Abstract
Granulomatosis with polyangiitis (GPA) is a systemic inflammatory disease, characterized by the presence of necrotizing vasculitis of small and medium-sized vessels, granulomatous inflammation and anti-neutrophil cytoplasmic antibodies (ANCAs). The diagnosis can be challenging due to the variable clinical presentation and possible involvement of virtually all organ systems. A correct diagnosis is indispensable for a timely start of medical treatment and to avoid unnecessary surgery. Therefore, cooperation with and the input of the pathologist is crucial. We report a case of a woman presenting with suspected metastatic cancer. The diagnosis of GPA was made mainly based on breast biopsy, and the patient was treated accordingly, with full recovery. This report provides a case description and a brief review of the literature.
Keywords: Granulomatosis with polyangiitis, granulomatous mastitis, breast
Introduction
Granulomatosis with polyangiitis (GPA) is a granulomatous inflammatory disease that can affect small- and medium-sized blood vessels of virtually all organ systems, but mainly the upper and lower respiratory tract and the kidneys (1). Cytoplasmic antineutrophil cytoplasmic autoantibodies (c-ANCAs) with proteinase-3 (PR3) or myeloperoxidase (MPO) specificity are found in the majority of patients (2). Granulomatous inflammation, vasculitis, and extensive stromal necrosis are histologic hallmarks of GPA. Although these pathological features are well known, the diagnosis can be easily missed when the disease is encountered in an unusual clinical setting. Indeed, GPA can also present in the peripheral or central nervous system, and also show urogenital, ocular, gastro-intestinal, cardiac, cutaneous, and even breast involvement (3, 4). Detailed clinical information is therefore of pivotal importance when consulting the pathologist in these cases. We present a case of multi-organ GPA with breast involvement and mimicking disseminated carcinoma.
Case Presentation
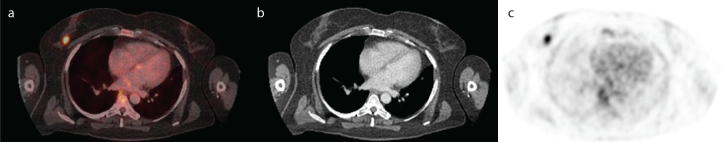
A 44-year-old woman with a previous history of Stage I papillary thyroid cancer and viral meningitis was referred to our hospital for further diagnostic workup for suspected metastatic disease. During the 7 years after thyroidectomy followed by radio-ablation, she had been in a healthy condition, although in retrospect, she had been complaining of vague migrating arthralgia in several peripheral joints for a longer time. A spontaneous rupture of a gluteal abscess during the empirical treatment with clindamycin was recorded 3 months before, followed 1 month later by a symmetrical polyarthritis. Based on the negative anticyclic citrullinated peptide (anti-CCP), negative rheumatoid factor (RF), and normal X-rays of the hands, the diagnosis of RF-negative rheumatoid arthritis was made. The patient was treated with corticosteroids at a low dose (methylprednisolone), without a significant therapeutic effect. Her symptomatology progressed further, with abdominal pain and an unexplained weight loss of 7 kg, when she was eventually admitted to our clinic. Abdominal and thoracic computed tomography (CT) scans demonstrated two gluteal abscesses, an ill-defined mass in the pelvis adjacent to the right iliac artery, and several pulmonary nodules with a diameter of up to 15 mm. A hybrid positron-emission tomography (PET) with an 18F-fluorodeoxyglucose and CT scan (18F-FDG-PET/CT) was performed, revealing several strongly FDG-avid lesions in the right breast (Figure 1), the posterior mediastinum, both lungs, the lower pole of the right kidney, the abdomen, and the pelvis; and also a moderate FDG-uptake in the gluteal abscess (5).
Figure 1. a–c.
Transaxial fused PET-CT (a), CT (b), and PET (c) images show a strongly hypermetabolic nodular lesion in the right breast.
