Abstract
Background:
Cardiac surgery and cardiopulmonary bypass (CPB) are associated with alterations in blood pressure in the perioperative period, which if uncontrolled, can result in end organ damage or dysfunction. Microvessels, significant contributors to blood pressure, both in the myocardium and peripheral skeletal muscle, have diminished responsiveness to major mediators of vascular tone, including thromboxane and serotonin after CPB. Responsiveness of these vessels to β-adrenergic stimulation, a major mediator of vascular tone, has not yet been studied. Here, we will investigate the role of β-adrenergic receptors in vascular tone regulation in human skeletal muscle microvessels before and after CPB.
Methods:
Skeletal muscle microvessels were isolated from patients undergoing cardiac surgery before and after CPB. Vessels were exposed in an ex-vivo model to the β-adrenergic agonist isoproterenol, or the direct adenylyl cyclase activator, forskolin, and the selective β-receptor antagonist ICI18.551 hydrochloride plus isoproterenol. Immunofluorescence of β-receptors and western blotting were also performed.
Results:
Microvessels showed diminished responsiveness to isoproterenol (10−6 - 10−4M) after CPB (n = 8/group, p = 0.01). Pretreatment with the selective β−2 blocker ICI18.551 (10-6M) prevented isoproterenol-induced microvascular relaxation (p = 0.001). Forskolin-induced relaxation response was also significantly diminished post CPB (n = 4/group, p < 0.05 vs. pre CPB). No significant changes in the total protein expression of β−1, β−2 and β−3 receptors were detected by western blotting or immunofluorescence.
Conclusions:
Microvessels isolated from human skeletal muscle show diminished responsiveness to isoproterenol and its downstream activator forskolin after CPB, suggesting there is an alteration in β-adrenergic receptor responsive in adenylate cyclase. The relaxation response to isoproterenol was via activation β−2 receptors without changes in β-adrenergic receptor abundance.
INTRODUCTION:
Cardiac surgery and CPB are associated with dysregulation of vascular tone and subsequent alterations in blood pressure in the perioperative period1,2. These alterations, if uncontrolled, may result in hypotension and end organ damage or dysfunction. It has been reported that after CPB there is significant reduction in the contractile responsiveness of peripheral microvessels to major mediators of microvascular tone including, serotonin3, substance P3,4, and a-adrenergic stimulation5,6,7,8. Despite these changes, a-adrenergic receptors do not show significant changes at the protein level with CPB, suggesting other mediators may be implicated in alterations in vasomotor tone9.
An important mediator of vascular tone, β-adrenergic stimulation, has not yet been investigated in vascular tissue from patients in the context of CPB. β-adrenergic receptors are abundant in peripheral microvasculature and are responsible for vasodilation in skeletal muscle microvasculature10. β−2-adrenergic receptors initiate a G-protein coupled cascade, ultimately resulting in relaxation of smooth muscle though increased production of cAMP. Given the prevalence of hypotension and vasoplegia in CPB11, it is possible over-activity or increased sensitivity of β-receptors may be implicated. Alternatively, microvessels may display a global lack of responsiveness to both the aforementioned vasopressive agents, as well as to vasodilatory β-agonists.
In this study, we interrogate the responsiveness of human skeletal muscle arteriolar microvessels to β-adrenergic stimulation in an ex-vivo model and compare their reactivity before and after CPB. We tested the non-selective β-agonist, isoproterenol in the presence or absence of β−2 receptor antagonist, as well as its downstream effector, forskolin, which directly increases cAMP. We then examined the relative abundance of β-adrenergic receptors using western blotting and immunofluorescence.
METHODS:
Case Selection
Patients undergoing either coronary artery bypass grafting or valve replacement were recruited from Rhode Island Hospital. Exclusion criteria included patients with aortic cross clamp time over 120 minutes or total CPB time over 180 minutes. Informed consent was obtained prior to study involvement consistent with protocols approved by the Institutional Review Board of Rhode Island Hospital and the Alpert Medical School of Brown University.
