Abstract
Zygapophyseal, or facet, joints are complicated biomechanical structures in the spine, with a complex three-dimensional (3D) anatomy, variable mechanical functions in different spinal movements, and effects on the overall spine mechanical behavior. The 3D morphology of the facet joint is linked to its biomechanical function. Failure of the biomechanical function of the facet joint leads to osteoarthritic changes in it and is implicated in other spinal disorders such as degenerative spondylolisthesis. Facet joints and intervertebral disk are part of an entity called the spinal motion segment, the three-joint complex, or the articular triad. Functioning together, the structures in the spinal motion segments provide physiological spinal motion, while protecting the spine by preventing activities that can be injurious. Loss of intervertebral disk height associated with disk degeneration affects the mechanical behavior of facet joints. Axial compressive load transmission through the tip of the inferior articular process can occur in the extended position, especially with reduced disk height, which may cause capsular impingement and low back pain. The 3D curvature of the articular surfaces and capsular ligaments play important roles in different spinal positions. In this review article, we will summarize the anatomy of the lumbar facet joint relevant to its biomechanical function and biomechanical behavior under different loading conditions.
Keywords: Lumbar spine, Facet joint, Functional anatomy, Biomechanics
Introduction
Facet joints, also known as zygapophyseal or apophyseal joints, are true synovial joints that can undergo degenerative changes in a fashion similar to that of other synovial joints. The unique morphology of facet joints is linked to their biomechanical function. Failure of the biomechanical function of a facet joint leads to osteoarthritic changes in it and is implicated in other spinal disorders such as degenerative spondylolisthesis. In this review article, we will summarize the anatomy of the lumbar facet joint relevant to its biomechanical function and biomechanical behaviors of the lumbar facet joint under different loading conditions.
Anatomy of the Lumbar Facet Joint
Facet joints comprise the inferior and superior articular processes, which are bony protuberances that arise vertically from the junction of pedicles and laminae behind the transverse processes, sometimes described as “bony articular pillars”1). In the lumbar region, the articular processes are completely incorporated into the laminae, so that the loads passing from superior to inferior articular facets diffuse into the lamina2). Trabecular tracts were recognized running obliquely from the superior process downward to the inferior endplate and from the inferior process upward to the superior endplate in the sagittal plane3,4). Furthermore, a preferred trabecular orientation mainly perpendicular to the articular surface in the transverse plane has been recognized in the superior process5,6).
The superior and inferior articular processes are covered with articulating cartilage primarily facing posteromedial and anterolateral directions, respectively. However, it has been reported that this cartilage layer does not extend all the way to the tips of the processes7), which sometimes makes the definition of the articular cartilage contours difficult. The orientation and distribution of the articulating cartilage in the processes appear to be suitable to transmit the forces in the transverse plane parallel to the endplates, rather than transmitting the vertical force applied to the posterior element of the spinal column. However, Hadley observed that, in cadaveric specimens, the articular cartilage extended beyond the limits of bony contact, which enlarged the joint space extending around to the posterior surface of the articular process8).
It has been generally described that the articular surfaces on the superior articular processes are concave, whereas those on the inferior articular processes are convex. The term “zygapophyseal” is derived from the Greek words “physis”, meaning outgrowth, and “zygos”, meaning yoke or bridge. Therefore, the original meaning of “zygapophyseal” is “bridging of outgrowths” (i.e., bridging adjacent vertebrae)9). On the other hand, the anatomical definition of the term “facet” is a smooth flat circumscribed anatomical surface. Since the articular surfaces of the facet joints are effectively not flat, the term “facet joint” may not well represent the geometrical characteristics of the joint. Beresford et al. stated in their article that the term “facet joint” was actually a misnomer, although they did not disagree with the usage of the term “facet joint” because it had been the most commonly used one. The curvature of the superior articular joint surface has been reported in the literature. The facet joint in the transverse plane parallel to the intervertebral disk space approximates a “C” or a “J” shape. In the upper lumbar spine, approximately 80% of the facet joints are curved and 20% are flat. In the lower lumbar spine, these numbers are reversed10). In the sagittal plane, Hadley showed the existence of concave articular surfaces in the inferior facet and convex articular surfaces in the superior facet, which are opposite curvatures in the transverse plane and conflict with the generally considered geometry of the facet joint surfaces8). The surface inversely curved in two perpendicular directions (i.e., concave in one and convex in the other) is described as a “saddle” shape, or in mathematical terms a parabolic hyperboloid. In fact, Steindler classified the lumbar facet joint as a saddle joint in addition to the carpometacarpal joint in his textbook4).
