Abstract
Background & Aims:
The association between prevalence of celiac disease and geographic region is incompletely understood, but the occurrence of several autoimmune disorders has been found to vary along a North–South gradient. We examined geographic, demographic, and clinical factors associated with prevalence of celiac disease and gluten-free diet (GFD) in the United States (US).
Methods:
In a population-based study, we analyzed data on gluten-related conditions from the US National Health and Nutrition Examination Survey, from 2009 through 2014, on 22,277 participants 6 years and older. We identified persons with celiac disease, based on results of serum tests for immunoglobulin A against tissue transglutaminase and endomysium or on both a health care provider diagnosis and adherence to a GFD. Gluten-avoidance without celiac disease was defined as adherence to a GFD without a diagnosis of celiac disease. We compared mean serum levels of biochemical and nutritional markers based on status of gluten-related conditions.
Results:
We found 0.7% of participants to have celiac disease and 1.1% of participants to avoid gluten without celiac disease. Celiac disease was more common among individuals who lived at latitudes of 35–39° North (odds ratio, 3.2; 95% CI, 1.4–7.1) or at latitudes of 40° North or more (odds ratio, 5.4; 95% CI, 2.6–11.3) than individuals who lived at latitudes below 35° North independent of race or ethnicity, socioeconomic status, and body mass index. Gluten-avoidance without celiac disease was more common among individuals who lived at latitudes of 40° North or more, independent of demographic factors and body mass index. Participants with undiagnosed celiac disease (identified by positive results from serologic tests) had lower mean levels of B12 and folate (data collected from 2009 through 2012) than persons without celiac disease. Participants with a health-care provider diagnosis of celiac disease had a lower mean level of hemoglobin than persons without celiac disease. Mean levels of albumin, calcium, iron, ferritin, cholesterol, vitamin B6, and vitamin D (data collected from 2009 through 2010) did not differ between participants with gluten-related conditions and those without.
Conclusions:
In the US population, a higher proportion of persons living at latitudes of 35° North or greater have celiac disease and/or avoid gluten than persons living south of this latitude, independent of race or ethnicity, socioeconomic status, or body mass index. Mean levels of B12 and folate are lower in individuals with undiagnosed celiac disease, and levels of hemoglobin are lower in participants with a diagnosis of celiac disease, compared to individuals without celiac disease.
Keywords: celiac disease, gluten-free diet, gluten-related disorder, latitude, National Health and Nutrition Examination Survey, small intestine, autoimmune, epidemiology, population-based, tTG, EMA
Celiac disease is a chronic small intestinal immune-mediated enteropathy caused by intolerance to dietary glutens in genetically susceptible individuals.1 The prevalence of celiac disease varies widely across and within different countries.2 It is common in Europe, the United States, North Africa, the Middle East, and India and believed to be uncommon in Sub-Saharan Africa and East Asia.3, 4 These differences may be related to variation in human leukocyte antigens (HLA) or other genetic factors, dietary factors, infant feeding practices, gastrointestinal infections, socioeconomic status, hygiene, or other unknown factors.5, 6 Moreover, several studies in autoimmune diseases showed North–South gradients in disease occurrence in genetically similar populations, including inflammatory bowel disease, multiple sclerosis, and rheumatoid arthritis.7-10 It is possible that similar geographic variation exists for celiac disease as an autoimmune condition based on differential impact of environmental factors on susceptible individuals. A leading explanation for this ‘North–South’ gradient in the risk of autoimmune diseases may be differences in exposure to sunlight or UVB radiation, which is generally lower in northern latitudes predisposing to vitamin D deficiency. There is evidence that vitamin D plays an important role in immunomodulation in celiac disease and other autoimmune diseases.11 The availability of and adherence to a gluten-free diet, the treatment for celiac disease, may also vary by geographic location.
Following an NIH Consensus Conference on celiac disease in June 2004, a population-based study to estimate prevalence of celiac disease across ethnic groups in the United States was initiated.13 In the first report using data from the National Health and Nutrition Examination Survey (NHANES) 2009-2010 celiac disease components, the prevalence of celiac disease was 0.71% and most cases were undiagnosed.14 Celiac disease was more common among non-Hispanic whites (1%) than among minorities in whom it was rare. The prevalence of persons who reported following a gluten-free diet was 0.63% and most did not have a diagnosis of celiac disease. A more recent report using data from NHANES 2009-2012 celiac disease components, as well as earlier NHANES, suggested more than doubling of celiac disease prevalence between 1988 and 2012.15 A higher prevalence of persons maintaining a gluten-free diet in the absence of a diagnosis of celiac disease was found in blacks (1.2%). In a recently published report from our group using NHANES celiac disease components through 2014, the prevalence of celiac disease was 0.7% overall and was higher among non-Hispanic whites (1.0%) than among other race-ethnicities (0.2%).16 The prevalence of gluten-avoidance among persons without celiac disease was 1.1% overall and did not differ among racial-ethnic groups.
Celiac disease and gluten-related disorders along with the gluten-free diet are receiving increasing attention in the United States; however, their clinical significance and public health impact are incompletely understood. NHANES is the only nationally representative survey that has measured celiac disease serology coupled with medical condition questions. Six years of serology data integrated with geocoding and other health measures are now available and provide the opportunity to explore relationships with potential risk stratification or identification of protective factors. We examined geographic, demographic, and clinical factors associated with celiac disease and the gluten-free diet in this U.S. national population-based study.
METHODS
The NHANES is conducted in the United States by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) and since 1999, has been a continuous survey in 2-year cycles.17 The survey consists of cross-sectional interview, examination, and laboratory data collected from a complex multistage, stratified, clustered probability sample representative of the civilian, noninstitutionalized population with oversampling of non-Hispanic blacks, Hispanics, Asians (2011-2014), low income whites, and persons age 80 years or older. The survey was approved by the CDC ethics review board, and all participants provided written informed consent to participate. The current analysis utilized survey data collected from 2009 through 2014, the years during which gluten-related disorders were assessed by interview and serology for participants 6 years or older nation-wide.
Of 35,189 sampled persons age 6 years and older in NHANES 2009-2014, 24,703 (70%) attended a study visit at a mobile examination center. We excluded participants who were missing celiac disease serology (n=2,425) or celiac disease questionnaire responses (n=1) resulting in an analysis sample of 22,277, including 6,454 children and adolescents age 6-19 years and 15,823 adults age 20 years or older.
