blotting
Western blotting analysis was performed as described previously (51, 63) on plasma membrane/cytosolic fractions, biotinylated cell surface proteins, or on whole cell lysates treated ±siRNA as described below, ±lentivirus as described below, ± 50 μM TRPV4 antagonist RN-1734 (1 h), or ± 2ng/ml TGF-β (3-24 h), and developed with an enhanced chemiluminescence system (Amersham ECL Prime Western Blotting Detection Reagent). Western blots were quantified using VisionWorks acquisition and analysis software, version 8.19.17027.9424 (Analytik Jena).
siRNA-mediated knockdown
All siRNAs were transfected into human lung fibroblasts (19Lu) using siLentFect lipid reagent (BioRad) according to the manufacturer’s instructions. TRPV4-specific siRNA and control scrambled siRNA duplexes were purchased from Origene and used at the indicated concentrations (48 h transfection). siRNA duplexes were created from human PI3Kα and PI3Kγ cDNA sequences using Ambion or Qiagen siRNA design programs. The target 21-mer sequence for PI3Kα was AACTGAGCAAGAGGCTTTGGA, while for PI3Kγ it was AAGGATATTCCCGAAAGCCAA (regions 777 to 795 bp). The PI3Kα and PI3Kγ siRNA as well as the All-Stars negative control siRNA were synthesized by and purchased from Qiagen and used at a concentration of 100 nM (72 h transfection). After transfection, cells were washed with serum free-media and transferred to 1% BSA-containing serum-free MEM ± TGF-β (2ng/ml, 3h), and Western blotting or calcium assays were performed as indicated.
Isolation of Plasma Membranes
Plasma membranes were prepared as published (51). Human lung fibroblasts (19 Lu) or mouse lung fibroblasts (MLF) were treated ±100 MOI lentivirus as below, ± RN-1734 (50 μM, 1h), ± TGF-β (2 ng/mL, 3-24 h) and lysed with osmotic lysis buffer (25 mM Tris-HCL pH 7.4, 5 mM EDTA + protease and phosphatase inhibitors). Cells were homogenized, toggled at 4°C for 20 minutes, and centrifuged at 1000 x g at 4°C for 5 minutes to remove cell debris/nuclei. Then, the supernatant was centrifuged at 37,000 x g at 4°C for 30 minutes to pellet the membrane, which was resuspended in Triton lysis buffer (0.8% Triton X-100, 20 mM Tris-HCl pH 7.4, 300 mM NaCl, 20% glycerol + protease and phosphatase inhibitors). The supernatant contains the cytosolic fraction, which includes all intracellular membranes/organelles/cytoskeletal proteins, whereas the pellet contains plasma membrane-associated proteins (18). Human lung fibroblast (19 Lu) plasma membranes were further subjected to immunoprecipitation and lipid kinase assays as below, while mouse lung fibroblast plasma membranes were subjected to Western blotting. Alternatively, a cell surface protein isolation kit used for the biotinylation/isolation of cell surface proteins was purchased and used according to the manufacturer’s instructions (ThermoFisher).
Lipid Kinase Assays
Lipid kinase assays were performed as published (64). PI3Kγ or α isoforms were immunoprecipitated from a solution containing Protein A/G plus agarose beads (Thermo Scientific), PI3Kγ or PI3Kα antibody (diluted 1:100), and 100 μg of lysate from human lung fibroblast (19 Lu) plasma membrane fractions diluted in Triton lysis buffer as above. After washing, the beads were resuspended in 50 μl reaction buffer (10 mM Tris-Cl pH 7.4, 150 mM NaCl, 5 mM EDTA, 100 μM sodium-orthovanadate) with 10 μl of 100 mM MgCl. Then, 20 μl PtdIns (2.5 mg/ml, 50 μg total) was dissolved in TE buffer (10 mM Tris-HCl, pH 7.4, 1 mM EDTA) and added to each reaction. The reactions were initiated by adding 1uL of a mixture of 10 ul 440 uM cold ATP and 10 μCi 32P-γ-ATP, and the reactions were incubated at 25°C with continuous agitation for 10 min. The reactions were stopped by adding 20 μl 6N HCl. Lipids were extracted by adding 160 ul of 1:1 chloroform:methanol, vortexing, and centrifuging. 30 μl of the organic phase (bottom phase) was spotted onto 200 μm silica-coated TLC plates (Selecto-flexible; Fisher Scientific) pre-coated with 1% potassium oxalate. The plate was resolved via chromatography with 1:1.87 2N glacial acetic acid:1-propanol for approximately 6 hours. The plates were then dried, exposed, and lipid phosphorylation was visualized with autoradiography.
