Abstract
Objective
The clinical impact of repeat renal biopsies in patients with lupus nephritis (LN) is still debatable. The aim of this retrospective analysis was to assess whether repeat renal biopsy is a reliable tool in guiding therapeutic decisions.
Methods
Laboratory and histological parameters and therapeutic changes in 35 patients with LN and repeat renal biopsies were retrospectively analyzed. Biopsies were performed in the presence of clinical evidence of an active glomerular disease. Biopsy specimens were retrospectively re-assessed by two renal pathologists and were compared according to the last International Society of Nephrology/ Renal Pathology Society classification.
Results
Thirty-five patients had two, 13 had three, 5 had four, 4 had five, and 1 had six renal biopsies. Fifty-eight comparisons of renal biopsies were made. Median times between the first and second, second and third, third and fourth, and fourth and fifth biopsies were 31, 27, 34, and 28 months, respectively. The mean activity indices from the first to the fifth biopsy were 8.7, 6.6, 7.8, 9.4, and 4.7, whereas the mean chronicity indices were 1.7, 2.3, 4.3, 5.2, and 7.7, respectively. Conversion was observed in 65.5% of cases with the most frequent (21%) being between classes III and IV. Conversion to a more severe type of nephritis occurred in 19% of cases. There was no correlation of laboratory parameters to the type of nephritis upon conversion. In 79% of cases, immunosuppressive therapy was modified after repeat biopsy.
Conclusion
Repeat biopsy is a reliable tool for monitoring the activity and chronicity status of LN and for tailoring immunosuppressive therapy to the needs of the patient, especially late in the course of the disease.
Keywords: Conversion, lupus nephritis, monitoring, relapses, repeat biopsies, therapeutic decisions
Introduction
Lupus nephritis (LN) is a major cause of morbidity in systemic lupus erythematosus (SLE) and a predictor of poor long-term patient survival (1). Up to 60% of patients with a diagnosis of SLE will develop nephritis at some time point in the course of the disease (2). Moreover, the disease is characterized by remissions and relapses (3).
The role of kidney biopsy is essential for the diagnosis and classification of LN, and kidney biopsy is recommended, according to the recently published European Dialysis and Transplant Association/European Renal Association—European League Against Rheumatism guidelines, in all patients with clinical evidence of active LN previously untreated, unless strongly contraindicated (4).
However, the value of repeat kidney biopsy as a tool for monitoring LN still remains debatable. Although renal flares and conversion from one class of LN to another are common, the need of a repeat biopsy is not clearly established.
It has been our clinical practice for the last 15 years to perform a renal biopsy both at clinical suspicion of LN and at diagnosis, as well as at relapse during follow-up.
The aim of the present study was to determine the role of repeat renal biopsies performed by clinical indication for monitoring of LN longitudinally and its utility for the guidance of therapeutic decisions.
Methods
A total of 35 patients with SLE who had at least two renal biopsies performed between 2000 and 2012 were reviewed. Inclusion criteria were a clinical and laboratory diagnosis of LN flare. The study was approved by the ethics committee of the Laiko Hospital according to the Declaration of Helsinki (20-02-2014/140). Written consent was obtained from all patients.
Clinical data collection
Clinical data were collected by chart review, including demographics, time of first diagnosis of SLE, and time of first renal involvement. Renal function, proteinuria, and urinary sediment were assessed. Renal function was assessed by estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease equation, whereas proteinuria was determined by 24-hour urine collection. Active urine sediment was defined as glomerular hematuria with >5 RBC/hpf and/or cellular casts. All patients with evidence of active glomerular disease were biopsied. Further laboratory parameters that were examined were complement levels (C3 and C4) and anti-double strand DNA antibody (anti-ds DNA) titer.
Assessment of renal histology
Biopsy samples were considered as appropriate for the study only if the number of glomeruli was ≥10. For the purpose of the study, a total of 93 kidney biopsy specimens were re-evaluated by two experienced renal pathologists. Biopsies were examined by light microscopy, including calculation of activity and chronicity indices, and were categorized according to the newer International Society of Nephrology/ Renal Pathology Society (ISN/RPS) classification. This classification was used to compare the histological findings of repeat biopsies. For patients with more than two biopsies, each new biopsy was compared with the previous, i.e., the second and third, the third and fourth, and the fourth and fifth were paired. Thus, the biopsy performed before each repeat biopsy served as the reference.