Participants were asked the following 2 interview questions: 1) Has a doctor or other health professional ever told you that you have celiac disease, also called sprue? and 2) Are you on a gluten-free diet?18 Serum specimens were shipped to the Celiac Disease Research Laboratory at Mayo Clinic, Rochester, MN, for sequential serological testing. Serum was tested for tissue transglutaminase immunoglobulin A (tTG IgA) as a screening test (sensitivity, ~98%) with an enzyme-linked immunosorbent assay that uses human recombinant tTG (Inova Diagnostics, San Diego, CA), and results were categorized as positive (>10 U/ml), weakly positive (4-10 U/ml), or negative (<4.0 U/ml).14, 19 Persons with a positive or weakly positive tTG IgA were tested for endomysial antibody (EMA IgA) as a confirmatory test (specificity, 99-100%) by indirect immunofluorescence using the reticulin component of the endomysium of the smooth muscle in monkey esophagus tissue (Inova Diagnostics, San Diego, CA), and results were considered positive if fluorescence was observed at a dilution of 1:≥5. Sequential serology has been shown by one of the authors (JAM) to be effective at detecting undiagnosed celiac disease cases in the general population.20, 21
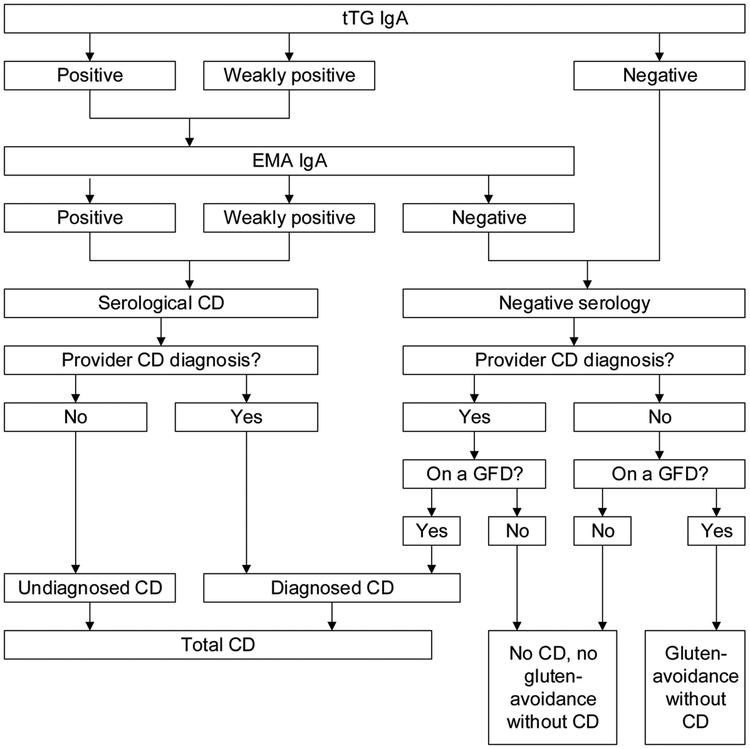
We identified participants as having celiac disease if they met at least one of the following two criteria: 1) double positive (or weakly positive) serology for tTG IgA followed by EMA IgA, or 2) both a self-reported health care provider diagnosis of celiac disease and adherence to a gluten-free diet. Participants were considered to have diagnosed celiac disease if they had a clinical (provider) diagnosis and either positive serology or adherence to a gluten-free diet, and undiagnosed celiac disease if they had positive serology without a clinical diagnosis. Gluten-avoidance without celiac disease was defined as adherence to a gluten-free diet without a diagnosis of celiac disease. These definitions are diagramed in Figure 1 for reference. tTG IgA titers (U/mL) were also analyzed as a continuous measure for this study through the NCHS Research Data Center.22
Figure 1.
Celiac disease and gluten-avoidance without celiac disease definitions
tTG IgA, tissue transglutaminase immunoglobulin A; EMA, endomysial antibodies; CD, celiac disease; GFD, gluten-free diet.
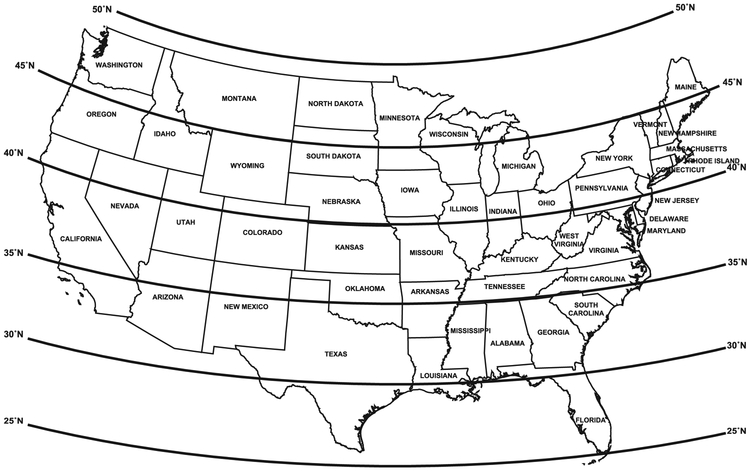
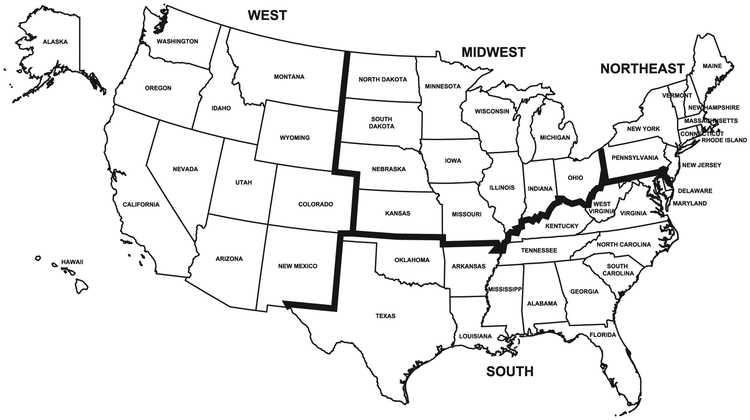
Data were collected on demographic and clinical characteristics and examined in relation to gluten-related disorders:23, 24 age (years), sex, race-ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), education, income, latitude, region, and body mass index (BMI). Latitude (° North) was geocoded by NCHS from the participant’s residential address and categorized for this analysis as <35, 35-<40, and ≥40 (Figure 2).25 Region was categorized based on the 4 census regions: Northeast, Midwest, South, and West (Figure 3). Geocoding data were used through the NCHS Research Data Center.22 For adults, the highest grade or level of school completed, or the highest degree received, was reported and categorized as less than high school graduation, a high school degree or GED or equivalent, or education beyond high school. For participants 6-19 years, a household education level was calculated as the higher of the education level of a ‘household reference person’ or household reference person’s spouse. The household reference person was defined as the first household member 18 years of age or older listed on the household member roster that owns or rents the residence where household members reside.23 Income was measured by the poverty income ratio (PIR; ratio of family income to poverty threshold) and categorized as tertiles (cut-points, <1.7, 1.7-<4.0, ≥4.0). PIRs of 1.7 and 4.0 represent family incomes of 1.7 times and 4 times, respectively, the poverty threshold for a given family size and year. BMI was categorized for adults in kg/m2 as normal weight (<25), overweight (25-<30), or obese (≥30) and for children/adolescents using the 2000 CDC sex-specific BMI-for-age growth chart percentiles as normal weight (<85th), overweight (85th-<95th), or obese (≥95th).26, 27 Serum was tested for hemoglobin, albumin, calcium, iron, ferritin, total cholesterol, vitamin B6, vitamin B12, folate and vitamin D as previously described.28
Figure 2.
