Abstract
Supported by GAVI Alliance, measles-rubella vaccination was introduced in Vietnam in 2014, involving a mass campaign among 1–14 year olds and routine immunization of children aged 9 months. We explore the impact on the incidence of Congenital Rubella Syndrome (CRS) during 2013–2050 of this strategy and variants involving women aged 15–35 years. We use an age and sex-structured dynamic transmission model, set up using recently-collected seroprevalence data from Central Vietnam, and also consider different levels of transmission and contact patterns. If the serological profile resembles that in Central Vietnam, the planned vaccination strategy could potentially prevent 125,000 CRS cases by 2050 in Vietnam, despite outbreaks predicted in the meantime. Targeting the initial campaign at 15–35 year old women with or without children aged 9 months–14 years led to sustained reductions in incidence, unless levels of ongoing transmission were medium-high before vaccination started. Assumptions about contact greatly influenced predictions if the initial campaign just targeted 15–35 year old women and/or levels of ongoing transmission were medium-high. Given increased interest in rubella vaccination, resulting from GAVI Alliance funding, the findings are relevant for many countries.
Keywords: rubella, Congenital Rubella Syndrome, measles-rubella vaccination, mathematical modelling, Vietnam
Introduction
In Vietnam, rubella outbreaks continue to occur regularly, with over 60% of rubella cases in 2009 being of child-bearing age.1 Rubella is a mild illness involving fever and a rash, but if infection occurs during pregnancy, the child may be born with Congenital Rubella Syndrome (CRS), which is associated with mortality and lifelong disability. Since 2013, GAVI Alliance has supported the introduction of measles-rubella (MR) vaccination in eligible countries, allocating funding for an initial mass-vaccination campaign, with the cost of routine vaccination being covered locally. Campaigns are important for preventing outbreaks among women of child-bearing age in the long-term. However, the optimal age groups for targeting vaccination are unclear and need to be elucidated, as many countries are expected to introduce vaccination with GAVI Alliance support (49 by 2020).2
In 2014, MR vaccination, supported by GAVI Alliance, was introduced in Vietnam, with the initial mass campaign targeting children aged 1–14 years.3 Recently, a relatively high proportion (30%) of women of child-bearing age were found to be seronegative for rubella antibodies, and therefore at risk of infection, in Central Vietnam.4 Given the high risk of a child being born with CRS if the mother is infected while pregnant, the initial campaign could potentially prevent an increased number of cases of CRS if it also included adult women. The impact of vaccination and the number of CRS cases that it prevents is complicated by other factors including the vaccination coverage, extent of ongoing transmission and contact patterns between adults and children. Since the birth rate also influences the impact of vaccination,5 predictions from one setting are not necessarily generalisable to other settings. These effects are difficult to study using epidemiological studies, but can be studied using modeling.
We here use a mathematical model developed to describe the transmission dynamics of rubella in Vietnam, using serological and demographic data from Vietnam, to explore how different vaccination strategies affect the CRS incidence and number of cases prevented until 2050. We also explore the sensitivity of model predictions to assumptions about the amount of ongoing transmission and contact between people, as these 2 factors may differ regionally in Vietnam.
Materials and Methods
Data sources
Demography
Both the female population size for Vietnam in 5-year age groups for each year for the period 2000–2050 and fertility rates in 5-year age groups for the period 2000–2010, were compiled from Vietnamese census data and projections.6 The fertility rate after 2010 was fixed at the level estimated for 2010. The total number of live births in Vietnam by year and age group of the mother for the period 2000–2050 was calculated by multiplying the age-specific fertility rate by the corresponding female population size. Per capita birth rates and age and sex-specific mortality rates were extracted from UN population databases.7
Numbers of rubella and CRS cases
Age-specific estimates of the number of rubella cases were compiled from nationwide case-based surveillance, which is integrated into measles surveillance. A suspected case of measles/rubella case is defined as any patient who is suspected to have measles/rubella by a health worker or a patient with fever, maculopapular rash and one of 5 sign/symptoms (cough, coryza, conjunctivitis, adenopathy (cervical, sub-occipital, post-auricular) or arthralgia/arthritis). Suspected cases are investigated, with blood samples taken and tested for measles and rubella IgM in the 2 WHO-accredited national laboratories in Vietnam.
The number of CRS cases born in Vietnam in 2011 were compiled from hospital-based sentinel sites, involving 3 central hospitals, located in Hanoi and Ho Chi Minh City. Enrolled cases had to meet the WHO's case definition.8 Suspected cases were investigated, with blood samples tested for rubella IgM antibody using ELISA. Confirmed cases included clinically-confirmed cases (defined as cases who had either 2 of the complications in group A or one complication from each of groups A and B in the WHO guidelines). Laboratory-confirmed cases were defined as clinically-confirmed cases who were positive for rubella-specific IgM.
