Abstract
As the HIV population continues to live longer as a result of antiretroviral therapy, liver-related mortality has become one of the leading causes of non-AIDS related death in this patient population. The liver possesses a remarkable regenerative capacity but undergoes complex biological changes in response to aging and inflammation that result in decreased cellular regeneration and a tipping of the scales towards fibrogenesis. Patients with HIV infection have serological evidence of ongoing inflammation, with elevations in some biomarkers persisting despite adequate virologic control. In addition, HIV-co-infected patients have markers of advanced age on liver biopsy and increased prevalence of fibrosis as compared to an age-matched HCV mono-infected cohort. In this review, we will discuss the biology of aging, age- related changes in the liver, and the relevant mechanisms by which HIV causes inflammation in the context of accelerated aging, fibrosis of the liver, and other viral co-infection.
Introduction
The advent of antiretroviral therapy (cART) represents one of the greatest interventions of our time, curbing an epidemic and resulting in millions of years of life saved in the USA alone [1]. One of the greatest public health achievements of the past two decades is that our HIV population is surviving longer [2, 3]. It is estimated that 40 % of all HIV-infected individuals in the USA are currently over the age of 50 [4]. This increase in survival has also resulted in changes in the epidemiology of morbidity and mortality in the HIV population [5]. As we push to- wards higher rates of virologic suppression in an aging population, we are faced with new challenges and questions that continue to evolve. Chief among these concerns is that individuals with HIV exhibit age-associated disease with higher prevalence and earlier onset than their non- infected counterparts [6–8].
In the context of this changing epidemiology, the question of how HIV affects the liver has become increasingly relevant.
Liver-related disease including chronic viral hepatitis now accounts for 13–18 % of all-cause mortality in HIV-infected patients and is one of the leading causes of non-AIDS- related death, competing closely with non-AIDS-related can- cer [2, 3, 9]. In the HCV/HIV co-infected population, HIV is known to accelerate the natural history of liver disease, with co-infected individuals demonstrating accelerated progression to hepatic fibrosis, cirrhosis, and increased risk for hepatocellular carcinoma [10–12]. More recently, data from the CFAR Network of Integrated Clinical Systems (CNICS) study cohort demonstrates that poorly controlled HIV mono-infection is an independent risk factor for liver fibrosis [13]. This review explores the cellular and molecular biology of the aging liver in the context of the inflammation and immune dysregulation that results from HIV infection.
Biology of Aging
Aging has been defined as the progressive loss of function accompanied by decreasing fertility and increasing mortality and disability [14]. In general, the aging process is viewed as cumulative subcellular damage that eventually results in macroscopic effects [15]. While the theories for such a process are varied, Sahin et al. proposed what is now the most widely accepted theory of aging, arguing that telomere attrition leads to multiple downstream effects on cellular signaling pathways that have been implicated in the aging process [16]. At the heart of this model is the concept that telomere loss and dysfunction leads to decreased expression of key regulatory proteins that affect multiple metabolic pathways including mitochondrial biogenesis, hepatic lipid synthesis, and lipoprotein production [17]. This in turn results in downstream repression of genes controlling oxidative defense, creating a positive feedback loop that accelerates telomere loss through increased oxidative stress [18].
This has been expanded to the cellular senescence theory of aging as a further attempt to bridge the gap be- tween molecular signaling and the aging phenotype [15, 19]. Inherent to this theory is the concept that cells trade their pluripotency and cellular immortality for differentiation and gain of function. The need for cellular diversity causes the germ cell to become a highly specialized somatic cell with a specific function in the body, but also a limited number of cell cycles [20, 21]. As the differentiated somatic cell goes through multiple cycles of division, it accumulates intracellular damage, resulting in the loss of replication of nuclear DNA in addition to elevated and sustained expression of tumor suppressor proteins p16ink4a, p53, and p21 as demonstrated in murine models [22–24]. This oncogene activation then leads to downstream inhibition of multiple different cyclin- dependent kinase (CDK) pathways, including E-Cdk2, D-Cdk4, and D-Cdk-6, ultimately leading to inhibition of Rb and cell cycle arrest [22, 25]. These changes in intracellular signaling result in the loss of the original cellular function and an arrest in replication that is now recognized as the senescence phenotype.
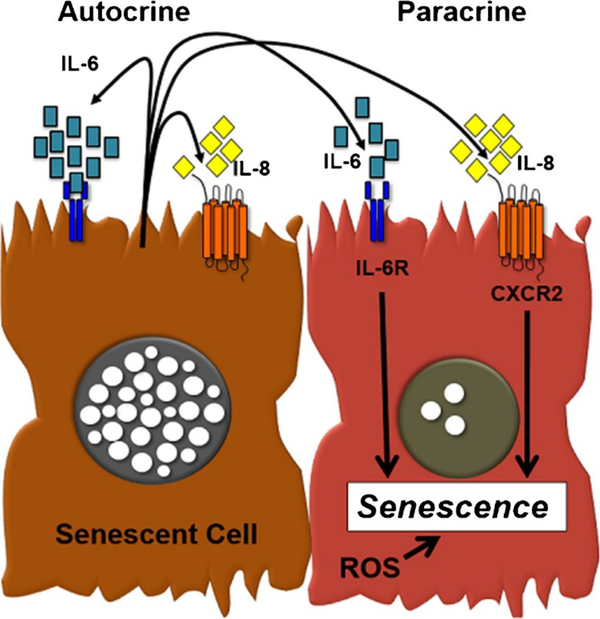
Perhaps the most crucial aspect of the senescent phenotype is the expression of new proteins and enzymes including pro- inflammatory factors, matrix metalloproteinases, and nitric oxide [26, 27]. This secretome has been termed the senescence-associated secretory phenotype (SASP) or senescence-messaging secretome (SMS) [27, 28] (Fig. 1). Chief among the pro-inflammatory cytokines are the intracellular molecules interleukin (IL)-1α, IL-6, IL-8, and chemokine CXCR2 [29–31]. The secretion of these biomarkers has been described as a method of inducing the senescent phenotype in neighboring cells, essentially resulting in infectious senescence [25].