Latitude of the contiguous United States
Figure 3.
Census regions of the United States
Statistical analysis
Characteristics of participants were compared by latitude using a chi-square (χ2) test and linear regression. The prevalence of celiac disease and gluten-avoidance without celiac disease was compared by demographic and BMI subgroups using a chi-square (χ2) test and linear regression. Odds ratio (OR) estimates and 95% CI for celiac disease and gluten-avoidance without celiac disease were calculated using logistic regression analysis (SUDAAN PROC MULTILOG) to control for the effects of age, sex, race-ethnicity, education, income, latitude or region, and BMI.
Unadjusted mean (standard deviation (SD)) tTG IgA titers were compared by demographic and BMI subgroups using analysis of variance. The relationship of demographic characteristics and BMI with tTG IgA titers was further examined using linear regression analysis (SUDAAN PROC REGRESS) to calculate (least squares) mean estimates adjusted for all other factors and for use of a gluten-free diet by survey participants. Finally, means of hemoglobin and serum concentrations of albumin, calcium, iron, ferritin, cholesterol, vitamin B6, vitamin B12, folate, and vitamin D were compared among gluten-related conditions using analysis of variance to better understand the impact of gluten-related disorders on these clinical markers. Multivariate analyses excluded persons with missing values for any factor included in the model. P-values were two-sided, and a P-value of <0.05 was considered to indicate statistical significance. All analyses utilized sample weights that accounted for unequal selection probabilities and nonresponse. All variance calculations accounted for the design effects of the survey using Taylor series linearization.29
RESULTS
The contiguous United States is encompassed by latitudes of approximately 25 to 50° North (Figure 2). Among 22,277 participants 6 years of age or older with celiac disease serology, the percentage residing within latitudes (° North) <35, 35-<40, and ≥40, was 27%, 31%, and 42%, respectively. Compared with persons living at <35° North, those residing at 35-<40° North or ≥40° North were older, more likely to be non-Hispanic white, had more education and a higher income, were less likely to be obese, and were more likely to be on a gluten-free diet (Table 1).
Table 1.
Characteristics* of NHANES examination participants 6+ years by latitude (°N), United States, 2009-2014
| <35 (N=7,476) |
35-<40 (N=6,624) |
≥40 (N=8,175) |
|
|---|---|---|---|
| Age (years) | |||
| 6-19 | 20.5 | 18.6 | 17.81 |
| 20-39 | 32.0 | 28.21 | 28.91 |
| 40-59 | 28.7 | 31.41 | 31.71 |
| ≥60 | 18.9 | 21.81 | 21.51 |
| Sex | |||
| Male | 48.8 | 48.0 | 49.6 |
| Female | 51.2 | 52.0 | 50.42 |
| Race-ethnicity | |||
| Non-Hispanic white | 43.2 | 67.51 | 77.31,2 |
| Non-Hispanic black | 16.1 | 13.6 | 6.71,2 |
| Hispanic | 32.0 | 10.61 | 9.61 |
| Other | 8.7 | 8.3 | 6.5 |
| Education (years)† | |||
| <12 | 23.2 | 16.31 | 13.21 |
| 12 | 20.9 | 21.3 | 20.8 |
| >12 | 56.0 | 62.51 | 65.91 |
| Income‡ | |||
| PIR 0-<1.7 | 41.8 | 32.91 | 29.31 |
| PIR 1.7-4.0 | 33.6 | 33.0 | 32.8 |
| PIR ≥4.0 | 24.6 | 34.11 | 37.91 |
| BMI§ | |||
| Normal weight | 34.3 | 37.3 | 37.4 |
| Overweight | 28.8 | 30.0 | 31.41 |
| Obese | 36.9 | 32.71 | 31.21 |
| Gluten-free diet | 0.9 | 0.8 | 2.01,2 |
NHANES, National Health and Nutrition Examination Survey; PIR, poverty income ratio; BMI, body mass index.
p<0.05 compared with <35 °N.
p<0.05 compared with 35-<40 °N.
Statistics are percentages.
For persons 6-19 years, education level was the higher of the household reference person or that person’s spouse.
PIR is family income to poverty threshold ratio. Cut-points were tertiles.
For persons 6-19 years, BMI was defined in age- and sex-specific percentiles as normal weight, <85th, overweight, 85th-<95th, and obese, ≥95th; for persons 20+ years, BMI was defined in kg/m2 as normal weight, <25, overweight, 25-<30, and obese, ≥30.
Celiac disease was present in 0.7% (95% CI, 0.6%-0.9%; N=109) of the sampled U.S. population. Celiac disease was more common among participants residing at higher latitude. Prevalence increased from 0.2% to 0.6% to 1.2% as latitude (° North) increased from <35 to 35-<40 to ≥40 (Table 2). After adjustment for demographic factors and BMI, celiac disease remained associated with higher latitude. Compared with <35° North, the odds of celiac disease were over 3 times higher at 35-<40° North and over 5 times higher at ≥40° North (Table 2). Celiac disease prevalence was higher with education beyond high school and the association remained significant after adjustment for persons with 12 years of education (Table 2). Normal-weight individuals had a higher celiac disease prevalence compared with overweight or obese persons and this association remained with adjustment for multiple factors (Table 2). Non-Hispanic black race was independently associated with a lower celiac disease prevalence; there was no relationship with age or sex (Table 2). When adults 20+ years were analyzed separately, the directions of relationships with celiac disease were similar to those for all participants and associations with celiac disease were stronger for higher latitude and higher education (Supplemental Table 1). Children and adolescents 6-19 years were not analyzed separately due to the smaller number of celiac disease cases.
Table 2.
