Abstract
Objective
To evaluate whether there is any difference between radiographic axial spondyloarthritis (r-axSpA), also termed ankylosing spondylitis (AS), and non-radiographic (nr-) axSpA, with respect to subclinial myocardial dysfunction using speckle tracking echocardiography (STE).
Methods
This was a cross-sectional case control study. We included 72 patients with AS, 38 patients with nr-axSpA, and 56 age-matched healthy subjects. Patients with cardiac disease and cardiac risk factors affecting STE were excluded. The disease burden evaluated by the BASDAI, BASFI, BAS-G, and ASAS-HI scores were comparable in both the r- and nr-axSpA groups. A detailed echocardiographic examination including the M-mode, Doppler, and STE was applied to whole study population.
Results
Duration of the disease, the use of an anti-TNFα agent, and CRP levels were higher in patients with AS. Although the AS, nr-axSpA, and control groups had similar ejection fraction values (59±5.2, 60±4.6, 60±4.6, respectively, and p=0.499), the global longitudinal peak systolic strain (GLS) (20.5±3.3, 21.1±3.5, and 22.3±2.4, respectively, and p<0.05) was different between the groups. In a post-hoc analysis, GLS was not different between the nr-axSpA and control groups, and it was significantly lower in patients with AS. In the univariate analysis, peripheral arthritis (p=0.035) and age (p=0.032) were correlated with GLS. A multivariate regression analysis demonstrated that peripheral arthritis (p=0.009) was the only independent GLS predictor.
Conclusion
Subclinical myocardial dysfunction as assessed by GLS was present in AS, but not in nr-ax-SpA patients. Thus, GLS could be used as a differentiating factor between radiographic and nr-axSpA patients.
Keywords: Speckle tracking echocardiography, axial spondyloarthritis, ankylosing spondylitis, echocardiography, myocardial dysfunction
Introduction
Axial spondyloarthritis (axSpA) is a group of chronic inflammatory diseases affecting mainly the axial skeleton, including the sacroiliac joints and spine. There are two subtypes of axSpA: a non-radiographic form (nr-axSpA), which is defined as the absence of definite sacroiliitis on conventional radiographs, and a radiographic form (r-axSpA), which is better known as ankylosing spondylitis (AS). In addition to radiographic manifestations, a higher female predominance and lower serum C-reactive protein (CRP) levels might be other characteristics differentiating patients with nr-axSpA from those with AS. However, clinical disease activity measures and response rates to tumor necrosis factor inhibitors (TNFi) were found to be comparable in both patients axSpA groups (1).
The relationship of chronic inflammation with the development of cardiovascular diseases (CVD), especially atherosclerosis, has been demonstrated. Inflammatory rheumatic diseases may cause an increased frequency of CVD in otherwise traditional risk factors. It has recently been demonstrated that the frequency of major cardiovascular events was similar in chronic inflammatory diseases such as rheumatoid arthritis, psoriatic arthritis, and in patients with axSpA (2). CVD have also been reported to be a prominent cause of mortality in axSpA (3). It has been suggested that a 10-year risk of CVD may be related to both the radiographic grade of sacroiliitis and spinal radiographic damage in axSpA (4). In accordance with these data, a previous study by the current authors showed that myocardial functions were also impaired in axSpA when they assessed using conventional echocardiographic studies (5).
Speckle tracking echocardiography (STE) is a highly sensitive and accurate method for detecting subclinical ventricular dysfunction before the development of clinical myocardial dysfunction as assessed by conventional two-dimensional echocardiography (6). Recent studies using STE have shown that patients with axSpA have subtle left ventricular myocardial dysfunction (7, 8). However, these studies have focused on the whole group of patients with axSpA, and no clear statement can be made about whether there is a difference between AS and nr-axSpA patients regarding to subclinical myocardial dysfunction. Therefore, in this study, we aim to evaluate left ventricular myocardial function using advanced STE in patients with and without radiographic axSpA and to determine the risk factors for myocardial dysfunction in those patients.
Methods
Study population
This study was conducted on patients with ax-SpA aged >18 years, who were followed up in a rheumatology clinic between July 2017 and March 2018. A total of 72 consecutive patients with AS and 38 patients with nr-axSpA according to the Assessment of Spondyloarthritis International Society (ASAS) criteria for axial spondyloarthritis (9) were included in the study. A control group was formed of 56 age- and gender-matched healthy subjects. Patients with hypertension, diabetes mellitus, known cardiac diseases (e.g., coronary artery disease, acute and chronic heart failure, cardiac pacemaker, moderate and severe valve disease, atrial fibrillation) and those who had a history of stroke or transient ischemic attack, renal failure, liver disease, and poor echogenity that could affect STE were excluded from the analysis.
