Background:
Tendon grafts play an important role in flexor tendon reconstruction. This study was an investigation of the effects of surface modification of allograft intrasynovial tendons with carbodiimide-derivatized hyaluronic acid and gelatin in an in vivo canine model. To mimic the actual clinical situation, a novel and clinically relevant model of a failed primary flexor tendon repair was used to evaluate the flexor tendon grafts.
Methods:
Twenty-eight flexor digitorum profundus tendons from the second and fifth digits of fourteen dogs were lacerated and repaired in zone II in a first-surgery phase. The dogs were allowed free active motion postoperatively. In a second phase, six weeks later, the tendons were reconstructed with use of a flexor digitorum profundus allograft. In each dog, one graft was treated with carbodiimide-derivatized hyaluronic acid and gelatin (the CHG group) and the other was treated with saline solution, as a control. The dogs were restricted from free active motion, but daily therapy was performed beginning on postoperative day 5 and continued until six weeks after the operation, when the animals were killed. The outcomes were evaluated on the basis of digit work of flexion, gliding resistance, healing at the distal attachment, graft cell viability, histological findings, and findings on scanning electron microscopy.
Results:
In the first phase, all twenty-eight repaired tendons ruptured, with scar and adhesion formation in the repair site. Six weeks after allograft reconstruction, the mean work of flexion was 0.37 and 0.94 N-mm/degree in the CHG group and the saline-solution control group, respectively; these values were significantly different (p < 0.05). The gliding resistance in the CHG group was also significantly less than that in the saline-solution control group (0.18 versus 0.28 N) (p < 0.05), but no difference between groups was observed with regard to the distal tendon-bone pullout strength. Histological analysis showed that tenocytes in the host tendon proliferated and migrated toward the acellular allograft.
Conclusions:
This primary repair failure model was reproducible and reliable, with a uniform failure pattern, and provides an appropriate and clinically relevant animal model with which to study flexor tendon reconstruction. The surface modification of allografts with carbodiimide-derivatized hyaluronic acid and gelatin improved digital function and tendon gliding ability.
Hand injuries are common, affecting approximately seven in 1000 individuals per year. In a Swedish population-based study, patients with hand injuries represented about 12% of the patients seen in the emergency department1. Tendon injuries are among the most severe hand injuries, and they occur almost exclusively in a young, working-age population, with resultant disability2-4. Restoration of function of a digit with severe damage to the flexor tendon—particularly in zone II, where two flexor tendons pass through a narrowing fibro-osseous sheath—is difficult, with high rates of complications, such as adhesion formation and primary tenorrhaphy rupture5-7.
Tendon grafts still play an important role in reconstruction to restore finger function8-11. Although flexor tendons in zone II include a synovially lined sheath12,13, most tendon autografts are derived from sources without synovium. Clinical and animal models have shown that extrasynovial tendon autografts are associated with more adhesions to the surrounding tissue than are intrasynovial tendon autografts14-16.
Potential sources of intrasynovial tendons for use as tendon autografts are limited. Chemical modification of hyaluronic acid with use of the carbodiimide (EDC, or 1-ethyl-3-[(3-dimethylaminopropyl) carbodiimide hydrochloride]) reaction to create new biopolymers, crosslinking with other biological molecules such as collagen, has been widely investigated for use in applications such as anti-adhesion membranes17,18, biodegradable scaffolds19,20 for tissue regeneration, or timed-release drug-delivery vehicles21,22. Recent studies have demonstrated that extrasynovial autografts whose surface was modified with carbodiimide-derivatized hyaluronic acid and gelatin improved tendon gliding ability and decreased postoperative adhesions23,24; however, the digital function following reconstruction with such treated extrasynovial autografts still is not normal25.
Although donor sites for intrasynovial tendon autografts are scarce, sources of intrasynovial tendon allografts are readily available and are frequently used for anterior cruciate ligament (ACL) reconstruction26-28. The advantages of allografts include a decrease in surgical morbidity, time, and cost by eliminating the graft harvesting procedure29 and the ability to match the graft tissue to the size of the injured tendon30. A decellularized allograft is less immunogenic31,32. Preserved allografts also maintain their mechanical properties and offer the convenience of being available at a desired time33-35.
Like ACL allografts, flexor tendon allografts require, in addition to mechanical strength and appropriate tendon-to-bone healing, a smooth gliding surface with low frictional properties. Although surface modification of an extrasynovial autograft improved the outcomes following flexor tendon reconstruction in an in vivo animal model25, it is not known if surface modification would have the same beneficial effects, or even better effects, when used for intrasynovial allografts.