Unadjusted prevalence and multivariate-adjusted odds ratios for celiac disease and gluten-avoidance without celiac disease among NHANES participants 6+ years (N=22,277), United States, 2009-2014
| Celiac disease |
Gluten-avoidance without celiac disease |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | Unadjusted prevalence (%) |
Multivariate-adjusted* |
N | Unadjusted prevalence (%) |
Multivariate-adjusted |
|||
| OR† | 95% CI | OR | 95% CI | |||||
| Total population | 109 | 0.72 | -- | -- | 213 | 1.11 | -- | -- |
| Latitude (°N) | ||||||||
| <35 | 16 | 0.15 | 1.0 | 60 | 0.81 | 1.0 | ||
| 35-<40 | 26 | 0.581 | 3.18 | 1.43-7.07 | 55 | 0.67 | 0.74 | 0.43-1.28 |
| ≥40 | 67 | 1.181,2 | 5.45 | 2.63-11.30 | 98 | 1.631,2 | 1.85 | 1.11-3.08 |
| Age (years) | ||||||||
| 6-19 | 30 | 0.74 | 0.85 | 0.52-1.37 | 29 | 0.60 | 0.47 | 0.27-0.82 |
| 20-39 | 26 | 0.70 | 1.0 | 63 | 1.271 | 1.0 | ||
| 40-59 | 28 | 0.70 | 0.93 | 0.50-1.73 | 67 | 1.341 | 1.02 | 0.59-1.75 |
| ≥60 | 25 | 0.75 | 0.99 | 0.50-1.94 | 54 | 1.01 | 0.78 | 0.39-1.56 |
| Sex | ||||||||
| Male | 40 | 0.55 | 1.0 | 95 | 0.83 | 1.0 | ||
| Female | 69 | 0.88 | 1.51 | 0.93-2.45 | 118 | 1.381 | 1.72 | 1.18-2.51 |
| Race-ethnicity | ||||||||
| Non-Hispanic white | 75 | 0.97 | 1.0 | 84 | 1.18 | 1.0 | ||
| Non-Hispanic black | 6 | 0.121 | 0.19 | 0.07-0.52 | 53 | 1.13 | 1.35 | 0.85-2.14 |
| Hispanic | 19 | 0.311 | 0.66 | 0.31-1.41 | 43 | 0.80 | 1.08 | 0.71-1.64 |
| Other | 9 | 0.281 | 0.32 | 0.12-0.87 | 33 | 1.17 | 1.13 | 0.61-2.11 |
| Education (years)‡ | ||||||||
| <12 | 15 | 0.32 | 0.54 | 0.24-1.19 | 35 | 0.74 | 0.74 | 0.38-1.45 |
| 12 | 17 | 0.37 | 0.51 | 0.27-0.99 | 35 | 0.73 | 0.60 | 0.33-1.10 |
| >12 | 77 | 0.951,2 | 1.0 | 142 | 1.351,2 | 1.0 | ||
| Income§ | ||||||||
| PIR 0-<1.7 | 40 | 0.57 | 0.94 | 0.50-1.76 | 75 | 0.78 | 0.58 | 0.32-1.03 |
| PIR 1.7-<4.0 | 28 | 0.62 | 0.78 | 0.46-1.33 | 59 | 1.11 | 0.81 | 0.46-1.40 |
| PIR ≥4.0 | 35 | 1.01 | 1.0 | 64 | 1.521 | 1.0 | ||
| BMI∥ | ||||||||
| Normal weight | 60 | 1.08 | 1.0 | 89 | 1.32 | 1.0 | ||
| Overweight | 23 | 0.601 | 0.52 | 0.30-0.91 | 51 | 0.94 | 0.67 | 0.40-1.15 |
| Obese | 24 | 0.421 | 0.43 | 0.22-0.85 | 68 | 1.02 | 0.77 | 0.51-1.17 |
NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; CI, confidence interval; PIR, poverty income ratio; BMI, body mass index.
p<0.05 compared with first-listed group.
p<0.05 compared with second-listed group.
Adjusted for all other factors listed (N=20,178).
Estimated using logistic regression analysis.
For persons 6-19 years, education level was the higher of the household reference person or that person’s spouse.
PIR is family income to poverty threshold ratio. Cut-points were tertiles.
For persons 6-19 years, BMI was defined in age- and sex-specific percentiles as normal weight, <85th, overweight, 85th-<95th, and obese, ≥95th; for persons 20+ years, BMI was defined in kg/m2 as normal weight, <25, overweight, 25-<30, and obese, ≥30.
We also conducted analyses substituting region for latitude (Figure 3). The percentage of participants residing within the Northeast, Midwest, South, and West was 18%, 24%, 34%, and 24%, respectively. When region was substituted for latitude, the prevalence of celiac disease was higher in the Northeast (1.11%) and Midwest (0.94%) compared with the South (0.37%) and did not differ in the West (0.70%) from other regions. With multivariate-adjustment, the celiac disease odds remained over twice as high for the Northeast compared with the South (OR, 2.3; 95% CI, 1.1-4.7, p=0.026), but were not statistically significantly different for the Midwest and West compared with the South (Supplemental Table 2). Similar to analyses that included latitude, non-Hispanic black race and overweight and obesity were independently associated with a lower celiac disease prevalence; more education was non-significantly associated with a higher celiac disease prevalence.
Gluten-avoidance without celiac disease was present in 1.1% (95% CI, 0.8%-1.4%; N=213) of the sampled U.S. population. Gluten-avoidance without celiac disease was twice as common among participants living at a latitude (° North) of ≥40 (1.6%), compared with those residing at latitudes of <35 (0.8%) or 35-<40 (0.7%) (Table 2). After adjustment for demographic factors and BMI, gluten-avoidance without celiac disease remained associated with higher latitude. The odds of gluten-avoidance without celiac disease were over 80% higher at ≥40° North compared with <35° North (Table 2). Gluten-avoidance without celiac disease prevalence was higher with education beyond high school and among the highest family income tertile; however, these relationships were no longer statistically significant after adjustment for multiple factors (Table 2). Gluten-avoidance without celiac disease was independently associated with female sex and was less common among children and adolescents; there was no relationship with race-ethnicity (Table 2). When adults 20+ years were analyzed separately, the directions of relationships with gluten-avoidance without celiac disease were similar to those for all participants and associations with gluten-avoidance without celiac disease were similar for latitude and stronger for higher income and lower BMI (Supplemental Table 1). Children and adolescents 6-19 years were not analyzed separately due to the smaller number of gluten-avoidance without celiac disease cases.
When region was substituted for latitude, the prevalence of gluten-avoidance without celiac disease was higher in the Northeast (1.88%) compared with the South (0.63%) and did not differ in the Midwest (0.97%) or West (1.36%) from other regions. With multivariate-adjustment, the gluten-avoidance without celiac disease odds remained almost 3 times as high for the Northeast compared with the South (OR, 2.9; 95% CI, 1.6-5.1, p<0.001), and a higher odds of gluten-avoidance without celiac disease emerged in the West compared with the South (OR, 2.5; 95% CI, 1.3-4.8, p=0.008) (Supplemental Table 2). Similar to analyses that included latitude, female sex was independently associated with a higher gluten-avoidance without celiac disease prevalence and age 6-19 years was independently associated with a lower prevalence.