A written informed consent form was obtained from all subjects. This study was reviewed and approved by the Institutional Review Board (Decision number: 299/2017)
Clinical, laboratory, and radiologic evaluation
All patients underwent a detailed evaluation. Patients’ socioeconomic and demographic characteristics, including age, gender, cigarette consumption, age at the onset of symptoms, and age at diagnosis, were obtained using a standard questionnaire. At the time of evaluation, all patients completed the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (10, 11), Bath Ankylosing Spondylitis Functional Index (BASFI) (12), Ankylosing Spondylitis Quality of Life (ASQoL) (13), Assessment of Spondyloarthritis International Society—Health Index (ASAS-HI) (14), the Bath Ankylosing Spondylitis Patient Global Score (BAS-G) (15), and a health assessment questionnaire (HAQ). The ankylosing spondylitis disease activity score (ASDAS)-CRP (16) was also calculated and obtained. The HLA-B27 status was obtained from the patient’s medical records, if available. Laboratory parameters including a complete blood count, sedimentation rate, CRP, and lipid profile were also obtained. Radiographic images of the pelvis and the lumbar and cervical spine were assessed by a single experienced rheumatologist. The radiographic images were evaluated according to the modified Stoke AS Spine Score (mSASSS) (17) and prior intra-observer intra-class coefficient and inter-observer ICC values for mSASSS scoring were estimated as 0.932 and 0.933–0.948, respectively, for the reader. All radiographs were also evaluated regarding whether there was a hip involvement and syndesmophyte by the same reader. Kappa (κ) value for the existence of syndesmophyte was estimated as 0.467 (%95 CI; 0.401–0.559).
Echocardiographic examination
All subjects underwent a detailed evaluation with two-dimensional transthoracic echocardiography, including M-mode color Doppler, tissue Doppler, and strain imaging studies. A commercially available ultrasound machine EPIQ 7C (Philips Medical Imaging, Eindhoven, the Netherlands) was used in all echocardiographic examinations. Standard two-dimensional and Doppler echocardiographic examinations were performed based on the American Society of Echocardiography/ European Association of Echocardiography guidelines (18, 19). Two independent and experienced echocardiographers, blinded to the patients’ clinical data such as diagnosis, symptoms, and treatment of axial spondyloarthritis, performed and examined the echocardiographic recordings. Left ventricular ejection fraction (LVEF) was calculated by the biplane Simpson’s method. Mitral inflow Doppler velocities, and the peak early (E-wave) and late filling (A-wave), were measured by using a pulsed-wave Doppler after placing the sample volume at the leaflets’ tips. Left ventricle tissue Doppler velocities, systolic annular velocity (s’), diastolic early (e’) and late (a’) lateral-basal and septal-basal annular velocities, were calculated after placing the sample volume of the pulsed-wave Doppler at the lateral and septal sides of the mitral annulus, respectively. Tricuspid systolic jet velocity was calculated from the apical four-chamber view by obtaining the highest Doppler velocity aligned with continuous wave. Left ventricular diastolic functions were evaluated using the E velocity, E/e’ (E/mean lateral and septal-basal e’ velocity) ratio, left atrial volume, and deceleration time (DT) in accordance with the American Society of Echocardiography/ European Association of Echocardiography guidelines (19).
Speckle tracking echocardiography
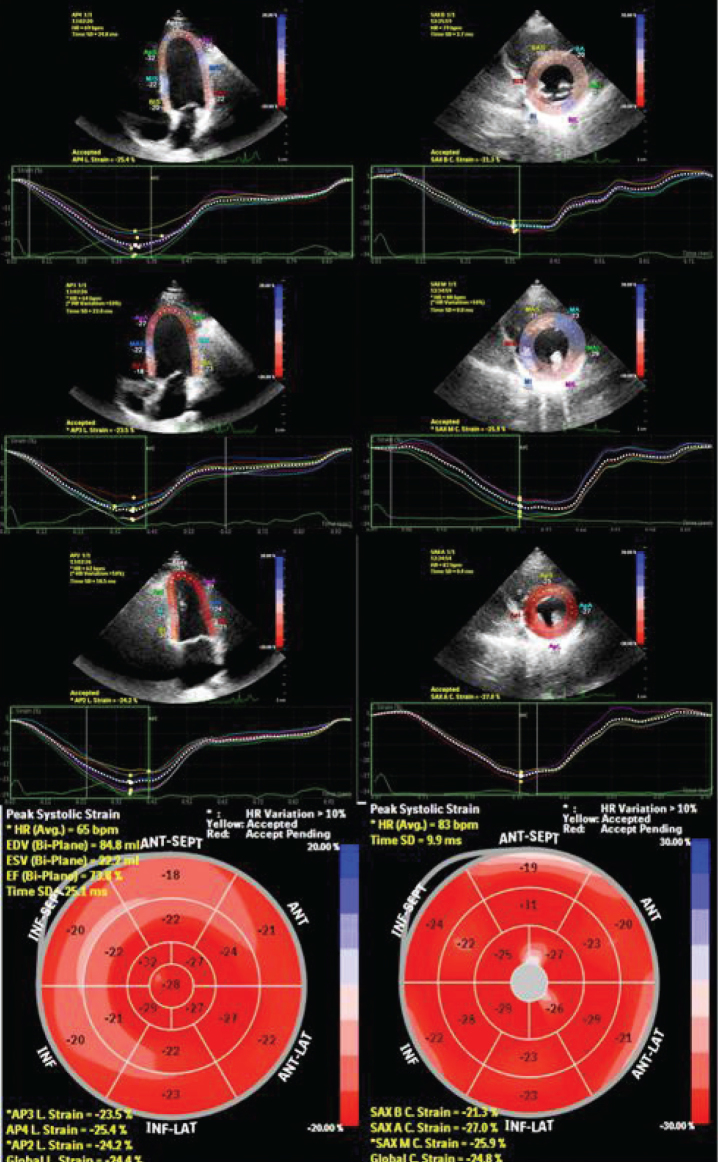