In vitro interaction assay
Baculovirus expression constructs of TRPV4, WT PI3Kγ, N-term PI3Kγ, and N-del PI3Kγ were generated by subcloning the relevant sequences downstream of the 6X His tag sequence into pFastBac donor plasmids (Life Technologies), which were sequenced to confirm the in frame subcloning. The donor plasmids were used to produce recombinant bacmid DNA by transposition using competent DH10Bac E.coli cells (Kemp Bio). The recombinant bacmid DNA was isolated and transfected into SF9 insect cells to generate recombinant baculovirus particles (Kemp Bio.). Recombinant proteins were then expressed by infecting insect cells with viral particles (Kemp Bio.) and purified using Ni-NTA affinity resin (Qiagen). Binding assays of Ni-NTA bead immobilized 6-His-WT PI3Kγ (wild type/ full length), 6-his-N-term PI3Kγ (only AA 1-142), or 6-his-N-del PI3Kγ (Δ AA 1-142 and Δ AA 948-981 (ATP binding site)) with fibroblast lysate were performed in 1%NP-40 lysis buffer. The reactions were incubated overnight at 4°C with toggling, and then the beads were washed 3x with PBS. Washed Ni-NTA beads were heated to 95°C for 5 min in 2x Laemmli sample buffer, and the supernatant was subjected to SDS–polyacrylamide gel electrophoresis. Binding assays of immobilized 6-his-TRPV4 with pure WT PI3Kγ were performed in sf9 lysis buffer [(20mM HEPES, pH 7.4 (5.21g), 50 mM KCl (3.73g), 300 mM NaCl (17.53g), 10% glycerol (167 ml of 60%), 14 mM 2-ME (1.1ml), protease inhibitor]. The reactions were incubated for 30 min at room temperature with shaking, and the beads were washed four times with PBS. Washed beads were heated to 95°C for 5 min in 2x Laemmli sample buffer and subjected to SDS–polyacrylamide gel electrophoresis.
Surface Plasmon Resonance (SPR) assay
Biomolecular interaction analysis was performed using a Biacore 3000 (GE Healthcare Life Sciences) in the Molecular Biotechnology Core of Lerner Research Institute, Cleveland Clinic. Binding was measured by recording response units (RU) over time. Purified 6-his-TRPV4 (NM_021625) was immobilized on Sensor Chip CM5 (GE Healthcare Life Sciences) in pH 5.5 10 mM acetate buffer. Purified 6-his-PI3Kγ or N-term PI3Kγ was used as the soluble analyte, and was injected at different concentrations over the immobilized TRPV4 in SPR buffer (0.01 M HEPES pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% surfactant P-20, GE Healthcare Life Sciences, Biacore Inc.). Binding experiments were performed at 25°C in SPR buffer with a flow rate of 20 μl/min. Data was normalized against an empty reference channel. Analysis and fitting were performed with BIAevaluation software, version 4.0.1 (Biacore Inc.), with the option for calculating the association constant (Ka/Kd). Global fitting was used for fitting sensogram data, and a 1:1 Langmuir model was used to calculate the Ka and Kd values. The distribution and magnitude of the residuals, as well as χ2 values, were used to determine fit quality (51).