Clinical follow-up
After the first renal biopsy, all patients were followed up for at least 16 months. The therapeutic regimen for induction therapy in proliferative (III and IV) and mixed (III/IV+V) classes of LN consisted of either intravenous cyclophosphamide pulses or mycophenolic acid (MPA; mycophenolate mofetil or mycophenolate sodium), both in combination with steroids, whereas for maintenance, the majority of patients continued with MPA. Maintenance therapy was individualized in patients with frequent relapses and/or cumulative toxicity from prior courses of therapy. In a minority of cases, 9% (5/58) of patients received azathioprine, whereas 40% (23/58) of patients were treated with the monoclonal anti-CD20 antibody Rituximab, whereas calcineurin inhibitors (cyclosporine) were added in 7% (4/58), primarily for persistent nephrotic syndrome. Analysis of treatment regimens for classes II and V or particular subgroups of patients as those with rapidly progressive glomerulonephritis and refractory or frequently relapsing cases is beyond the scope of the present study and thus were excluded.
Repeat biopsies were always performed by clinical indication of either relapse (n=27, 47%), ongoing activity (n=16, 28%), partial remission (n=6, 10%), or deterioration (n=9, 15%).
Definitions
Complete remission was defined as normal serum creatinine and albumin levels, inactive urine sediment, and 24-hour urinary protein <0.5 g.
Relapse was defined as the presence of any of the following in at least two measurements with 1–2 weeks interval:
increase in proteinuria by >1 g/day
activation of previously inactive urinary sediment
decrease in GFR by 25%.
Ongoing activity was defined as persistently active urinary sediment and proteinuria >1 g/day.
Partial remission was defined as >50% improvement in all parameters that were abnormal at baseline.
Deterioration was defined as worsening of renal function of >25% and/or nephrotic range proteinuria and/or presence of active urinary sediment.
Statistical analysis
Data are expressed as mean±standard deviation for continuous variables and as percentage (%) for categorical data. Owing to the sample size (n>30), normality has been assumed for all variables. However, the application of non-parametric tests was preferred. Mann-Whitney test was performed in continuous variables to identify the possible statistically significant differences between the mean values of the various biopsies. Chi-square test (with Fisher’s correction) was used for categorical variables. Wilcoxon’s test was used for comparison between serial biopsies between the groups. All statistical tests were examined in a 5% level of significance. In other words, null hypothesis is rejected with >95% confidence interval (2-tailed p<0.5).
Results
Demographic and clinical characteristics of the patient population
A total of 35 patients with LN who underwent repeat renal biopsies were evaluated. The study population consisted of 28 (80%) female and 7 (20%) male patients. The mean age of the patients was 27 years at the time of first renal biopsy. The mean time of SLE duration before onset of nephritis was 31.5 months. Overall, there were 93 biopsies, with 35 patients with two, 13 with three, 5 with four, 4 with five, and 1 with six biopsies. The number of repeat biopsies was 58.
Histological parameters and pattern of pathology at reference and repeat renal biopsies
The mean number of glomeruli in our biopsy specimens ranged from 20±7 to 29±14. The mean renal activity indices on the first, second, third, fourth, and fifth biopsies were 8.7, 6.6, 7.8, 9.4, and 4.7, respectively, whereas the mean chronicity indices were 1.7, 2.3, 4.3, 5.2, and 7.7, respectively. The mean time intervals between subsequent biopsies were 31, 27, 34, and 28 months, respectively.
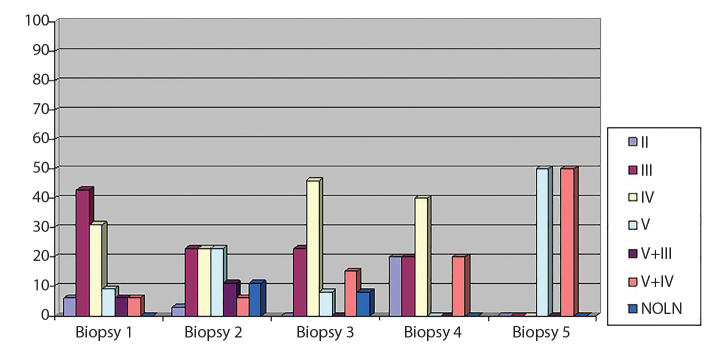
LN class at first and repeat renal biopsies is shown in Figure 1.
Figure 1.
Classification of lupus nephritis at first and repeat renal biopsies.
Pattern of histological conversion on repeat biopsies
The histological conversions on repeat biopsies are shown in Table 1.
Table 1.
Histological conversions on repeat biopsies (ISN/RPS 2003 classification of lupus nephritis).