Fig. 1.
Senescent cells develop the senescent-associated secretory phenotype (SASP). Senescent cells secrete significant amounts of IL-6 and IL-8, cytokines which can act in both an autocrine or paracrine manner to promote cellular senescence. IL-6 through IL-6 receptor (IL-6R) and/or IL-8 (through CXCR2) act in concert with cellular stress induced by reactive oxidative species (ROS) to induce the senescent phenotype
HIV and Inflammation
As the HIV population ages, it has become increasingly clear that diseases traditionally associated with aging occur at a younger age and with higher prevalence in this population [32, 33]. Despite adequate virologic control, the risk of non- AIDS-related morbidity is higher among HIV-infected patients as compared to the general population [3, 9]. Liver dis- ease, renal disease, bone loss, diabetes mellitus, and non- AIDS-related cancers are all more common in the HIV- infected population [34–36].
Chronic inflammation and immune dysregulation are central to any discussion about increased morbidity in HIV- infected patients. In the elderly population, mild levels of chronic inflammation, in the absence of overt infection, have been described as a highly significant risk factor for both morbidity and mortality [37–39]. With respect to HIV, immune activation, even in the face of virologic suppression, has been described as a key component of infection [40, 41] and the immunological mechanisms underlying chronic immune activation appear to be multifactorial. In the context of aging, HIV-infected patients often have immunologic profiles of patients three to four decades their senior [33, 40, 42]. It is believed that the chronic inflammation associated with HIV infection is the primary driver of the premature aging phenotype seen in HIV patients [41].
Microbial Translocation and Immune Activation
HIV effects on the microbiome and gut translocation also play a crucial role in the HIV pathophysiology of inflammation [43]. In acute infection, HIV has been demonstrated to preferentially target gut lymphoid tissue, heavily depleting multiple different lymphoid cell populations including the CD22 and the CD4 subset, TH17 [44–46]. The depletion of TH17 and CD22 cells is believed to play a critical role in the failure to maintain the intestinal epithelium, leading to the promotion of microbial translocation [46, 47]. In addition to direct infection, HIV viral proteins cause increased production of multiple inflammatory cytokines from the gut epithelium, resulting in increased intestinal epithelial cell apoptosis and disruption of the gut tight junctions [47–49]. In vitro models of colonic epithelial cells have demonstrated a pro-inflammatory response to HIV envelope protein gp120. In response to gp120 exposure, these cells release TNF-α, IL-6, and IL-8, ultimately resulting in tight junction and barrier function impairment [49]. Clayton et al. have developed an in vitro model of HIV- mediated tight junction dysfunction [43, 50] where HIV co- receptor Bob/GPR15 binds HIV gp120 with high affinity. In other cellular models, Bob/GPR15 receptor activation leads to downstream calcium signaling and subsequent microtubular loss that disrupt the integrity of epithelial tight junctions.
As a result of these changes to the colonic mucosa, HIV patients have been shown to have increased levels of inflammatory markers associated with microbial translocation even after initiation of cART [51–53]. Hattab et al. have conducted multiple studies looking at levels of different soluble inflammatory markers in both acutely infected HIV patients and those who have been virologically suppressed on cART. While multiple markers, including systemic IL-6, normalized after initiation of cART, sCD14, a scavenger molecule and surrogate for levels of bacterial lipopolysaccharide (LPS), remained elevated [51, 52, 54]. This finding has been replicated in multiple different studies of soluble inflammatory markers, with the implication that cART does not completely restore the gastrointestinal barrier function and that even those HIV patients with up to 2 years of virologic suppression demonstrate elevated levels of bacterial translocation as compared to non-infected controls. Soluble CD163 (sCD163) has also been noted as a bio- marker of great interest. Although little is known about its function as a soluble protein, it is most commonly expressed on monocytes and serves as a marker of alternate macrophage (M2) activation [55]. This protein is involved in multiple functions including endocytosis of the haptoglobin-hemoglobin complex, but also plays a role in the sCD-14 and LPS pathway in the liver [55, 56]. In this context of this pathway, cellular CD-163 has been identified as another innate immune sensor for bacteria, with the ability to induce local immunity through chemokine secretion.
Endotoxin-mediated shedding via the TLR pathway is believed to be the primary source of soluble sCD-163, similar to sCD-14 [57]. In a study of 933 patients with HIV-1, elevated levels of sCD163 have been associated with more rapid progression to AIDS and mortality. Based on these results, it is postulated that increased mortality in HIV patients is associated with higher levels of alternate macrophage activation and systemic inflammation [58, 59].
The Aging Liver
Aging-related liver changes occur at the level of the organ and the cell. These collective aging effects result in diminished function and a liver more prone to acute insults and vulnerable to chronic disease. This is important to consider as the liver plays a pivotal role in maintaining homeostasis in the aging person through interplay with other organs.
Organ and Structural Level Changes
Multiple organ and structural level changes occur in the liver with aging. Several ultrasound studies demonstrate that the adult liver volume gradually decreases by 20–40 % across the adult human lifespan, a finding more marked in females [60–63]. In addition to a decline in volume, the functional liver cell mass measured by galactose elimination capacity has also been shown to decline in the elderly [64]. This has been further investigated by scintigraphy studies using radiolabeled galactosylalbumin that suggest a decline in functional liver mass more so than total hepatic mass [65]. Blood flow to the liver also declines with age by 35–50 %, particularly evident after age 75, and has been suggested to contribute to reduced functional capacity to metabolize drugs [60, 66]. Despite having diminished hepatic blood flow, rat models suggest sinusoidal perfusion rates are relatively preserved throughout the lifespan [67].