Speckle tracking echocardiography was performed using customized software (MVQ, QLAB, Philips, Andover, MA). For the calculation of global longitudinal strain (GLS) and global circumferential strain (GCS), digital cineloops from the apical four-, three-, and two-chamber views, and parasternal short-axis views at the basal, midventricular, and apical levels were acquired at a frame rate of 50–90 frames/ sec at end-expiration from the peak of the R-wave. The averages of three cardiac cycles were used in the analysis. For the circumferential strain analysis, the sampling points were placed manually along the endocardium at the LV base, middle and apex at short-axis levels. For the longitudinal strain analysis, three sampling points were placed manually at the septal mitral annulus, mitral lateral annulus, and apical endocardium during end-diastole at apical two-, three-, and four-chamber views. The software tracked the endocardial contour, automatically generating a region of interest. The quality of myocardial speckle tracking was controlled visually, and the process was replied or manually corrected if unacceptable tracking was obtained. The graphics of longitudinal and circumferential strain parameters of each myocardial segment were then automatically demonstrated, and the average peak systolic strain values were obtained. The global longitudinal and circumferential strain values were assessed as the average of the segmental values (Figure 1). A normal GLS value was accepted as 20%, as determined by current guidelines (18).
Figure 1.
The assessment of global longitudinal strain from the apical four-chamber, three-chamber and two-chamber views and global circumferential strain from basal, mid and apical short axis views. Each colored line represents corresponding myocardial segmental strain and the white dotted line represents the mean strain that was measured as changes of the whole myocardium of each view. The global longitudinal strain and global circumferential strain values are calculated as the average of the peak strain values of three apical views and three short axis views respectively.
Statistical analysis
Statistical analysis was performed using the IBM Statistical Package for Social Sciences, Statistics for Windows, Version 21.0. (IBM Corp.; Armonk, NY, USA. Released 2012). The normality distribution patterns of variables were evaluated using histograms, probability plots, and analytical methods (Kolmogorov-Smirnov). Unless otherwise stated, normally distributed variables and not normally distributed variables were showen as mean±standard deviation and median (interquartile range), respectively. Categorical variables were presented as percentages. The AS and nr-axSpA groups were compared using the independent Student’s t-test and the Mann-Whitney U test, according to normality analyses for continuous variables. Continuous data was compared between the AS, nr-axSpA, and control groups using the one-way analysis of variance, and Tukey’s HSD was used in post-hoc comparisons. Categorical variables were compared using the chi-squared test. Spearman’s rank correlation test was used for bivariate correlations between variables and GLS. Significantly correlated variables were tested in a multivariate linear regression analysis to determine the independent risk factors for strain values. All tests were two-tailed, and the p-value <0.05 was accepted as statistically significant.
Results
Patients’ baseline clinical characteristics are presented in Table 1. The BASDAI, BAS-G, HAQ-S, BASFI, and ASAS-HI scores were found to be similar in patient groups. The mSSASS, duration of disease, and use of an anti-TNFα agent were higher in patients with AS. In addition, serum CRP levels were higher in the AS group, and other laboratory parameters were similar in the groups.
Table 1.