Pathological transition from the reference biopsy occurred in 65.5% and did not occur in 34.5% of the repeat biopsies The most frequent types of conversion were from one pure proliferative form to another (class III to class IV or vice versa) (21%) and from class III or IV to mixed nephritis (V+III/IV) ( 15.5%).
| Repeat biopsy (class) | Reference biopsy (class) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| I | II | III | IV | V | VI | III+V | IV+V | |
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| II | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| III | 0 | 1 | 5 | 5 | 0 | 0 | 0 | 1 |
| IV | 0 | 1 | 7 | 9 | 0 | 0 | 1 | 1 |
| V | 0 | 0 | 2 | 1 | 4 | 0 | 1 | 1 |
| VI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| III+V | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 |
| IV+V | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 2 |
| NO LN | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 |
On repeat biopsy, no shift to another class was found in only 20 (34.5%) of the 58 repeat biopsies. The total rate of transition was 65.5% (38/58). The most frequent transitions were those from class III to IV and vice versa (12/58, 21%). Some investigators suggest that this type of conversion, i.e., between proliferative classes, should not be considered as “true” conversion, since both classes are treated as the same entity and differ only in the percentage of the affected glomeruli. In our study, when this type of class transformation was “with-drawn,” the total rate of conversions decreased to 36% (21/58) and involved predominantly the transition from class III or IV to mixed nephritis (V+III/IV) (15.5%, 9/58).
Classification of biopsies according to the presence of a proliferative pattern
Assessment of conversion on repeat biopsies
When the reference biopsies were classified as pure proliferative (n=45), non-proliferative (n=6), and mixed nephritis (n=7), the histological pattern in the repeat biopsy changed in 42% (19/45), 33% (2/6), and 71% (5/7), respectively (Table 2).
Table 2.
Conversion according to proliferative and non-proliferative lesions.
For the reference biopsies that showed pure proliferative, non-proliferative and mixed nephritis, histological change was recorded in 42%, 33% and 71% respectively. A transition to a nonproliferative pattern was observed in 12% of the repeat biopsies.
| Reference Biopsy | ||||
|---|---|---|---|---|
|
| ||||
| Pure proliferative (III, IV) | Non proliferative (V, II) | Mixed (V+III/IV) | Total Number | |
| Repeat Biopsy | Pure proliferative | 26 | 2 | 3 31 |
| Non proliferative | 5 | 4 | 2 11 | |
| Mixed | 9 | 0 | 2 11 | |
| NO LN | 5 | 0 | 0 5 | |
| Total Number | 45 | 6 | 7 58 | |
Since proliferative classes (III and IV) have worse prognosis than non-proliferative classes (II and V) and mixed (V+III/IV) probably even worse, conversion from either class II or V to III/IV±V, as well as from pure III or IV to mixed (V+III/IV), was considered as conversion to a more severe class of LN. This was observed in 19% (11/58) of repeat biopsies.
Switch from non-proliferative lesions on reference biopsy to proliferative (pure or mixed) on repeat biopsy, which is considered by certain authors (5) as the most clinically relevant switch, occurred in only 3.4% (2/58) of cases. In both cases, conversion was from class II to either class III or IV. Interestingly, no switch from pure membranous (class V) to proliferative nephritis (class III/IV) was seen in our study population. The opposite, i.e., switch from proliferative (pure or mixed) to non-proliferative classes, occurred in 12% (7/58) of cases.
In 5/58 (9%) cases with pure proliferative nephritis on reference biopsy, the repeat biopsy revealed non-LN. Two of them showed non-specific lesions with negative immunofluorescence, and the other three showed focal segmental glomerulosclerosis (FSGS).
Assessment of laboratory and histological parameters between serial biopsies
Renal (serum creatinine, eGFR, and proteinuria) and serological (C3, C4, and anti-ds DNA) parameters between serial biopsies were compared to determine if there was any laboratory parameter that could predict conversion. The only differences observed were in C3 levels between the first and second biopsies (p=0.012) and in anti-ds DNA titer between the fourth and fifth biopsies (p=0.019) (Table 3). The fact that there was a significant increase in C3 levels between the first and second biopsies could be an indicator of lower activity at the first relapse. In addition, the lower anti-ds DNA titer between the fourth and fifth biopsies probably indicates the lower activity for relapses that occur late in the course of the disease.
Table 3.
Descriptive statistics for laboratory parameters per biopsy and Mann Whitney results between serial renal biopsies.