Structural microenvironment changes are also evident with age. The lobular area, measured as the distance between adjacent central veins (terminal hepatic venules), increases with age in rat models [67]. In addition to these changes, reduction in drug metabolism capacity also appears to be related to diminished cytochrome P450 content. Data from in vitro and in vivo drug metabolism studies suggest ∼30 % reduction in drug metabolism after age 70 related to this mechanism of clearance [68]. On the other hand, albumin concentration, a serum marker of synthetic function, appears to only minimally decrease with age, possibly reflecting relatively preserved overall function. After adjusting for gender, BMI, systolic blood pressure, alcohol use, waist-hip ratio, diabetes, fasting glucose, total-HDL ratio, triglycerides, and adiposity markers (leptin, adiponectin, ghrelin, IL-6), albumin decreased from 4.5 to 4.2 g/dL in young (age 30–62) compared to old (age 78–93) in a cross-sectional study of 2364 patients [69].
Regenerative Response to Injury
Age appears to be a risk factor for fibrosis related to chronic liver disease. The two general patterns of chronic liver injury that lead to fibrosis are hepatocellular and cholestatic. Chronic hepatocellular injury refers to prolonged damage to hepatocytes, as seen in chronic viral hepatitis, alcoholic liver disease, drug-related liver injury, and non-alcoholic fatty liver disease. Chronic cholestatic injury results from prolonged biliary obstruction, as seen in primary sclerosing cholangitis and primary biliary cholangitis. Rodent models for chronic hepatocellular injury utilize chronic CCl4 administration. There have been conflicting age-related results in this regard. One study showed that oxidative stress markers between old (15 month) and young (8 week) mice were equivalent in response to chronic CCl4 administration [70]. A more recent study demonstrated that older mice had a significantly greater fibrogenic response to chronic CCl4 assessed by col1α1 mRNA expression, morphometric analysis, and hydroxyproline measurement [71]. Further investigation has shown that age-associated alterations of C/EBP proteins result in differential fibrotic response to chronic injury from CCl4, and this likely plays a pivotal role in fibrosis progression in aged livers [72].
Protracted chronic injury can lead to fibrosis and ultimately liver architectural destruction and cirrhosis. Accumulating clinical and epidemiological evidence has demonstrated that aging appears to be a risk factor for fibrosis progression across multiple etiologies of liver disease. In patients with chronic HCV infection, studies have demonstrated that accelerated fibrosis occurs in age >50 years [73]. In a cross-sectional analysis of participants in the NASH Clinical Research Network, elderly patients (age ≥65) were more likely to have NASH and advanced fibrosis compared to nonelderly [74]. Old age, furthermore, has been established as a poor prognostic indicator for patients with alcoholic hepatitis [75].
The Impact of Chronic HIV Infection on the Aging Liver
The importance of characterizing the ways in which HIV interacts with the aging liver has increased dramatically in the past decade. Although the natural history of advanced liver disease in HCV was well described early in the history of co- infection, elucidation of the specific role of HIV has only recently become more relevant. It is clear that poorly con- trolled HIV, without hepatitis C, is a risk factor for both NASH and cirrhosis [13, 76, 77]. In addition, the advent of DAA agents for the treatment of HCV and the aging of the HIV population may bring about a shift in the epidemiology of liver disease in HIV patients, with NASH and NASH- related cirrhosis on the rise [78]. Understanding the ways in which HIV interacts with the liver is becoming an increasingly important area of research.
HIV, the Gut-Liver Axis, and Inflammation
The model of indirect liver damage for alcoholic hepatitis shares many similarities in the alteration of the gut microbiome and increase in microbial translocation with HIV. While alcohol is well described to exert direct liver toxicity [79, 80], it is believed that there are also multiple indirect mechanisms through which alcohol is able to cause injury. Alcohol consumption has been demonstrated to disrupt the intestinal epithelial barrier and alter the gut flora, thus leading to increased gut permeability and microbial translocation, similar to the end effects of HIV on gut epithelia [47, 81, 82]. In addition, biomarkers of bacterial translocation such as lipopolysaccharide (LPS), sCD-14, and sCD-163 are similarly elevated in both HIV patients as well as patients with acute and chronic alcohol consumption.
These findings suggest that HIV infection is able to exert continued pressure on both the gut endothelium and the gut- liver axis, potentially in the face of adequate virologic control. It is possible that these mechanisms share a similar pathogenesis with respect to downstream effects of these biomarkers, specifically the activation of TLR-4 via LPS. This model of liver injury caused by alcoholic hepatitis, as described by Greuter et al. and Szabo et al., is characterized by neutrophilic infiltration and hepatic stellate cell (HSC) activation leading to in- creased liver injury and fibrosis [81–83]. Increased levels of LPS entering the liver have been shown to increase inflammation through three primary mechanisms. LPS induces recruitment and activation of inflammatory cells including Kupffer cells and HSCs. In addition, microbial products indirectly induce systemic immune responses and promote local hepatocyte cell death, resulting in a pro-fibrotic state [84, 85]. Finally, LPS and other down- stream pro-inflammatory cytokines induce production of multiple different acute phase reactants in hepatocytes including TGF-β1, IL-6, and IL-10 [81, 86].
HIV and Liver Macrophages
Liver macrophages play a key role in liver homeostasis and housekeeping. In the normal liver, Kupffer cells are the most prominent type of liver macrophage and are responsible for activation of the local inflammatory response and hepatocellular repair [87]. Hepatic stellate cells on the other hand are a different macrophage sub-population that are involved primarily in retinoid metabolism in addition to activating different aspects of the local inflammatory response [88, 89]. Activated hepatic stellate cells have been increasingly de- scribed as a central pathway to hepatic fibrogenesis [88]. Both of these liver-predominant macrophages are a target for direct HIV infection and activation via viral proteins.