Baseline and demographic characteristics of patients (Continuous and categorical variables were presented as mean±standard deviation and percentages respectively).
| Ankylosing spondylitis | Nr-axSpA patients | ||
|---|---|---|---|
| N:72 | N:38 | p | |
| Height (cm) | 170±10 | 170±11 | 0.718 |
| Weight (kg) | 73±13 | 72±12 | 0.721 |
| BMI ( kg/m2) | 24.9±4.3 | 24.1±2.5 | 0.445 |
| Age at onset of disease | 25.2±10 | 27±8.5 | 0.373 |
| Duration of disease | 13±8.1 | 8±7.8 | 0.003 |
| Smoking | 0.057 | ||
| Current smoker | 34 (47%) | 19 (50%) | |
| Ex-smoker | 23 (32%) | 5 (13%) | |
| Non-smoker | 14 (19%) | 13 (34%) | |
| Family history of SpA | 17 (24%) | 11 (29%) | 0.870 |
| HLA B27 positive | 37 (51%) | 14 (37%) | 0.055 |
| Sacroiliitis on X-ray | 15 (21%) | 3(8%) | 0.054 |
| Syndesmophytes | 25 (35%) | 5 (13%) | 0.018 |
| Peripheral arthritis | 12 (17%) | 10 (26%) | 0.540 |
| Sacroiliitis | 2 (3%) | - | 0.122 |
| İnflammatory bowel disease | 1 (1%) | 1 (3%) | 0.368 |
| Psoriasis | - | - | - |
| Dactylitis | 1 (1,4 %) | - | 1 |
| Uveitis | 5 (7%) | 5 (13%) | 0.524 |
| MSASSS | 7.5±13.7 | 1±2 | 0.019 |
| HAQ-S | 0.97±0.61 | 0.78±0.69 | 0.188 |
| BAS-G | 3.5±2.6 | 4±2.3 | 0.364 |
| BASFI mean | 2.6±2.5 | 2.2±1.9 | 0.349 |
| BASDAI mean | 2.8±2.2 | 3±1.7 | 0.622 |
| ASQoL mean | 6.7±5.3 | 7.3±4.9 | 0.540 |
| ASAS-HI | 6±4 | 6.6±4.4 | 0.479 |
| Patients on Anti TNF treatment | 42 (58%) | 13 (34%) | 0.016 |
| Patients on NSAID treatment | 49 (68%) | 27 (71%) | 0.260 |
| ESR (mm/h) | 24.7±20.6 | 17±15.2 | 0.075 |
| CRP (mg/L) | 16.5±31 | 2.8±3.1 | 0.013 |
| Hemoglobin, g/L | 13.5±1.8 | 13.9±1.8 | 0.302 |
| Leukocyte count X109/L | 8.3±2.1 | 7.6±1.2 | 0.150 |
| Platelets X109/L | 298±75 | 277±53 | 0.154 |
| Creatinine mg/dL | 0.76±0.1 | 0.76±0.1 | 0.827 |
| Triglycerides(mg/dL) | 99±59 | 135±149 | 0.114 |
| Total cholesterol (mg/dL) | 182±33 | 192±43 | 0.227 |
| HDL cholesterol (mg/dL) | 46±9 | 49±15 | 0.503 |
| LDL cholesterol (mg/dL) | 117±20 | 119±34 | 0.812 |
ASQoL: Ankylosing Spondylitis Quality of Life Questionnaire; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index BASFI: Bath Ankylosing Spondylitis Functional Index; BAS-G Bath Ankylosing Spondylitis Global Score BMI: Body mass index. CRP: C- reactive protein; ESR: erythrocyte sedimentation rate; HAQ-S: The Health Assessment Questionnaire for Spondyloarthritis; HDL: High density cholesterol; LDL: Low density cholesterol; mSASS: modified Stoke AS Spine Score SpA: Spondyloarthritis; TNF: Tumor necrosis factor.
N: number.
Echocardiographic parameters
The echocardiographic parameters of patients with axSpA and control groups are presented in Table 2. There was no difference between the three groups in respect of left ventricular dimensions, volume, and ejection fraction (p>0.05). With the exception of deceleration time, the parameters representing diastolic functions including the left atrial volume, E velocity, and E/e’ ratio were comparable between the groups.
Table 2.
Echocardiographic findings of the study population (Continuous and categorical variables were presented as mean±standard deviation and percentages respectively).