Human Subjects and Tissue Harvesting
Samples of skeletal muscle were taken from intercostal muscles adjacent to the left internal mammary artery (LIMA) before the initiation of CPB. After CPB separation skeletal muscle samples were harvested from intercostal muscles distant from the LIMA. Samples were either snap frozen in liquid nitrogen for western blotting, fixed in 10% formalin for 24 hours followed by parafinization and sectioning (6 m) for immunohistochemistry, or stored in in Krebs buffer for ex-vivo analysis. This is consistent with previously published methods9,3. CPB included a Medtronic affinity integrated hollow fiber oxygenator and cardiotomy reservoir with trillium coating (Medtronic, Minneapolis, MN), and an arterial 38-mg filter (Medtronic Affinity, Minneapolis, MN) with 61 trillium coating.
Ex-vivo skeletal muscle microvessel analysis
Microvessels, 90–180 m internal diameter, harvested from human skeletal muscle from 14 patients, stored in Krebs buffer were isolated using a dissecting microscope and cannulated with glass micropipettes on either end (40201380 m in diameter). Vessels were secured using 10–0 nylon microfilament sutures. Microvessels were placed in a chamber with continuously circulating, oxygenated (95% oxygen, 5% CO2) Krebs buffer at physiological temperature (37°C). Microvessels were pressurized using a burette manometer filled with Krebs buffer. Using an inverted microscope (40–200 ×, Olympus CK2, Olympus Optical) connected to a video camera the internal dimension of the vessels were analyzed using an electronic dimension analyser (Living Systems, Burlington, VT). Vessels were allowed to equilibrate at physiologic conditions for 30 minutes prior to application of vasoactive mediators. After equilibration, vessels were pre-contracted by the thromboxane A2 (TXA-2) analog U46619 (2× 10−7M) to 25% of the baseline diameter. The increasing concentrations of the b-adrenergic receptor activator, isoproterenol (Sigma-Aldrich) or the adenylate cyclase activator, forskolin (Sigma-Aldrich), starting at 10−9M and increasing to 10−4M, were applied to the vessels and the diameter was measured at each concentration. Isoproterenol and forskolin solutions were made fresh directly prior to each experiment. Additional experiments were performed with the selective b-2 receptor blocking agent ICI18.551 (10−6M). This procedure was performed as previously described3,9,12,13,14.
Western Blotting
Skeletal muscle from 6 patients, pre and post CPB, were collected, connective tissue was removed and samples were snap frozen. Tissue was then dissolved in SDS-PAGE (Thermo-fisher, Waltham MA). Protein was fractionated on a graduated SDS-PAGE gel (8–16%) and transferred to a polyvinylidene difluoride membrane (Millipore)15,16. Membranes were incubated for one hour in a 1:500 dilution of individual antibodies (β−1, β−2, β−3, GAPDH) (AbCam, Cambridge MA). Densitometric analysis of band intensity was performed using ImageJ software.
Immunofluorescence
Formalin fixed skeletal muscles were deparaffinized in xylene and rehydrated in increasing concentration of Ethanol (diluted with phosphate buffered saline (PBS)). Antigen retrieval was performed using Dako Target Retrieval Solution at (Thermo-fisher, Waltham MA), rinsed in PBS, incubated in 3% hydrogen peroxide, washed again with PBS, and blocked with 5% goat serum in PBS for 2 hours at room temperature. Samples were again washed with PBS and incubated overnight at 4°C with rabbit polyclonal primary antibodies to either β−1, β−2, or β−3 adrenergic receptors (AbCam, Cambridge MA), with concomitant goat primary antibodies to α smooth muscle actin (αsma) (AbCam, Cambridge MA). Dilutions of 1:500 in 5% goat serum were used for β-adrenergic antibodies, a 1:250 dilution of asma was used. Negative controls of αsma with respective secondary antibodies were also performed. Sections were washed in PBS and incubated with Alexa fluor secondary antibodies (AbCam, Cambridge MA), green fluorophores were used for β adrenergic detection, while red fluorophores were used for αsma detection. Fluorescent microscopy was performed using a Zeiss LSM510 confocal system in a manner similar to previously reported studies3,9,15,16.