The purpose of the current study was to investigate the effects of modification of allograft intrasynovial tendons with carbodiimide-derivatized hyaluronic acid and gelatin for flexor tendon reconstruction in an in vivo animal model. We hypothesized that this modification would improve outcomes compared with those following use of intrasynovial allografts without such treatment. To mimic the actual clinical situation, a novel, clinically relevant canine in vivo model of a failed primary flexor tendon repair was used.
Materials and Methods
Study Design
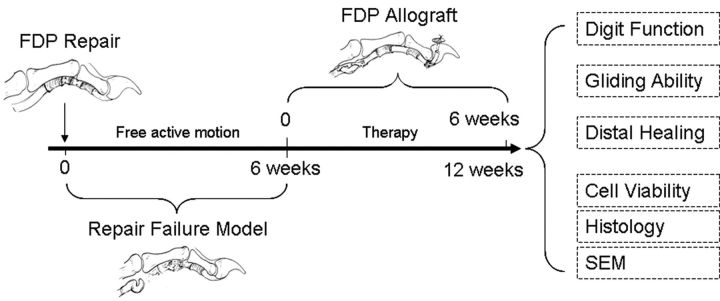
Fourteen mongrel dogs weighing 20 to 25 kg were used. The study was approved by our Institutional Animal Care and Use Committee. The flexor digitorum profundus tendons from the second and fifth digits of right and left forepaws (with the sides randomly allocated) were lacerated and repaired. Free cage activity was allowed after five days of immobilization. This protocol has been found to produce a 100% rate of breakage of the flexor tendon repair in this canine model, with dense scarring at the site of the disrupted repair. Six weeks after the primary tendon repair, an intrasynovial allograft reconstruction was performed. One of the surgically treated digits was randomly selected for treatment with a graft coated with carbodiimide-derivatized hyaluronic acid and gelatin (the CHG group) and the other digit was treated with a graft soaked in saline solution (the control group). Six weeks after reconstruction, the dogs were killed, and the surgically treated paws were harvested for evaluation of digital function, tendon gliding ability, healing strength, and histological characteristics (Fig. 1).
Fig. 1.
Study design flow chart. FDP = flexor digitorum profundus, and SEM = scanning electron microscopy.
Preparation of Intrasynovial Allograft Tendons
Twenty-eight flexor digitorum profundus tendons were obtained from dogs that had been killed for other Institutional Animal Care and Use Committee-approved protocols. The tendons were immediately immersed in liquid nitrogen for one minute and then thawed for five minutes in warmed saline solution at 37°C. This procedure was repeated five times to induce tenocyte necrosis. This protocol has been previously shown to produce necrosis of 97% to 100% of all viable tenocytes36. The tendons were then lyophilized with a custom-made lyophilizer. Each graft tendon was put in a separate sealed plastic bag and stored at room temperature until use. The graft was moved to a special plastic bag for gas sterilization one week before the graft was used. After sterilization, the graft was rehydrated in a 0.9% NaCl bath in a closed, sterilized container for twenty-four hours in an incubator at 37°C prior to graft surgery.
Creation of Flexor Tendon Primary Repair Failure Model
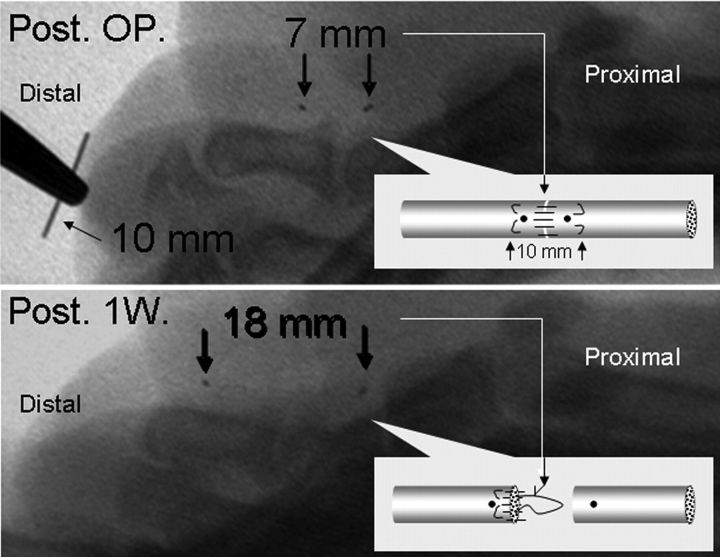
The dogs were prepared for flexor tendon laceration and repair surgery as previously described33-35. Briefly, the flexor digitorum profundus tendon was lacerated at the level of the proximal phalanx and repaired with use of a two-strand modified Kessler technique with 3/0 Ethibond suture (Ethicon, Somerville, New Jersey) and a simple running suture with 6/0 nylon. Following tendon repair, two small metal markers (2/0 surgical steel suture; Ethicon) were embedded into the repaired tendon with the repair site in the middle. The distance between the two metal markers was measured with use of a fluoroscope (Mini 6600 Digital Mobile C-Arm; OEC Medical Systems, Salt Lake City, Utah). The incisions were closed in layers. Intramuscular antibiotics were administered perioperatively. The operatively treated paw was immobilized with a custom-made canine jacket to maintain it underneath the chest without weight-bearing for five days. Then, the jacket was removed and the animals were allowed cage activity without any protection. One week after the surgery, the dogs were restrained with a custom-made sling, and the metal markers were evaluated fluoroscopically. This procedure was repeated every week until the distance between the two markers had increased by 10 mm as compared with the distance immediately after the repair; this increase indicated tendon rupture.