No significant difference was recorded in renal parameters of disease activity between biopsies. Serum C3 levels increased significantly between the first and the second biopsy (p=0.012). Antids DNA titer decreased between the fourth and the fifth biopsy (p=0.019).
| Variable | Biopsy-1 | Biopsy-2 | Biopsy-3 | Biopsy-4 | Biopsy-5 |
|---|---|---|---|---|---|
| N = 35 | N=35 | N=13 | N=5 | N=4 | |
| Cr (mg/dL) | 1.21±0.81 | 1.05±0.77 | 1.27±1.08 | 1.86±1.30 | 1.03±0.32 |
| Z-test* | −1.635 | −0.466 | −0.529 | −1.169 | |
| p | 0.102 | 0.641 | 0.597 | 0.243 | |
| e GFR (mL/min/1.73m2 ) | 77±30.52 | 89.44±32.74 | 76.19±30.84 | 65.20±56.91 | 74.50±19.23 |
| Z-test* | −1.78 | −0.517 | −0.522 | −1.155 | |
| p | 0.075 | 0.605 | 0.602 | 0.248 | |
| Proteinuria (g/24h) | 3.02±2.65 | 3.73±3.23 | 4.55±2.88 | 5.30±2.59 | 2.23±1.85 |
| Z-test* | −0.876 | −0.136 | −0.419 | −0.289 | |
| p | 0.381 | 0.892 | 0.675 | 0.773 | |
| C3 (mg/dL) | 51.02±29.49 | 73.76±40.99 | 69.46±28.23 | 73.70±32.91 | 73.43±30.23 |
| Z-test* | −2.52 | −0.396 | −0.245 | 0.000 | |
| p | 0.012** | 0.692 | 0.806 | 1.000 | |
| C4 (mg/dL) | 8.95±6.36 | 13.32±9.67 | 11.28±7.63 | 6.44±2.98 | 10.03±6.82 |
| Z-test* | −1.94 | −0.89 | −1.225 | −1.443 | |
| p | 0.052 | 0.373 | 0.221 | 0.149 | |
| ds DNA (IU/mL) | 47.01±41.55 | 41.01±39.56 | 52.84±42.37 | 54.60±42.81 | 28.73±25.29 |
| Z-test* | −0.47 | −1.005 | −0.768 | −2.337 | |
| p | 0.638 | 0.315 | 0.443 | 0.019** |
Data are expressed as mean±SD.
Mann Whitney test vs previous biopsy, Confidence interval:
95%,
99% Normal values of C3:75–140mg/dL, C4:10–40mg/dL, dsDNA: 0–7IU/mL
Our biopsies were performed by indication of relapse, ongoing activity, partial remission, or deterioration of renal function. The presence of these clinical indications raises the suspicion of an active disease, which was confirmed by the calculation of the activity index and the fact that it did not differ significantly between serial biopsies. Thus, the glomerular disease was active at the time of the biopsy in all cases. On the other hand, the mean chronicity index increased over time reaching significant difference between the fourth and fifth biopsies (Table 4). This fact indicates cumulative chronic, irreversible damage regardless of the close monitoring and the appropriate immunosuppression.
Table 4.
Descriptive statistics for histological parameters per biopsy and Mann Whitney results between serial renal biopsies.
Histological activity index was not significantly different between serial biopsies. Chronicity index showed a progressive rise over time with a significant increase between biopsies 4 and 5 (0.017).
| Variable | Biopsy-1 | Biopsy-2 | Biopsy-3 | Biopsy-4 | Biopsy-5 |
|---|---|---|---|---|---|
| N=35 | N=35 | N=13 | N=5 | N=4 | |
| Number of glomeruli | 23.54±16.67 | 21.14±12.12 | 20.15±7.09 | 29.60±13.94 | 26.75±12.09 |
| Activity Index | 8.77±5.37 | 6.64±4.55 | 7.82±4.6 | 9.40±5.13 | 4.75±3.86 |
| Z-test* | −1.385 | −0.1 | −0.105 | −1.169 | |
| p | 0.166 | 0.921 | 0.917 | 0.243 | |
| Chronicity Index | 1.73±1.80 | 2.29±2.58 | 4.36±2.50 | 5.20±0.45 | 7.75±0.96 |
| Z-test* | 0.347 | −0.778 | −1.342 | −2.381 | |
| p | 0.729 | 0.436 | 0.180 | 0.017** | |
| Time interval (months) | 31 | 27 | 34 | 28 |
When renal and serological parameters between reference and repeat biopsy for the group of patients with histological conversion were compared, a statistically significant increase in C3 levels was found (Table 5). C3 levels were also significantly higher at the time of repeat biopsy than at the reference biopsy for the group without histological conversion (Table 6). The results were the same even when switches between proliferative classes were not considered as true conversions and were, therefore, included in the groups of no conversion (data not shown).