Lentiviral Constructs
Lentiviral constructs for HA-tagged WT PI3Kγ (full length), HA-N-terminal PI3Kγ (N-term, contains only AA 1-142), HA-N-terminal deleted PI3Kγ (N-del, missing the N-terminal (ΔAA 1-142) and the ATP binding site (ΔAA 948-981)), and GFP-tagged full length TRPV4 were produced by Cyagen Biosciences. For transduction, subconfluent PI3Kγ KO or TRPV4 KO mouse lung fibroblast monolayers were exposed to 100 MOI of one of the above lentiviral constructs (or a control lentivirus) for 48 hours in complete MEM medium supplemented with polybrene (8 ug/ml, Santa Cruz Biotechnology). Cells were washed 3x with serum free media (SFM), and transferred to 1%BSA/SFM ± TGF-β for 24 hours. The cells were then either lysed in 1%NP-40 lysis buffer and Western blotted for αSMA protein as above, subjected to plasma membrane isolation as above, or fixed and fluorescently stained as below. Transfection efficiency was determined by Western blotting for PI3Kγ and TRPV4.
Immunofluorescence staining
Primary mouse lung fibroblasts (MLFs) were plated on fibronectin-coated (10 μg/ml) glass-bottom plates or on polyacrylamide gels of pathophysiological range stiffness (Matrigen) and maintained in MEM supplemented with 1% FBS for 24 hours, followed by 0.4% FBS for 24 hours. Cells were then transferred to 1% BSA/serum free MEM ± 2ng/ml TGF-β for 24 hours. The attached cells were fixed with 4% paraformaldehyde, permeabilized by 0.05% Triton X-100, and blocked with 5% normal goat serum. To examine the αSMA-incorporated cytoskeletal fibers (indicative of fully differentiated myofibroblasts), the fibroblasts were incubated with primary anti-αSMA antibody (1:1000) followed by Alexa Fluor-488 secondary antibody (1:500), while in some cases, F-actin was stained with Alexa Fluor 594-phalloidin (1:40). To examine TRPV4 and PI3Kγ plasma membrane translocation, fibroblasts were incubated with primary anti-TRPV4 or anti- PI3Kγ antibodies (1:100) followed by Alexa Fluor-488 secondary antibody (1:500). Images were acquired using a Leica DMIRB inverted microscope (Leica Microsystems) equipped with a Retiga SRV camera and QCapture Pro software (Qimaging), or with a Leica TCS-SP8-AOBS inverted confocal microscope using LAS X v3.1.5 with HyD detectors (Leica Microsystems, GmbH). Digital fluorescence microscopic images were analyzed by ImageJ software.
Traction Force Microscopy
Polyacrylamide substrates (shear moduli 25 kPa) embedded with 0.2 μm yellow/green fluorospheres (Matrigen) were incubated with 10 ug/ml fibronectin for 2 hours. Wild-type mouse lung fibroblasts (MLF), TRPV4 KO MLF, or PI3Kγ KO MLF were plated on the gels and allowed to attach in 10% serum-containing media for one hour. The media was aspirated and replaced with 1% BSA in serum free media ± 2 ng/ml TGF-β for 48 hours. Images of gel surface-conjugated fluorescent beads were acquired from 10 different locations before and after trypsinization using a Leica DMIRB inverted microscope (Leica Microsystems) equipped with a Retiga SRV camera and QCapture Pro software (Qimaging) at 10x original magnification. Bead displacement and resultant traction force was calculated using TractionsForAll v1.0 software (Mayo Clinic) as previously described (65–68).
Measurement of intracellular Ca2+
Calcium influx in both human (19Lu) and primary mouse lung fibroblasts was measured in relative fluorescence units (RFU), where RFU is internally referenced, and varies in a linear relationship to calcium concentration, using Calcium 5 dye fluorescence on intact fibroblast monolayers (FlexStation 3, Molecular Devices) as previously published (16). Fibroblasts were plated in 96-well plates coated with fibronectin, and treated ± siRNA as indicated above or ± TGF-β (2ng/ml, 24h) in 1% BSA-containing serum-free MEM as indicated. For the calcium assay, cells were incubated for 45 min at 37°C with Calcium 5 dye in 1X HBSS solution (pH 7.4) containing 20 mM HEPES (pH 7.4) and 2.5 mM probenecid. Calcium influx was induced by the TRPV4 agonist, GSK1016790A (10 nM in human lung fibroblasts (19 Lu) and as indicated in mouse lung fibroblasts). Cytoplasmic calcium increases (Ca2+ influx) were recorded by measuring ΔF/F (Max-Min) and shown as relative fluorescence units (RFU).