Panjabi et al. reported a clear tendency toward an increase in the cartilaginous area of the facets in the lower lumbar segment11). Similarly, the inner capsular area was also shown to display a pattern of increase from L1-2 to L5-S1; thus, larger facets were more likely to carry wider capsules12). Otsuka et al. found that the facet joint surface area increased with age and attributed this surface area increase to larger load bearing in the lower lumbar segments and facet joint degeneration13).
Similar to the other major synovial joints, the facet joint is surrounded by a capsule. This capsule consists of an outer layer made of densely packed parallel bundles of collagen fibers and an inner layer of irregularly oriented wavy elastic fibers14). Putz identified these as firm “transverse strengthening ligaments”5). Yamashita et al. described that collagenous fibers formed the strong connective tissue of the outer layer of the fibrous capsule14). In a recent study on the detailed lumbar capsular structures by Gorniak and Conrad, it was reported that the outer layer of the joint capsule exhibits three bands of fibers: curved superior fibers, middle horizontal running fibers, and curved inferior fibers15). The superior curved fibers form a distinct “dome-like band” that crosses the superior and superior-posterior parts of the joint. The middle horizontal fibers generally run horizontally but may also slant slightly downward, in a mediolateral direction. The curved inferior fibers form a distinct “hammock-like band” that crosses the inferior and posterior-inferior part of the facet joint15).
Since the articular cartilage extends beyond the posterior surface of the articular process as described earlier, the capsule is not attached to the margins of the joints but rather is reflected around to the outer surfaces of the articular processes8). The capsule and joint space extend by a variable distance from the margins along the superior or inferior articular process16). Attachment of the capsule at a certain distance from the margin of the joint surface causes the “wrap-around” effect of the capsule, which can create compressive forces and compressive stress within the capsule, thus probably forming fibrocartilage in the capsule17).
The thickness of the lumbar facet capsule has been measured in a study using 260 facet joints obtained from 26 embalmed vertebral columns18). In this investigation, regional variations of the thickness were reported (2.0 mm in the posterior region and 2.4 mm in the anterior region), whereas in the superior and inferior regions, as much as a 3.2 mm thickness has been reported in the anterior region, although the authors stated that thick capsules in the anterior region are partly caused by difficulty in separation between the ligamentum flavum and joint capsule during dissection18). Regional variations of the capsule thickness were also noted in the aforementioned study14). The inner layer in the inferior part was thicker than that in the superior and middle parts of the joint; however, this finding was only qualitative.
Proper knowledge of the anatomical geometry of the facet joint surface and capsular structures is important for biomechanical studies that seek to elucidate the normal function and biomechanical factors causing osteoarthritis of the facet joint and spinal disorders caused by facet joint dysfunction.
