Abstract
Objective
It has been documented that several major components of air pollution, including trace elements and polycyclic aromatic hydrocarbons, are associated with the prevalence of systemic lupus erythematosus (SLE). However, the impact of air pollution on the SLE disease activity is still elusive. In this paper, we review the current evidence investigating the link between air pollution, especially when measured as PM2.5, and SLE severity and activity.
Methods
A detailed literature search was applied a priori to the Ovid MEDLINE In-Process and Other Non-Indexed Citation 1986 to present. Presented abstracts from the European League Against Rheumatism and American College of Rheumatology (ACR)/Association for Rheumatology Health Professionals (ARHP) Annual Meetings (2011–2018) were also screened.
Results
Out of a total of 1354 papers retrieved from search and references list for detailed evaluation, data from 652 patients with SLE from three studies were analyzed. Two studies had an observational longitudinal design, counting for 348 patients with a follow-up of 24 months and 79 months. Retrieved studies differed for disease activity assessment and air pollution quantifications.
Conclusion
Current evidence suggests that variations in air pollution may influence the disease activity in patients with SLE. However, the sample size, methodological biases, and differences across the studies make further research mandatory. Understanding the increased burden of SLE and its complications, not only from a medical, but also from a socio-demographic perspective, including an exposure to pollutants, should have implications for resource allocation and access to subspecialty care.
Keywords: Air pollution, autoimmunity, environment, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease of unknown origin. Its prevalence has been estimated as high as 1 in 2,500 women in the general population, with extreme heterogeneity across studies and countries (1, 2). Potentially, any organ can be affected and can present with life-threatening manifestations (3, 4). The underlying mechanism driving SLE is complex, but a growing body of evidence is supporting a role of specific exogenous triggers. Various possible environmental triggers have been considered, but at present, there are few relevant studies (2, 5–7), and essentially no work has systematically investigated the effect of air pollution on SLE manifestations and diseases activity, to the best of our knowledge. This gap represents a major unmet need because there is a growing concern about harmful air pollution exposure, especially related to small particulate matter pollution with a diameter smaller than 2.5 and 10 micrometers (PM2.5 and PM10, respectively) and its effects on human health. Particulate air pollution has effects on the immune system similar to those of inhaling cigarette smoke and silica, and it has been linked to asthma, chronic bronchitis, cardiovascular disease, and lung and laryngeal cancers (8–15). However, while particulate air pollution has been linked to the development of systemic autoimmune rheumatic diseases (SARD) (5, 16), the debate on its role on SLE is still ongoing. In addition, it is vital for physicians and health care specialists to look at socio-demographic aspects when reviewing impacts of particulate air pollution. Thus, we aim to perform a systematic review of the available literature, intending to address a question often raised by patients with SLE during routine consultations: Is air pollution affecting my disease?
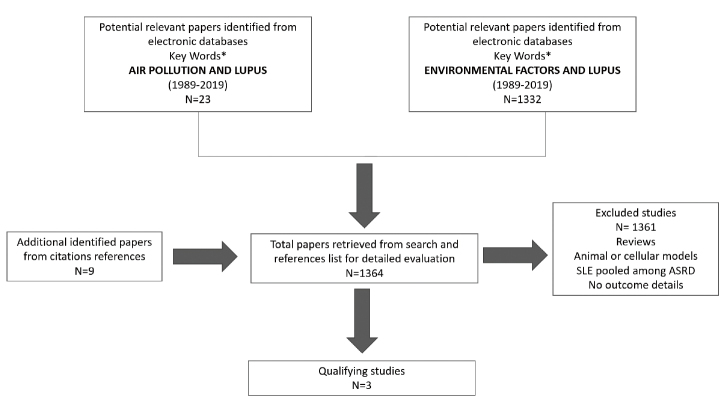
To find an answer to this question, a literature analysis has been performed to identify the most robust information reported on the impact of air pollution on disease activity in patients with SLE. Inclusion criteria were the following: (a) studies including patients with a confirmed SLE diagnosis according to current classification criteria (17); (b) a detailed description of a definition of the air pollution exposure; and (c) details on the SLE activity and inflammation assessment, including validated activity score indexes and/or laboratory biomarkers. Keywords and subject terms were listed as supplementary materials. The literature search strategy is presented in Figure 1. The present study has been performed according to the PRISMA guidelines.