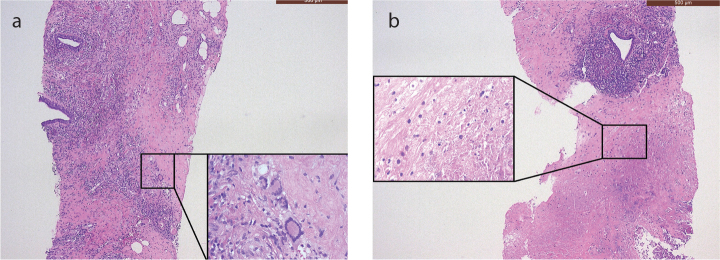
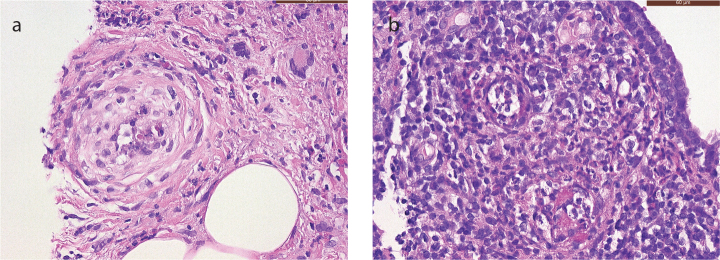
On admission to our hospital, the patient was afebrile, and the clinical examination was unremarkable. Biochemical analysis showed high CRP levels (325 mg/L, normal ≤5 mg/L). The urine and blood cultures were negative. Microscopic hematuria was found on urinalysis. Protein electrophoresis showed evidence of chronic inflammation, but no monoclonal fraction was detected. Shortly after admission, we observed acute renal failure with a rise in creatinine from normal to 4.5 mg/dL (normal range, 0.51–0.95 mg/dL). The first histological examinations done on two lymph nodes (right iliac and retroperitoneal, respectively) and on a fine-needle aspiration cytology of the pelvic mass were negative, showing only benign findings. A biopsy taken from the lesion in the right breast suggested the solution to this intriguing clinical case, showing the areas of necrosis, adjacent to the granulomatous inflammatory infiltrate with a large number of foamy histiocytes and some multinucleated giant cells (Figure 2), involving also the adjacent adipose tissue. Furthermore, a mixed inflammatory cell population was present, which focally surrounded and infiltrated the wall of small blood vessels. Few microthrombi and focal fibrinoid necrosis of vessel walls were noticed (Figure 3). No microorganisms were detected. The histological findings were strongly suggestive of primary vasculitis involving the breast. Based on these findings, c-ANCAs and PR3 were tested, and they returned positive (45 U/mL, reference <2 U/mL). To further investigate the pathophysiology of the acute renal failure, a renal biopsy was taken. The biopsy revealed a pauci-immune crescentic glomerulonephritis, corroborating the primary suspected diagnosis (Figure 4). The GPA diagnosis was made mainly on breast biopsy, in correlation with the clinical and radiological findings, and supported by c-ANCA serology tests and compatible renal involvement on renal biopsy. Treatment was initiated with high-dose intravenous steroids, plasmapheresis, and intravenous rituximab, followed by a spectacular therapeutic effect and full recovery of the patient. There was no relapse during a follow-up period of 3 years under immunosuppressive therapy.
Figure 2. a, b.
Core needle biopsy of the breast. The H&E stained section (50× magnification) shows (a) mammary parenchyma diffusely infiltrated by a dense, mixed inflammatory infiltrate involving the adipose tissue and sparing the ducts, and the presence of some multinucleated giant cells (Touton cells), localized mostly at the periphery of the inflammatory infiltrate and (b) extensive zones of infarct and necrosis of the breast parenchyma.
Figure 3. a, b.
Core needle biopsy of the breast. The H&E stained section (400× magnification) shows (a) involvement of a small artery in a zone of fat necrosis, with thickening of the vessel wall, infiltration by inflammatory cells and focal fibrinoid necrosis, and (b) two small veins showing thickening of the vessel wall and infiltration by a mixed inflammatory infiltrate.
Figure 4.