Table 5.
Comparison of laboratory parameters between reference and repeat biopsy for the group of patients with histological conversion.
No significant difference was recorded in renal parameters of disease activity between reference and repeat biopsy. Among serological parameters, C3 levels were significantly increased at the time of repeat biopsy.
| Variables | Z-test | p |
|---|---|---|
| Cr (mg/dL) | −0.540 | 0.589 |
| eGFR (mL/min/1.73m2) | −0.454 | 0.650 |
| Proteinuria (g/24h) | −0.113 | 0.910 |
| C3 (mg/dL) | −3.425 | 0.001 |
| C4 (mg/dL) | −1.494 | 0.135 |
| dsDNA (IU/mL) | −0.280 | 0.779 |
Table 6.
Comparison of laboratory parameters between reference and repeat biopsy for the group of patients without histological conversion.
No significant difference was recorded in renal parameters of disease activity between reference and repeat biopsy. Among serological parameters, C3 levels were significantly increased at the time of repeat biopsy.
| Variables | Z-test | p |
|---|---|---|
| Cr (mg/dL) | 0.000 | 1.000 |
| eGFR (mL/min/1.73m2) | −0.308 | 0.758 |
| Proteinuria (g/24h) | −1.374 | 0.169 |
| C3 (mg/dL) | −1.977 | 0.048 |
| C4 (mg/dL) | −1.665 | 0.096 |
| dsDNA (IU/mL) | −0.902 | 0.367 |
The group of patients with histological conversion between reference and repeat biopsy was further divided into those with conversion to a “worse” class nephritis (II or V→III/IV±V, III or IV→III/IV+V) and those with conversion to a nephritis of “milder” class (III/IV±V→II or V). For the group with conversion to a “worse” class, there was no difference in renal and serological parameters between the reference and the repeat biopsy (data not shown), whereas in the group of conversion to a “milder” class, there were significant higher C3 levels and lower proteinuria (data not shown).
Repeat renal biopsies and impact on therapy
Histological findings, such as class conversion and changes in activity and chronicity indices, are important factors that determine therapeutic decisions. Accordingly, the impact of repeat biopsies on therapy was further examined. As previously mentioned, repeat biopsies were performed only by clinical indication.
As already mentioned, there was a rather long time interval between the first and second biopsies (31 months), suggesting that at the time of repeat biopsy, most patients either were on maintenance immunosuppression or had completed therapy. In the majority of instances (67%), patients were still under immunosuppression. In 17% (10/58) of cases, repeat biopsy was performed during tapering of immunosuppression, whereas in 16% (9/58), immunosuppression had been discontinued.
No change in the therapeutic regimen occurred in only 21% (12/58) of instances. In all other cases (79%), treatment was altered in means of either increase or decrease of immunosuppression.
In 7% (4/58) of cases, after the repeat biopsy, the previous immunosuppressive regimen was continued at higher doses or for longer time, whereas in 24% (14/58), a new agent was added. Finally, in 29% (17/58), a different immunosuppressive regimen was applied, whereas in 12% (7/58), there was reintroduction of previously discontinued immunosuppression. Overall, in 72% (42/58) of cases, immunosuppressive therapy was enhanced.
Immunosuppression was reduced only in 5% (3/58) of patients, whereas in 1 (2%) patient, it was completely discontinued due to irreversible chronic damage.
Clinical status at the end of follow-up
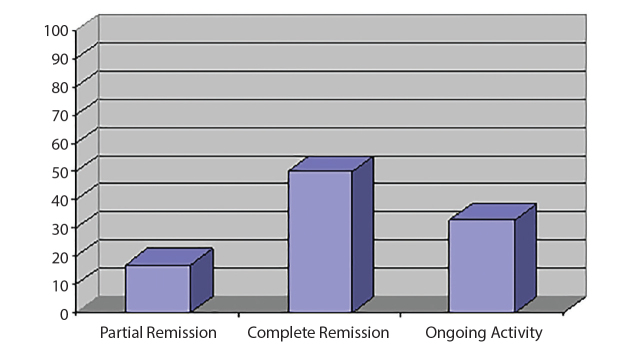
From the 35 patients, 11 (31%) were lost to follow-up. Until the end of follow-up, from the remaining 24 patients, 12 (50%) were in complete remission, 4 (17%) had achieved partial remission, and 9 (33%) had persistent activity (Figure 2).
Figure 2.