Statistics
All data are expressed as means ± standard error (SEM) unless otherwise indicated. Statistical comparisons between control and experimental groups were performed using SigmaPlot software. Student’s t-test was used for two-group comparisons, while one-way ANOVA was used for comparisons between more than two groups. A Student-Newman-Keuls test was used to adjust for multiple comparisons. Values of p ≤ 0.05 were considered significant.
Acknowledgements:
We thank Smarajit Bandyopadhyay PhD of the Cleveland Clinic Lerner Research Institute Molecular Biotechnology Core for his valuable help with the planning and execution of the surface plasmon resonance (Biacore) experiments. We also acknowledge the assistance of the Cleveland Clinic Lerner Research Institute Imaging Core in providing microscopy services.
Funding: This work was supported by National Institutes of Health grants to M. A. Olman (HL119792), M. A. Olman and S. V. Naga Prasad (HL133721), S. V. Naga Prasad (HL089473), B. D. Southern (HL132079), and R. G. Scheraga (HL133380).
Footnotes
Competing Interests: The authors declare that they have no competing interests.
Data and Materials Availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
References and Notes
- 1.Rockey DC, Bell PD, Hill JA, Fibrosis - A common pathway to organ injury and failure. N Engl J Med 372, 1138–1149 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA, Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 4, 583–594 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR, Fibrosis: ultimate and proximate causes. J Clin Invest 124, 4673–4677 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toldo S, Abbate A, The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 15, 203–214 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Darby IA, Zakuan N, Billet F, Desmouliere A, The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol Life Sci 73, 1145–1157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micallef L, Vedrenne N, Billet F, Coulomb B, Darby IA, Desmouliere A, The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair 5, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliére A, Varga J, De Wever O, Mareel M, Gabbiani G, Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180, 1340–1355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendall RT, Feghali-Bostwick CA, Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 5, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marinkovic A, Mih JD, Park J-A, Liu F, Tschumperlin DJ, Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-B responsiveness. Am J Physiol Lung Cell Mol Physiol 303, L169 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humphrey JD, Dufresne ER, Schwartz MA, Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15, 802–812 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaalouk DE, Lammerding J, Mechanotransduction gone awry. Nat Rev Mol Cell Biol 10, 63–73 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle AD, Carvajal N, Jin A, Matsumoto K, Yamada KM, Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat Commun 6, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Ouyang M, Van den Dries K, McGhee EJ, Tanaka K, Anderson MD, Groisman A, Goult BT, Anderson KI, Schwartz MA, Talin tension sensor reveals novel features of focal adhesion force transmission and mechanosensitivity. J Cell Biol 213, 371–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Hayre M, Degese MS, Gutkind JS, Novel insights into G protein and G protein-coupled receptor signaling in cancer. Curr Opin Cell Biol 27, 126–135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White JPM, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I, TRPV4: molecular conductor of a diverse orchestra. Physiol Rev 96, 911 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Rahaman SO, Grove LM, Paruchuri S, Southern BD, Abraham S, Niese KA, Scheraga RG, Ghosh S, Thodeti CK, Zhang DX, Moran MM, Schilling WP, Tschumperlin DJ, Olman MA, TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest 124, 5225–5238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS, Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell 23, 781–791 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA, Agonist-dependent Recruitment of Phosphoinositide 3-Kinase to the Membrane