Biomechanical Function of the Facet Joints
Interaction between the facet joint and intervertebral disk
Facet joints and intervertebral disk are part of an entity called the spinal motion segment, the three-joint complex, or the articular triad19,20). Functioning together, the structures in the spinal motion segments provide physiological spinal motion, while protecting the spine by preventing activities that can be injurious. While the intervertebral disk has usually been considered to transmit mostly axial (vertical) compressive loads placed on the back, the facet joints have been traditionally considered to primarily function in guiding and stabilizing the motion segment2,21-23). Fortunately, in order to characterize this loading scenario, the relative magnitude of the axial compressive force passing through the intervertebral disk and facet joints has been studied by several investigators, thus demonstrating that, in fact, there is some type of load transmission through the facet joints. Adams and Hutton7) showed that 16% of the whole spine load is transmitted through the facet joints when the lumbar spine is in a slight extension of 2°, as in an erect standing position, and after the intervertebral disk height has been reduced by a period of axial compressive loading, whereas no load is transmitted via the facet joint in slight flexion, as in an erect sitting posture. The same research group measured the contact pressure between facet joint surfaces with pressure-sensitive paper (Fujifilm Prescale) under different postures and reduced disk height by nucleotomy and showed increased peak pressure with disk height loss and increasing extension7,24). Yang and King estimated that 3%-25% of the axial compressive load is carried via the normal facets by an indirect method using cadaveric lumbar specimens25). In a recent study using the nucleotomy model, Ivicsics et al. also demonstrated significantly greater load transmission via the facet joint from a median of 8.6% of the applied external force to 15.8% after nucleotomy with the intact capsule26).
Mechanisms of axial compressive load transmission through the facet joint
Although the aforementioned studies demonstrated axial compressive load transmission through the facet joint, the exact mechanism of load transmission was not delineated. There can be complex mechanisms by which facet joints transfer the axial compressive load because the lumbar facet joints form a synovial joint with low friction and almost vertical articulating surfaces. The three possible mechanisms are (1) through the articular joint surfaces, (2) through capsular ligaments, and (3) through direct contact between the tips of articular processes and the neural arch the lamina or the pars interarticularis.
Pressure-sensitive paper or film-like flat thin sensors have been used to directly measure the contact pressure between the facet articular cartilage surfaces. As described earlier, Dunlop et al. measured the contact pressure in a human cadaveric lumbar facet joint with a compressive load of 1,000 N and a shear load of 200-400 N and reported a contact pressure of 6.1 MPa in the central-medial and central-inferior regions of the articular surface near its periphery with 6° of extension24). Direct pressure measurements using the flat pressure sensors, however, require capsule transection in order to insert the sensor in the facet joint, which may alter the mechanical behavior of the facet joint because the capsule plays an important role in transmitting the applied load and constraining the movement of the facet joint, as described later in this review.
Information on the axial compression load transmission through the capsular ligaments is limited in the literature. Ivicsics et al., in their aforementioned study, measured load transmission through the facet joint with and without capsular ligaments under 700 N of axial compression in extension-flexion (neutral position ±5° with 0.25° increments). The authors demonstrated that the transmitted force supported by the capsular ligament was a median of 1.2% of the applied force with the intact intervertebral disk and increased to 5.1% after nucleotomy over the full extension-flexion cycle. They also found that the capsular ligaments transfer tensile forces mainly in the caudal-posterior direction during extension26).
Axial compressive load transmission through direct contact between the tips of articular processes and the neural arch (i.e., the lamina or the pars interarticularis) is important when the lumbar spine is extended, and/or the height of the intervertebral disk is reduced because the distance between the tips of the articular processes and the neural arch becomes narrower in such circumstances. In a histological study by Hadley, an articulation was demonstrated between the tip of the superior articular process and the pedicle of the adjacent superior vertebra or between the tip of the inferior articular process and the lamina or the pars interarticularis of the adjacent inferior vertebra caused by telescoping or imbrication of the facet articulations in the lumbar segment with intervertebral disk height loss. In these facet joints, the original facet articular cartilage layers do not register exactly opposite each other and the development of a fibrocartilage bumper due to intermittent pressure was noted at the tips of the articular processes8). Yang and King25) claimed that the bottoming-out of the tip of the inferior facet on the pars interarticularis of the vertebra below is likely to propitiate a lever-like mechanism of load transmission. Dunlop et al.24) also stated that substantial loads may be transmitted from the tips of the facets directly to the lamina below, or to the pars interarticularis above. In the experiment by Dunlop et al., extra-articular impingement was only seen in maximum extension at full disk height, but when the intervertebral disks presented with significant decreases in disk height it was found in all postures. The load transmission mechanism across the facet joint through bony contact was proven by direct measurement of the facet tip contact pressure using a pressure transducer implemented at the tip of a 13-gage steel tube that was placed at the tip of the inferior articular process through the bone part of the process27). The results from the aforementioned recent study by Ivicsics et al., however, did not indicate direct load transmission through the tip of the articular process and the neural arch26).