In a macaque model, Ahsan et al. demonstrated significantly and dramatically increased levels of Kuppfer cells in both acutely SIV-infected macaques and macaques with AIDS [90]. They inferred that increased liver macrophage turnover played a role in SIV pathogenesis. In opposition to this finding, Balagopal et al. noted that in the liver tissue from HIV-HCV co-infected patients, there was a direct linear relationship be- tween CD4 lymphocyte count and the Kuppfer cell density. In addition, for those patients who were initiated on cART, Kuppfer cell density increased with virologic suppression [91]. In the aging liver, Kupffer cells appear to increase in number and overall activation [92, 93]. Although generally considered to play a strong role in the regenerative capacity of the liver, inappropriate and prolonged activation has been felt to drive fibrogenesis in multiple models of liver disease [81]. In the HIV-infected patient, it has been postulated that decreased levels of Kupffer cells as a result of direct infection by HIV may result in a decreased ability to clear the products of microbial translocation, leading to increased systemic levels of LPS, sCD-14, and sCD163 [94–96]. However, the effect of direct HIV infection on the Kupffer cell inflammatory cytokine secretion profile remains unknown.
With respect to hepatic stellate cells, the effects of HIV infection are well described. As a defining cell type in fibrogenesis of the liver, HSCs have received a significant amount of research attention. HSCs express the TLR-4 and CD-14 receptor complex and therefore can be activated by bacterial LPS. As noted above, HIV enteropathy results in increased microbial translocation, and subsequently, more bacterial cell wall products are present in the portal and systemic circulation [51, 52, 54]. In addition, Del Corno et al. recently described a mechanism of TLR-4-mediated HSC activation via HIV viral protein gp120 [97]. Although the exact cellular kinetics of HSC activation in the setting of acute and chronic HIV are not yet known, this research would imply that patients with HIV infection would have higher levels of HSC activation as compared to their seronegative counterparts. Normally, HSC activation is an important part of the local inflammatory response and plays an important role in liver homeostasis and maintenance [98, 99]. However, excessive activation of HSCs results in over-production of TGF-β, an inflammatory cytokine that activates multiple downstream pathways of fibrogenesis and further propagates the inflammatory response [98, 99].
With that said, HSC activation via LPS receptor complex activation alone does not necessarily produce a directly pro- fibrogenic HSC [88]. HSCs activated via TLR-4 certainly propagate an inflammatory response through secretion of IL- 6, TGF-β, and MCP-1, but are only considered to be indirectly fibrogenic because they do not demonstrate increased transcription of collagen I. HIV infection may result in an alteration in the behavior and gene expression of activated HSCs [100]. In vitro modeling of HSC and HIV infection revealed that HSCs can be directly infected by HIV via a CD4+ independent pathway. In addition, when analyzing changes in the inflammatory cytokine profile of HIV-infected HSCs, Tuyama et al. noted that activated HSCs infected by HIV had significant increases in both collagen I and MCP-1 expression [100]. Collagen I deposition is the central mechanism of liver fibrogenesis and MCP-1 is one of the primary chemoattractant proteins for monocyte-derived macrophages to migrate to the liver. When taken together, these findings would imply that in the setting of HIV infection, HSCs are more potent activators of the local immune response and more fibrogenic (Table 1).
Table 1.
Recent studies linking age and clinical outcome in NAFLD and HCV in addition to key articles discussing the cellular mechanisms of liver aging
| Topic | Authors | Year | Summary | Ref |
|---|---|---|---|---|
| NAFLD and NASH | Angulo et al. | 1999 | Older age is a risk factor for fibrosis in patients with NASH | [101] |
| Ratziu et al. | 2000 | Age >50 is a risk factor for NAFLD | [102] | |
| Hossain et al. | 2009 | Old age is a risk factor for fibrosis in NAFLD | [103] | |
| Fierbinteanu-Braticevici et al. | 2011 | Older age is a risk factor for NASH | [104] | |
| Stepanova et al. | 2013 | Older age is a risk factor for increased mortality in NAFLD | [105] | |
| Bhala et al. | 2013 | Older age is a risk factor for fibrosis in patients with NAFLD | [106] | |
| HCV | Pradat et al. | 2007 | Older age of HCV infection is associated with increased progression to fibrosis | [107] |
| Kirk et al. | 2013 | Prevalence of fibrosis in HIV and HCV co-infected patients | [108] | |
| Butt et al. | 2015 | Age is associated with increased risk of cirrhosis and hepatic decompensation in HCV infection | [109] | |
| Rueger | 2015 | Age of infection contributes to the rate of fibrosis progression in HCV | [110] | |
| Cellular mechanisms of liver aging | Videla et al. | 2001 | Mouse model of Kupffer cell aging | [111] |
| Friedman et al. | 2004 | A review of the mechanisms of hepatic fibrosis | [112] | |
| Hilmer et al. | 2007 | The effect of age on Kupffer cell activity | [93] | |
| Mahrouf-Yorgov et al. | 2010 | Increased susceptibility to liver fibrosis and inflammation | [70] | |
| Collins et al. | 2013 | Murine model describing liver injury, fibrosis, and the role of liver macrophages | [71] | |
| Hong et al. | 2014 | C/EBP Proteins in the context of liver aging and regeneration | [72] |
Conclusion
As the liver ages, it undergoes a decrease in size that is ac- companied by architectural and cellular changes. In addition, the remarkable regenerative capacity of the liver is reduced as a result of changes in gene expression and altered response to growth factors. Using the model of HIV/HCV co-infection, it is clear that HIV infection tips this scale away from regeneration and towards fibrogenesis in the setting of chronic liver damage. The exact mechanisms by which HIV is able to bring about the phenotype of accelerated fibrogenesis remain un- known. However, there are many promising directions of re- search, specifically at the intersection between inflammation, microbial translocation, and chronic immune activation.