| Ankylosing spondylitis N:72 |
Nr-axSpA patients N:38 |
Control group N:56 |
p | |
|---|---|---|---|---|
| Age, years | 39.2±9.6 | 37±11 | 41±7.2 | 0.137 |
| Gender, female | 20 (28%) | 16 (42%) | 26 (46%) | 0.076 |
| LA volume, mL | 33±4.3 | 32±4.2 | 31±3.8 | 0.124 |
| IVST mm | 9.4±1.5 | 9±1.7 | 9.3±1.4 | 0.450 |
| PWT mm | 9.1±1.4 | 8.5±1.5 | 9.1±1.3 | 0.062 |
| LVDD mm | 43.2±.5 | 44±4.3 | 42.5±3 | 0.301 |
| LVSD mm | 26±3.5 | 26±4 | 26±3.5 | 0.746 |
| LVEDV mm | 93±33 | 95±26 | 86±19 | 0.212 |
| LVESV mm | 38±5 | 38±12 | 37±12 | 0.871 |
| EF % mean | 59±5.2 | 60±4.6 | 60±4.6 | 0.499 |
| E velocity cm/s | 92±22 | 91±16 | 94±16 | 0.760 |
| A velocity cm/s | 76±16 | 75±17 | 81±14 | 0.124 |
| E/A ratio | 1.2±0.3 | 1.2±0.3 | 1.2±0.15 | 0.232 |
| E/e’ ratio | 7.8±2.3 | 7.3±1.8 | 7.9±1.9 | 0.421 |
| DT ms | 184±48 | 181±38 | 165±38 | 0.042 |
| Ls’ velocity cm/sn | 12±3.5 | 11.9±2.8 | 10.9±2.9 | 0.226 |
| Le’ velocity cm/sn | 13.8±3.9 | 15.3±4.8 | 13.8±2.4 | 0.108 |
| La’ velocity cm/sn | 11.1±3.5 | 12.3±4.8 | 10.9±2.3 | 0.172 |
| Ss’ velocity cm/sn | 9.3±2.3 | 8.7±1.6 | 8.7±2.6 | 0.262 |
| Se’ velocity cm/sn | 11±3.4 | 11±2.4 | 10.4±2.2 | 0.423 |
| Sa’ velocity cm/sn | 10.1±2.3 | 10±2.3 | 9.5±3.2 | 0.379 |
| TR velocity m/sn | 2.4±0.4 | 2.24±0.4 | 2.38±0.37 | 0.127 |
| GLS % | 20.5±3.3 | 21.1±3.5 | 22.3±2.4 | 0.004 |
| GCS % | 21.2±5.5 | 21.2±4.4 | 21.2±4.1 | 0.839 |
A: Peak late diastolic velocity; DT: Deceleration time; E: Peak early diastolic velocity; EF: Ejection frac; LA: Left atrium; La’: Late diastolic velocity at basal lateral annulus Le’: Early diastolic velocity at basal lateral annulus; Ls’: Myocardial systolic velocity at basal lateral annulus LVDD: Left ventricular diastolic diameter; LVEDV: Left ventricular end diastolic volume; LVESV: Left ventricular end systolic volume; LVSD: Left ventricular systolic diameter; PWT: posterior wall thickness; Ss’: Myocardial systolic velocity at basal septal annulus; Se’: Early diastolic velocity at basal septal annulus; Sa’: Late diastolic velocity at basal septal annulus; TR: tricuspid regurgitation.
Table 3.
Predictors of global longitudinal strain in univariate and multivariate lineer regression analysis.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| r | p | β [95% CI] | p | |
| Age | r:−0.253 | 0.032 | −0.064 [(−0.148) - (−0.021)] | 0.138 |
| Peripheral arthritis | r.:−0.233 | 0.038 | −2.28 [ (−4.49) - (−0.67)] | 0.009 |
Although GCS was similar (p=0.839), GLS was different between the groups (p=0.004). Posthoc tests demonstrated that GLS was comparable in the nr-axSpA and control groups However, it was significantly lower in patients with AS. The GLS distribution per group is presented in Figure 2. Of the study population, 76% AS and 24% nr-axSpA patients had lower GLS values than the normal reference values (Figure 3).
Figure 2.
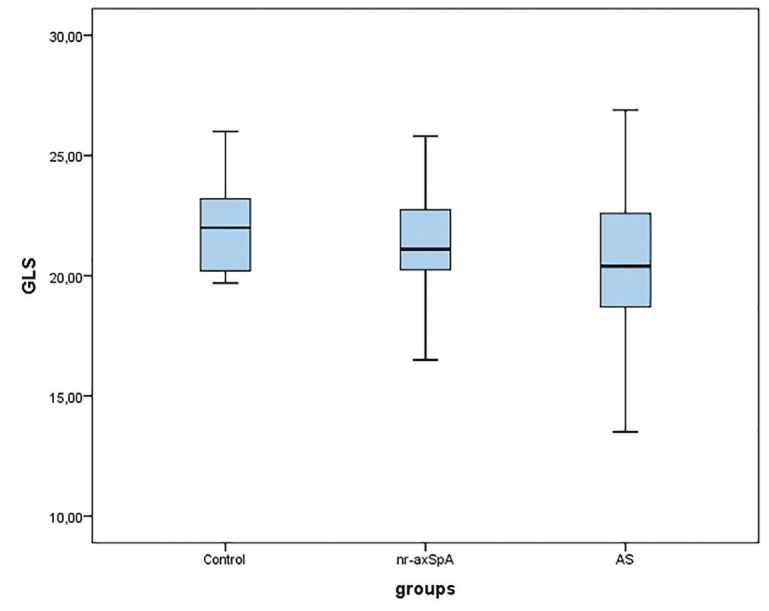
Illustration of the global longitudinal strain distribution for per group in boxplots.