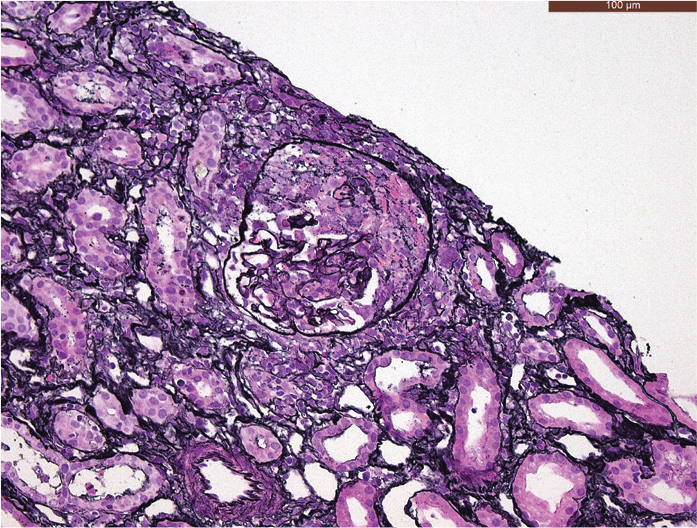
Kidney core needle biopsy. Jones’ staining (methenamine silver-Periodic acid- Schiff stain; 200× magnification) shows a crescent formation within the glomerulus.
Literature Review
Breast involvement in GPA is very uncommon. To the best of our knowledge, there have been 36 cases of breast involvement in GPA published in the English literature since the first description by Elsner and Harper in 1969 (4, 6–16), with a slightly increasing number of cases reported in the last decade. GPA with breast involvement can occur in both women and men, most often between the third and seventh decades. In most cases, breast lesions develop synchronous or metachronous in a multi-organ systemic disease, in which upper- or lower-airway symptoms are usually present. More rarely, unilateral or bilateral breast lesions can be the first or only symptom of the disease. Breast involvement can present as a painless breast lump or as a painful mass with redness, induration, and sometimes ulceration (4). Less frequently, the lesion can mimic breast carcinoma (4, 14).
In our case, there was no history of GPA, and upper- or lower-airway symptoms were absent. The breast lesion was detected simultaneously with the other lesions, and therefore, a disseminated malignant tumor seemed the most likely diagnosis. A biopsy of the breast lesion was therefore of utmost importance. This biopsy showed necrotizing granulomatous mastitis. These histopathological findings can be seen in many conditions, including the granulomatous reaction and necrosis in association with carcinoma, Zuska’s disease, various granulomatous infections, sarcoidosis, idiopathic granulomatous mastitis, systemic histiocytosis, and primary vasculitides. Among the primary vasculitides, GPA, giant cell arteritis, and polyarteritis nodosa are the more frequently encountered types in the breast. A recent publication stressed the importance of tissue biopsy in the diagnostic process of and the differentiation between these diseases (17).
However, because of the overlapping histological features shared by the aforementioned entities, subtle histopathological clues, such as focal vasculitis affecting small-sized arteries, can be easily overlooked if no clinical information is provided. We believe that a pathological tissue examination plays a crucial role in the diagnostic process, but correlation with the clinical setting and radiological manifestations is essential to make the diagnosis, since the diagnosis can be very challenging in cases with an atypical presentation.
Conclusion
Although GPA of the breast is rare, it should be actively considered, especially whenever necrotizing granulomatous mastitis is associated with clinical, biochemical, or radiological evidence of a systemic disease. By reporting this case, we want to stress the importance of a multidisciplinary approach with good communication between the clinician, the radiologist, and the pathologist to establish the diagnosis.
Main Points.
GPA lesions can occur in virtually all organ systems, including the breast.
GPA should actively be considered in patients presenting with necrotizing granulomatous mastitis.
The diagnosis of GPA requires a multidisciplinary approach and histopathological confirmation.