The importance of understanding the interactions between chronic HIV infection and the aging liver cannot be overstated. As the epidemiology of liver disease in the setting of HIV infection shifts away from viral hepatitis towards NASH-related cirrhosis, there will be a greater need for more efficient diagnostic methods and effective therapies. Our success in the treatment of HIV and the revolutionary new therapies for HCV has presented exciting new challenges and new questions to answer.
References
- 1.Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, Sax PE, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194(1):11–9. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral ther- apy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet (London, England). 2014;384(9939):241–8. doi: 10.1016/s0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 4.HIV Surveillance Report 2014. In: HIV surveillance report. CDC division of HIV/AIDS Prevention. 2015. http://www.cdc.gov/hiv/library/reports/surveillance/. Accessed 7/15/2016 26.
- 5.May MT, Ingle SM, Costagliola D, Justice AC, de Wolf F, Cavassini M, et al. Cohort profile: Antiretroviral Therapy Cohort Collaboration (ART-CC). Int J Epidemiol. 2014;43(3): 691–702. doi: 10.1093/ije/dyt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, et al. Aging and infectious diseases: workshop on HIV infec- tion and aging: what is known and future research directions. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2008;47(4):542–53. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2011;53(11):1120–6. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 9.Smith C, Sabin CA, Lundgren JD, Thiebaut R, Weber R, Law M, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS (London, England). 2010;24(10):1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 10.Macias J, Berenguer J, Japon MA, Giron JA, Rivero A, Lopez- Cortes LF, et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology (Baltimore, Md). 2009;50(4): 1056–63. doi: 10.1002/hep.23136. [DOI] [PubMed] [Google Scholar]
- 11.Tovo CV, Becker SC, Almeida PR, Galperim B, Chaves S. Progression of liver fibrosis in monoinfected patients by hepatitis C virus and coinfected by HCV and human immunodeficiency virus. Arq Gastroenterol. 2013;50(1):19–22. [DOI] [PubMed] [Google Scholar]
- 12.Benhamou Y, Di Martino V, Bochet M, Colombet G, Thibault V, Liou A, et al. Factors affecting liver fibrosis in human immuno- deficiency virus-and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology (Baltimore, Md). 2001;34(2):283–7. doi: 10.1053/jhep.2001.26517. [DOI] [PubMed] [Google Scholar]
- 13.Kim N, editor. Poorly controlled HIV infection is a risk factor for liver fibrosis in CNICS Cohort. Congress on Retroviruses and Opportunistic Infection; 2016. 2/25/2016; Boston. [Google Scholar]
- 14.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120(4):437–47. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 15.Bhatia-Dey N, Kanherkar RR, Stair SE, Makarev EO, Csoka AB. Cellular senescence as the causal nexus of aging. Front Genet. 2016;7:13. doi: 10.3389/fgene.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, et al. Telomere dysfunction induces metabolic and mitochondrial com- promise. Nature. 2011;470(7334):359–65. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C, Lin JD. PGC-1 coactivators in the control of energy me- tabolism. Acta Biochim Biophys Sin. 2011;43(4):248–57. doi: 10.1093/abbs/gmr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–70. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senes- cence in aging and age-related disease: from mechanisms to ther- apy. Nat Med. 2015;21(12):1424–35. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. [DOI] [PubMed] [Google Scholar]
- 21.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pit- falls and uncertainties. Lessons for and from the crypt. Development (Cambridge, England). 1990;110(4):1001–20. [DOI] [PubMed] [Google Scholar]
- 22.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–46. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker DJ, Perez-Terzic C, Jin F, Pitel KS, Niederlander NJ, Jeganathan K, et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat Cell Biol. 2008;10(7):825–36. doi: 10.1038/ncb1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker DJ, Weaver RL, van Deursen JM. p21 both attenuates and drives senescence and aging in BubR1 progeroid mice. Cell Rep. 2013;3(4):1164–74. doi: 10.1016/j.celrep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15(7):397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 26.Green MR. Senescence: not just for tumor suppression. Cell. 2008;134(4):562–4. doi: 10.1016/j.cell.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS- ing cellular stress. Nat Rev Cancer. 2009;9(2):81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 28.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence- associated secretory phenotype: the dark side of tumor suppres- sion. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133(6):1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 30.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6): 1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 31.Maier JA, Voulalas P, Roeder D, Maciag T. Extension of the life- span of human endothelial cells by an interleukin-1 alpha anti- sense oligomer. Science (New York, NY). 1990;249(4976): 1570–4. [DOI] [PubMed] [Google Scholar]
- 32.Ipp H, Zemlin AE, Erasmus RT, Glashoff RH. Role of inflammation in HIV-1 disease progression and prognosis. Crit Rev Clin Lab S ci. 2014;5 1(2):98–1 1 1. doi: 10.3109/10408363.2013.865702. [DOI] [PubMed] [Google Scholar]