Description of the Model
Overview
We estimated the impact of different vaccination strategies on the CRS incidence per 100,000 live births and the numbers of CRS cases averted using a dynamic, age and sex-structured compartmental model, adapting previous models,9,10 including one used for GAVI Alliance projections of the impact of funding MR campaigns.11 The population is stratified into those with maternal immunity, pre-infectious (infected but not yet infectious), infectious and immune, with newborns having maternal immunity lasting 6 months from birth. The Supplemental File provides further details, including the model equations.
Demographic assumptions
The model population, stratified by sex into single year age strata in the range 0–74 years, was described using a realistic age structure (RAS).12 Newborns enter the population on the 31st August each year, facilitating tracking the exact time when they are aged 6 months, when they lose maternal immunity. Following standard approaches,12 each age stratum moves to the subsequent age stratum on the 31st August each year and leaves the model once reaching age 75 years.
For simplicity, the age distribution was fixed over time by holding the birth and mortality rates in the model constant. The birth rate equalled the value estimated for Vietnam for 2010;7 age- and sex-specific mortality rates were based on lifetables for the period 2005–2010 from UN population databases.7 To adjust for differences between the modeled and observed number of births, census-based projections of the actual number of livebirths were used when calculating the numbers of CRS cases (see below).
Scenarios for the pre-vaccination epidemiology of rubella
In the base-case model, the prevaccination epidemiology of rubella was assumed to be similar to that implied by the only seroprevalence survey in Vietnam to date, which came from Khanh Hoa province, Central Vietnam.4 In this study, approximately 50% of women were still susceptible to rubella by age 18 years (Fig. 1) and the force of infection (rate at which susceptibles are infected) was 5%/year (95% CI: 4.7–5.8%) and 4%/year (95% CI: 3.5–4.4%/year),4 for those aged <13 and ≥13 years respectively. Khanh Hoa province comprises a population of 1.2 million, making up 1.3% of the entire population in Vietnam. Its population density was 229 people/km2, as compared with 271 people/km2 for the whole of Vietnam in 2013).13 The birth and mortality rates in Khanh Hoa province are comparable to those overall in Vietnam (mortality rates: 6.6 vs 7.0 per 1000 respectively, birth rates: 16.4 vs 16.9 per 1000 respectively in 2012).13
Figure 1.
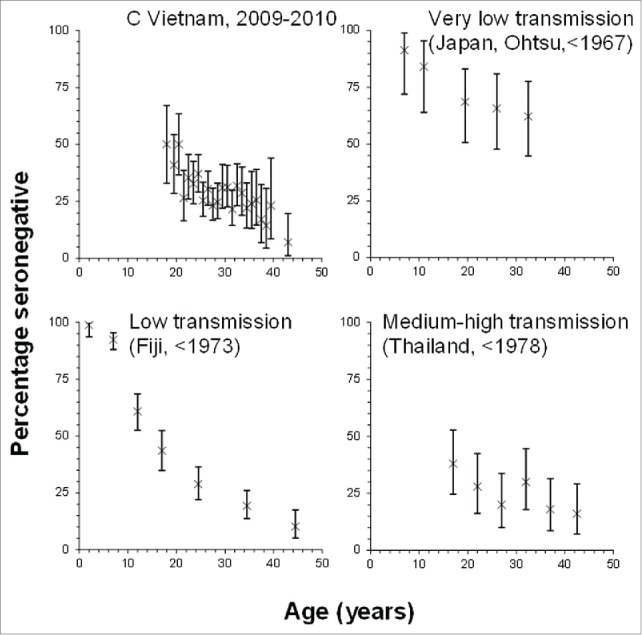
Summary of the age-specific proportions of individuals who were found to be seronegative in the serological data that were used to characterize the epidemiology of rubella in Vietnam[4,14,15,16] prior to the introduction of vaccination.
Since the population density in Central Vietnam is slightly lower than that overall in Vietnam, it is plausible that there is less ongoing transmission of rubella in Central Vietnam than elsewhere in Vietnam. Similarly, the population density, and, potentially, the amount of ongoing transmission, in some parts of Vietnam is less than that in Central Vietnam (<100 per km2 in some areas of the northern midlands, vs 229/km2 in Khanh Hoa province in 2013).13
In sensitivity analyses, we therefore explored the effect of different assumptions about the amount of ongoing-transmission, namely that this was very low, low and medium-high. To characterize the epidemiology of very low, low and medium transmission settings, we used data from settings in which the percentage of women that were still susceptible by the time they reached child-bearing age was high (>50%), low (40–50%) and very low (<40%) respectively. These data are shown in Figure 1 and come from seroprevalence studies from Japan,14 Fiji,15 and Thailand,16 (Fig. 1).