Facet biomechanical functions at different spinal positions
Facet joints fulfill different biomechanical functions in different spinal positions. In this section, we will review the biomechanical behaviors in extension, forward flexion, axial rotation, and lateral bending (Fig. 1).
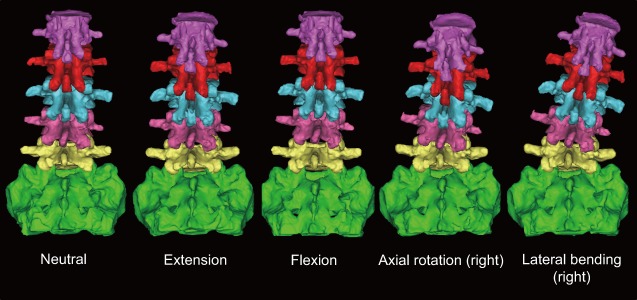
Figure 1.
3D lumbar CT models showing facet movements in different lumbar positions in a lumbar cadaveric specimen. Five different lumbar positions (neutral, flexion, extension, lateral bending, and axial rotation) were determined by load-control kinematic testing using a modified servohydraulic material testing frame (Instron 8874) and a six-camera (Eagle 4; Motion Analysis Corp.) optoelectronic motion capturing system. The specimen was fixed in a Stewart parallel platform (hexapod) frame in each position using the kinematic data and CT-scanned in each position.
Extension
In lumbar extension, the inferior articular processes move inferiorly in reference to the superior articular process of the lower level28). Kozanek et al. measured the range of motion of the lumbar facet joint (L2/L3-L4/L5) in vivo in healthy volunteers and reported that the facet joints rotated primarily along the mediolateral axis (average: 2°-6°) and were translated in the cephalad-caudad direction (average: 2-4 mm) from full flexion to full extension movements of the trunk with more mobility in the cranial levels29). Prasad et al. applied +Gz (caudocephalad) impact acceleration to embalmed whole human cadavers sitting on a load cell equipped seat pan and measured strain in the pedicle and lamina and load transmitted through the vertebral body in order to estimate the load transmission through the facet joint30). The results of that study demonstrated that both tensile and compressive loads can be transmitted via the facets or the posterior structures of the lumbar vertebrae and that hyperextension of the spine transfers more load to the facets30). The pressure measurement using the pressure-sensitive film by Dunlop et al. showed that the contact area of the articular surface of the facet joint moved to the lower margin of the facet in full extension24). In this study, it was estimated that the facet joint carried 10%-40% of the applied compressive force when in 4° of extension with 1 mm disk height loss24). A finite element analysis by Schendel et al. also showed that the facet contact site on the inferior articular process of L1 moved inferiorly to a position of tip impingement near the lamina as extension moments increased and large loads were transmitted through the facet joint during extension (205 N at a 10-N·m moment and 190 N axial load)31). A cadaveric study with an intact facet joint capsule by Ivicsics et al. showed that the capsular ligaments carry tensile force in the caudal-posterior directions, especially under the reduced intervertebral disk height condition, in addition to the compressive force transmission through the joint surface in the lumbar extended posture26).
Contact between the tips of the inferior articular process and the lamina or the pars interarticularis can occur in lumbar extension, especially under intervertebral disk height loss conditions, as described earlier in this review. Impingement of the capsule due to this bony contact has been postulated to be a cause of pain associated with lumbar extension by many authors7,14,15,21,24). Yang and King found that overloading of the facet joint resulted in rearward rotation of the inferior facets, the tip of which pivoted about the lamina below in compressive testing of the isolated posterior element of the lumbar spine25). The results of finite element analysis simulations also postulated that hyperextension activities will cause impingement of the inferior process31,32).