Chemicals
U46619, Isoproterenol, forskolin, and ICI18.551 Hydrochloride were purchased from Sigma Aldrich (St. Louis, MO).
Data Analysis
Data are presented as the mean ± SD or mean± SEM. Patients are used as their own controls pre vs post CPB so analysis of patient character between pre and post CPB groups is unnecessary. Microvessel responses are analyzed as a percent change compared to their baseline diameter. 2-factor ANOVA for repeated measures was performed to assess vessel reactivity to isoproterenol and forskolin pre vs post CPB. Paired t-test was also used to assess changes in protein abundance by western blot, and receptor localization with immunofluorescence in pre vs post CPB.
RESULTS:
Patient characteristics
Patient characteristics are displayed in Table 1. Samples from 14 patients were collected for this study. Patient demographic are reported as mean ± standard deviation. Mean age was 73.14 ± 7.50, 36% of patients were female (5), mean BMI was 31.3 ± 5.60, 57% of patients had a diagnosis of hypertension (8). Mean HbA1c was 6.09 ± 0.98. Duration of CPB was 101.3 ± 29.75 minutes, while cross clamp time was 73.3 ± 21.36 minutes. 50% of patients underwent valve repair or replacement (7), while 50% underwent coronary artery bypass grafting (7).
Table 1 -.
Patient characteristics
| Age | 73.14 ± 7.50 |
| Female | 5 (36%) |
| BMI | 31.3 ± 5.60 |
| Hypertension | 8 (57%) |
| HbAlc | 6.09 ± 0.98 |
| Duration of Cross Clamp (min) | 73.3 ± 21.36 |
| Duration of CPB (min) | 101.3 ± 29.75 |
| Valve Repair / Replace | 7 (50%) |
| CABG | 7 (50%) |
BMI = Body Mass Index CABG = coronary artery bypass graft, Mean ± standard deviation Hgb1Ac = hemoglobin A1c
Microvascular reactivity
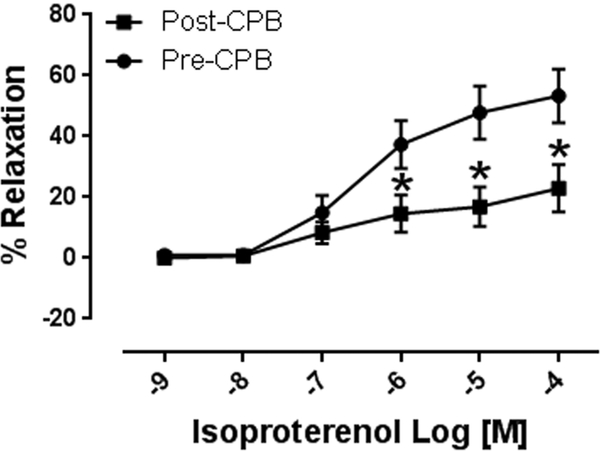
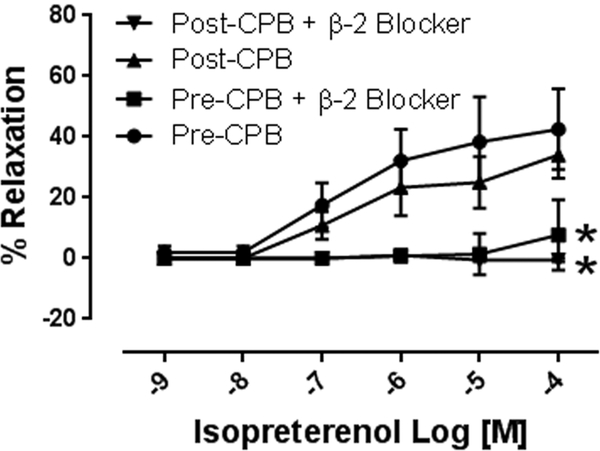