Figure 3.
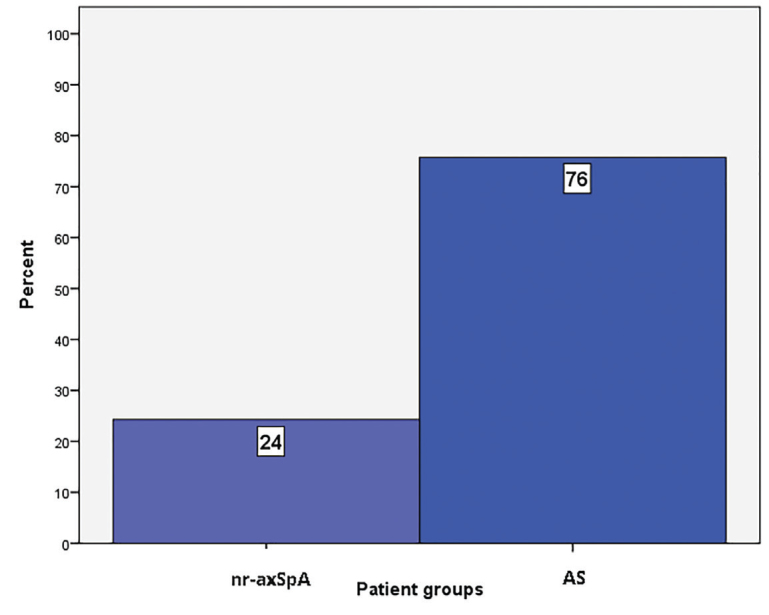
Percentages of patients under the normal value of global longitudinal strain.
In a univariate analysis, GLS was correlated with an increasing age (p=0.032) and the presence of peripheral joint involvement (p=0.035). In the multivariate regression analysis peripheral joint involvement was shown to be the only independent predictor of GLS (p=0.009) (Table 4).
Discussion
It is clearly known that axSpA, especially AS consisting of the majority of axSpA, has a direct relationship with myocardial dysfunction. To the best of our knowledge, this is the first study to have compared patients with AS and nr-axSpA regarding the subclinical myocardial dysfunction using STE. The main finding of this study was that patients with AS had subclinical myocardial systolic dysfunction as assessed by two-dimensional STE compared to the control group. However, myocardial function does not seem to differ between patients with nr-axSpA and control subjects.
There are some well-known differences between AS and nr-axSpA, including mobility, function, inflammatory activity, and the gender ratio (20). Patients with AS have less mobility and function, and a higher male-to-female ratio compared to patients with nr-axSpA. Furthermore, patients with AS have a higher inflammatory activity and number of inflammatory spinal lesions.
Systemic inflammation is implicated in the risk of myocardial dysfunction in individuals with chronic inflammatory arthritis (4). It is thought that the inflammatory burden in AS leads to myocardial dysfunction. In a post-mortem study, it was shown that patients with AS had a diffuse increase of interstitial connective tissue as a means of increasing reticulin fibers without a history of CVD such as hypertension, or ischemic or valvular disease. In the above-mentioned study, the authors did not report any inflammatory changes or amyloid deposition (21). In this study, a direct relationship between inflammatory markers such as CRP and decreased GLS values could not be shown.
Previous studies using conventional echocardiographic methods have demonstrated myocardial dysfunction, especially impaired diastolic function, in axSpA (5, 22, 23). In a meta-analysis, using TTE, AS patients had a higher prevalence of left ventricular diastolic dysfunction compared to general population (24). The parameters, including a lower E/A ratio, prolonged mean deceleration time, and prolonged interventricular relaxation time, were higher in patients with AS (24). In the current study, DT was higher in r-axSpA patients compared to the control group. However, there was no difference between the groups in respect of E/e’ ratio, TR velocity, E/A ratio, and LA volume, which are recommended in the latest guidelines for the diagnosis of left ventricle dysfunction.