by β-Adrenergic Receptor Kinase 1: A ROLE IN RECEPTOR SEQUESTRATION. Journal of Biological Chemistry 276, 18953–18959 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Mohan ML, Naga Prasad SV, Scaffolding function of PI3Kgamma emerges from enzyme’s shadow. J Mol Biol 429, 763–772 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodyga M, Cambridge E, Karvonen HM, Pakshir P, Wu B, Boo S, Kiebalo M, Kaarteenaho R, Glogauer M, Kapoor M, Ask K, Hinz B, Cadherin-11–mediated adhesion of macrophages to myofibroblasts establishes a profibrotic niche of active TGF-β. Science Signaling 12, eaao3469 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Herrera J, Forster C, Pengo T, Montero A, Swift J, Schwartz MA, Henke CA, Bitterman PB, Registration of the extracellular matrix components constituting the fibroblastic focus in idiopathic pulmonary fibrosis. JCI Insight 4, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilchrist CL, Leddy HA, Kaye L, Case ND, Rothenberg KE, Little D, Liedtke W, Hoffman BD, Guilak F, TRPV4-mediated calcium signaling in mesenchymal stem cells regulates aligned collagen matrix formation and vinculin tension. Proceedings of the National Academy of Sciences 116, 1992 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cayouette S, Boulay G, Intracellular trafficking of TRP channels. Cell Calcium 42, 225–232 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Gombedza F, Kondeti V, Al-Azzam N, Koppes S, Duah E, Patil P, Hexter M, Phillips D, Thodeti CK, Paruchuri S, Mechanosensitive transient receptor potential vanilloid 4 regulates Dermatophagoides farinae—induced airway remodeling via 2 distinct pathways modulating matrix synthesis and degradation. FASEB J, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oancea E, Wolfe JT, Clapham DE, Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res 98, 245–253 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Cuajungco MP, Grimm C, Oshima K, D’hoedt D, Nilius B, Mensenkamp AR, Bindels RJM, Plomann M, Heller S, PACSINs Bind to the TRPV4 Cation Channel - PACSIN3 Modulates the Subcellular Localization of TRPV4. J Biol Chem 281, 18753–18762 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Wang, X YF; Gaiser S; Kottgen M; Kramer-Zucker A; Walz G; Wegierski T, OS-9 regulates the transit and polyubiquitination of TRPV4 in the endoplasmic reticulum. J Biol Chem 282, 36561–36570 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Shin SH, Lee EJ, Chun J, Hyun S, Kang SS, Phosphorylation of TRPV4 serine 824 regulates interaction with STIM1. Open Biochem J 9, 24–33 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamande SR, Yuan Y, Gresshoff IL, Rowley L, Belluoccio D, Kaluarachchi K, Little CB, Botzenhart E, Zerres K, Amor DJ, Cole WG, Savarirayan R, McIntyre P, Bateman JF, Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nature Genet 43, 1142–1146 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Fu Y, Tian W, Cohen DM, Glycosylation of the osmoresponsive transient receptor potential channel TRPV4 on Asn-651 influences membrane trafficking. Am J Physiol Renal Physiol 290, F1103–F1109 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Southern BD, Grove LM, Rahaman SO, Abraham S, Scheraga RG, Niese KA, Sun H, Herzog EL, Liu F, Tschumperlin DJ, Egelhoff TT, Rosenfeld SS, Olman MA, Matrix-driven myosin II mediates the pro-fibrotic fibroblast phenotype. J Biol Chem 291, 6083–6095 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA, Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3, 349–363 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Hinz B, The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol 47, 54–65 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Goswami C, Kuhn J, Heppenstall PA, Hucho T, Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS One 5, e11654 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin SH, Lee EJ, Hyun S, Chun J, Kim Y, Kang SS, Phosphorylation on the Ser 824 residue of TRPV4 prefers to bind with F-actin than with microtubules to expand the cell surface area. Cell Signal 24, 641–651 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Suzuki M, Hirao A, Mizuno A, Microfilament-associated protein 7 increases the expression of trasient receptor potential vanilloid 4 (TRPV4). J Biol Chem 278, 51448–51453 