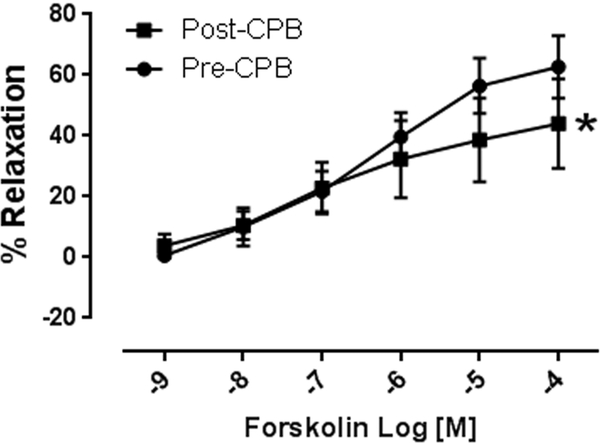
Isoproterenol (10−9 to 10−4M) induced concentration dependent relaxation of microvessels. This relaxation response was significantly attenuated at concentrations of 10−6 −10−4M in post CPB vessels compared to pre CPB, (n = 8/group, p < 0.01, Figure 1A). In addition, inclusion of the selective b-2 antagonist ICI18.551 (10−6M) significantly abolished isoproterenol-induced relaxation at dose dependent manner in both pre and post CPB vessels (10−9 to 10−4M, n = 4/group, P = 0.0001, Figure 1B). The direct adenylate cyclase activator, forskolin, (10−9 to 10−4M) also induced concentration dependent relaxation of microvessels, that was significantly diminished post CPB (n = 8/group, p < 0.05, Figure 1C).
Figure 1.
A. Dose dependent relaxation response to the b-adrenergic receptor agonist isoproterenol (10−9-10−4M) pre and post CPB; *p<0.05 vs. pre CPB; B. Dose dependent relaxation response to isoproterenol in the presence or absence of the selective b-2 receptor antagonist ICI18.551 (10−6M) pre- and post CPB; p<0.05 vs. pre or post CPB alone; C. Dose dependent relaxation response to the direct adenylyl cyclase activator, forskolin pre and post CPB; n =8/group; Mean ± SEM, *p<0.05 vs.pre CPB.
Western Blotting and Immunohistochemistry
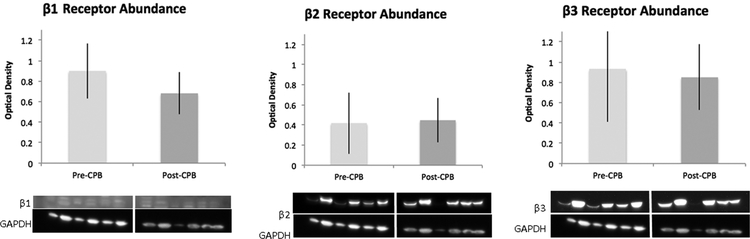
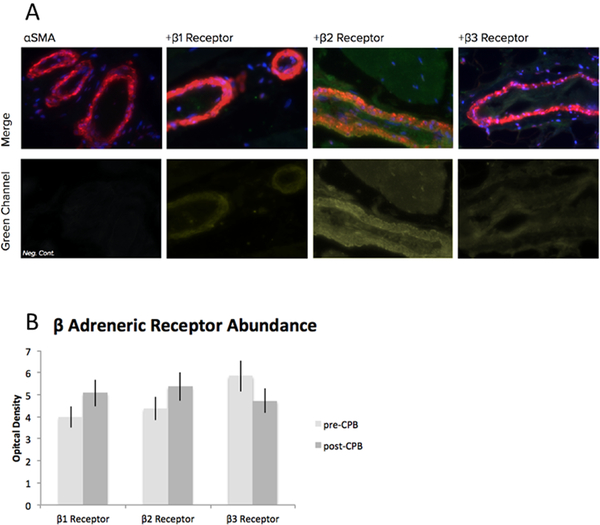
There was no significant difference in the abundance of β−1, β−2, or β−3 receptors in harvested skeletal muscle pre vs post CPB as assayed by western blot, respectively (n = 6/group, p > 0.05, Figure 2). Similarly, there was no change in receptor abundance as assayed by immunohistochemistry (n = 6/group, p > 0.05). Nor was there a change in receptor localization to vessels as assayed by co-localization analysis to αsma (n = 6/group, p > 0.05, Figure 3).