STE, determining the relative length change in a certain part of the myocardium between end-diastole and end-systole, can demonstrate subtle changes in myocardial systolic function. It is less operator dependent and more sensitive than conventional TTE in the demonstration of subtle myocardial dysfunction without clinical signs and symptoms. However, STE is vendor dependent, and there is no clear determination of normal GLS reference values. Current guidelines arbitrarily accept 20% as a normal GLS range (18). There are few studies that have investigated the myocardial function using STE in patients with axSpA. Ustun et al. (8) demonstrated reduced myocardial systolic function in patients with AS, even without CVD as assessed by STE. However, that study did not include patients with nr-axSpA. Chen et al. (7) also showed that patients with axSpA had subtle systolic left ventricular dysfunction using advanced 2-STE analysis, even with an apparently normal LVEF assessed by conventional echocardiography. However, the majority (78%) of patients with axSpA comprised AS patients. In our study, despite mean GLS values of patients with AS within normal limits, majority of the AS patients had lower GLS values than the accepted normal GLS value (18). The current study findings were comparable to those of previous studies. Similarly, AS itself may reduce myocardial functions using STE despite a normal EF, even without cardiovascular risk factors. The results of this study further demonstrated that myocardial functions, using advanced STE and conventional echocardiography, were comparable between patients with nr-axSpA and the healthy population. An additional important finding was that peripheral arthritis was determined as an independent predictor of reduced GLS. Although it is not exactly known how peripheral arthritis is related to impaired myocardial systolic function, CVD occurrence is more frequent in those patients with long-term AS and with peripheral arthritis (25). Patients with AS and peripheral arthritis tend to have a higher inflammatory activity than those without peripheral arthritis, which may contribute to myocardial dysfunction (26, 27).
The main limitations of the present study are that it had the single-center, cross-sectional design and a relatively small number of patients. There is a need for multicenter studies with a larger sample size to be able to reach a more solid conclusion about whether GLS can be used as a differentiating factor between AS and nr-axSpA.
This study indicated that even in the absence of CVD and cardiovascular risk factors, GLS was reduced in patients with AS but was unchanged in patients with nr-axSpA. In addition to conventional echocardiographic methods, STE may add valuable information regarding myocardial dysfunction in axSpA. Therefore, reduced GLS may be used as another distinctive factor between radiographic and nr-axSpA patients.
Main Points.
Speckle tracking echocardiography can demonstrate subtle myocardial dysfunction even having normal ejection fraction in patients with spondyloarthropathies.
Ankylosing Spondylitis poses a higher risk for myocardial dysfunction among spondyloarthropathies.
Speckle tracking echocardiography is a promising screening method which may also be used to differentiate radiographic and non-radiographic axial spondyloarthropathies.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of the Katip Çelebi University (Decision Date: December 20, 2017; Decision Number: 299).
Informed Consent: Written informed consent was obtained from the parents of the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.A.; Design - S.V.E.; Supervision - M.T.; Resources - O.G., D.S., G.K., E.Ç.Ş.; Materials - O.G., D.S., G.K., S.A., E.Ç.Ş.; Data Collection and/or Processing - S.V.E., E.Ö.; Analysis and/or Interpretation - S.A, Z.E., Ö.K.; Literature Search - S.A, D.S, Ö.G, M.T.; Writing Manuscript - S.V.E.; Critical Review - M.T., Z.E.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that thi study has received financial no financial support.
References
- 1.Poddubnyy D, Sieper J. Similarities and differences between nonradiographic and radiographic axial spondyloarthritis: a clinical, epidemiological and therapeutic assessment. Curr Opin Rheumatol. 2014;26:377–83. doi: 10.1097/BOR.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 2.Lauper K, Courvoisier DS, Chevallier P, Finckh A, Gabay C. Incidence and prevalence of major adverse cardiovascular events in rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis. Arthritis Care Res. 2018;70:1756–63. doi: 10.1002/acr.23567. [DOI] [PubMed] [Google Scholar]
- 3.Prati C, Puyraveau M, Guillot X, Verhoeven F, Wendling D. Deaths Associated with Ankylosing Spondylitis in France from 1969 to 2009. J Rheumatol. 2017;44:594–8. doi: 10.3899/jrheum.160942. [DOI] [PubMed] [Google Scholar]
- 4.Kang KY, Her YH, Ju JH, Hong YS, Park SH. Radiographic progression is associated with increased cardiovascular risk in patients with axial spondyloarthritis. Modern Rheumatol. 2016;26:601–6. doi: 10.3109/14397595.2015.1119348. [DOI] [PubMed] [Google Scholar]