The force of infection based on these data (see Supplemental File), as estimated elsewhere using catalytic models,10 were used to calculate the contact parameters in the model (see below).
Contact patterns
Contact between individuals was assumed to differ between the ages <13 and ≥13 years (“younger” and “older” individuals respectively) according to the following matrix of Who Acquires Infection From Whom:
Here, the rate at which those aged ≤13 years effectively contact (a contact sufficient to lead to transmission if it occurs between a susceptible and infectious person),17 each other (β1) differs from the rate at which older individuals effectively contact each other (β2). The rate at which younger and older individuals come into effective contact is assumed to be 70% of the rate at which older individuals effectively contact each other, consistent with empirical data from middle-income settings[18] and overall in Vietnam[19] (Supplemental File). This age-stratification is consistent with analyses elsewhere and changes in school attendance [10,20] and therefore exposure to rubella infection. Given the similar force of infection among those aged <13 and ≥13 years (Table S3), an alternative age stratification will give similar results.
In sensitivity analyses, we explored the effect of pessimistic assumptions about contact between younger and older individuals, whereby the effective contact rate between younger and older individuals, equals 30% of that at which older individuals effectively contact each other. Such contact patterns are probably atypical for Vietnam, where the age-dependency in contact appears to be similar to that in Europe.19 However, they might occur in areas where parents spend a reduced amount of time with their children and extended family, if, for example, they travel far to work.
For each assumption about contact, one hundred sets of plausible values for the contact parameters were generated using rejection sampling from the force of infection estimated for each dataset. The model was run with each of the 100 sets of plausible parameter values to generate the 95% range in the CRS incidence per 100,000 live births and the numbers of CRS cases prevented.
Calculating the CRS Incidence
Following previous work,20 the CRS incidence per 100,000 live births was calculated for 5-year age groups (15–19, 20–24, 25–29, 30–34, 35–39 and 40–44 years) for each year during 2000–2050, assuming a 65% risk of a child being born with CRS if infection occurs during the first 16 weeks of pregnancy. The CRS incidence per live birth for each year in the model for mothers in each 5 year age group was then multiplied by the estimated number of live births to calculate the number of CRS cases born each year. The number of CRS cases averted by each vaccination strategy by a given year was then calculated as the difference between the cumulative number of cases predicted without vaccination and that with the given vaccination scenario. These calculations were repeated for each assumed value for the contact parameter, generated through rejection sampling and the average and 95% range of the values from all 100 rejection samples was calculated.
The average CRS cumulative incidence ratio was calculated as the ratio between the number of cases predicted by a given time with vaccination and that without vaccination, calculated for each rejection sample and averaged over the values obtained for all rejection samples.
Comparisons Between Model Predictions and the Observed Pre-Vaccination Data
Before exploring the impact of the different vaccination scenarios, model predictions of the age distribution of rubella cases and the CRS incidence were compared against available data.
Vaccination
We explored the impact of 4 vaccination scenarios on the CRS incidence per 100,000 live births, the number of CRS cases and the number of CRS cases prevented during 2000–2050 in Vietnam.
-
1.
One dose routine immunisation for children aged 9 months
-
2.
Catch-up campaign for children aged 9 months–14years, followed by one dose routine immunisation for children aged 9 months, identical to the planned strategy for 2014, with GAVI Alliance support.
-
3.
Catch-up campaign for women of child-bearing age (15–35 years), followed by one dose of MR vaccine for children aged 9 months in the routine schedule.
-
4.
Catch-up campaign for children aged 9 months–14 years and women (aged 15–35 years), followed by one dose routine immunisation for children aged 9 months.
The vaccine efficacy and coverage are assumed to be 95% and 90% respectively. For each scenario, vaccination occurs on the same day each year (31st August) from 2013.
Results
Comparison between model predictions and the observed prevaccination data
Table 1 compares model predictions of the CRS incidence and the percentage of rubella cases who were aged <15 years before vaccination was introduced against the observed data. The CRS incidence predicted using the seroprofile for Central Vietnam was 208 per 100,000 live births (95% range: 154–248), ranging between 101 (95% range: 4–204) and 256 (95% range: 242–271) per 100,000 live births assuming the medium-high (Thailand) and low (Fiji) transmission seroprofiles respectively. The number of CRS cases predicted using each of the seroprofiles was 5–20-fold greater than that observed (207 in 2011), for example, 3836 (95% range: 2827–4566) and 1861 (95% range: 72–3761) for the Central Vietnam and medium-high (Thailand) transmission seroprofiles respectively. Similarly, the number of rubella cases predicted during the period 2005–2011 for each assumed seroprofile and that reported differed greatly (1.1–1.4 million/year vs 15,579 and 6,927 reported during the period 2005–7 and in 2011 respectively). The predicted and observed percentage of rubella cases who were aged <15 years were similar (51–62% vs 55% respectively) for each seroprofile, excepting that for the medium-high (Thailand) transmission setting, for which it was >80% .