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Storch U, Forst A-L, Pardatscher F, Erdogmus S, Philipp M, Gregoritza M, Schnitzler MM, Gudermann T, Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol. Proc Nat Acad Sci USA 114, E37–E46 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Graaf SFJ, Hoenderop JGJ, van der Kemp AWCM, Gisler SM, Bindels RJM, Interaction of the epithelial Ca2+ channels TRPV5 and TRPV6 with the intestine- and kidney-enriched PDZ protein NHERF4. Pflugers Arch - Eur J Physiol 452, 407–417 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Vanhaesebroeck B, Whitehead MA, Pineiro R, Molecules in medicine mini-review: isoforms of PI3K in biology and disease. J Mol Med 94, 5–11 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Conte E, Fruciano M, Fagone E, Elisa G, Caraci F, Iemmolo M, Crimi N, Vancheri C, Inhibition of PI3K prevents the proliferation and differentiation of human lung fibroblasts into myofibroblasts: the role of class I P110 isoforms. PLoS One, e24663 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei X, Han J, Chen Z, Qi B, Wang G, Ma Y, Zheng H, Luo Y, Wei Y, Chen L, A phosphoinositide 3-kinase-gamma inhibitor, AS605240 prevents bleomycin-induced pulmonary fibrosis in rats. Biochem Biophys Res Commun 397, 311–317 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Russo RC, Garcia CC, Barcelos LS, Rachid MA, Guabiraba R, Roffe E, Souza AL, Sousa LP, Mirolo M, Doni A, Cassali GD, Pinho V, Locati M, Teixeira MM, Phosphoinositide 3-kinase gamma plays a critical role in bleomycin-induced pulmonary inflammation and fibrosis in mice. J Leukoc Biol 89, 269–282 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Kral JB, Kuttke M, Schrottmaier WC, Birnecker B, Warszawska J, Wernig C, Paar H, Salzmann M, Sahin E, Brunner JS, Osterreicher C, Knapp S, Assinger A, Schabbauer G, Sustained PI3K activation exacerbates BLM-induced lung fibrosis via activation of pro-inflammatory and pro-fibrotic pathways. Sci Rep 6, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conte E, Gili E, Fruciano M, Korfei M, Fagone E, Iemmolo M, Lo Furno D, Giuffrida R, Crimi N, Guenther A, Vancheri C, PI3K p110γ overexpression in idiopathic pulmonary fibrosis lung tissue and fibroblast cells: in vitro effects of its inhibition. Lab Invest 93, 566–576 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Wymann MP, Marone R, Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr Opin Cell Biol 17, 141–149 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Shymanets A, Prajwal, Vadas O, Czupalla C, LoPiccolo J, Brenowitz M, Ghigo A, Hirsch E, Krause E, Wetzker R, Williams RL, Harteneck C, Nürnberg B, Different inhibition of Gβγ-stimulated class I(B) phosphoinositide 3-kinase (PI3K) variants by a monoclonal antibody: Specific function of p101 as a Gβγ-dependent regulator of PI3Kγ enzymatic activity. Biochem. J 469, 59–69 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nurnberg B, Gierschik P, Seedorf K, Hsuan JJ, Waterfield MD, Wetzker R, Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science 269, 690 (1995). [DOI] [PubMed] [Google Scholar]
- 48.Walker EH, Perisic O, Ried C, Stephens L, Williams RL, Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature 402, 313–320 (1999). [DOI] [PubMed] [Google Scholar]
- 49.Naga Prasad SV, Laporte SA, Chamberlain D, Caron MG, Barak L, Rockman HA, Phosphoinositide 3-kinase regulates β2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/β-arrestin complex. J Cell Biol 158, 563–575 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perino A, Ghigo A, Ferrero E, Morello F, Santulli G, Baillie George S, Damilano F, Dunlop Allan J., Pawson C, Walser R, Levi R, Altruda F, Silengo L, Langeberg Lorene K., Neubauer G, Heymans S, Lembo G, Wymann Matthias P., Wetzker R, Houslay Miles D., Iaccarino G, Scott John D., Hirsch E, Integrating Cardiac PIP3 and cAMP Signaling through a PKA Anchoring Function of p110γ. Molecular Cell 42, 84–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohan ML, Jha BK, Gupta MK, Vasudevan NT, Martelli EE, Mosinski JD, Naga Prasad SV, Phosphoinositide 3-kinase gamma inhibits cardiac GSK-3 independently of Akt. Sci Signal 6, ra4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vadudevan NT, Mohan ML, Gupta MK, Martelli EE, Hussain AK, Qin Y, Chandrasekharan UM, Young D, Feldman