Footnotes
Informed Consent: Written informed consent for the publication of this article was given by the patient prior to its submission.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - L.G., L.H., K.E.G., O.G., E.L., T.R., D.B., G.F.; Supervision - L.G., L.H., K.E.G., O.G., E.L., T.R., D.B., G.F.; Writing Manuscript - L.G.; Critical Review - L.G., L.H.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Savage CO, Harper L, Adu D. Primary systemic vasculitis. Lancet. 1997;349:553–8. doi: 10.1016/S0140-6736(97)80118-3. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Kallenberg CG. ANCA-associated vasculitides-advances in pathogenesis and treatment. Nat Rev Rheumatol. 2010;6:653–64. doi: 10.1038/nrrheum.2010.158. [DOI] [PubMed] [Google Scholar]
- 3.Comarmond C, Cacoub P. Granulomatosis with polyangiitis (Wegener): clinical aspects and treatment. Autoimmun Rev. 2014;13:1121–5. doi: 10.1016/j.autrev.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Allende DS, Booth CN. Wegener’s granulomatosis of the breast: a rare entity with daily clinical relevance. Ann Diagn Pathol. 2009;16:351–7. doi: 10.1016/j.anndiagpath.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt WA, Blockmans D. Investigations in systemic vasculitis - The role of imaging. Best Pract Res Clin Rheumatol. 2018;32:63–82. doi: 10.1016/j.berh.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Elsner B, Harper FB. Disseminated Wegener’s granulomatosis with breast involvement. Report of a case. Arch Pathol. 1969;87:544–7. [PubMed] [Google Scholar]
- 7.Comas AG, Diana CA, Crespo CC, Cebollada MM, Liñán MA, Vila JV. Wegener’s granulomatosis presented as recurrent breast abscess. Breast J. 2010;16:82–4. doi: 10.1111/j.1524-4741.2009.00851.x. [DOI] [PubMed] [Google Scholar]
- 8.Kandiah DA. Development of granulomatosis with polyangiitis (Wegener): listen to the patient. J Clin Rheumatol. 2011;17:275–7. doi: 10.1097/RHU.0b013e31822890fc. [DOI] [PubMed] [Google Scholar]
- 9.Szabo-Moskal J. Wegener’s granulomatosis of the breast: A case report. Pol J Radiol. 2014;79:117–9. doi: 10.12659/PJR.889917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgescu R, Podeanu MD, Colcer I, Grigorescu G, Coros MF, Moldovan C, et al. Wegener’s granulomatosis of the breast with peculiar radiological aspect mimicking breast carcinoma. Breast J. 2015;21:550–2. doi: 10.1111/tbj.12458. [DOI] [PubMed] [Google Scholar]
- 11.Bataduwaarachchi VR, Galappaththi R, Tissera N. Blindness in a Sri Lankan woman with bilateral breast lumps: a case report. J Med Case Rep. 2015;9:296. doi: 10.1186/s13256-015-0792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallais Sérézal I, Jennische K. Image Gallery: Symmetrical necrosis of the breast as an inaugural manifestation of granulomatosis with polyangiitis (Wegener granulomatosis) Br J Dermatol. 2016;175:e132. doi: 10.1111/bjd.14984. [DOI] [PubMed] [Google Scholar]
- 13.Ryba M, Konieczny A, Hruby Z. Breast involvement in anti-neutrophil cytoplasmic antibodies positive granulomatosis with polyangiitis in a 64-year-old female patient. Arch Rheumatol. 2017;32:358–60. doi: 10.5606/ArchRheumatol.2017.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mengoli MC, Ragazzi M, Lococo F, Mengoli MA, Balli MC, Marchioni A, et al. Breast granulomatosis with polyangiitis mimicking breast cancer. Pathologica. 2017;109:405–7. [PubMed] [Google Scholar]
- 15.Jarrot PA, Pelletier ML, Brun M, Penicaud M, Mazodier K, Benyamine A, et al. Bilateral Breast Ulcers: Granulomatosis with Polyangiitis. Am J Med. 2019;132:179–81. doi: 10.1016/j.amjmed.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Lluch J, Montserrat Pérez-Tapia L, Taco-Sánchez MDR, Narváez J. Breast involvement in granulomatosis with polyangiitis. Joint Bone Spine. 2019;86:263–4. doi: 10.1016/j.jbspin.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Ren J, Liu J, Su J, Zhang J, Zhao J. Systemic vasculitis involving the breast: a case report and literature review. Rheumatol Int. 2019;39:1447–55. doi: 10.1007/s00296-019-04294-9. [DOI] [PubMed] [Google Scholar]