Figure 1.
Literature search strategy for the systematic review of the literature (1989–2019).
ASRD: Autoimmune Systemic Rheumatic Diseases.
*Listed as supplementary materials.
Available evidence
Data from 652 patients with SLE from three studies were analyzed. The studies differed in their design and sample sizes. Two studies had an observational longitudinal design, including 348 patients with a follow-up of 24 months in the study by Alves et al. (18) and 79 months in the study by Bernatsky et al. (19). In brief, the main conclusions from these studies can be summarized as follows:
Bernatsky et al. (19) evaluated the association between PM2.5 and annual disease activity assessed by the SLEDAI-2K score in adult patients with SLE. It was found that anti-dsDNA and the presence of renal casts were significantly associated with PM2.5 levels at 24–48 h before the visits, even if the disease activity was not associated with PM2.5 levels.
Almes et al. (18) investigated 108 consecutive appointments with pediatric SLE patients without respiratory diseases and found that an increase in the risk of SLEDAI-2K ≥8 was associated with PM2.5 7-day moving average. They also observed an increase in the airway inflammation markers (nitrogen dioxide, cytokines of exhaled breath condensate—EBC [interleukins 6, 8, 17, and tumoral necrose factor-α], fractional exhaled NO) associated with the daily exposure to PM2.5. This was assessed by individual real-time monitoring.
A third study by Lanata et al. (20) showed results from preliminary genome-wide assessment of DNA methylation. This study showed that SLE patients who reside close to a highway are more likely to present with hypomethylation of the UBE2U gene, which encodes an enzyme that is involved in the ubiquitination of proteins and histones, as well as DNA repair. If confirmed, these observations raise the possibility that these epigenetic changes might be associated with SLE.
Medical and socio-demographic considerations
Outdoor air pollution caused 3.2 million premature deaths worldwide in 2015 (12). While the link between cardiovascular (14) and respiratory conditions (13) and air pollution is well known, recent studies has provided an increasing body of evidence that polluted air, predominantly air with high levels of particulate matter with a diameter <2.5 micrometers (PM2.5), can have a range of negative impacts on health, both in terms of mortality and morbidity. A number of conditions, including chronic kidney disease progression (11), bone fracture (21), premature birth and low birth weight (22), hypertension (23) and psychological distress (24), among others, have been observed with a higher prevalence in polluted areas. Albeit the level of evidence is still low, and our preliminary findings suggested that air pollution might trigger an SLE flare.
We retrieved two observational studies for our analysis, with only one supporting an association between air pollution and the SLE disease activity, when expressed by SLEDAI. However, even if they failed to find a significant association with SLEDAI levels, Bernatsky et al. (19) observed a strong association between PM2.5 levels at 24 and 48 hours before the visits and diseases activity measured by anti-dsDNA and presence of renal casts, traditional biomarkers associated with diseases flare. A third long-term cohort study investigating the exposure to traffic-related air pollution and SLE confirmed that higher levels PM2.5 are significantly associated with the risk of SLE. In line with these observation, a recent Taiwanese study showed that the exposure to gaseous air pollutants (NO2 and CO) that is lower than current National Ambient Air Quality Standards is associated with SLE.