We also conducted analyses of tTG IgA titers. The median (interquartile range) tTG IgA titer (U/mL) in the total population was 0.74 (0.62, 0.86) and the range from the 1st percentile to 99th percentile was 0.41-2.66. The unadjusted mean (SD) titer (U/mL) was higher at a latitude (° North) of 35-<40 [1.2 (6.8)] or ≥40 [1.2 (7.0)], compared with <35 [0.8 (2.3)] (Table 3). The association of higher latitude with higher titers remained after adjustment for age, sex, race-ethnicity, education, income, BMI, and use of a gluten-free diet (Table 3). Age 60 years or older and non-Hispanic black race were independently associated with a lower tTG IgA titer.
Table 3.
Unadjusted mean and multivariate-adjusted regression coefficients and means for tTG IgA titers (U/mL) among NHANES participants 6+ years (N=22,273), United States, 2009-2014
| Unadjusted |
Multivariate-adjusted* |
||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Beta† | SE | p-value | Mean | SE | |
| Total population | 1.11 | 6.1 | --- | --- | --- | ||
| Latitude (°N) | |||||||
| <35 | 0.83 | 2.3 | ref | 0.89 | 0.043 | ||
| 35-<40 | 1.191 | 6.8 | 0.33 | 0.15 | 0.028 | 1.23 | 0.14 |
| ≥40 | 1.211 | 7.0 | 0.31 | 0.095 | 0.002 | 1.20 | 0.094 |
| Age (years) | |||||||
| 6-19 | 1.13 | 6.4 | −0.099 | 0.16 | 0.54 | 1.18 | 0.15 |
| 20-39 | 1.23 | 7.9 | ref | 1.28 | 0.12 | ||
| 40-59 | 1.11 | 5.2 | −0.15 | 0.18 | 0.39 | 1.12 | 0.11 |
| ≥60 | 0.912 | 3.4 | −0.40 | 0.14 | 0.006 | 0.87 | 0.062 |
| Sex | |||||||
| Male | 1.11 | 5.9 | ref | 1.12 | 0.074 | ||
| Female | 1.11 | 6.2 | 0.015 | 0.14 | 0.91 | 1.14 | 0.10 |
| Race-ethnicity | |||||||
| Non-Hispanic white | 1.21 | 7.0 | ref | 1.22 | 0.089 | ||
| Non-Hispanic black | 0.871 | 2.8 | −0.31 | 0.12 | 0.014 | 0.91 | 0.067 |
| Hispanic | 0.911 | 3.4 | −0.23 | 0.12 | 0.060 | 0.99 | 0.074 |
| Other | 0.931 | 5.7 | −0.32 | 0.16 | 0.050 | 0.91 | 0.12 |
| Education (years)‡ | |||||||
| <12 | 0.95 | 3.7 | −0.16 | 0.12 | 0.19 | 1.05 | 0.087 |
| 12 | 0.93 | 3.9 | −0.26 | 0.14 | 0.065 | 0.95 | 0.080 |
| >12 | 1.211 | 7.1 | ref | 1.21 | 0.093 | ||
| Income§ | |||||||
| PIR 0-<1.7 | 1.08 | 6.1 | 0.092 | 0.21 | 0.66 | 1.18 | 0.12 |
| PIR 1.7-<4.0 | 1.11 | 5.9 | 0.030 | 0.18 | 0.87 | 1.12 | 0.12 |
| PIR ≥4.0 | 1.18 | 6.8 | ref | 1.09 | 0.13 | ||
| BMI∥ | |||||||
| Normal weight | 1.13 | 6.3 | ref | 1.12 | 0.095 | ||
| Overweight | 1.23 | 7.4 | 0.14 | 0.15 | 0.33 | 1.26 | 0.12 |
| Obese | 0.98 | 4.3 | −0.092 | 0.13 | 0.48 | 1.02 | 0.090 |
| Gluten-free diet | |||||||
| No | 1.10 | 6.1 | ref | 1.17 | 0.32 | ||
| Yes | 1.19 | 4.5 | −0.045 | 0.32 | 0.89 | 1.13 | 0.059 |
tTG IgA, tissue transglutaminase immunoglobulin A; NHANES, National Health and Nutrition Examination Survey; SD, standard deviation; SE, standard error; PIR, poverty income ratio; BMI, body mass index.
p<0.05 compared with first-listed group.
p<0.05 compared with second-listed group.
Adjusted for all other factors listed (N=20,175).
Estimated using linear regression analysis.
For persons 6-19 years, education level was the higher of the household reference person or that person’s spouse.
PIR is family income to poverty threshold ratio. Cut-points were tertiles.
For persons 6-19 years, BMI was defined in age- and sex-specific percentiles as normal weight, <85th, overweight, 85th-<95th, and obese, ≥95th; for persons 20+ years, BMI was defined in kg/m2 as normal weight, <25, overweight, 25-<30, and obese, ≥30.
Lastly, we examined the relationships of diagnosed and undiagnosed celiac disease and of gluten-avoidance without celiac disease with serum concentrations of nutrition-related factors. Mean albumin, calcium, iron, ferritin, total cholesterol, vitamin B6, and vitamin D did not differ with either celiac disease or gluten-avoidance without celiac disease compared with participants without either condition (Table 4). Based on currently available data, participants with undiagnosed celiac disease had lower vitamin B12 and folate (2009-2012) levels and those with diagnosed celiac disease had lower hemoglobin levels compared with persons with neither condition. Folate and vitamin D data from the most recent surveys will be released pending completion of laboratory quality control checks.
Table 4.