Flexor Tendon Reconstruction with Intrasynovial Allograft
Six weeks after the primary repair, flexor tendon reconstruction was performed in the forelimb that had been previously operated on. The distal portion of the flexor digitorum profundus tendon was approached through the previous incision. The normal tendon-bone attachment was exposed, and the tendon was cut 5 mm from its insertion site. The distal 5-mm tendon stump with its insertion was preserved. A bone tunnel beneath the flexor digitorum profundus tendon attachment was created for the distal attachment of the tendon graft with a 3-mm-diameter drill bit. Then, dissection along the distal portion of the flexor digitorum profundus tendon toward the proximal repair site was carefully performed, and the repair status and adhesions were observed, noted, and photographed. The proximal portion of the repaired tendon was exposed through a separate incision made longitudinally at the mid-metacarpal level. A tunnel was created by removing the previously repaired flexor digitorum profundus tendon along with scar and adhesion tissues and preserving the proximal pulley in zone II. Following the preparation of the recipient digit, the prepared allograft was pulled through the proximal tunnel. The distal tendon-bone junction and proximal tendon-tendon junction were repaired with previously described techniques33-35.
One of the two allograft flexor digitorum profundus tendons was randomly selected for treatment with carbodiimide-derivatized hyaluronic acid and gelatin. It was immersed in 1% sodium hyaluronate (95%; Acros, Geel, Belgium), 10% gelatin (from porcine skin; Sigma Chemical, St. Louis, Missouri), 1% 1-ethyl-3-([3-dimethylaminopropyl] carbodiimide hydrochloride) (EDC) (Sigma), and 1% N-hydroxysuccinimide (NHS) (Sigma) in 0.1 M NaCl (pH 6.0) and 0.9% phosphate-buffered saline solution (PBS) for five minutes37. The treated tendon was loosely wrapped with a section of the elastic bandage used to exsanguinate the limb, in order to prevent the carbodiimide-derivatized hyaluronic acid and gelatin from being removed from the tendon surface by the moistened gauze surrounding the rubber sheet. The control tendon was immersed in 0.9% NaCl solution and similarly wrapped.
Following the tendon grafting, a proximal radial neurectomy was performed through a lateral humeral incision in the selected recipient forelimb, in order to denervate the triceps muscle and prevent elbow extension and thus weight-bearing38,39. The operatively treated paw was immobilized with a custom-made canine jacket to maintain it underneath the chest. As a result of the radial neurectomy and sling fixation, the dogs were unable to bear weight on the operatively treated limb. On postoperative day 5, rehabilitation was started, with a synergistic wrist-digit-motion protocol performed once daily until the animal was killed39,40.
Evaluation of Digital Function According to Work of Flexion
Digital function and adhesion formation after tendon grafting were evaluated with use of a previously developed and established method25. The second and fifth digits from the contralateral, untreated paw were also tested, as a normal control. Briefly, the proximal end of the tendon graft was dissected free, divided, and sutured to a cable that was connected to a load transducer. The entire digit was mounted on a custom-made testing device by a Kirschner wire that was inserted longitudinally through the metacarpal bone to fix the metacarpophalangeal joint in extension.
A motion analysis system with two CCD cameras (Motion Analysis Corporation, Santa Rosa, California) was used for the proximal interphalangeal and distal interphalangeal joint-motion measurements. A 0.5-N weight was attached to the extensor tendon to ensure full extension of the digit as a starting position and to apply an initial tension to the graft. A 0.1-N preload was applied to the proximal part of the graft before testing. The actuator pulled the tendon proximally at a rate of 2 mm/sec, causing digit flexion. Data from the linear potentiometer and the proximal load transducer were recorded at 20 Hz along with the joint motion data captured by the motion analysis cameras. The work of flexion was measured until the distal interphalangeal joint angle reached 40° of flexion, as was done in a previously published study25. Work-of-flexion data were calculated from the tendon displacement versus tendon loading curve during digital flexion and then were normalized by the total proximal interphalangeal and distal interphalangeal joint angles; this was called normalized work of flexion.