Small particulate matter pollution (PM2.5 and PM10) has serious health effects even at very low concentrations. Particles with a diameter ≤10 microns (≤PM10) are harmful to human health as they can penetrate the airways of a smaller caliber within the lungs. However, it is now known that particles with a diameter ≤2.5 microns (≤PM2.5) are associated with the higher risk of damage, contributing to the development of cardiovascular and respiratory diseases, as well as lung cancer (25). Because PM2.5 are smaller in size, these particles can penetrate the lung barrier and easily enter the circulation. Our findings are in line with these observations, supporting that PM2.5 might affect the SLE activity, assessed either with SLEDAI or biomarkers. In addition to the serious risks from exposure to particulate matter, exposure to other harmful pollutants, such as ozone (O3), nitrogen dioxide (NO2), and sulfur dioxide (SO2), pose human health risks. However, the retrieved studies did not take these aspects into full account, pointing to the need for a future specifically designed study. In addition, the level of air pollution should be discussed.
To date, there is no threshold of particular matter identified to express the damage to health. Countries around the world have their own air quality indexes corresponding to different national air quality standards, which also shows differing “safe” levels for particulate matter. For example, India’s National Air Quality Index ranks levels <60 μg/m3 of PM2.5 as satisfactory levels within a 24-hour period, whereas satisfactory levels of PM2.5 in the United Kingdom are <53 μg/m3, and the United States ranks 35.4 μg/m3 within a 24-hour period. The World Health Organization’s (WHO) Air Quality Guidelines (2005) estimates that the lowest concentration of PM2.5 at safe levels is 25 μg/m3 for the 24-hour mean (not to be exceeded for >3 days/year) and 10 μg/m3 for the annual average (26). The WHO advises to reduce the annual average particulate matter (PM2.5) concentrations from 35 μg/m3, which is common in many developing cities, to the WHO guideline level of 10 μg/ m3. By doing so, this could reduce air pollution related deaths by approximately 15%. However, despite the fact that many European cities comply with the WHO guideline for levels PM concentrations, it has been speculated that the average life expectancy is 8.6 months lower than it would otherwise be, due to the exposure to PM from human sources (26).
Interestingly, the association between the disease activity and SLE was reported in the study by Almes et al. (18), including patients (mainly form a pediatric population) exposed to higher levels of pollutants. On the contrary, the association was not observed in the Canadian study (19), probably also because the PM2.5 levels infrequently exceeded the national goal of 30 μg/m3 during the observation time, posing the question if only a higher value of PM2.5 might significantly trigger disease exacerbations.
A further question is to investigate how air pollution can induce a disease flare. In a genome-wide analysis investigating DNA methylation in relation to residential proximity to highways, Lanata et al. (20) suggested that specific methylation profiles (such as hypomethylation of UBE2U) are more frequently found in patients with SLE residing close to traffic areas. While primary, these observations come from a large Lupus Genetics Project. If confirmed, they will pave the way to further investigations to determine whether epigenetic modification related to the exposure to pollutants are associated with the SLE severity and outcome.
Our analysis has some limitations. First, the evidence is relatively new for a potential association of the SLE disease activity with ambient PM2.5. Only a handful of studies have investigated the association of particulate matter and its impact on autoimmune diseases (27).
Second, investigating the quality of the evidence concerning environmental exposure and the SLE risk depend on a critical analysis of available epidemiologic research. Traditional approaches, such as retrospective case-control studies, might suffer from the recall bias and misclassification error. Occupational cohort studies and population-based investigations might have some shortcomings when dealing with low incidence rates. Besides, challenges in discriminating SLE based on self-reporting, which is sensitive but non-specific, might represent another bias. Conversely, using other techniques to confirm cases may introduce yet another selection bias (e.g., when filtering for specific medications). Similarly, it is not possible to exclude heterogeneity in consent to a medical records review, potentially varying in major demographic subgroups (e.g., by age, gender, or other social factors). Taken all together, the challenges in case definition also reflect the challenges in disease diagnosis, including a potential for incomplete clinical manifestations and overlap with other autoimmune features.