Figure 2.
Immunoblots of human skeletal muscle for β1, β2, and β3 receptors, pre and post CPB. Densitometric analysis shows no significant difference between groups for any receptor between pre and post CPB, n = 6/group, p > 0.05; mean ± standard deviation (SD).
Fig. 3:
A. Representative immunofluorescence of human skeletal muscle for β1, β2, and β3 receptors, alpha smooth muscle actin as a marker of smooth muscle, DAPI for nuclear staining. B. Optical density of fluorescence. No significant difference between pre and post CPB, n = 6/group, p > 0.05; mean ± standard deviation (SD).
DISCUSSION:
We have previously reported that CPB alters the responsiveness of microvasculature to various vasoactive mediators, such as phenylephrine9, endothelin-117, and serotonin3. Here, we demonstrate a continuation of this pattern of diminished responsiveness following CPB. Using an ex-vivo model of human skeletal muscle microvessels, we have shown there is dose-dependent diminished reactivity of these microvessels to the non-selective β-agonist, isoproterenol, post CPB vs pre CPB in matched patient samples. This relaxation response was abolished in the presence of the selective b-2 receptor blocker ICI18.551 hydrochloride. Interestingly, the same diminished reactivity in post-CPB vs pre CPB vessels to the direct cAMP activator forskolin was also observed. Of note, there was not a change observed in β-adrenergic receptor abundance in these vessels as assayed by western blot or by immunofluorescence, suggesting changes in sensitivity are not regulated at the level of total receptor protein abundance.
β-adrenergic receptors work through a G-protein coupled cascade; binding of the receptor stimulates release of the alpha subunit into the cytosol, resulting in activation of adenylyl cyclase. Subsequent increases in the concentration of cAMP then causes activation of protein kinase A. In smooth muscle, this causes inhibition of myosin light chain kinase and subsequent relaxation, leading to increased vessel diameter (Figure 4). Though we do not observe changes at the protein level with CPB, given the diminished responsiveness to isoproterenol that we observe, it is still possible alterations in the β-adrenergic receptor signaling occur with CPB. This is likely the case, as we continue to observe a significant decreased relaxation response to the direct adenylyl cyclase activator, forskolin, after CPB, suggesting that the downregulation of β-adrenergic receptor signaling may contribute to CPB-induced microvascular dysfunction.
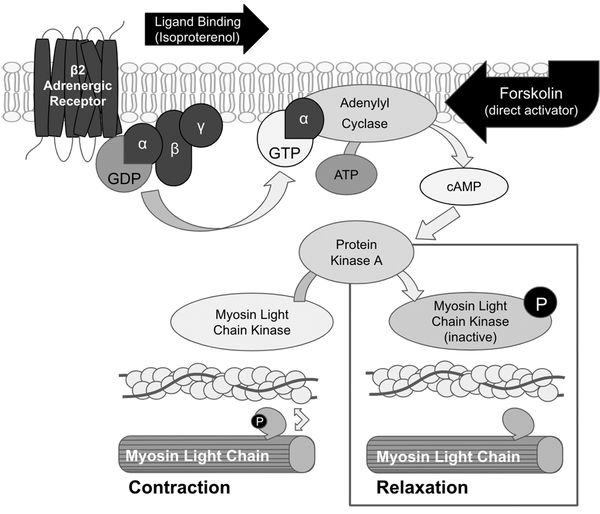
Fig. 4:
b-2 adrenergic signaling cascade in smooth muscle. The b-2 receptor is a trans membrane Gs protein coupled receptor. When stimulated by ligands, such as isoproterenol, the alpha subunit’s associated GDP is exchanged for GTP, allowing the subunit to be relased from the receptor complex and associate with adenylyl cyclase. Adenylyl cyclase converts ATP into cyclic AMP, a positive regulator of protein kinase A. Protein kinase A phosphorylates myosin light chain kinase, inactivating it and preventing its phosphorylation of myosin light chain (MLC). In the absence of phosphorylation, MLC is unable to form cross links with actin and the muscle relaxes. In the vasculature, this results in an increased vessel diameter.