Forward flexion
In lumbar forward flexion, the inferior articular processes move superiorly in reference to the superior articular process of the lower level. The contact areas are located on the upper tip in the superior articular surface and on the upper and central regions in the inferior articular surface in large flexion32). Facet joints play an important role in maintaining lumbar stability in forward flexion. During forward flexion, the inferior articular process glides upward and forward upon the superior articular process of the inferior vertebra and the articular surfaces separate at the lower margins of the joint8). Ivicsics et al. calculated the facet joint force vectors in the sagittal plane under a 700 N compressive load applied to the motion segment with an intact intervertebral disk26). The magnitudes and orientation angles of the vectors in reference to the cranial-caudal axis (positive, posterior) in flexion of 5° were 31.2 N at ‒48.4° through the total facet joint, 46.1 N at ‒39.4° through bony contact, and 14.9 N at 158.2° through the capsular ligament. These values after nucleotomy were 52.4 N at ‒61.3° through the total facet joint, 59.7 N at ‒49.8° through bony contact, and 7.3 N at ‒178.6° through the capsular ligament26). Ianuzzi et al. measured lumbar facet joint capsule strains during physiological motions using cadaveric spines and reported that the mean principal strains of the joint capsules increased monotonically from full extension to full flexion of the lumbar spine33). Claeson and Barocas elucidated the existence of in-plane and through-plane shear deformations of the capsular ligament during flexion by finite element models of the lumbar facet joint. In this study, the magnitudes of stress and strain were largest across the ligaments between the attachments to the articular facets (i.e., over the joint space). The authors noted that the largest tensile strains were a function of unconstrained motion (i.e., motion in the anterior direction) and the largest tensile stresses were a function of fiber direction of the capsular ligament34). Beyond the limit of normal physiologic flexion, the inferior articular process is forced over the superior process, and the compressive loads carried by the facets increase again compared to those carried in the neutral position32). This phenomenon has been considered as a possible mechanism to cause degenerative spondylolisthesis as described below.
The mechanism of anterior slippage has been already proposed in the first paper in the English literature on degenerative spondylolisthesis by Macnab in 195035). The author described that the facet joints hook round anteriorly, like the letter J, and form bars resisting forward displacement, although they may be sagittal posteriorly. He hypothesized that the anterior slippage is unusual, because the posterior joints seldom lie in a true sagittal plane. Therefore, he proposed an “overriding” mechanism of the facet joint during flexion to explain the anterior slippage. In this mechanism, the facet joints lying in an oblique or in the coronal plane act as bony bars preventing dislocation, which can occur only by overriding or fracture of the facets. With the advent of clinical computed tomography (CT), assessment of facet orientation in transverse planes was of interest among investigators of degenerative spondylolisthesis. However, the majority of investigations focused on the overall sagittal orientation at the level of the superior endplate of the caudad vertebra, and only a few studies paid attention to the “bony bar” or “hook” resisting forward displacement described by Macnab36-38).
Axial rotation
Lumbar facet joints have been considered important in preventing the axial rotation of the motion segment since the beginning of the 20th century39,40). In lumbar axial rotation, the articular surfaces of the facet joint compress together on one side and tend to open on the other. For example, with right axial rotation, the left inferior articular process impacts the left superior articular process of the lower vertebra and the joint space width in the right facet joint increases. The impaction of the facet joint surfaces limits the range of axial movement and protects against excessive torsion of the intervertebral disk. The facets were found to carry large loads during axial rotation (65 N at a 10 Nm moment and a 150 N axial load) and cause the resultant contact force to lie at the posterior edge of the articulating surface31).