Clinical status at the end of follow up.
Discussion
This retrospective analysis investigated the role of repeat renal biopsies as a useful tool for monitoring LN longitudinally. The frequency and types of conversion and the changes in activity and chronicity in the long term, as well as the impact of histological findings on the guidance of our therapeutic decisions, were analyzed. Last but not the least, a number of patients whose repeat biopsy revealed non-LN were reported.
Conversion from one histological class to another is the main feature of LN. A high frequency of transitions (25%–50%) has been described in previous studies using the older World Health Organization classification (6). More recent studies with the newer ISN/RPS 2003 classification report an even higher conversion rate of 49%–75% (5, 7, 8). In our study, the total rate of transition was 57%.
An interesting observation of the present study is the types of transition that occur in patients with LN in the long term. We, similar to other authors, found a predominance of switches between proliferative and mixed classes of LN, i.e., 21% transition rate between classes III and IV and vice versa with or without additional lesions of class V. Indeed, when biopsies are performed by indication of a flare, this type of conversion is the most frequent, varying in recent studies from 20% to 54% (5, 6). Some argue that these conversions have no additive value on treatment decisions since switch between classes III and IV may simply reflect a sample error given the fact that the differentiation between the two classes is simply the cut-off of the involvement of 50% of glomeruli in the biopsy specimen. Moreover, treatment guidelines do not distinguish between the two proliferative classes, and therapy is not even influenced by the addition or disappearance of class V lesions in the repeat biopsy. However, even after withdrawing this type of conversion, the transition rate remains high in our study (36%).
It has been suggested that the presence of proliferative lesions in the reference biopsy should prompt for immediate initiation of induction treatment during a renal flare. In these cases, the need of a repeat biopsy has been questioned (5). Thus, for additional clarity of our analysis, the histological patterns were categorized into pure proliferative, non-proliferative, and mixed nephritis, and the frequency and type of transition from one pathology pattern to another were examined. A 15% rate of transition from proliferative (III or IV) to mixed (III/IV+V) classes was found. In a recent report from China, in a large cohort of 156 patients with LN with 412 repeat biopsies, this was the most common transition (20% of cases) (7). Although without direct therapeutic implication, this transition is not considered as clinically irrelevant. Mixed LN, consisting of a proliferative class along with membranous lesions, is an aggressive form of LN and has long been considered as having worse prognosis (9). Even though not universally accepted, there is some indication that this type of LN responds better to alternative “multitarget” treatment consisting of tacrolimus, mycophenolate mofetil, and steroids (9).
On the other hand, transition from proliferative to non-proliferative nephritis, though rare, has an obvious clinical impact. In our study, only in 12% of instances was there a conversion from classes III/IV±V to classes II/V consistently with the findings by other authors. From studies with biopsies performed by clinical indication, only the study by Lu et al. (7) reports a 50% rate of this type of transition. The other studies with a high frequency of switches from proliferative to non-proliferative classes of LN, varying from 50% to 65%, are all with protocol biopsies (10–12). In these studies, the repeat biopsies were performed “by protocol,” i.e., at predetermined time points regardless of the clinical status of the patient, a practice which may lead to different results than those drawn when performing repeat biopsies by clinical indication of a flare.
In addition to conversion, another important issue is the prognostic value of repeat biopsies. In the case of LN, it is not unusual for histological activity to be related to clinical quiescence, whereas patients with a suspected renal flare may not exhibit ongoing active disease on renal biopsy. Especially in diffuse proliferative LN, achievement of clinical remission is not a sufficient target for the prediction of long-term outcome. Many attempts have been made to define histological features associated with disease progression at initial, as well as at repeat, biopsy. There is increasing evidence that individual morphological variables as crescents, infiltration by macrophages, electron dense deposits, and membranoproliferative glomerulonephritis features have prognostic value and correlate with clinical parameters only on repeat but not on initial biopsies (13).
The predictive ability of activity and chronicity indices at first and at repeat biopsies has also been the subject of debate with conflicting results over the years. In the study by Hill et al. (13), a modified biopsy index with activity and chronicity parameters correlated with doubling of serum creatinine only at second biopsy. In a recent study by Alsuwaida et al. (14) with protocol biopsies at 12–18 months, the activity index was predictive of renal survival only at repeat biopsy. In our study, activity index did not differ significantly between serial biopsies. This finding simply reflects the histological evidence of a renal flare. The mean chronicity index was relatively low at the initial biopsy indicating a prompt referral and intervention at the first diagnosis of LN. It worsened over time reaching statistical significance between the fourth and fifth repeat biopsies. The subsequent increase in chronicity index confirms the severity of LN, since irreversible damage progressed over time despite close monitoring and immunosuppression.