Table 1.
Comparison between the observed data and the predicted percentage of rubella cases that were aged <15 years, and the CRS incidence, obtained for different assumptions about the susceptibility profile. Model predictions reflect the average value and, where calculated, the 95% range in parentheses
| Average CRS incidence per 100,000 live births (95% range) | Number of CRS cases in 2011 (95% range) | Average % of rubella cases aged <15 years during 2005–2011 | |
|---|---|---|---|
| Observed | — | 232 | 47 |
| Central Vietnam | 208 (154–248) | 3836 (2827–4566) | 62 |
| Very low transmission (Japan seroprofile) | 123 (14–219) | 2269 (267–4027) | 51 |
| Low transmission (Fiji seroprofile) | 256 (242–271) | 4714 (4452–4992) | 53 |
| Medium-high transmission (Thailand seroprofile) | 101 (4–204) | 1861 (72–3761) | 82 |
The impact of vaccination on the CRS incidence – base-case model
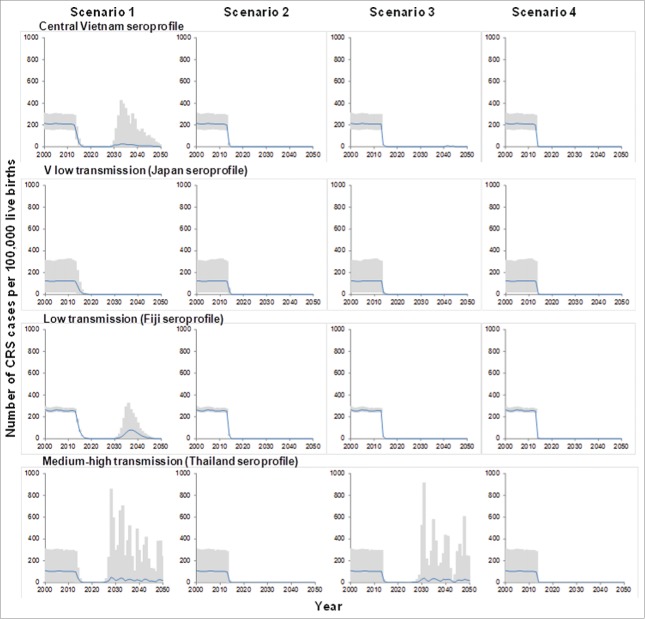
Figure 2 summarizes predictions of the impact of each vaccination strategy on the CRS incidence for the base-case model. For each assumed seroprofile, excepting that from a very low transmission setting (Japan), introducing a single dose of vaccine for children aged 9 months in 2013 (scenario 1) did not lead to sustained reductions in the CRS incidence, with outbreaks predicted by 2028 .
Figure 2.
Predictions of the number of CRS cases per 100,000 live births in Vietnam until the year 2050, for the 4 vaccination scenarios, introduced in 2013, obtained for the base-case assumptions about contact between individuals. The black line shows the average predictions, based on 100 rejection samples; the gray areas show the 95% range of the predictions.
However, if an initial mass campaign occurred either among children aged 9 months–14 years (scenario 2, equivalent to that planned for 2014), women aged 15–35 years (scenario 3) or both children aged 9 months-15 years and women aged 15–35 years (scenario 4) sustained reductions in the CRS incidence were predicted for each assumed seroprofile, excepting that from the medium-high transmission setting (Thailand). For a given assumed seroprofile, each vaccination strategy led to similar numbers of CRS cases prevented by 2050, for example, 125,000 cases (95% range: 94,000–146,000) using the serological profile from Central Vietnam (Table 2)
Table 2.
Summary of the numbers of CRS cases prevented during the period 2013–2050 by the introduction of MR vaccination in 2013 through 4 vaccination scenarios, assuming that the age-specific proportion susceptible follows the pattern seen in serological data from Central Vietnam, Japan, Fiji and Thailand (see Fig. 1) and using the base-case assumptions about contact between individuals. The numbers in parentheses reflect the 95% range of model estimates, obtained through rejection sampling
| Serological profile |
||||
|---|---|---|---|---|
| Scenario | Central Vietnam | V low transmission (Japan) | Low transmission (Fiji) | Medium-high transmission (Thailand) |
| 1. One dose routine immunisation for children aged 9 months | 117862 (88937, 139180) | 71566 (8714, 115909) | 139323 (128520, 148684) | 50241 (−15988, 105867) |
| 2. Catch-up campaign for children aged 9mths–14years, followed by one dose routine immunisation for children aged 9 months | 125105 (93837, 146215) | 73612 (8830, 120255) | 153284 (151589, 154208) | 60391 (2307, 121906) |
| 3. Catch-up campaign for women of child-bearing age (15–35 years), followed by one dose routine immunisation for children aged 9 months | 124323 (93526, 146222) | 73695 (8764, 120676) | 153427 (151681, 154392) | 51990 (−16051, 118611) |
| 4. Catch-up campaign for children aged 9 months–14 years and women (aged 15–35 years), followed by one dose routine immunisation for children aged 9 months | 125455 (94055, 146661) | 73951 (8845, 120920) | 153834 (152109, 154774) | 60504 (2309, 122204) |
.