Mean (standard deviation) serum biochemical and nutritional markers by gluten-related disorders among NHANES participants 6+ years (N=22,277), United States, 2009-2014
| Diagnosed celiac disease |
Undiagnosed celiac disease |
Gluten-avoidance without celiac disease |
No celiac disease, no gluten-avoidance without celiac disease |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | Mean | SD | |
| Hemoglobin (g/dL) | 28 | 13.51 | 1.1 | 79 | 14.1 | 1.6 | 213 | 13.9 | 1.4 | 14.1 | 1.4 |
| Albumin (g/dL)* | 27 | 4.3 | 0.4 | 63 | 4.3 | 0.4 | 197 | 4.3 | 0.3 | 4.3 | 0.3 |
| Calcium (mg/dL)* | 27 | 9.5 | 0.4 | 63 | 9.4 | 0.4 | 197 | 9.5 | 0.4 | 9.5 | 0.4 |
| Iron (ug/dL)* | 27 | 90.0 | 29.7 | 63 | 79.8 | 32.2 | 197 | 89.5 | 35.2 | 86.5 | 36.1 |
| Ferritin (ng/mL)† | 0 | -- | -- | 7 | 34.1 | 36.6 | 14 | 73.3 | 61.5 | 54.9 | 63.0 |
| Total cholesterol (mg/dL) | 28 | 191.3 | 39.8 | 79 | 180.9 | 41.0 | 213 | 194.0 | 47.6 | 186.9 | 41.6 |
| Vitamin B6 (nmol/L)‡ | 6 | 126.0 | 100.2 | 29 | 67.5 | 40.8 | 48 | 91.7 | 90.4 | 69.7 | 76.1 |
| Vitamin B12 (pg/mL)§ | 5 | 688.0 | 401.3 | 20 | 454.01 | 169.8 | 57 | 962.3 | 922.8 | 609.9 | 507.7 |
| Folate (ng/mL)∥ | 12 | 19.7 | 17.5 | 56 | 17.81 | 9.0 | 118 | 27.3 | 28.3 | 20.5 | 12.5 |
| Vitamin D (nmol/L)¶ | 6 | 61.0 | 17.5 | 29 | 75.9 | 24.8 | 49 | 71.2 | 48.4 | 67.7 | 25.7 |
SD, standard deviation; NHANES, National Health and Nutrition Examination Survey.
p<0.05 compared to no celiac disease and no gluten-avoidance without celiac disease.
Measured on participants 12+ years.
Measured on females 12-49 years in NHANES 2009-2010.
Measured in NHANES 2009-2010.
Measured on participants 20+ years in NHANES 2011-2014.
Measured in NHANES 2009-2014; currently available for NHANES 2009-2012.
Measured in NHANES 2009-2014; currently available for NHANES 2009-2010.
DISCUSSION
In the U.S. population, both celiac disease and gluten-avoidance without celiac disease were associated with higher northern latitude which was not completely explained by differences in age, sex, race-ethnicity, socio-economic status, and BMI. We are not aware of other reports of the relationship of celiac disease with latitude of residence in the U.S. population. The prevalence of celiac disease varies widely across and within different countries.3, 4 These differences are likely related to both genetic and environmental factors. Celiac disease is characterized by a high heritability.30 Genetic variation in the major histocompatibility complex (MHC) region at the HLA-DQA1 and HLA-DQB1 genes, specifically the common haplotypes DQ2.5, DQ2.2, and DQ8, is well established as the major risk factor for celiac disease.31 Altogether, 43 predisposing loci have been implicated in celiac disease and collectively explain approximately 50% of the genetic variation.31, 32 Recently, fine mapping in the MHC region identified 5 new loci, independent of the HLA-DQA1 and HLA-DQB1 alleles, which account for an additional 18% of heritability.33 These HLA class I genes could provide the genetic link to intraepithelial lymphocytes which have been shown to exacerbate celiac disease and are restricted to MHC class I recognition.33 In addition, studies of gene function at celiac disease loci have confirmed the regulatory nature of autoimmune disease-associated SNPs and the well-established role for T cells, while also suggesting that B cells play a role in celiac disease.34, 35 These studies also identified 4 genes in relation to defective intestinal barrier function in celiac disease and a genetic network involved in interferon-gamma signaling and linked to celiac disease.34
Environmental factors other than gluten may also modulate celiac disease risk. A North–South gradient in disease occurrence in genetically similar populations has been shown in studies of autoimmune diseases, including inflammatory bowel disease, multiple sclerosis, and rheumatoid arthritis.7-10 Differences in exposure to sunlight or UVB radiation, which are generally lower in northern latitudes and predispose to vitamin D deficiency, could explain this ‘North–South’ gradient in the frequency of autoimmune diseases.11 Susceptibility to latitude-dependent autoimmune diseases may be mediated by the vitamin D receptor.36 The vitamin D receptor is activated by binding of 1,25-dihydroxy vitamin D3 and functions as a transcription factor that regulates gene expression in many cell types, including immune cells. The molecular pathways controlled by the vitamin D receptor appear to be important in maintaining immune tolerance. Serum vitamin D was measured in NHANES 2009-2014, but is currently available only for 2009-2010 which did not provide sufficient numbers for analyses.
A few studies have examined geographic region in relation to celiac disease prevalence. In a study from India, celiac disease was more prevalent in the north compared with the south and correlated with greater wheat intake, but not with genetic background.37 In the current study, there were no data on HLA-DQ 2/8 genotype. While we could speculate that there may be latitude-dependent differences in gluten intake, dietary data collection instruments were not designed to quantify gluten intake. In contrast to our findings, a recent large Swedish study reported a higher celiac disease incidence among children born in southern Sweden compared with the north.38 However, these ‘opposite’ latitude gradients may result from important differences between the two studies. Sweden lies between latitudes of 55 and 69° North and the southern border is approximately 350 miles north of the contiguous United States which lies below 50° North. In addition, the two populations differ with regard to racial-ethnic composition and the Swedish study was restricted to children and examined latitude of birth region (versus latitude of residence at the time of the study) in relation to incident biopsy-verified celiac disease (versus prevalent clinically- or serologically-diagnosed celiac disease). Further studies are needed of lifestyle and environmental factors that mediate the association of celiac disease with latitude, as well as integration with genetic data to account for potential interaction with genetic risk factors.
Celiac disease was independently associated with a higher education level. A relationship of celiac disease with socio-economic status has been suggested. Celiac disease prevalence was lower in Russian Karelia compared with Finland, 2 adjacent regions with partially shared genetic ancestry and equal exposure to grain products. This suggested that the lower economic status and less hygienic environment in Russian Karelia may protect against celiac disease.5, 6 Variation in hygiene standards, frequency of microbial infections, and early childhood feeding practices among socioeconomic environments may lead to differences in programming of the immune system in young children. Socioeconomic status may also influence health-seeking behavior and access to health care that increase the opportunity for a diagnosis; however, this would not completely explain the association we found with total celiac disease cases, the majority of which were serologically diagnosed.
A higher prevalence of gluten-avoidance without celiac disease among non-Hispanic blacks compared with non-Hispanic whites was suggested by a previous report based on four years of NHANES celiac disease data;15 however, no racial-ethnic differences were seen with two additional years of data (Table 2). This fluctuation in frequency was within the expected range given the small numbers with gluten-related conditions within the nationally representative survey cycles and the population-based sampling strategy dictated by the study design. Among adults, gluten-avoidance without celiac disease was independently associated with a higher income level. This was not unexpected, considering the higher cost and lesser availability of gluten-free foods. In one study conducted in 5 regions of the U.S., gluten-free products cost 240% more, on average, than their wheat-based counterparts (p<0.05).39 Availability of gluten-free products varied between venues; 36% of products studied were found at regular grocery stores compared to 41% at upscale markets, 94% at health food stores, and 100% on the internet.