As changes were not ascertained at the total protein level, other mechanisms may underlie the reported findings. Sensitization and desensitization of the β-adrenergic receptor are well noted phenomena18. After the receptor is stimulated, it internalizes and requires a recycling process mediated by protein phosphatase 2A18,19. It is Possible a perturbation of this recycling process underlies vascular dysfunction in CPB; as such, further investigation into this sensitization and desensitization process may reveal the mechanism of reduced β-adrenergic receptor responsiveness that is observed with CPB.
Limitations
This study is limited by a small sample size; such studies with limited samples sizes are often confounded by variation in patient factors. However, as our study uses the same patient in pre CPB vs post CPB analysis, this confounding factor is substantially mitigated. Additionally, comorbidities of patients is often cited as a concern in small studies, however, the patients studied represent those that generally undergo cardiac surgery who often have many comorbidities, giving this study a high degree of external validity and general applicability. Furthermore, a particular strength of this paper comes from its demographics; 36% of the patients are female. A prior analysis of NHLBI funded projects revealed fewer than ⅓ of subjects are women20, more recent data suggest this continues to be the case21.
In conclusion, the b-adrenergic receptor agonist isoproterenol caused peripheral microvascular relaxation via b-2 receptor activation. Both Isoproterenol- and forskolin-induced relaxation response was significantly diminished post CPB, suggesting that CPB results in generalized defect in the b-adrenoceptor downstream adenylate cyclase pathway in human peripheral microvasculature.
Acknowledgements:
We would like to thank the many nurses, physician’s assistants, perfusionists, and other members of the operating room staff for facilitating the collection of patient samples.
Funding/Support: This research project was supported by the National Institute of Health (NIH) 1R01HL127072–01A1, 1R01 HL136347–01, National Institute of General Medical Science (NIGMS) of the NIH [5P20-GM103652 (Pilot Project and CORE)] and AHA-Grant-in-Aid (#15GRNT25710105) to J.F. This work was supported in part by R01-HL46716 and RO1HL128831 to F.W.S; and NHLBI T35 training grant:-T5T34HL09430809 through Brown University to O.Z.; and Brown University Medical Student Summer Research Award to K.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI/Disclosure: We have no conflicts of interest
Presentation: 14th Annual Academic Surgical Congress Abstract Submission, Feb. 5–7, 2019 in Houston, TX.
References:
- 1.Ruel M Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg. 2004;26(5):1002–14. [DOI] [PubMed] [Google Scholar]
- 2.St. André AC, DelRossi A. Hemodynamic management of patients in the first 24 hours after cardiac surgery. Crit Care Med. 2005;33(9):2082–93. [DOI] [PubMed] [Google Scholar]
- 3.Sabe SA, Feng J, Liu Y, Scrimgeour LA, Ehsan A, Sellke FW. Decreased contractile response of peripheral arterioles to serotonin after CPB in patients with diabetes. Surgery. 2018. August;164(2):288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Métais C, Bianchi C, Li J, Li J, Simons M, Sellke FW. Serotonin-induced human coronary microvascular contraction during acute myocardial ischemia is blocked by COX-2 inhibition. Basic Res Cardiol. 2001. February;96(1):59–67. [DOI] [PubMed] [Google Scholar]
- 5.Feng J, Liu Y, Singh AK, Dobrilovic N, Feng WC, Chu LM, et al. Impaired contractile response of human peripheral arterioles to thromboxane A-2 after cardiopulmonary bypass. Surgery. 2011;150(2):263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang SY, Friedman M, Johnson RG, Weintraub RM, Sellke FW. Adrenergic regulation of coronary microcirculation after extracorporeal circulation and crystalloid cardioplegia. Am J Physiol. 1994. December;267(6 Pt 2):H2462–70. [DOI] [PubMed] [Google Scholar]