Model predictions for the medium-high transmission seroprofile (Thailand) suggested that outbreaks could occur about 15 years after introducing vaccination scenario 3 (initial campaign targeting only women aged 15–35 years, with children aged 9 months subsequently vaccinated routinely). However, for the same assumed seroprofile, vaccination scenarios 2 and 4 (initial campaign for all children, with or without women aged 15–35 years, with children aged 9 months subsequently vaccinated routinely), were predicted to lead to sustained reductions in the CRS incidence until 2050.
Sensitivity analyses
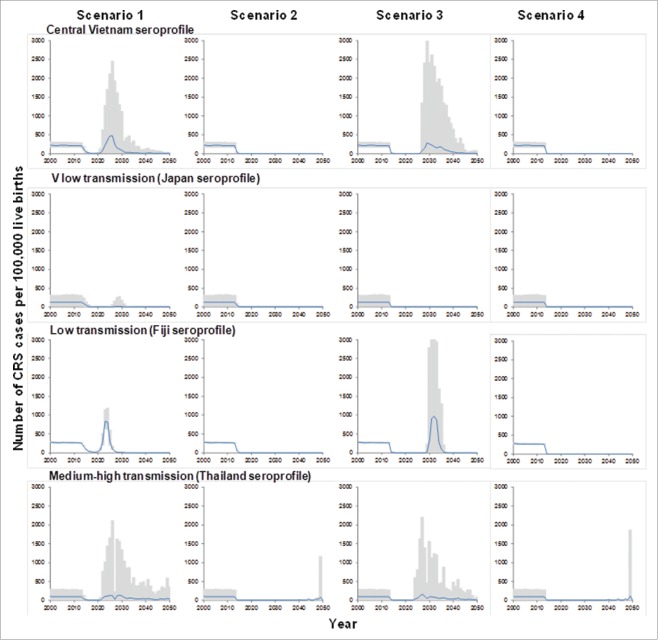
When assuming that contact between older and younger individuals was reduced (30% of that between older individuals), then as for the base-case model, for each assumed susceptibility profile, introducing vaccination just for children aged 9 months into the routine schedule did not lead to sustained reductions in incidence, with outbreaks predicted to occur 10 or more years thereafter (Fig. 4) .
Figure 4.
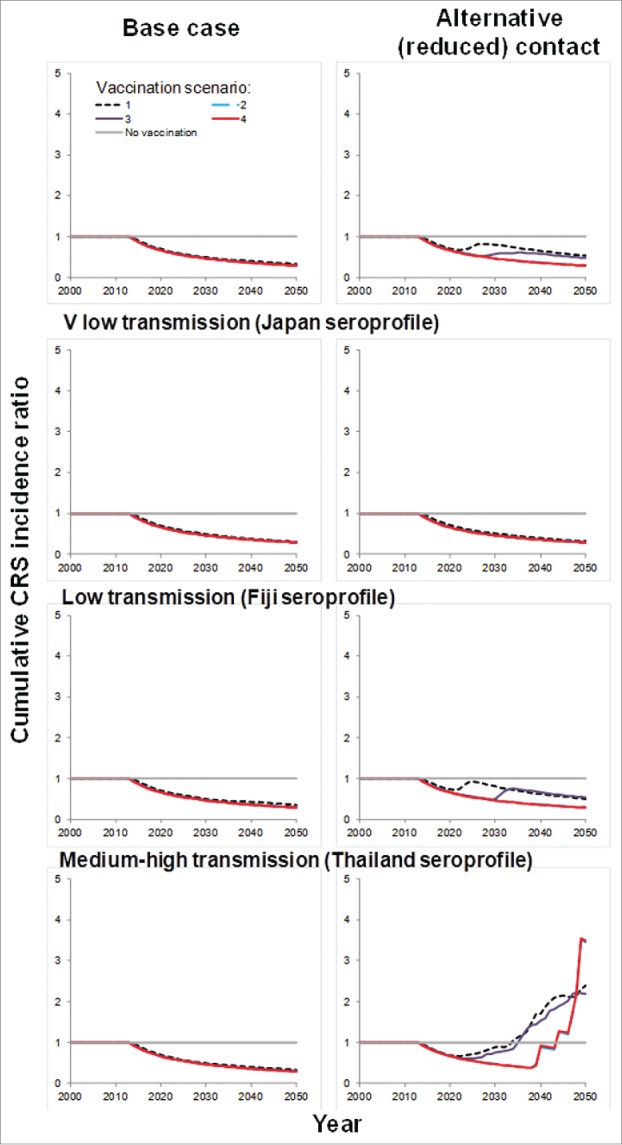
Summary of predictions of the average cumulative CRS incidence ratio for each assumed serological profile and assumptions about contact between individuals.