Evaluation of Graft Gliding Ability According to Gliding Resistance
Following the work-of-flexion measurement, the tendon graft was further dissected, with the proximal pulley kept intact. The gliding resistance between the tendon graft and the proximal pulley was then measured with use of a custom tendon-pulley friction-testing device, as previously described25,41. The flexor digitorum profundus tendon, proximal pulley, and proximal phalanx were preserved. A custom-made ring load transducer was connected to the distal portion of the flexor digitorum profundus tendon, and a load of 4.9 N was applied. The other transducer was connected to the proximal portion of the flexor digitorum profundus tendon. The flexor digitorum profundus tendon was driven by a motor with a potentiometer that was connected to the proximal transducer to simulate flexion and extension. The difference in force recorded by the two transducers was used to calculate the gliding resistance25,41. The flexor digitorum profundus tendons from the second and fifth digits in the contralateral, untreated paw were also tested, as a normal control.
Evaluation of Graft Healing According to Mechanical Strength of Distal Graft Attachment
The testing of the mechanical strength of the distal graft attachment was conducted according to previously published protocols42. Briefly, the distal 10 mm of the graft tendon with its attachment to the distal phalanx was isolated from the digit. The distal portion of the tendon graft was gripped, and the distal phalanx was then secured in the materials testing machine (MTS, Minneapolis, Minnesota). The tendon was pulled at a rate of 20 mm/min until failure, and the ultimate force and stiffness (the slope of force versus displacement) at the insertion were measured to assess the mechanical quality of healing of the distal graft juncture.
Cell Viability, Histological Analysis, and Scanning Electron Microscopy
Two tendon grafts from each group were harvested with the animal under general anesthesia, before it was killed, in order to examine the cell viability. The graft tendons were immediately divided into three sections. The proximal section was assessed for cell viability by measuring levels of calcein AM and ethidium homodimer43, to evaluate intracellular esterase activity and plasma membrane activity, respectively. The graft sections were immersed in minimal essential medium (MEM) with Earle’s salts (GIBCO, Grand Island, New York) containing 5 μM calcein AM and 5 μM ethidium homodimer-1 (LIVE/DEAD Viability/Cytotoxicity Kit; Molecular Probes, Eugene, Oregon) for thirty minutes. Then, the grafts were longitudinally observed with a confocal microscope (LSM310; Carl Zeiss, Gutenberg, Germany). The middle section of the graft was submitted for scanning electron microscopy in our institution’s Scanning Electron Microscopy Core Facility44. The distal section was sent to our institution’s Histology Core Facility for paraffin embedding and subsequent sectioning for hematoxylin and eosin (H & E) and Masson trichrome staining.
Statistical Methods
All quantitative measurements, including work of flexion, gliding resistance, and mechanical strength of the distal attachment, were presented as a mean and standard deviation. The data were analyzed with use of one-way analysis of variance, followed by the Tukey studentized range (honestly significant difference [HSD]) post hoc test to detect the effect of surface modification on each dependent variable (work of flexion, gliding resistance, and maximal failure strength and stiffness). In all cases, a level of p < 0.05 was considered to be significant.
Source of Funding
This study was funded by the Musculoskeletal Transplant Foundation (MTF).
Results
Creation of Primary Flexor Repair Failure Model
The wounds all healed primarily, without infection. After the tendon laceration and repair, all fourteen dogs were able to bear weight on the operatively treated limb after the jacket was removed at day 5 postoperatively. The distance between the two metal markers in all repaired tendons was increased by >10 mm within two weeks, indicating that the repaired tendon had ruptured (Fig. 2). At six weeks following the primary repair, the joints in the operatively treated digits, including the metacarpophalangeal, proximal interphalangeal, and distal interphalangeal joints, all had maintained a normal passive range of motion compared with the contralateral, untreated digits.
Fig. 2.
Upper panel: Fluoroscopic view demonstrating the distance between the two metal markers (thick black arrows) immediately after tendon repair (Post. OP.). Lower panel: Fluoroscopic image showing the same markers (black arrows) one week after tendon repair (Post 1W.). The distance was >10 mm greater than the distance seen initially after the repair, indicating that the repaired tendon had ruptured.