AM, Sen S, Dorn G. W. n., DiCorleto PE, Naga Prasad SV, Gßy-independent recruitment of G-protein coupled receptor kinase 2 drives tumor necrosis factor a-induced cardiac ß-adrenergic receptor dysfunction. Circulation 128, 377–387 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campa CC, Ciraolo E, Ghigo A, Germena G, Hirsch E, Crossroads of PI3K and Rac pathways. Small GTPases 6, 71–80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campa CC, Silva RL, Margaria JP, Pirali T, Mattos MS, Kraemer LR, Reis DC, Grosa G, Copperi F, Dalmarco EM, Lima-Júnior RCP, Aprile S, Sala V, Dal Bello F, Prado DS, Alves-Filho JC, Medana C, Cassali GD, Tron GC, Teixeira MM, Ciraolo E, Russo RC, Hirsch E, Inhalation of the prodrug PI3K inhibitor CL27c improves lung function in asthma and fibrosis. Nat Commun 9, 5232 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mercer PF, Woodcock HV, Eley JD, Platé M, Sulikowski MG, Durrenberger PF, Franklin L, Nanthakumar CB, Man Y, Genovese F, McAnulty RJ, Yang S, Maher TM, Nicholson AG, Blanchard AD, Marshall RP, Lukey PT, Chambers RC, Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax 71, 701–711 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM, Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell 110, 737–749 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Huang SK, Wettlaufer SH, Chung J, Peters-Golden M, Prostaglandin E2 Inhibits Specific Lung Fibroblast Functions via Selective Actions of PKA and Epac-1. Am J Respir Cell Mol Biol 39, 482–489 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Xu H, Geng Y, Xu D, Zhang L, Yang Y, Wei Z, Zhang B, Li S, Gao X, Wang R, Zhang X, Brann D, Yang F, Dibutyryl-cAMP attenuates pulmonary fibrosis by blocking myofibroblast differentiation via PKA/CREB/CBP signaling in rats with silicosis. Respir Res 18, 38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lambers C, Boehm PM, Karabacak Y, Samaha E, Benazzo A, Jaksch P, Roth M, Combined Activation of Guanylate Cyclase and Cyclic AMP in Lung Fibroblasts as a Novel Therapeutic Concept for Lung Fibrosis. BioMed Res Int 2019, 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding Q, Cai G. q., Hu M, Yang Y, Zheng A, Tang Q, Gladson CL, Hayasaka H, Wu H, You Z, Southern BD, Grove LM, Rahaman SO, Fang H, Olman MA, FAK-related nonkinase is a multifunctional negative regulator of pulmonary fibrosis. Am J Pathol 182, 1572–1584 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizuno A, Matsumoto N, Imai M, Suzuki M, Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol 285, C96–C101 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Nienaber JJ, Tachibana H, Prasad SK, Espisito G, Wu D, Mao L, Rockman HA, Inhibition of receptor-localized PI3K preserves cardiac beta-adrenergic receptor function and ameliorates pressure overload heart failure. J Clin Invest 112, 1067–1079 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grove LM, Southern BD, Jin TH, White KE, Paruchuri S, Harel E, Wei Y, Rahaman SO, Gladson CL, Ding Q, Craik CS, Chapman HA, Olman MA, Urokinase-type plasminogen activator receptor (uPAR) ligation induces a raft-localized integrin signaling switch that mediates the hypermotile phenotype of fibrotic fibroblasts. J Biol Chem 289, 12791–12804 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naga Prasad SV, Jayatilleke A, Madamanchi A, Rockman HA, Protein kinase activity of phosphoinositide 3-kinase regulates β-adrenergic receptor endocytosis. Nat Cell Biol 7, 785–796 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Marinkovic A, Mih JD, Park JA, Liu F, Tschumperlin DJ, Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am J Physiol Lung Cell Mol Physiol 303, L169–L180 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mih JD, Sharif AS, Liu F, Marinkovic A, Symer MM, Tschumperlin DJ, A multiwell platform for studying stiffness-dependent cell biology. PLoS One 6, e19929 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Butler JP, Tolić-Nørrelykke IM, Fabry B, Fredberg JJ, Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282, C595–C605 (2002). [DOI] [PubMed] [Google Scholar]
- 68.Tolić-Nørrelykke IM, Butler JP, Chen J, Wang N, Spatial and temporal traction response in human airway smooth muscle cells. Am J Physiol Cell Physiol 283, C1254–C1266 (2002). [DOI] [PubMed] [Google Scholar]