- 33.Aberg JA. Aging, inflammation, and HIV infection. Top Antiviral Med. 2012;20(3):101–5. [PMC free article] [PubMed] [Google Scholar]
- 34.Abraham AG, Althoff KN, Jing Y, Estrella MM, Kitahata MM, Wester CW, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2015;60(6):941–9. doi: 10.1093/cid/ciu919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS- defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2015;60(4):627–38. doi: 10.1093/cid/ciu869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vance DE, Mugavero M, Willig J, Raper JL, Saag MS. Aging with HIV: a cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care: JANAC. 2011; 22(1):17–25. doi: 10.1016/j.jana.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69 Suppl 1:S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 38.Varadhan R, Yao W, Matteini A, Beamer BA, Xue QL, Yang H, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci. 2014;69(2):165–73. doi: 10.1093/gerona/glt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ottinger ME, Monaghan SF, Gravenstein S, Cioffi WG, Ayala A, Heffernan DS. The geriatric cytokine response to trauma: time to consider a new threshold. Surg Infect. 2014;15(6):800–5. doi: 10.1089/sur.2013.235. [DOI] [PubMed] [Google Scholar]
- 40.Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep. 2010;7(1):4–10. doi: 10.1007/s11904-009-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506–16. doi: 10.1097/CCO.0b013e328355e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appay V, Fastenackels S, Katlama C, Ait-Mohand H, Schneider L, Guihot A, et al. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected pa- tients. AIDS (London, England). 2011;25(15):1813–22. doi: 10.1097/QAD.0b013e32834640e6. [DOI] [PubMed] [Google Scholar]
- 43.Assimakopoulos SF, Dimitropoulou D, Marangos M, Gogos CA. Intestinal barrier dysfunction in HIV infection: pathophysiology, clinical implications and potential therapies. Infection. 2014;42(6):951–9. doi: 10.1007/s15010-014-0666-5. [DOI] [PubMed] [Google Scholar]
- 44.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, et al. Primary HIV-1 infection is associated with prefer- ential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200(6):761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim CJ, McKinnon LR, Kovacs C, Kandel G, Huibner S, Chege D, et al. Mucosal Th17 cell function is altered during HIV infec- tion and is an independent predictor of systemic immune activa- tion. J Immunol (Baltimore, Md: 1950). 2013;191(5):2164–73. doi: 10.4049/jimmunol.1300829. [DOI] [PubMed] [Google Scholar]
- 46.Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, et al. A role for mucosal IL-22 production and Th22 cells in HIV- associated mucosal immunopathogenesis. Mucosal Immunol. 2012;5(6):670–80. doi: 10.1038/mi.2012.72. [DOI] [PubMed] [Google Scholar]
- 47.Dandekar S, George MD, Baumler AJ. Th17 cells, HIV and the gut mucosal barrier. Curr Opin HIV AIDS. 2010;5(2):173–8. doi: 10.1097/COH.0b013e328335eda3. [DOI] [PubMed] [Google Scholar]
- 48.Canani RB, Cirillo P, Mallardo G, Buccigrossi V, Secondo A, Annunziato L, et al. Effects of HIV-1 Tat protein on ion secretion and on cell proliferation in human intestinal epithelial cells. Gastroenterology. 2003;124(2):368–76. doi: 10.1053/gast.2003.50056. [DOI] [PubMed] [Google Scholar]
- 49.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6(4):e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clayton F, Kotler DP, Kuwada SK, Morgan T, Stepan C, Kuang J, et al. Gp120-induced Bob/GPR15 activation: a possible cause of human immunodeficiency virus enteropathy. Am J Pathol. 2001;159(5):1933–9. doi: 10.1016/s0002-9440(10)63040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hattab S, Guihot A, Guiguet M, Fourati S, Carcelain G, Caby F, et al. Comparative impact of antiretroviral drugs on markers of inflammation and immune activation during the first two years of effective therapy for HIV-1 infection: an observational study. BMC Infect Dis. 2014;14:122. doi: 10.1186/1471-2334-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppres- sion on circulating markers of inflammation and immune activa- tion. AIDS (London, England). 2015;29(4):463–71. doi: 10.1097/qad.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorenec L, Zidovec Lepej S, Grgic I, Planinic A, Iscic Bes J, Vince A, et al. The comparison of Th1, Th2, Th9, Th17 and Th22 cytokine profiles in acute and chronic HIV-1 infection. Microb Pathog. 2016;97:12 5–30. doi: 10.1016/j.micpath.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Hattab S, Guiguet M, Carcelain G, Fourati S, Guihot A, Autran B, et al. Soluble biomarkers of immune activation and inflammation in HIV infection: impact of 2 years of effective first-line combina- tion antiretroviral therapy. HIV Med. 2015;16(9):553–62. doi: 10.1111/hiv.12257. [DOI] [PubMed] [Google Scholar]
- 55.Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47(7–8):1650–60. doi: 10.1016/j.molimm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, et al. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113(4):887–92. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- 57.Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA, et al. Endotoxin induces rapid metalloproteinase- mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol. 2002;72(4):711–7. [PubMed] [Google Scholar]
- 58.Hunt PW. HIV and aging: emerging research issues. Curr Opin HIV A IDS. 2014;9(4):3 02–8. d o i: 10.1097/coh.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knudsen TB, Ertner G, Petersen J, Moller HJ, Moestrup SK, Eugen-Olsen J, et al. Plasma CD163 independently predicts all- cause mortality from HIV-1 infection. J Infect Dis. 2016. doi: 10.1093/infdis/jiw263. [DOI] [PubMed] [Google Scholar]