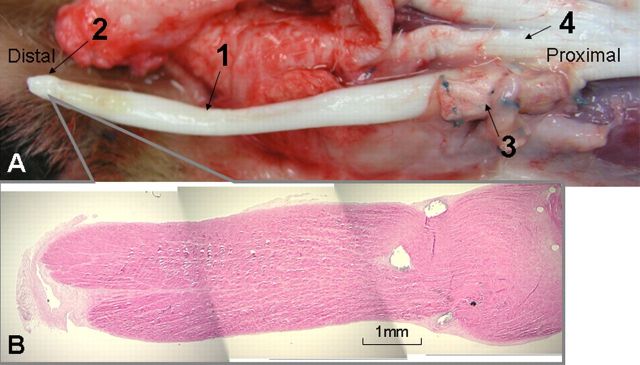
Six weeks after the initial tendon repair, surgical exploration showed that all of the flexor digitorum profundus repairs had ruptured by suture pullout from the proximal tendon stump. At the distal ruptured-tendon stump, scar formation and adhesions were observed between the tendon and the tendon bed, the tendon and the pulley, and the tendon and the surrounding soft tissues (Fig. 3, A). The proximal tendon stump had retracted into the paw.
Fig. 3.
Typical observations during surgical exploration and histological analysis six weeks after flexor digitorum profundus tendon repair, indicating failure of the repair with a scarred digit. A: The distal ruptured-tendon stump (2) and scar and adhesion formation at the repaired tendon end (1). A1: Histological specimen of the distal tendon stump with scar tissue (H & E, ×20). B: The proximal end of the ruptured repaired tendon was free from adhesions and was folded and enlarged. B1: Histological specimen of the proximal tendon stump (H & E, ×20).
Because of the retraction of the proximal portion of the ruptured tendons, a separate incision was made longitudinally at the mid-metacarpal level to expose the proximal flexor digitorum profundus tendon stump. The proximal end of the ruptured repaired flexor digitorum profundus was easily pulled out. No adhesion was observed at the proximal tendon end. The proximal tendon stump was folded and enlarged in diameter (Fig. 3, B).
Outcomes of Allograft Reconstruction
After the tendon reconstruction, the wounds all healed without infection. Four grafts in the CHG (carbodiimide-derivatized hyaluronic acid and gelatin) group and two grafts in the saline-solution control group were ruptured at the distal tendon-bone junction. The distal graft stump in all ruptured cases was retracted proximally without adhesions (Fig. 4). There were no ruptures at the proximal tendon repair site in either group. These digits with a distal rupture were considered to be an experimental failure and were excluded from the biomechanical and biological evaluations. Therefore, eight samples in the CHG group and ten samples in the saline-solution group were assessed for work of flexion, gliding resistance, and distal attachment strength, and two samples in each group were assessed for cell viability, histologically, and with scanning electron microscopy.
Fig. 4.
A: In some dogs, rupture of the flexor digitorum profundus tendon occurred at the distal tendon-bone junction with graft pullout from the bone tunnel six weeks after use of an allograft treated with carbodiimide-derivatized hyaluronic acid and gelatin. The graft tendons were retracted proximally without adhesions. 1 = tendon allograft six weeks after surgery, 2 = distal portion of the graft, 3 = proximal graft-host tendon junction, and 4 = normal neighboring flexor digitorum profundus tendon (in zone III). B: Histological specimen of the ruptured graft tendon (H & E, ×40).
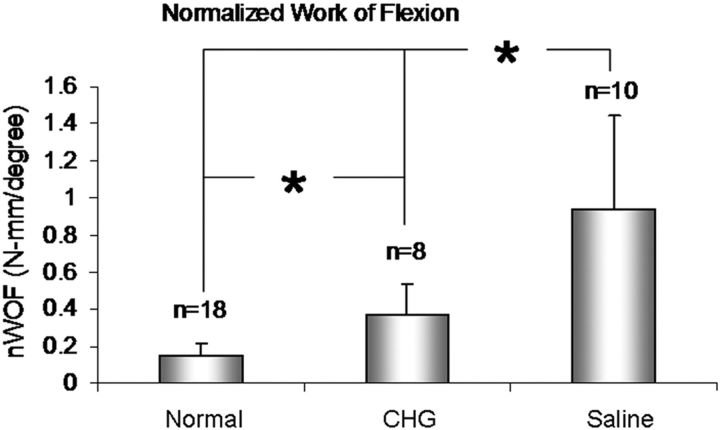
The normalized work of flexion was 0.14 ± 0.07, 0.37 ± 0.16, and 0.94 ± 0.51 N-mm/degree in the normal digits, CHG group, and saline-solution group, respectively (Table I). The normalized work of flexion in both the CHG and the saline-solution group was significantly higher than that in the normal digits (p < 0.05). The normalized work of flexion in the CHG group was significantly lower than that in the saline-solution group (p < 0.05) (Fig. 5).