- 7.Wang SY, Stamler A, Li J, Johnson RG, Sellke FW. Decreased myogenic reactivity in skeletal muscle arterioles after hypothermic cardiopulmonary bypass. J Surg Res. 1997. April;69(1):40–4. [DOI] [PubMed] [Google Scholar]
- 8.Sodha NR, Feng J, Clements RT, Bianchi C, Boodhwani M, Ramlawi B, et al. Protein kinase C alpha modulates microvascular reactivity in the human coronary and skeletal microcirculation. Surgery. 2007. August;142(2):243–52. [DOI] [PubMed] [Google Scholar]
- 9.Sellke N, Gordon C, Lawandy I, Gorvitovskaia AY, Scrimgeour LA, Fingleton JG, et al. Impaired coronary contraction to phenylephrine after cardioplegic arrest in diabetic patients. J Surg Res. 2018. October;230:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundvall J, Järhult J. Beta Adrenergic Dilator Component of the Sympathetic Vascular Response in Skeletal Muscle Influence on the micro-circulation and on transcapillary exchange. Acta Physiol Scand. 1976;96(2):180–92. [DOI] [PubMed] [Google Scholar]
- 11.Fischer GW, Levin MA. Vasoplegia during cardiac surgery: current concepts and management. Semin Thorac Cardiovasc Surg. 2010. Summer;22(2):140–4. [DOI] [PubMed] [Google Scholar]
- 12.Feng J, Chu LM, Dobrilovic N, Liu Y, Singh AK, Sellke FW. Decreased coronary microvascular reactivity after cardioplegic arrest in patients with uncontrolled diabetes mellitus. Surgery. 2012. August;152(2):262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng J, Chu LM, Robich MP, Clements RT, Khabbaz KR, Hagberg R, et al. Effects of cardiopulmonary bypass on endothelin-1-induced contraction and signaling in human skeletal muscle microcirculation. Circulation. 2010. September 14;122(11 Suppl):S150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng J, Liu Y, Chu LM, Clements RT, Khabbaz KR, Robich MP, et al. Thromboxane-induced contractile response of human coronary arterioles is diminished after cardioplegic arrest. Ann Thorac Surg. 2011. Sep;92(3):829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng J, Bianchi C, Sandmeyer JL, Li J, Sellke FW. Molecular indices of apoptosis after intermittent blood and crystalloid cardioplegia. Circulation. 2005. August 30;112(9 Suppl):I184–9. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Sellke EW, Clements RT, Sodha NR, Liu Y, Khabbaz KR, et al. 3. Calcium Activated Potassium Channels Contribute to Cardiopulmonary Bypass Related Microvascular Endothelial Dysfunction of Human Skeletal Muscle Arterioles. J Surg Res. 2008;144(2):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng J, Liu Y, Khabbaz KR, Hagberg R, Sodha NR, Osipov RM, et al. Endothelin-1-induced contractile responses of human coronary arterioles via endothelin-A receptors and PKC-α signaling pathways. Surgery. 2010;147(6):798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasudevan NT, Mohan ML, Goswami SK, Naga Prasad SV. Regulation of β-adrenergic receptor function: an emphasis on receptor resensitization. Cell Cycle. 2011. November 1;10(21):3684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ. The Role of Sequestration in G Protein-coupled Receptor Resensitization. J Biol Chem. 1997;272(1):5–8. [DOI] [PubMed] [Google Scholar]
- 20.Vidaver RM, Lafleur B, Tong C, Bradshaw R, Marts SA. Women subjects in NIH-funded clinical research literature: lack of progress in both representation and analysis by sex. J Womens Health Gend Based Med. 2000. June;9(5):495–504. [DOI] [PubMed] [Google Scholar]
- 21.Epstein S Inclusion: The Politics of Difference in Medical Research (Chicago Studies in Practices of Meaning) (Large Print 16pt). ReadHowYouWant.com; 2010428 p.