Third, this study was not aiming to investigate if exposure to PM2.5 might trigger the new onset of SLE or any other autoimmune diseases. Some studies have investigated the topic already (28–32). However, when analyzing this aspect, Chau-Ren Junget et al. (28) recently conducted a population-based cohort study in Taiwan to examine the associations of air pollution with newly diagnosed SLE cases. They retrieved a total of 682,208 individuals aged 18–70 years from the National Health Insurance Research Database. When applying the 1-km-resolution land use regression and satellite-based models to estimate the air pollution concentrations from 2001 to 2010, the authors found a positive association of SLE with the exposure to several air pollutants. They observed a 9.76 ppb increase in nitrogen dioxide (NO2), a 0.20 ppm increase in carbon monoxide (CO), and a 10.2 μg/m3 increase in PM2.5 in the areas with a higher incidence of newly diagnosed SLE.
Socio-economic perspectives on air pollution and health
A multifaceted relationship between genetic, hormonal, environmental, and socio-economic factors likely contributes to the onset and severity of SLE. Other variations, including gender, race/ethnicity, and income level, can also be contributing factors (33). Additionally, genetic differences by ethnicity have been examined to explain the younger age of onset of SLE among African Americans, and a higher prevalence of serologic abnormalities in African Americans compared to Caucasians (1, 33). However, ethnic differences often hide more society-based causes that are generating diseases. For example, in the case of obesity-related illnesses, it is difficult to understand if it is the diet or genetic configurations that affect them (34). Similarly, lack of access to healthy foods in low-income communities, also referred to as “food deserts,” has been seen as the most important cause of obesity and food-related diseases. Occupational and environmental exposures, in addition to genetics, may influence the course of SLE, affecting the disease activity (35). People of a lower socio-economic status often live in closer proximity to hazardous wastes and might be expose to higher levels of air pollution (35), and the workplace exposure, particularly to silica dust, is more likely from manual labor jobs. In the case of air pollutants exposure in the urban environment, it is clear that at least all city dwellers are exposed to the risk. However, given that the risk is not universal and that it does not transform into real disease for everybody, the problems are the vulnerabilities that differentiate among people on the basis of social variables, namely income, lifestyle, or work conditions. A recent nationwide study led in the United States found that communities living below the poverty line have a 35% higher burden from PM emissions than the general population. Non-Caucasian communities had a 28% higher health burden, with African Americans specifically having a 54% higher burden than the overall population (12, 35). These data show that by having polluting facilities in low-income neighborhoods, marginalized communities experience higher rates of health problems, including asthma, heart attacks, or premature death. The lasting social-economic effects that come with those health issues, such as children being absent from school, create a cycle of poverty. This also leads to a continuous lack of access for people in low-income communities in the United States and around the world.
Understanding the increased burden of SLE and its complications from a socio-demographic perspective, including exposure to pollutants, should have implications for resource allocation and access to subspecialty care. Although more studies are needed to confirm these findings, physicians, public health officials, and patients need to continue the discussion of the potentially serious adverse health effects of harmful air pollution related to SLE.
Conclusion
In conclusion, our analysis, although preliminary, suggests that higher levels of air pollution may influence the disease activity in patients with SLE. Our observation aligns with concerns that pollution, especially particulate matter, may be an important trigger of inflammation and autoimmunity. It is imperative that we employ effective measures to adapt to an increasing air pollution exposure. Certainly, different measures of adaptation (including policies for improving the air quality and low carbon technologies) are already being implemented in some areas, but in a piecemeal manner.
A strategic approach is needed to ensure that effective adaptation measures are being implemented across all communities—from low income to affluent—ensuring at the same time coherency across different social sectors and levels of governance. Ultimately, from a physician’s standpoint, adaptation is required to make a concrete impact on the care of patients.
Main Points.
There is a growing concern regarding the pollution emissions exposure, especially related to particulate matter, and its effects on human health.
The involvement of environmental factors has been speculated to a role in the SLE etiology.