Fig. 5.
Normalized work of flexion (and standard deviation) of the normal digits, the digits with an allograft treated with carbodiimide-derivatized hyaluronic acid and gelatin (CHG), and the digits with an allograft treated with saline solution. An asterisk indicates that the difference was significant.
TABLE I.
Study Results*
| Measurement | Normal | CHG† | Saline Solution |
| Normalized work of flexion (N-mm/degree) | 0.14 ± 0.07‡ | 0.37 ± 0.16‡ | 0.94 ± 0.51‡ |
| Gliding resistance (N) | 0.07 ± 0.03‡ | 0.18 ± 0.08‡ | 0.28 ± 0.13‡ |
| Maximal strength (N) | 22.2 ± 12.8 | 27.4 ± 16.4 |
The values are given as the mean and standard deviation.
CHG = carbodiimide-derivatized hyaluronic acid and gelatin.
The value in the saline solution group was significantly higher than that in the CHG group, which was significantly higher than that in the normal group (p < 0.05).
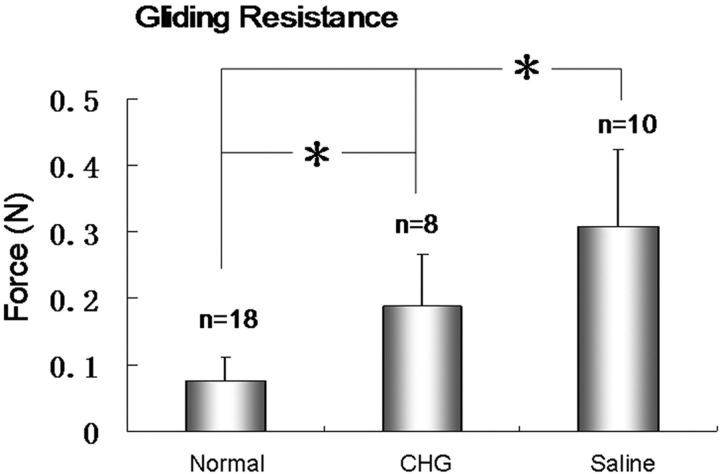
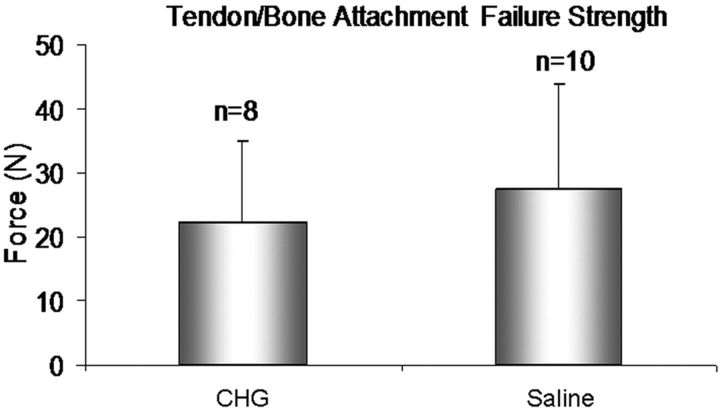
The gliding resistance was 0.07 ± 0.03, 0.18 ± 0.08, and 0.28 ± 0.13 N in the normal flexor digitorum profundus tendons, CHG group, and saline-solution group, respectively (Table I). The gliding resistance of the allograft tendons in both the CGH and saline-solution groups was significantly higher than that of the normal flexor digitorum profundus tendons from the untreated, contralateral digits (p < 0.05). The gliding resistance in the CHG group was significantly lower than that in the saline-solution group (p < 0.05) (Fig. 6). The failure strength at the distal tendon-bone junction was 22.2 ± 12.8 N and 27.4 ± 16.4 N in the CHG and saline-solution groups, respectively (Table I); there was no significant difference between these values (p > 0.05) (Fig. 7).
Fig. 6.
Gliding resistance (and standard deviation) of normal flexor digitorum profundus tendons, the allografts treated with carbodiimide-derivatized hyaluronic acid and gelatin (CHG), and the allografts treated with saline solution. An asterisk indicates that the difference was significant.
Fig. 7.
Maximal failure strength (and standard deviation) at the distal tendon-bone junction of the allografts treated with carbodiimide-derivatized hyaluronic acid and gelatin (CHG) and the allografts treated with saline solution.