Inflammation and destruction of the small intestinal mucosa that occurs in celiac disease lead to loss of absorptive surface and malabsorption of nutrients. Decreased serum levels of vitamins and minerals are common in untreated celiac disease patients. Removal of gluten from the diet leads to histological recovery in most patients with normalization of serum micronutrient levels. We found decreased serum vitamin B12 and folate levels among persons with undiagnosed celiac disease celiac disease celiac disease. Dietary B12 binds with intrinsic factor in the duodenum and the complex is absorbed in the terminal ileum. Although the terminal ileum is relatively spared in celiac disease, inadequate vitamin B12 levels are not uncommon among untreated celiac disease patients and were reported in 5-41% in a recent review.40 Folate is absorbed mostly in the duodenum and upper jejunum. Folate deficiency has been reported in 8%-85% of adult celiac disease patients and 10%-40% of children with celiac disease.40 Vitamin B12 and folate deficiencies can lead to megaloblastic anemia and to neurological symptoms. Among persons with diagnosed celiac disease in our study, vitamin B12 and folate levels did not differ from those of healthy participants. In contrast to our findings among participants with undiagnosed celiac disease, community-based studies in Olmsted County, Minnesota found no difference in vitamin B12 or folate levels among persons 50 years and over or under 50 years with serology-positive celiac disease compared with seronegative controls.41, 42 However, total cholesterol and ferritin levels were lower with undiagnosed celiac disease in both of these studies. We did not find significant differences in either total cholesterol or ferritin in the NHANES population, though ferritin was measured only in a select subset, so numbers with gluten-related conditions were small. Iron-deficiency anemia is the most common extra-intestinal manifestation of celiac disease and usually resolves with adherence to a gluten-free diet.40 We did not find lower iron or hemoglobin levels among participants with undiagnosed celiac disease compared to persons with neither celiac disease or gluten-avoidance without celiac disease. Mean hemoglobin level was, however, lower with diagnosed celiac disease. This may have been due to the low amounts of iron contained in gluten-free cereal products which are generally not enriched or fortified and are frequently made from refined flour and/or starch.43 The lower serum vitamin B12 and folate levels among participants with undiagnosed celiac disease, while statistically significant, are of unclear clinical significance. However, they are concerning because the majority of celiac disease cases in the United States are undiagnosed. Potential nutritional deficiencies associated with a gluten-free diet are also of concern, especially because the majority of persons following a gluten-free diet do not have a clinical diagnosis. These population-based survey findings warrant further study in larger samples with longitudinal follow-up.
A limitation of using NHANES to study celiac disease is the inability to confirm the diagnosis by small intestinal histology. However, upper endoscopy with small intestinal biopsy would be difficult to perform on the general U.S. population. We defined celiac disease based on a self-reported health care provider diagnosis and adherence to a gluten-free diet or on positive sequential serology. Sequential serology to detect undiagnosed celiac disease has been validated by one of the authors (JAM) and others.20, 21, 44 The accuracy of a clinical diagnosis among participants with negative serology is unknown. An additional limitation of the celiac disease definition is that serology may not detect celiac disease in persons with IgA deficiency. However, the prevalence of selective IgA deficiency is low, so few individuals with undiagnosed celiac disease should have been missed. Another limitation of the study was the small number of cases with gluten-related disorders despite 6 years of NHANES testing. However, NHANES is the first nationally representative survey to collect celiac disease serology. Thirdly, latitude was geocoded based on a participant’s address at the time of the survey, and data were unavailable on residential history. Finally, it is possible that the association we found between latitude and celiac disease and gluten-avoidance without celiac disease may be attributable to some factor(s) not measured in NHANES. These limitations are balanced by the benefits of a large, national, population-based sample, particularly avoidance of ascertainment bias found in clinical studies of selected patients and the ability to generalize the results to the U.S. population.
In conclusion in the U.S. population, both celiac disease and gluten-avoidance without celiac disease were associated with higher northern latitude, and with higher socioeconomic status, which was not completely explained by differences in race-ethnicity or BMI. BMI was lower among participants with celiac disease, serum vitamin B12 and folate levels were decreased with undiagnosed celiac disease, and hemoglobin was decreased with diagnosed celiac disease; there was no difference with regard to serum albumin, calcium, iron, ferritin, cholesterol, vitamin B6, or vitamin D concentrations from participants without either condition.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the staff of the Celiac Disease Research Laboratory and the Immunodermatology Laboratory at Mayo Clinic for conducting the serological testing. The National Center for Health Statistics (NCHS) was the source of the National Health and Nutrition Examination Survey 2009-2014 geocoding data and tTG IgA titer data. All analyses, interpretations, and conclusions are those of the authors and not NCHS. The authors thank Robert Krasowski for assistance in using the NCHS Research Data Center.
Financial support: The work was supported by a contract from the National Institute of Diabetes and Digestive and Kidney Diseases (HHSN276201200161U).
Abbreviations:
- BMI
body mass index
- CD
celiac disease
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- EMA
endomysial antibodies
- GFD
gluten-free diet
- HLA
human leukocyte antigens
- IgA
immunoglobulin A
- MHC
major histocompatibility complex
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
- PIR
poverty income ratio
- SD
standard deviation
- tTG
tissue transglutaminase
Footnotes
Disclosures: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aynur Unalp-Arida, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services, Two Democracy Plaza, Room 6009, 6707 Democracy Blvd., Bethesda, MD 20892-5458
Constance E. Ruhl, Social & Scientific Systems, Inc., 8757 Georgia Avenue, 12th floor, Silver Spring, MD 20910.