Cramer et al. measured the facet joint gap distance in an axially rotated position (side-posture positioning) using magnetic resonance imaging and demonstrated that axial rotation increases the gap distance in the rotated side of the axial rotation (i.e., in the right facet during right axial rotation). The authors also demonstrated that side-posture manipulation further increases the gap distance41). Measurements of changes in the facet joint space width due to passive axial rotation using CT scanning demonstrated an increased gap distance in the rotated side and a decrease in the joint space distance in the opposite side42). In the gap opening side, the capsular ligament becomes tensed17). Putz identified the firm “transverse strengthening ligaments” to carry tensile load in the opening side of the facet joint and emphasized a mechanical role of capsular ligament during axial rotation5). The results of photoelastic experiments and the trabecular orientation observed in this study suggested that the superior articular processes are under bending stress during axial rotation by compression in the lateral direction and tension in the medial direction5).
Lateral bending
Limited information is available in the literature on the biomechanical behavior of the facet joint under lateral bending. In lateral bending, the inferior articular process glides in the superior direction in reference to the superior articular process of the inferior vertebra on the convex side of the spinal curve and opposite direction on the concave side28). Schendel et al. found that the facet joints carry large loads during lateral bending (78 N at a 3 Nm moment and a 160 N axial load) and the location of the resultant contact in the right inferior articular process moved from the posterior edge in the left lateral bending to the tip in the right lateral bending31). The authors also noted that the lateral bending motion was coupled with axial rotation (i.e., the left lateral bending was associated with axial rotation, which loads the right facet) and the facet resultant contact force location in the left lateral bending was in the same area as that for right axial torsion. Since coupled motions between lateral bending and axial rotation in the lumbar spine have been reported, for example, left lateral bending to be coupled with right axial rotation31,43,44), the authors suggested that the axial rotation component associated with lateral bending could be partially responsible for facet loading31). The coupled motions were also measured in flexion-extension and axial rotation in the aforementioned in vivo study29).
Conclusion
The lumbar facet joint exhibits a complex three-dimensional (3D) geometry that includes small components within this small joint, which are closely linked to the biomechanical functions of the facet joint and the motion segment in different spinal positions. Oversimplification of the facet joint as a flat joint may prevent the proper understanding of the facet joint functions. Despite the keen observations of the 3D facet joint geometry and consideration of the facet joint functions in 3D space reported many decades, even a century, ago, the development of CT scanning rather tends to limit investigators' thought to the transverse plane. Axial load transmission and forward translation in motion segments cannot be fully understood without consideration of the special relationships among the posterior elements of the lumbar spine. Current imaging modalities allow 3D modeling and reslicing of the model in the clinical setting; however, no valid information can be extracted without properly understanding the 3D geometry and function of the facet joint.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Acknowledgement
This study received funding from NIH grant NCCIH R01 AT006692.
References