Vaccination scenarios 2 and 4 (both including an initial campaign targeting those aged 9 months–14 years), were predicted to lead to sustained reductions in CRS incidence for all assumed seroprofiles, excepting that for a medium-high transmission setting (Thailand). However, if the initial campaign just targeted women aged 15–35 years, predictions obtained using all the assumed seroprofiles, excepting that from the very low transmission setting (Japan) suggested that outbreaks could occur 15–20 years after introducing vaccination.
For both assumptions about contact between individuals, fewer cases were predicted by a given year after introducing vaccination than if no vaccination occurred (Fig. 4), for all assumed serological profiles, excepting that from a medium-high transmission setting. For this assumption, more cases were predicted by 2050 after introducing vaccination if it was assumed that contact between younger and older individuals was reduced than without vaccination.
Discussion
Our analyses suggest that introducing rubella vaccination in Vietnam needs to be accompanied by an extensive catch-up campaign, such as the one that is currently being implemented, in order to reduce rubella transmission and the CRS burden. For the base-case model, with an initial campaign either among children aged 9 months–14 years and/or women aged 15–35 years, sustained reductions in the CRS incidence were predicted, except with medium-high levels of transmission. In this case, an initial vaccination campaign for children aged 9 months–14 years and women aged 15–35 years (scenario 4) was required to maintain a low CRS incidence, and, if contact between younger and older individuals was assumed to be reduced, the predicted number of CRS cases by 2050 with each vaccination strategy exceeded that without vaccination.
Our model includes several simplifications.
First, the epidemiology of rubella in the absence of vaccination is based on data from a recent seroprevalence survey in Central Vietnam, and it is unclear if it is representative of the rest of Vietnam. We accounted for this uncertainty by running the model using a wide range of contact parameters which were consistent with these data, based on rejection sampling. Also, we explored the effect of assuming alternative age-specific serological profiles, reflecting very low, low and medium-high transmission settings, and we used 2 different assumptions about contact. Using data from other settings for which the age-specific proportion of women of child-bearing age who were susceptible matched our criteria would not have affected conclusions.
Second, we assumed that the age-specific fertility rates after 2010 remained unchanged. If their decline continues in the way seen in recent decades in many countries, including Vietnam, we may have overestimated the numbers of CRS cases averted by 2050.
Third, the age distribution is assumed to remain unchanged over time. We have minimised the effect of demographic changes by calculating the numbers of CRS cases each year using the actual projected numbers of livebirths until 2050.
Fourth, for each scenario, vaccination occurs on the same day each year. This is unrealistic and may result in optimistic predictions of the speed at which the incidence decreases after introducing vaccination. However, it probably did not substantially affect predictions of the long-term impact.
We also note that the model does not include any effects of seasonality or metapopulation dynamics, such as those discussed in other recent models.5 Data on these effects are limited for Vietnam. Studies using TSIR (Time-series Susceptible-Infectious-Recovered) models applied to district-level rubella data from Mexico,21 found regional variation in the amount of transmission and the extent to which transmission varied seasonally. As shown in Figures 2–3, the amount of transmission influences both the impact of vaccination and the CRS incidence. Therefore, incorporating regional variation in the amount of transmission would have led to regional variation in the impact of vaccination in the CRS incidence. On the other hand, incorporating seasonal variation in transmission does not appear to affect the minimum level of vaccination coverage required to prevent increases in the burden of CRS,5 suggesting that incorporating its effect in our analyses would not have affected our conclusions.
Figure 3.
Predictions of the number of CRS cases per 100,000 live births in Vietnam until the year 2050, for the 4 vaccination scenarios, introduced in 2013, obtained for the alternative assumption about contact (the rate at which older individuals come into effective contact with younger individuals is 30% of the rate at which older individuals come into effective contact with other older individuals). The black line shows the average predictions, based on 100 rejection samples; the gray areas show the 95% range of the predictions.