It is important to mention that the progressive increase of the chronicity index may be associated with the time points of the repeat biopsies. Disease flares occurred rather late in the course of the disease, and repeat histology was performed accordingly: the first at 31 months after the onset of nephritis and the next after 27, 34, and 28 months. In 67% of instances, the repeat biopsy was performed during maintenance therapy and in 17%, during tapering of immunosuppression, whereas in 17%, immunosuppression had been discontinued. As it has been already mentioned, this indicates chronic, evolving renal injury despite the appropriate management of the patients.
To determine if a repeat biopsy could be avoided, we examined whether laboratory markers could predict renal morphology in patients with lupus with disease flare and renal exacerbation. We assessed biochemical and serological parameters and found that none of them could predict the type of renal flare, neither by comparing it between reference and repeat biopsy nor by comparing proliferative with non-proliferative conversions. This is consistent with other reports. In a series of 44 patients with 94 repeat biopsies, Wang et al. (8) found no difference in clinical characteristics in patients with and without conversion, whereas in the largest study until today with 412 repeat biopsies, Lu et al. (7) found no correlation of clinical and laboratory parameters and the prediction of flares. It is well known that in LN, at the initial diagnosis, as well as during flares, there is poor clinicopathological correlation, and this further emphasizes the necessity of repeat biopsies.
A rare but important histological entity is the finding of non-LN at repeat biopsy. Indeed, in some patients with SLE and LN at first biopsy, repeat biopsies reveal lesions that are pathogenetically and morphologically unrelated to SLE. This has also been reported by other authors. In a review of 252 biopsies from patients with a diagnosis of SLE, Baranowska-Daca et al. (15) report 13 cases with non-LN. Non-LN was found in five instances, i.e., 9% of repeat biopsies. Three of them had FSGS, and two had non-specific lesions with negative immunofluorescence. This is a rare entity, which occurs primarily late in the course of the disease, but we believe that it is of major therapeutic impact and supports the weakness of clinical parameters to predict histology.
One of the most important reasons to perform repeat biopsies is to decide on treatment strategies to adjust immunosuppression to each patient. Thus, this parameter was analyzed in our study. In the vast majority of cases (79%), treatment was altered after the repeat biopsy: in 72% with regard to enhancing immunosuppression and in 7% with regard to reducing or discontinuing it. In 39.6% of instances of mild to moderate proliferative flares, we added the monoclonal anti-CD20 antibody Rituximab to low-dose MPA with a slight increase on the dose of glucocorticoids. This therapeutic intervention has shown good long-term results, as described in a previous study of our department (16). In 7% of cases with severe nephrotic flares and no evidence of LN in histology, cyclosporine was given in combination either with steroids or with low-dose MPA plus steroids. In 5.2% of cases, therapy was reduced, and in one case, immunosuppression was discontinued due to chronic irreversible lesions.
Changes in immunosuppressive treatment after repeat biopsies have also been reported by other authors (5, 17). Some argue that therapy change itself Repeat renal biopsies are an essential tool for monitoring LN longitudinally. Currently, they represent the only reliable method for tailoring immunosuppression according to the disease status, which may result in the reduction of both the unwanted side effects of immunosuppression and the incidence of renal failure.
Main Points.
Lupus nephritis (LN) is a major cause of morbidity in systemic lupus erythematosus.
Reliable monitoring tools are mandatory for proper therapeutic management, avoidance of over-immunosuppression and delay of progression to chronic renal failure.