- 1.Jaumard NV, Welch WC, Winkelstein BA. Spinal facet joint biomechanics and mechanotransduction in normal, injury and degenerative conditions. J Biomech Eng. 2011;133(7):071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pal GP, Routal RV. Transmission of weight through the lower thoracic and lumbar regions of the vertebral column in man. J Anat. 1987;152:93-105. [PMC free article] [PubMed] [Google Scholar]
- 3.Gallois J, Japoit T. [Architecture interieure des vertebres]. Revue de Chirurgie. 1925;63:688-708. French. [Google Scholar]
- 4.Steindler A. Kinesiology of the human body under normal and pathological conditions. CC Thomas: Spring field; 1973. 63 p. [Google Scholar]
- 5.Putz R. The functional morphology of the superior articular processes of the lumbar vertebrae. J Anat. 1985;143:181-7. [PMC free article] [PubMed] [Google Scholar]
- 6.Drews S, Matsuura M, Putz R. The trabecular architecture of the superior articular process of the lumbar spine (L2-S1). Surg Radiol Anat. 2008;30:209-13. [DOI] [PubMed] [Google Scholar]
- 7.Adams MA, Hutton WC. The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. J Bone Joint Surg Br. 1980;62:358-62. [DOI] [PubMed] [Google Scholar]
- 8.Hadley LA. Anatomico-roentgenographic studies of the posterior spinal articulations. Am J Roentgenol Radium Ther Nucl Med. 1961;86:270-6. [PubMed] [Google Scholar]
- 9.Beresford ZM, Kendall RW, Willick SE. Lumbar facet syndromes. Curr Sports Med Rep. 2010;9:50-6. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz T, Smith RM. An anatomical, pathological and roentgenological study of the lumbar spine and of the sacroiliac joints. Am J Roentgenol. 1940;43:173-86. [Google Scholar]
- 11.Panjabi MM, Oxland T, Takata K, et al. Articular facets of the human spine. Quantitative three-dimensional anatomy. Spine (Phila Pa 1976). 1993;18:1298-310. [DOI] [PubMed] [Google Scholar]
- 12.Tanno I, Murakami G, Oguma H, et al. Morphometry of the lumbar zygapophyseal facet capsule and cartilage with special reference to degenerative osteoarthritic changes: an anatomical study using fresh cadavers of elderly Japanese and Korean subjects. J Orthop Sci. 2004;9:468-77. [DOI] [PubMed] [Google Scholar]
- 13.Otsuka Y, An HS, Ochia RS, et al. In vivo measurement of lumbar facet joint area in asymptomatic and chronic low back pain subjects. Spine (Phila Pa 1976). 2010;35:924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashita T, Minaki Y, Ozaktay AC, et al. A morphological study of the fibrous capsule of the human lumbar facet joint. Spine (Phila Pa 1976). 1996;21:538-43. [DOI] [PubMed] [Google Scholar]
- 15.Gorniak G, Conrad W. Lower lumbar facet joint complex anatomy. Austin J Anat. 2015;2:1-8. [Google Scholar]
- 16.Xu GL, Haughton VM, Carrera GF. Lumbar facet joint capsule: appearance at MR imaging and CT. Radiology. 1990;177:415-20. [DOI] [PubMed] [Google Scholar]
- 17.Boszczyk BM, Boszczyk AA, Putz R, et al. An immunohistochemical study of the dorsal capsule of the lumbar and thoracic facet joints. Spine (Phila Pa 1976). 2001;26:E338-43. [DOI] [PubMed] [Google Scholar]
- 18.Sato S, Oguma H, Murakami G, et al. Morphometrical study of the joint surface and capsule of the lumbar zygapophysial joint with special reference to their laterality. Okajimas Folia Anat Jpn. 2002;79:43-53. [DOI] [PubMed] [Google Scholar]
- 19.Gellhorn AC, Katz JN, Suri P. Osteoarthritis of the spine: the facet joints. Nat Rev Rheumatol. 2013;9:216-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twomey L, Taylor J. Age changes in lumbar intervertebral discs. Acta Orthop Scand. 1985;56:496-9. [DOI] [PubMed] [Google Scholar]
- 21.Adams MA, Hutton WC. The mechanical function of the lumbar apophyseal joints. Spine (Phila Pa 1976). 1983;8:327-30. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed AM, Duncan NA, Burke DL. The effect of facet geometry on the axial torque-rotation response of lumbar motion segments. Spine (Phila Pa 1976). 1990;15:391-401. [DOI] [PubMed] [Google Scholar]