Rok Seon Choung, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN
Tricia L. Brantner, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN
Joseph A. Murray, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN
REFERENCES
- 1.Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut 2013;62:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology 2006; 131:1981–2002. [DOI] [PubMed] [Google Scholar]
- 3.Lionetti E, Gatti S, Pulvirenti A, et al. Celiac disease from a global perspective. Best Pract Res Clin Gastroenterol 2015;29:365–379. [DOI] [PubMed] [Google Scholar]
- 4.Kang JY, Kang AH, Green A, et al. Systematic review: worldwide variation in the frequency of coeliac disease and changes over time. Aliment Pharmacol Ther 2013;38:226–245. [DOI] [PubMed] [Google Scholar]
- 5.Rubio-Tapia A, Murray JA. Celiac disease. Curr Opin Gastroenterol 2010;26:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondrashova A, Mustalahti K, Kaukinen K, et al. Lower economic status and inferior hygienic environment may protect against celiac disease. Ann Med 2008;40:223–231. [DOI] [PubMed] [Google Scholar]
- 7.Kurtzke JF, Beebe GW, Norman JE Jr. Epidemiology of multiple sclerosis in US veterans: III. Migration and the risk of MS. Neurology 1985;35:672–678. [DOI] [PubMed] [Google Scholar]
- 8.Khalili H, Huang ES, Ananthakrishnan AN, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut 2012;61:1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieira VM, Hart JE, Webster TF, et al. Association between residences in U.S. northern latitudes and rheumatoid arthritis: A spatial analysis of the Nurses' Health Study. Environ Health Perspect 2010;118:957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson S Jr., Blizzard L, Otahal P, et al. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry 2011;82:1132–1141. [DOI] [PubMed] [Google Scholar]
- 11.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis 2007;66:1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NIH Consensus Statement on Celiac Disease. NIH Consens State Sci Statements. 2004. June 28-30; 21(1) 1–22. [PubMed] [Google Scholar]
- 13.National Institutes of Health Consensus Development Conference Statement on Celiac Disease, June 28-30, 2004. Gastroenterology 2005;128:S1–9. [DOI] [PubMed] [Google Scholar]
- 14.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012;107:1538–1544; quiz 1537, 1545. [DOI] [PubMed] [Google Scholar]
- 15.Choung RS, Ditah IC, Nadeau AM, et al. Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: findings from the National Health and Nutrition Examination Surveys from 1988 to 2012. Am J Gastroenterol 2015;110:455–461. [DOI] [PubMed] [Google Scholar]
- 16.Choung RS, Unalp-Arida A, Ruhl CE, et al. Less Hidden Celiac Disease But Increased Gluten Avoidance Without a Diagnosis in the United States: Findings From the National Health and Nutrition Examination Surveys From 2009 to 2014. Mayo Clin Proc 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NCHS. National Health and Nutrition Examination Survey (NHANES). http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed January 2017.
- 18.NCHS. NHANES 2009-2010 Sample Person Questionnaire Medical Conditions. http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/mcq_f.pdf. Accessed January 2017.
- 19.NCHS. NHANES 2009-2010 Laboratory Procedure Manual - Tissue Transglutaminase Assay (IgA-TTG) & IgA Endomyseal Antibody Assay (IgA EMA). http://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/TGEMA_F.htm. Accessed January 2017.
- 20.Walker MM, Murray JA, Ronkainen J, et al. Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology 2010;139:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz KD, Rashtak S, Lahr BD, et al. Screening for celiac disease in a North American population: sequential serology and gastrointestinal symptoms. Am J Gastroenterol 2011;106:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NCHS. NCHS Research Data Center (RDC). http://www.cdc.gov/rdc/. Accessed January 2017.
- 23.NCHS. NHANES 2009-2010 Data Documentation, Codebook, and Frequencies. Demographic Variables and Sample Weights (DEMO_F). http://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/DEMO_F.htm#Component_Description. Accessed January 2017. [Google Scholar]
- 24.NCHS. NHANES 2009-2010 Examination Data - Body Measures. http://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/BMX_F.htm. Accessed January 2017.
- 25.NCHS. NHANES 1988-1994 Restricted Data - Geocoding. http://www.cdc.gov/nchs/data/nhanes/limited_access/N3_GEO.pdf. Accessed January 2017.
- 26.NCHS. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years). http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. Accessed January 2017.
- 27.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002:1–190. [PubMed] [Google Scholar]
- 28.NCHS. NHANES 2009-2010 Laboratory Data. http://wwwn.cdc.gov/nchs/nhanes/search/DataPage.aspx?Component=Laboratory&CycleBeginYear=2009. Accessed January 2017.
- 29.Breslow NE, Day NE. Statistical Methods in Cancer Research: the Design and Analysis of Cohort Studies: Lyon, France: International Agency for Research on Cancer, 1987:48–79. [PubMed] [Google Scholar]
- 30.Kuja-Halkola R, Lebwohl B, Halfvarson J, et al. Heritability of non-HLA genetics in coeliac disease: a population-based study in 107 000 twins. Gut 2016. [DOI] [PubMed] [Google Scholar]
- 31.Withoff S, Li Y, Jonkers I, et al. Understanding Celiac Disease by Genomics. Trends Genet 2016;32:295–308. [DOI] [PubMed] [Google Scholar]
- 32.Wijmenga C, Gutierrez-Achury J. Celiac disease genetics: past, present and future challenges. J Pediatr Gastroenterol Nutr 2014;59 Suppl 1:S4–7. [DOI] [PubMed] [Google Scholar]
- 33.Gutierrez-Achury J, Zhernakova A, Pulit SL, et al. Fine mapping in the MHC region accounts for 18% additional genetic risk for celiac disease. Nat Genet 2015;47:577–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar V, Gutierrez-Achury J, Kanduri K, et al. Systematic annotation of celiac disease loci refines pathological pathways and suggests a genetic explanation for increased interferon-gamma levels. Hum Mol Genet 2015;24:397–409. [DOI] [PubMed] [Google Scholar]
- 35.Gutierrez-Achury J, Zorro MM, Ricano-Ponce I, et al. Functional implications of disease-specific variants in loci jointly associated with coeliac disease and rheumatoid arthritis. Hum Mol Genet 2016;25:180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Booth DR, Ding N, Parnell GP, et al. Cistromic and genetic evidence that the vitamin D receptor mediates susceptibility to latitude-dependent autoimmune diseases. Genes Immun 2016;17:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramakrishna BS, Makharia GK, Chetri K, et al. Prevalence of Adult Celiac Disease in India: Regional Variations and Associations. Am J Gastroenterol 2016;111:115–123. [DOI] [PubMed] [Google Scholar]
- 38.Namatovu F, Lindkvist M, Olsson C, et al. Season and region of birth as risk factors for coeliac disease a key to the aetiology? Arch Dis Child 2016;101:1114–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee AR, Ng DL, Zivin J, et al. Economic burden of a gluten-free diet. J Hum Nutr Diet 2007;20:423–430. [DOI] [PubMed] [Google Scholar]
- 40.Caruso R, Pallone F, Stasi E, et al. Appropriate nutrient supplementation in celiac disease. Ann Med 2013;45:522–531. [DOI] [PubMed] [Google Scholar]
- 41.Godfrey JD, Brantner TL, Brinjikji W, et al. Morbidity and mortality among older individuals with undiagnosed celiac disease. Gastroenterology 2010;139:763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choung RS, Larson SA, Khaleghi S, et al. Prevalence and Morbidity of Undiagnosed Celiac Disease From a Community-based Study. Gastroenterology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson T. Folate, iron, and dietary fiber contents of the gluten-free diet. J Am Diet Assoc 2000;100:1389–1396. [DOI] [PubMed] [Google Scholar]
- 44.Walker MM, Murray JA. An update in the diagnosis of coeliac disease. Histopathology 2011;59:166–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.