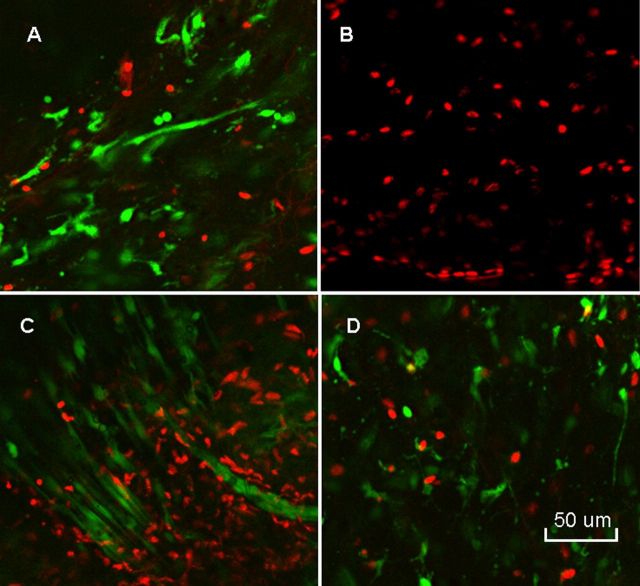
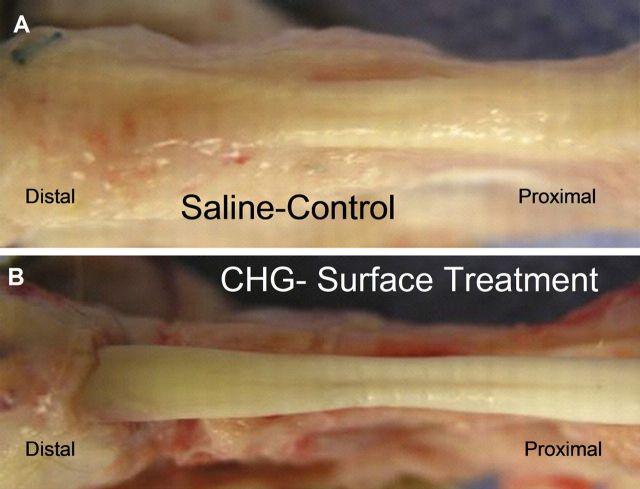
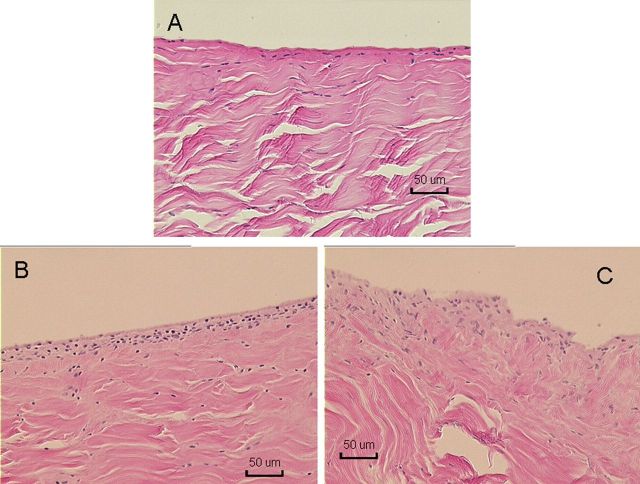
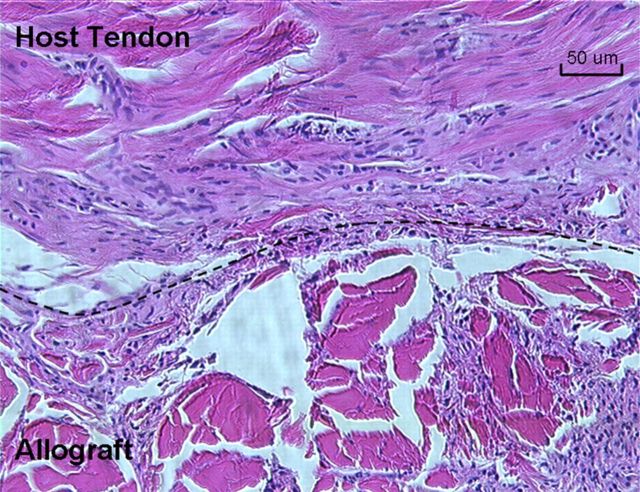
Non-quantitative evaluation of cell viability in two tendons from each group revealed a mixture of viable and dead cells in both the CHG and the saline-solution group. However, the penetration of the dyes and laser beam of the confocal microscope was limited to 50 μm, so cell viability could be assessed only to this depth (Fig. 8). With H & E staining, the grafts all showed collagen structure similar to that of the normal flexor digitorum profundus tendon, with no obvious difference between the CHG and saline-solution groups. Lymphocytes or other inflammatory cells were not observed in either the CHG or the saline-solution group. On the basis of observations during dissection, the CHG group seemed to have fewer adhesions along the graft itself than did the saline-solution group. The graft tendon surface within the flexor sheath was smooth and shiny in the CHG group but not in the saline-solution group (Fig. 9). The allografts all appeared to be structurally intact (Fig. 10). Histological analysis at the proximal tendon-tendon junction showed that tenocytes in the host tendon had proliferated and migrated toward the acellular allograft (Fig. 11). Qualitatively, there were no marked histological differences in this region between the two allograft groups. Under the scanning electron microscope, the surfaces of the allografts in the CHG and saline-solution groups displayed a similar morphology, while the saline-solution-treated grafts appeared to have a structure with fibrils or tendrils on the surface (see Appendix).