Repeat renal biopsies are an essential tool for monitoring LN longitudinally as they enable tailoring immunosuppression according to disease status.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Laiko Hospital (Decision Number: 140; Decision Date: February 20, 2014).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions:Concept - S.M, J.B., C.S.; Design - S.M., E.K.; Supervision - S.M., J.B.; Resources - J.B.; Materials - J.B.; Data Collection and/or Processing - E.K., C.S., K.K., G.L., H.G.; Analysis and/or Interpretation - S.M., E.K., K.K., G.L., H.G., C.S.; Literature Search - S.M., C.S, K.K.; Writing Manuscript - S.M, E.K., C.S.; Critical Review - S.M, J.B.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Doria A, Iaccarino L, Ghirardello A, Zampieri S, Arienti S, Sarzi-Puttini P, et al. Long-term prognosis and causes of death in systemic lupus erythematosus. Am J Med. 2006;119:700–6. doi: 10.1016/j.amjmed.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 2.Bihl GR, Petri M, Fine DM. Kidney biopsy in lupus nephritis: look before you leap. Nephrol Dial Transplant. 2006;21:1749–52. doi: 10.1093/ndt/gfl159. [DOI] [PubMed] [Google Scholar]
- 3.Sidiropoulos PI, Kritikos HD, Boumpas DT. Lupus nephritis flares. Lupus. 2005;14:49–52. doi: 10.1191/0961203305lu2059oa. [DOI] [PubMed] [Google Scholar]
- 4.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology guidelines for screening, treatment and management of lupus nephritis. Arthritis Care Res. 2012;64:747–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daleboudt GM, Bajema IN, Goemaere NN, van Laar JM, Brujin JA, Berger SP. The clinical relevance of a repeat biopsy in lupus nephritis flares. Nephrol Dial Transplant. 2009;24:3712–7. doi: 10.1093/ndt/gfp359. [DOI] [PubMed] [Google Scholar]
- 6.Moroni C, Pasquali S, Quaqlini S, Banfi G, Casanova S, Maccario M, et al. Clinical and prognostic value of serial renal biopsies in lupus nephritis. Am J Kidney Dis. 1999;34:530–9. doi: 10.1016/S0272-6386(99)70082-X. [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Tam LS, Lai FM, Kwan BC, Choi PC, Li EK, et al. Repeat renal biopsy in lupus nephritis: a change in histological pattern is common. Am J Nephrol. 2011;34:220–5. doi: 10.1159/000330356. [DOI] [PubMed] [Google Scholar]
- 8.Wang GB, Xu ZJ, Zhou QG, Zhou ZM, Jia N. Changes in pathological pattern and treatment regimens based on repeat renal biopsy in lupus nephritis. Chinese Med J. 2012;16:2890–4. [PubMed] [Google Scholar]
- 9.Bao H, Liu ZH, Xie XL, Hu WX, Zhang HT, Li LS. Successful treatment of class V+IV lupus nephritis with multitarget therapy. J Am Soc Nephrol. 2008;19:2001–10. doi: 10.1681/ASN.2007121272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunnarsson I, Sundelin B, Heimbürger M, Forslid J, van Vollenhoven R, Lundberg I, et al. Repeated renal biopsy in proliferative lupus nephritis-predictive role of serum C1q and albuminuria. J Rheumatol. 2002;29:693–9. [PubMed] [Google Scholar]
- 11.Esdaile J, Joseph I, MacKenzie T, Kashgarian M, Hayslett JP. The pathogenesis of lupus nephritis: information from repeat renal biopsy. Semin Arthritis Rheum. 1993;23:135–148. doi: 10.1016/S0049-0172(05)80019-8. [DOI] [PubMed] [Google Scholar]
- 12.Askenazi D, Myones B, Kamdar A, Warren R, Perez M, De Guzman M, et al. Outcomes of children with proliferative lupus nephritis: the role of protocol renal biopsy. Pediatr Nephrol. 2007;22:981–6. doi: 10.1007/s00467-007-0447-9. [DOI] [PubMed] [Google Scholar]
- 13.Hill GS, Delahousse M, Nochy D, Rémy P, Mignon F, Méry JP, et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 2001;59:304–16. doi: 10.1046/j.1523-1755.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- 14.Alsuwaida A, Husain S, Alghonaim M, AlOudah N, Alwakeel J, ullah A, et al. Strategy for second kidney biopsy in patients with lupus nephritis. Nephrol Dial Transplant. 2011;27:1472–8. doi: 10.1093/ndt/gfr517. [DOI] [PubMed] [Google Scholar]
- 15.Baranowska-Daca E, Choi YJ, Barrios R, Nassar G, Suki WN, Truong LD. Nonlupus nephritides in patients with systemic lupus erythematosus:a comprehensive clinicopathologic study and review of the literature. Hum Pathol. 2001;32:1125–35. doi: 10.1053/hupa.2001.28227. [DOI] [PubMed] [Google Scholar]
- 16.Boletis JN, Marinaki S, Lionaki SS, Iniotaki A, Sfikakis PP. Rituximab and mycophenolate mofetil for relapsing proliferative lupus nephritis: a long-term prospective study. Nephrol Dial Transplant. 2009;24:2157–60. doi: 10.1093/ndt/gfp002. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj S, Albert L, Gladman DD, Urowitz MB, Hallett DC, Ritchie S. Serial renal biopsy in systemic lupus erythematosus. J Rheumatol. 2000;27:2822–6. [PubMed] [Google Scholar]