- 5.Okan T, Sari I, Akar S, Cece H, Goldeli O, Guneri S, et al. Ventricular diastolic function of ankylosing spondylitis patients by using conventional pulsed wave Doppler, myocardial performance index and tissue Doppler imaging. Echocardiography. 2008;25:47–56. doi: 10.1111/j.1540-8175.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- 6.Gorcsan J, Tanaka H. Echocardiographic assessment of myocardial strain. J Am College Cardiol. 2011;58:1401–1413. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Chung HY, Zhao CT, Wong A, Zhen Z, Tsang HHL, et al. Left ventricular myocardial dysfunction and premature atherosclerosis in patients with axial spondyloarthritis. Rheumatology. 2014;54:292–301. doi: 10.1093/rheumatology/keu337. [DOI] [PubMed] [Google Scholar]
- 8.Ustun N, Kurt M, Nacar AB, Karateke HP, Guler H, Turhanoglu AD. Left ventricular systolic dysfunction in patients with ankylosing spondylitis without clinically overt cardiovascular disease by speckle tracking echocardiography. Rheumatol Int. 2015;35:607–11. doi: 10.1007/s00296-014-3130-z. [DOI] [PubMed] [Google Scholar]
- 9.Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83. doi: 10.1136/ard.2009.108217. [DOI] [PubMed] [Google Scholar]
- 10.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–91. [PubMed] [Google Scholar]
- 11.Akad K, Solmaz D, Sari I, Onen F, Akkoc N, Akar S. Performance of response scales of activity and functional measures of ankylosing spondylitis: numerical rating scale versus visual analog scale. Rheumatol Int. 2013;33:2617–23. doi: 10.1007/s00296-013-2789-x. [DOI] [PubMed] [Google Scholar]
- 12.Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21:2281–5. [PubMed] [Google Scholar]
- 13.Doward L, Spoorenberg A, Cook S, Whalley D, Helliwell P, Kay L, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis. 2003;62:20–6. doi: 10.1136/ard.62.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiltz U, Van Der Heijde D, Boonen A, Cieza A, Stucki G, Khan M, et al. Development of a health index in patients with ankylosing spondylitis (ASAS HI): final result of a global initiative based on the ICF guided by ASAS. Ann Rheum Dis. 2014;74:830–5. doi: 10.1136/annrheumdis-2013-203967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones SD, Steiner A, Garrett SL, Calin A. The Bath Ankylosing Spondylitis Patient Global Score (BAS-G) Br J Rheumatol. 1996;35:66–71. doi: 10.1093/rheumatology/35.1.66. [DOI] [PubMed] [Google Scholar]
- 16.Van der Heijde D, Lie E, Kvien TK, Sieper J, Van den Bosch F, Listing J, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68:1811–8. doi: 10.1136/ard.2008.100826. [DOI] [PubMed] [Google Scholar]
- 17.Creemers M, Franssen M, Van’t Hof M, Gribnau F, Van de Putte L, Van Riel P. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis. 2005;64:127–9. doi: 10.1136/ard.2004.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–71. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur J Echocardiogr. 2016;17:1321–60. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 20.Baraliakos X, Braun J. Non-radiographic axial spondyloarthritis and ankylosing spondylitis: what are the similarities and differences? RMD open. 2015;1(Suppl 1):e000053. doi: 10.1136/rmdopen-2015-000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brewerton D, Goddard D, Moore R, Revell P, Gibson D, Jones T, et al. The myocardium in ankylosing spondylitis: A clinical, echocardiographic, and histopathological study. Lancet. 1987;329:995–8. doi: 10.1016/S0140-6736(87)92268-9. [DOI] [PubMed] [Google Scholar]
- 22.Ercan S, Goktepe F, Kisacik B, Pehlivan Y, Onat AM, Yavuz F, et al. Subclinical cardiovascular target organ damage manifestations of ankylosing spondylitis in young adult patients. Modern Rheumatol. 2013;23:1063–8. doi: 10.3109/s10165-012-0791-x. [DOI] [PubMed] [Google Scholar]
- 23.Caliskan M, Erdogan D, Gullu H, Yilmaz S, Gursoy Y, Yildirir A, et al. Impaired coronary microvascular and left ventricular diastolic functions in patients with ankylosing spondylitis. Atherosclerosis. 2008;196:306–12. doi: 10.1016/j.atherosclerosis.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Heslinga SC, Van Dongen CJ, Konings TC, Peters MJ, Van der Horst-Bruinsma IE, Smulders YM, et al. Diastolic left ventricular dysfunction in ankylosing spondylitis-a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44:14–9. doi: 10.1016/j.semarthrit.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Ryall NH, Helliwell P. A critical review of ankylosing spondylitis. Critical Reviews™ in Physical and Rehabilitation Medicine. 1998;10 doi: 10.1615/CritRevPhysRehabilMed.v10.i3.40. [DOI] [Google Scholar]
- 26.Spoorenberg A, Dougados M, Mielants H. Relative value of erythrocyte sedimentation rate and C-reactive protein in assessment of disease activity in ankylosing spondylitis. J Rheum. 1999;26:980–4. [PubMed] [Google Scholar]
- 27.Peters MJ, Van der Horst-Bruinsma IE, Dijkmans BA, Nurmohamed MT. Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin Arthritis Rheum. 2004;34:585–92. doi: 10.1016/j.semarthrit.2004.07.010. [DOI] [PubMed] [Google Scholar]