According to routine surveillance of CRS in Vietnam, established in 2011,22 232 babies were born with CRS. Model predictions of the numbers of CRS cases for each seroprofile greatly exceeded those observed, although these were within the 95% range of predictions from the medium-high transmission profile. The discrepancy between the observed and estimated number of cases reflects difficulties in reporting and detecting CRS cases. For example, the reported number of cases included data from hospital-based sentinel sites, which could have missed some cases whose mothers did not access the health services and were born at home. Cases which were aborted spontaneously or with medical assistance would have also been missed in routine surveillance. Also, deafness, a common manifestation of CRS, is difficult to detect in young children, and will therefore usually be identified several years after infection.23 Thus, cases for whom deafness is the sole manifestation of CRS are unlikely to be included in surveillance statistics. Finally, rubella-specific IgM, which is used for laboratory confirmation, is thought to be detectable in about 60% of those aged 6–12 months.24
We have assumed that the amount of contact between younger and older individuals was 70% of that between older individuals, based on data from European contact surveys.18 Recent data,19 suggest that while the Vietnamese reported fewer daily numbers of contacts than their European counterparts, the age-dependency in contact was similar for both locations, suggesting that our assumption about reduced contact between children and adults may be pessimistic.
For each assumed seroprofile, if the initial mass campaign already includes children aged 9 months–14 years, including women aged 15–35 years did not increase predictions of the numbers of CRS cases averted. This follows from the relatively low force of infection assumed before vaccination is introduced for Vietnam, so that an initial campaign among children aged 9 months–14 years is predicted to reduce transmission to low levels in the population. Subsequently introducing routine vaccination for children aged 9 months is predicted to sustain the reduced levels of transmission and CRS incidence. However, in settings in which there is more ongoing transmission than we have assumed in our analyses, routine vaccination of children aged 9 months alone is unlikely to lead to sustained reductions in the CRS incidence. Therefore, it would be important to introduce vaccination of adolescent girls at the same time as vaccination of children aged 9 months, in order to prevent increases in the CRS incidence.
Our analyses support the vaccination strategy that is planned from 2014 in Vietnam, showing that introducing routine vaccination of children aged 9 months, with an initial campaign targeting children aged 9 months–14 years could result in sustained reductions in the CRS incidence, if the age-specific proportion of individuals that are susceptible to rubella matches that in Central Vietnam. Our findings are likely to be relevant for other countries, who, with GAVI Alliance support, are considering introducing rubella vaccination. The reduction in the force of infection following the introduction of vaccination leads to a reduced opportunity for exposure to infection. As a result, an increased proportion of women can reach child-bearing age still susceptible to infection, unless they have been vaccinated. If the vaccination coverage is sub-optimal, an increase in the proportion of women reaching child-bearing age still susceptible can be accompanied by an increase in the number of new infections among women of child-bearing age, and therefore an increase in the CRS incidence. If this occurs, introducing routine vaccination of children aged 9 months, with an initial campaign targeting children aged 9 months–14 years may lead to more CRS cases occurring by 2050 than that without vaccination. It is therefore important for countries that are considering introducing rubella vaccination to collect seroprevalence data before introducing rubella vaccination, to understand the existing epidemiology of rubella and the potential impact of vaccination.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was conducted with the support of Vietnam's National Institute of Hygiene and Epidemiology. We thank Dr. Susan Reef for helpful discussions about the results.
Disclaimer
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the institutions with which the authors are affiliated.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
This study was funded by the World Health Organization.
References
- 1.(2009) National EPI Review Report. 30 March to 10 April 2009 Review of Expanded Program of Immunization Vietnam 2009.
- 2.GAVI Alliance Measles and rubella. www.gavialliance.org/library/publications/gavi-fact-sheets/factsheet—rubelladisease/Rubella_factsheet_EN.pdf. Accessed 11/August/14 [Google Scholar]
- 3.GAVI Alliance. Vietnam. [08/08/2014] www.gavialliance.org/country/vietnam. Accessed 11/August/14 [Google Scholar]