- 60.Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, James OF. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology (Baltimore, Md). 1989;9(2):297–301. [DOI] [PubMed] [Google Scholar]
- 61.Le Couteur DG, McLean AJ. The aging liver. Drug clearance and an oxygen diffusion barrier hypothesis. Clin Pharmacokinet. 1998;34(5):359–73. doi: 10.2165/00003088-199834050-00003. [DOI] [PubMed] [Google Scholar]
- 62.Iber FL, Murphy PA, Connor ES. Age-related changes in the gas- trointestinal system. Effects on drug therapy. Drugs Aging. 1994;5(1):34–48. [DOI] [PubMed] [Google Scholar]
- 63.Zeeh J, Platt D. The aging liver: structural and functional changes and their consequences for drug treatment in old age. Gerontology. 2002;48(3):121–7. [DOI] [PubMed] [Google Scholar]
- 64.Marchesini G, Bua V, Brunori A, Bianchi G, Pisi P, Fabbri A, et al. Galactose elimination capacity and liver volume in aging man. Hepatology (Baltimore, Md). 1988;8(5):1079–83. [DOI] [PubMed] [Google Scholar]
- 65.Wakabayashi H, Nishiyama Y, Ushiyama T, Maeba T, Maeta H. Evaluation of the effect of age on functioning hepatocyte mass and liver blood flow using liver scintigraphy in preoperative estima- tions for surgical patients: comparison with CT volumetry. J Surg Res. 2002;106(2):246–53. [DOI] [PubMed] [Google Scholar]
- 66.Zoli M, Magalotti D, Bianchi G, Gueli C, Orlandini C, Grimaldi M, et al. Total and functional hepatic blood flow decrease in par- allel with ageing. Age Ageing. 1999;28(1):29–33. [DOI] [PubMed] [Google Scholar]
- 67.Vollmar B, Pradarutti S, Richter S, Menger MD. In vivo quantifi- cation of ageing changes in the rat liver from early juvenile to senescent life. Liver. 2002;22(4):330–41. [DOI] [PubMed] [Google Scholar]
- 68.Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther. 1997;61(3):331–9. doi: 10.1016/s0009-9236(97)90166-1. [DOI] [PubMed] [Google Scholar]
- 69.Dong MH, Bettencourt R, Barrett-Connor E, Loomba R. Alanine aminotransferase decreases with age: the Rancho Bernardo Study. PLoS One. 2010;5(12):e14254. doi: 10.1371/journal.pone.0014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahrouf-Yorgov M, Collin de l’Hortet A, Cosson C, Slama A, Abdoun E, Guidotti JE, et al. Increased susceptibility to liver fi- brosis with age is correlated with an altered inflammatory response. Rejuvenation Res. 2011;14(4):353–63. doi: 10.1089/rej.2010.1146. [DOI] [PubMed] [Google Scholar]
- 71.Collins BH, Holzknecht ZE, Lynn KA, Sempowski GD, Smith CC, Liu S, et al. Association of age-dependent liver injury and fibrosis with immune cell populations. Liver Int: Off J Int Assoc Study Liver. 2013;33(8):1175–86. doi: 10.1111/liv.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong IH, Lewis K, Iakova P, Jin J, Sullivan E, Jawanmardi N, et al. Age-associated change of C/EBP family proteins causes severe liver injury and acceleration of liver proliferation after CCl4 treatments. J Biol Chem. 2014;289(2):1106–18. doi: 10.1074/jbc.M113.526780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol. 2001;34(5):730–9. [DOI] [PubMed] [Google Scholar]
- 74.Noureddin M, Yates KP, Vaughn IA, Neuschwander-Tetri BA, Sanyal AJ, McCullough A, et al. Clinical and histological deter- minants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology (Baltimore, Md). 2013;58(5):1644–54. doi: 10.1002/hep.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54(8):1174–9. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hernandez MD, Sherman KE. HIV/hepatitis C coinfection natural history and disease progression. Curr Opin HIVAIDS. 2011;6(6): 478–82. doi: 10.1097/COH.0b013e32834bd365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guaraldi G, Lonardo A, Ballestri S, Zona S, Stentarelli C, Orlando G, et al. Human immunodeficiency virus is the major determinant of steatosis and hepatitis C virus of insulin resistance in virus- associated fatty liver disease. Arch Med Res. 2011;42(8):690–7. doi: 10.1016/j.arcmed.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 78.Verna E, editor. NAFLD and NASH in HIV infection. Boston: CROI; 2016. 2/24/2016. [Google Scholar]
- 79.Beier JI, McClain CJ. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391(11):1249–64. doi: 10.1515/bc.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang K Molecular mechanisms of liver injury: apoptosis or ne- crosis. Exp Toxicol Pathol: Off J Ges Toxikol Pathol. 2014;66(8): 351–6. doi: 10.1016/j.etp.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Szabo G Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–6. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greuter T, Shah VH. Hepatic sinusoids in liver injury, inflamma- tion, and fibrosis: new pathophysiological insights. J Gastroenterol. 2016. doi: 10.1007/s00535-016-1190-4. [DOI] [PubMed] [Google Scholar]
- 83.Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, et al. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15(Suppl): D20–5. [DOI] [PubMed] [Google Scholar]
- 84.Sacchi P, Cima S, Corbella M, Comolli G, Chiesa A, Baldanti F, et al. Liver fibrosis, microbial translocation and immune activation markers in HIV and HCV infections and in HIV/HCV co-infec- tion. Dig Liver Dis: Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2015;47(3):218–25. doi: 10.1016/j.dld.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 85.Page EE, Nelson M, Kelleher P. HIV and hepatitis C coinfection: pathogenesis and microbial translocation. Curr Opin HIV AIDS. 2011;6(6):472–7. doi: 10.1097/COH.0b013e32834bbc71. [DOI] [PubMed] [Google Scholar]
- 86.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science (New York, NY). 1990;249(4975): 1431–3. [DOI] [PubMed] [Google Scholar]