Current evidence, albeit scarce, suggests a link between air pollution, especially when measured as PM2.5, and SLE diseases severity and activity
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.S., D.R., C.P., W.G.G.; Design - S.S., W.G.G., N.F., M.R.; Supervision - D.R., C.P., S.M., M.J.C.; Data Collection and/or Processing - M.R., S.S., W.G.G., M.J.C.; Analysis and/or Interpretation - M.R., S.S., W.G.G.; Literature Search - M.R., M.J.C., W.G.G., S.S.; Writing Manuscript - S.S., N.E., W.G.G.; Critical Review - M.R., M.J.C., S.M., C.P., D.R., S.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support
References
- 1.Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605–20. doi: 10.1038/nrrheum.2016.137. [DOI] [PubMed] [Google Scholar]
- 2.Leffers HCB, Lange T, Collins C, Ulff-Møller CJ, Jacobsen S. The study of interactions between genome and exposome in the development of systemic lupus erythematosus. Autoimmun Rev. 2019;18:382–92. doi: 10.1016/j.autrev.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Sciascia S, Radin M, Roccatello D, Sanna G, Bertolaccini ML. Recent advances in the management of systemic lupus erythematosus. F1000Research. 2018;7:970. doi: 10.12688/f1000research.13941.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roccatello D, Sciascia S, Baldovino S, Rossi D, Alpa M, Naretto C, et al. A 4-year observation in lupus nephritis patients treated with an intensified B-lymphocyte depletion without immunosuppressive maintenance treatment-Clinical response compared to literature and immunological re-assessment. Autoimmun Rev. 2015;14:1123–30. doi: 10.1016/j.autrev.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Zhao CN, Xu Z, Wu GC, Mao YM, Liu LN, Wu Q, et al. Emerging role of air pollution in autoimmune diseases. Autoimmun Rev. 2019;18:607–14. doi: 10.1016/j.autrev.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Parks CG, de Souza Espindola Santos A, Barbhaiya M, Costenbader KH. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2017;31:306–20. doi: 10.1016/j.berh.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper GS, Parks CG, Schur PS, Fraser PA. Occupational and environmental associations with antinuclear antibodies in a general population sample. J Toxicol Environ Health A. 2006;69:2063–9. doi: 10.1080/15287390600746165. [DOI] [PubMed] [Google Scholar]
- 8.Bernatsky S, Smargiassi A, Barnabe C, Svenson LW, Brand A, Martin RV, et al. Fine particulate air pollution and systemic autoimmune rheumatic disease in two Canadian provinces. Environ Res. 2015;146:85–91. doi: 10.1016/j.envres.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Wang G, Chen N, Tao Lu, Nie S, Xu G, et al. Long-Term Exposure to Air Pollution and Increased Risk of Membranous Nephropathy in China. J Am Soc Nephrol. 2016;27:3739–46. doi: 10.1681/ASN.2016010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Argacha JF, Collart P, Wauters A, Kayaert P, Lochy S, Schoors D, et al. Air pollution and ST-elevation myocardial infarction: A case-crossover study of the Belgian STEMI registry 2009–2013. Int J Cardiol. 2016;223:300–5. doi: 10.1016/j.ijcard.2016.07.191. [DOI] [PubMed] [Google Scholar]
- 11.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. Particulate Matter Air Pollution and the Risk of Incident CKD and Progression to ESRD. J Am Soc Nephrol. 2018;29:218–30. doi: 10.1681/ASN.2017030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–71. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- 13.Mo Z, Fu Q, Zhang L, Lyu D, Mao G, Wu L, et al. Acute effects of air pollution on respiratory disease mortalities and outpatients in Southeastern China. Sci Rep. 2018;8:3461. doi: 10.1038/s41598-018-19939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah ASV, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, et al. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2015;350:h1295. doi: 10.1136/bmj.h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015;372:905–13. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farhat SCL, Silva CA, Orione MAM, Campos LMA, Sallum AME, Braga ALF. Air pollution in autoimmune rheumatic diseases: a review. Autoimmun Rev. 2011;11:14–21. doi: 10.1016/j.autrev.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 18.Alves AGF, de Azevedo Giacomin MF, Braga ALF, Sallum AME, Pereira LAA, Farhat LC, et al. Influence of air pollution on airway inflammation and disease activity in childhood-systemic lupus erythematosus. Clin Rheumatol. 2018;37:683–90. doi: 10.1007/s10067-017-3893-1. [DOI] [PubMed] [Google Scholar]