- 4.Miyakawa M, Yoshino H, Yoshida LM, Vynnycky E, Motomura H, Tho le H, Thiem VD, Ariyoshi K, Anh DD, Moriuchi H. Seroprevalence of rubella in the cord blood of pregnant women and congenital rubella incidence in Nha Trang, Vietnam. Vaccine 2014; 32:1192-8; PMID:24021315; http://dx.doi.org/ 10.1016/j.vaccine.2013.08.076 [DOI] [PubMed] [Google Scholar]
- 5.Metcalf CJ, Lessler J, Klepac P, Cutts F, Grenfell BT. Impact of birth rate, seasonality and transmission rate on minimum levels of coverage needed for rubella vaccination. Epidemiol Infect 2012; 140:2290-301; PMID:22335852; http://dx.doi.org/ 10.1017/S0950268812000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Government Statistical Office Projection of population in Vietnam 2011:2009-49. [Google Scholar]
- 7.UN Statistics Division , United Nations Population Division, (2008) World Population Prospects [Google Scholar]
- 8.World Health Organization (1999) Guidelines for surveillance of Congenital Rubella Syndrome and rubella. WHO/V&B/99.22 ed: World Health Organization. [Google Scholar]
- 9.Vynnycky E, Gay NJ, Cutts FT. The predicted impact of private sector MMR vaccination on the burden of Congenital Rubella Syndrome.Vaccine 2003; 21:2708-19; PMID:12798608; http://dx.doi.org/ 10.1016/S0264-410X(03)00229-9 [DOI] [PubMed] [Google Scholar]
- 10.Vynnycky E, Adams EJ, Cutts FT, Reef SE, Navar-Boggan AM, et al. (submitted) Using seroprevalence and immunisation coverage data to estimate the global burden of Congenital Rubella Syndrome, 1996-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee LA, Franzel L, Atwell J, Datta SD, Friberg IK, Goldie SJ, Reef SE, Schwalbe N, Simons E, Strebel PM, et al.. The estimated mortality impact of vaccinations forecast to be administered during 2011-2020 in 73 countries supported by the GAVI Alliance. Vaccine 2013; 31 Suppl 2:B61-72; http://dx.doi.org/ 10.1016/j.vaccine.2012.11.035 [DOI] [PubMed] [Google Scholar]
- 12.Schenzle D. An age-structured model of pre- and post-vaccination measles transmission. IMA J Math Appl Med Biol 1984; 1:169-91; PMID:6600102; http://dx.doi.org/ 10.1093/imammb/1.2.169 [DOI] [PubMed] [Google Scholar]
- 13.Department for Population and Labor Statistics (2010) The 2009 Vietnam population and housing census; Hanoi. [Google Scholar]
- 14.Rawls WE, Melnick JL, Bradstreet CM, Bailey M, Ferris AA, Lehmann NI, Nagler FP, Furesz J, Kono R, Ohtawara M, et al.. WHO collaborative study on the sero-epidemiology of rubella. Bull World Health Organ 1967; 37:79-88; PMID:5300057 [PMC free article] [PubMed] [Google Scholar]
- 15.Macnamara FN, Mitchell R, Miles JA. A study of immunity to rubella in villages in the Fiji islands using the haemagglutination inhibition test. J Hyg (Lond) 1973; 71:825-31; PMID:4520516; http://dx.doi.org/ 10.1017/S0022172400023081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desudchit P, Chatiyanonda K, Bhamornsathit S. Rubella antibody among Thai women of childbearing age. Southeast Asian J Trop Med Public Health 1978; 9:312-6; PMID:311950 [PubMed] [Google Scholar]
- 17.Abbey H. An examination of the Reed-Frost theory of epidemics. Hum Biol 1952; 24:201-33; PMID:12990130 [PubMed] [Google Scholar]
- 18.Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, Massari M, Salmaso S, Tomba GS, Wallinga J, et al.. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 2008; 5:e74; PMID:18366252; http://dx.doi.org/ 10.1371/journal.pmed.0050074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horby P, Pham QT, Hens N, Nguyen TT, Le QM, Dang DT, Nguyen ML, Nguyen TH, Alexander N, Edmunds WJ, et al.. Social contact patterns in Vietnam and implications for the control of infectious diseases. PLoS One 2011; 6:e16965; PMID:21347264; http://dx.doi.org/ 10.1371/journal.pone.0016965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutts FT, Vynnycky E. Modelling the incidence of congenital rubella syndrome in developing countries. Int J Epidemiol 1999; 28:1176-84; PMID:10661666; http://dx.doi.org/ 10.1093/ije/28.6.1176 [DOI] [PubMed] [Google Scholar]
- 21.Metcalf CJ, Bjornstad ON, Ferrari MJ, Klepac P, Bharti N, Lopez-Gatell H, Grenfell BT. The epidemiology of rubella in Mexico: seasonality, stochasticity and regional variation. Epidemiol Infect 2010: 139: 1029-38; PMID:20843389; http://dx.doi.org/ 10.1017/S0950268810002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toda K, Reef S, Tsuruoka M, Iijima M, Dang TH, Duong TH, Nguyen VC, Nguyen TH (submitted) Congenital Rubella Syndrome (CRS) in Vietnam 2011-2012. CRS epidemic after rubella epidemic in 2010-2011. Vaccine 2015; 33:3673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller E. Rubella in the United Kingdom. Epidemiol Infect 1991; 107:31-42; PMID:1879488; http://dx.doi.org/ 10.1017/S0950268800048652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chantler S, Evans CJ, Mortimer PP, Cradock-Watson JE, Ridehalgh MK. A comparison of antibody capture radio- and enzyme immunoassays with immunofluorescence for detecting IgM antibody in infants with congenital rubella. J Virol Methods 1982; 4:305-13; PMID:6752160; http://dx.doi.org/ 10.1016/0166-0934(82)90055-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.