- 23.Pal GP, Routal RV. A study of weight transmission through the cervical and upper thoracic regions of the vertebral column in man. J Anat. 1986;148:245-61. [PMC free article] [PubMed] [Google Scholar]
- 24.Dunlop RB, Adams MA, Hutton WC. Disc space narrowing and the lumbar facet joints. J Bone Joint Surg Br. 1984;66:706-10. [DOI] [PubMed] [Google Scholar]
- 25.Yang KH, King AI. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine (Phila Pa 1976). 1984;9:557-65. [DOI] [PubMed] [Google Scholar]
- 26.Ivicsics MF, Bishop NE, Puschel K, et al. Increase in facet joint loading after nucleotomy in the human lumbar spine. J Biomech. 2014;47:1712-7. [DOI] [PubMed] [Google Scholar]
- 27.el-Bohy AA, Yang KH, King AI. Experimental verification of facet load transmission by direct measurement of facet lamina contact pressure. J Biomech. 1989;22:931-41. [DOI] [PubMed] [Google Scholar]
- 28.Jegapragasan M, Cook DJ, Gladowski DA, et al. Characterization of articulation of the lumbar facets in the human cadaveric spine using a facet-based coordinate system. Spine J. 2011;11:340-6. [DOI] [PubMed] [Google Scholar]
- 29.Kozanek M, Wang S, Passias PG, et al. Range of motion and orientation of the lumbar facet joints in vivo. Spine (Phila Pa 1976). 2009;34:E689-96. [DOI] [PubMed] [Google Scholar]
- 30.Prasad P, King A, Ewing C. The role of articular facets during +Gz accelaration. J Appl Mech. 1974;41(2):321-6. [Google Scholar]
- 31.Schendel MJ, Wood KB, Buttermann GR, et al. Experimental measurement of ligament force, facet force, and segment motion in the human lumbar spine. J Biomech. 1993;26:427-38. [DOI] [PubMed] [Google Scholar]
- 32.Shirazi-Adl A, Drouin G. Load-bearing role of facets in a lumbar segment under sagittal plane loadings. J Biomech. 1987;20:601-13. [DOI] [PubMed] [Google Scholar]
- 33.Ianuzzi A, Little JS, Chiu JB, et al. Human lumbar facet joint capsule strains: I. During physiological motions. Spine J. 2004;4:141-52. [DOI] [PubMed] [Google Scholar]
- 34.Claeson AA, Barocas VH. Computer simulation of lumbar flexion shows shear of the facet capsular ligament. Spine J. 2017;17:109-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macnab I. Spondylolisthesis with an intact neural arch; the so-called pseudo-spondylolisthesis. J Bone Joint Surg Br. 1950;32-b:325-33. [DOI] [PubMed] [Google Scholar]
- 36.Grobler LJ, Robertson PA, Novotny JE, et al. Etiology of spondylolisthesis. Assessment of the role played by lumbar facet joint morphology. Spine (Phila Pa 1976). 1993;18:80-91. [PubMed] [Google Scholar]
- 37.Love TW, Fagan AB, Fraser RD. Degenerative spondylolisthesis. Developmental or acquired? J Bone Joint Surg Br. 1999;81:670-4. [DOI] [PubMed] [Google Scholar]
- 38.Toyone T, Ozawa T, Kamikawa K, et al. Facet joint orientation difference between cephalad and caudad portions: a possible cause of degenerative spondylolisthesis. Spine (Phila Pa 1976). 2009;34:2259-62. [DOI] [PubMed] [Google Scholar]
- 39.Fick R. Handbuch der Anatomie und Mechanik der Gelenke. Jena: Verlag Gustav Fischer; 1904. 512 p. [Google Scholar]
- 40.Nachemson A. Lumbar intradiscal pressure. Experimental studies on post-mortem material. Acta Orthop Scand Suppl. 1960;43:1-104. [DOI] [PubMed] [Google Scholar]
- 41.Cramer GD, Gregerson DM, Knudsen JT, et al. The effects of side-posture positioning and spinal adjusting on the lumbar Z joints: a randomized controlled trial with sixty-four subjects. Spine (Phila Pa 1976). 2002;27:2459-66. [DOI] [PubMed] [Google Scholar]
- 42.Yang KH, An HS, Ochia RS, et al. In vivo measurement of changes in lumbar facet joint width during torsion. Transactions of the 51st Annual Meeting of the Orthopaedic Research Society; 2005 Feb 20-23; Washington, D.C., 2005. 690 p. [Google Scholar]
- 43.Gregersen GG, Lucas DB. An in vivo study of the axial rotation of the human thoracolumbar spine. J Bone Joint Surg Am. 1967;49:247-62. [PubMed] [Google Scholar]
- 44.White AA, Panjabi MM. Clinical biomechanics of the spine. Philadelphia, Pa: J. B. Lippincott; 1978. 534 p. [Google Scholar]