- 19.Bernatsky S, Fournier M, Pineau CA, Clarke AE, Vinet E, Smargiassi A. Associations between ambient fine particulate levels and disease activity in patients with systemic lupus erythematosus (SLE) Environ Health Perspect. 2011;119:45–9. doi: 10.1289/ehp.1002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanata C, Nayak R, Nitiham J, Taylor K, Lisa F, Barcellos SAC, et al. Residential Proximity to Highways, DNA Methylation and Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015;67(Suppl 10) [Google Scholar]
- 21.Prada D, Zhong J, Colicino E, Zanobetti A, Schwartz J, Dagincourt N, et al. Association of air particulate pollution with bone loss over time and bone fracture risk: analysis of data from two independent studies. Lancet Planet Health. 2017;1:e337–47. doi: 10.1016/S2542-5196(17)30136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleischer NL, Merialdi M, van Donkelaar A, Vadillo-Ortega F, Martin RV, Betran AP, et al. Outdoor Air Pollution, Preterm Birth, and Low Birth Weight: Analysis of the World Health Organization Global Survey on Maternal and Perinatal Health. Environ Health Perspect. 2014;122:425–30. doi: 10.1289/ehp.1306837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang B-Y, Qian Z, Howard SW, Vaughn MG, Fan SJ, Liu KK, et al. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ Pollut. 2018;235:576–88. doi: 10.1016/j.envpol.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Sass V, Kravitz-Wirtz N, Karceski SM, Hajat A, Crowder K, Takeuchi D. The effects of air pollution on individual psychological distress. Health Place. 2017;48:72–9. doi: 10.1016/j.healthplace.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Facts Sheet.
- 26.WHO Ambient-(outdoor)-air-quality-and-health. https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health. Published 2005.
- 27.Bernatsky S, Smargiassi A, Johnson M, Kaplan GG, Barnabe C, Svenson L, et al. Fine particulate air pollution, nitrogen dioxide, and systemic autoimmune rheumatic disease in Calgary, Alberta. Environ Res. 2015;140:474–8. doi: 10.1016/j.envres.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung CR, Chung WT, Chen WT, Lee RY, Hwang BF. Long-term exposure to traffic-related air pollution and systemic lupus erythematosus in Taiwan: A cohort study. Sci Total Environ. 2019;668:342–9. doi: 10.1016/j.scitotenv.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Conde PG, Farhat LC, Braga ALF, Sallum AEM, Farhat SCL, Silva CA. Are prematurity and environmental factors determinants for developing childhood-onset systemic lupus erythematosus? Mod Rheumatol. 2018;28:156–60. doi: 10.1080/14397595.2017.1332508. [DOI] [PubMed] [Google Scholar]
- 30.Vidotto JP, Pereira LAA, Braga ALF, Silva AC, Sallum AM, Campos LM, et al. Atmospheric pollution: influence on hospital admissions in paediatric rheumatic diseases. Lupus. 2012;21:526–33. doi: 10.1177/0961203312437806. [DOI] [PubMed] [Google Scholar]
- 31.Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, et al. Systemic lupus erythematosus. Nat Rev Dis Prim. 2016;2:16039. doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 32.Goodarzi M. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018;6:223–36. doi: 10.1016/S2213-8587(17)30200-0. [DOI] [PubMed] [Google Scholar]
- 33.Adamkiewicz G, Zota AR, Fabian MP, Chahine T, Julien R, Spengler JDLJ. Moving environmental justice indoors: understanding structural influences on residential exposure patterns in low-income communities. Am J Public Health. 2011;101(Suppl 1):S238–45. doi: 10.2105/AJPH.2011.300119. [DOI] [PMC free article] [PubMed] [Google Scholar]