- 87.Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3(2):785–97. doi: 10.1002/cphy.c120026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair. 2010;3:21. doi: 10.1186/1755-1536-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahsan MH, Gill AF, Alvarez X, Lackner AA, Veazey RS. Kinetics of liver macrophages (Kupffer cells) in SIV-infected macaques. Virology. 2013;446(1–2):77–85. doi: 10.1016/j.virol.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balagopal A, Ray SC, De Oca RM, Sutcliffe CG, Vivekanandan P, Higgins Y, et al. Kupffer cells are depleted with HIV immunode- ficiency and partially recovered with antiretroviral immune recon- stitution. AIDS (London, England). 2009;23(18):2397–404. doi: 10.1097/QAD.0b013e3283324344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le Couteur DG, Warren A, Cogger VC, Smedsrod B, Sorensen KK, De Cabo R, et al. Old age and the hepatic sinusoid. Anat Rec (Hoboken, NJ: 2007). 2008;291(6):672–83. doi: 10.1002/ar.20661. [DOI] [PubMed] [Google Scholar]
- 93.Hilmer SN, Cogger VC, Le Couteur DG. Basal activity of Kupffer cells increases with old age. J Gerontol A Biol Sci Med Sci. 2007;62(9):973–8. [DOI] [PubMed] [Google Scholar]
- 94.Housset C, Boucher O, Girard PM, Leibowitch J, Saimot AG, Brechot C, et al. Immunohistochemical evidence for human im- munodeficiency virus-1 infection of liver Kupffer cells. Hum Pathol. 1990;21(4):404–8. [DOI] [PubMed] [Google Scholar]
- 95.Hufert FT, Schmitz J, Schreiber M, Schmitz H, Racz P, von Laer DD. Human Kupffer cells infected with HIV-1 in vivo. J Acquir Immune Defic Syndr. 1993;6(7):772–7. [PubMed] [Google Scholar]
- 96.Schmitt MP, Steffan AM, Gendrault JL, Jaeck D, Royer C, Schweitzer C, et al. Multiplication of human immunodeficiency virus in primary cultures of human Kupffer cells—possible role of liver macrophage infection in the physiopathology of AIDS. Res Virol. 1990;141(2):143–52. [DOI] [PubMed] [Google Scholar]
- 97.Del Corno M, Cappon A, Donninelli G, Varano B, Marra F, Gessani S. HIV-1 gp120 signaling through TLR4 modulates innate immune activation in human macrophages and the biology of hepatic stellate cells. J Leukoc Biol. 2016. doi: 10.1189/jlb.4A1215-534R. [DOI] [PubMed] [Google Scholar]
- 98.Fabregat I, Moreno-Caceres J, Sanchez A, Dooley S, Dewidar B, Giannelli G, et al. TGF-beta signalling and liver disease. FEBS J. 2016;283(12):2219–32. doi: 10.1111/febs.13665. [DOI] [PubMed] [Google Scholar]
- 99.Patel P, Khan N, Rani M, Gupta D, Jameel S. The expression of HIV-1 Vpu in monocytes causes increased secretion of TGF-beta that activates profibrogenic genes in hepatic stellate cells. PLoS One. 2014;9(2):e88934. doi: 10.1371/journal.pone.0088934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tuyama AC, Hong F, Saiman Y, Wang C, Ozkok D, Mosoian A, et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stel- late cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/ hepatitis C virus-induced liver fibrosis. Hepatology (Baltimore, Md). 2010;52(2):612–22. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md). 1999;30(6):1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 102.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118(6):1117–23. [DOI] [PubMed] [Google Scholar]
- 103.Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2009;7(11):1224–9. doi: 10.1016/j.cgh.2009.06.007.9.e1-2. [DOI] [PubMed] [Google Scholar]
- 104.Fierbinteanu-Braticevici C, Baicus C, Tribus L, Papacocea R. Predictive factors for nonalcoholic steatohepatitis (NASH) in pa- tients with nonalcoholic fatty liver disease (NAFLD). J Gastrointest Liver Dis: JGLD. 2011;20(2):153–9. [PubMed] [Google Scholar]
- 105.Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, et al. Predictors of all-cause mortality and liver- related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci. 2013;58(10):3017–23. doi: 10.1007/s10620-013-2743-5. [DOI] [PubMed] [Google Scholar]
- 106.Bhala N, Jouness RI, Bugianesi E. Epidemiology and natural his- tory of patients with NAFLD. Curr Pharm Des. 2013;19(29): 5169–76. [DOI] [PubMed] [Google Scholar]
- 107.Pradat P, Voirin N, Tillmann HL, Chevallier M, Trepo C. Progression to cirrhosis in hepatitis C patients: an age-dependent process. Liver Int: Off J Int Assoc Study Liver. 2007;27(3):335–9. doi: 10.1111/j.1478-3231.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 108.Kirk GD, Mehta SH, Astemborski J, Galai N, Washington J, Higgins Y, et al. HIV, age, and the severity of hepatitis C virus- related liver disease: a cohort study. Ann Intern Med. 2013;158(9): 658–66. doi: 10.7326/0003-4819-158-9-201305070-00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Butt AA, Yan P, Lo Re V 3rd, Rimland D, Goetz MB, Leaf D, et al. Liver fibrosis progression in hepatitis C virus infection after serocon- version. JAMA Intern Med. 2015;175(2):178–85. doi: 10.1001/jamainternmed.2014.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rueger S, Bochud PY, Dufour JF, Mullhaupt B, Semela D, Heim MH, et al. Impact of common risk factors of fibrosis progression in chronic hepatitis C. Gut. 2015;64(10):1605–15. doi: 10.1136/gutjnl-2014-306997. [DOI] [PubMed] [Google Scholar]
- 111.Videla LA, Tapia G, Fernandez V. Influence of aging on Kupffer cell respiratory activity in relation to particle phagocytosis and oxidative stress parameters in mouse liver. Redox Rep: Commun Free Radic Res. 2001;6(3):155–9. doi: 10.1179/135100001101536265. [DOI] [PubMed] [Google Scholar]
- 112.Friedman SL. Mechanisms of disease: mechanisms of hepatic fi- brosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1(2):98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]