Fig. 8.
Staining with calcein AM and ethidium homodimer displays living cells in green and dead cells in red. Normal flexor digitorum profundus tendon (A) showed mostly living cells with only a few dead cells. Lyophilized flexor digitorum profundus tendon at time 0 (B) showed no living cells. The graft tendon treated with carbodiimide-derivatized hyaluronic acid and gelatin (C) and that treated with saline solution (D) showed both living and dead cells after six weeks in vivo (×200).
Fig. 9.
The graft tendon surface within the flexor sheath was smooth and shiny in the CHG (carbodiimide-derivatized hyaluronic acid and gelatin) group but not in the saline-solution group.
Fig. 10.
Histological specimens of normal flexor digitorum profundus tendon (A) and of allografts in the CHG (carbodiimide-derivatized hyaluronic acid and gelatin) group (B) and the saline-solution group (C) after six weeks (H & E, ×100).
Fig. 11.
The junction of the host flexor digitorum profundus tendon and the carbodiimide-derivatized hyaluronic acid and gelatin-treated allograft at the proximal repair site. The top of the dashed line is the host tendon. The bottom of the dashed line is the allograft. Tenocytes were observed in the allografts (H & E, ×200).
Discussion
Flexor tendon reconstruction is indicated when the injured tendon cannot be repaired directly, the interval following the injury exceeds the time when delayed primary repair is possible, or severe soft-tissue injury has caused a scarred digit45-48. Flexor tendon grafts are also often used when a primary repair has failed and extensive scarring is present15,49,50.
Although the canine model has been widely used for flexor tendon graft research because of its similarity in anatomy, structure, and function to human flexor tendons25,51-53, an appropriate preconditioning of the digit that mimics the clinical indications for flexor tendon grafting—i.e., a previously injured or operated-on bed—has been lacking. Previous investigators have used a normal digit as the recipient site25,53. Unfortunately, such idealized models, without the problems of wound-healing and scar formation that occur following injury to the tendon and flexor sheath, do not truly represent the pathological and biomechanical conditions that confront the hand surgeon clinically. In the current study, we performed flexor tendon grafting in an animal model incorporating a failed primary repair with a scarred digit, which is both clinically important and relevant. Although quantitative assessment of the scarred digit was not feasible, surgical exploration of the failed repairs revealed a consistent pattern of repair rupture with scar and adhesion around the distal tendon stump and with the proximal tendon stump retracted and free of adhesion. We believe that this failed primary repair model is novel, reproducible, uniform, reliable, and clinically relevant to the study of flexor tendon grafts.
Lyophilization is commonly used for long-term storage of allograft tissue at room temperature54,55. Potenza and Melone used freeze-dried allograft tendons that had been stored for more than ten years and found no degeneration of the graft histologically51. Freeze-drying techniques also did not alter the mechanical properties of allograft tendons compared with those of autografts52. Because the freeze-thaw technique has been shown to produce necrosis of >97% of all viable tenocytes56, there would have been no, or very few, viable cells located within the allograft tendon at the time of implantation, so the cells noted in the retrieved grafts were the result of migration of host cells. Recently, several studies have demonstrated that surface modification with carbodiimide-derivatized hyaluronic acid and gelatin improves the gliding ability of tendons in vitro24,57 and in vivo25 without altering tendon mechanical properties. The current study, however, is the first to address the use of such modification of intrasynovial allografts.
The outcomes regarding postoperative adhesions and functional recovery in the current study were parallel to our previous findings in an autograft model25. In that study, modification with carbodiimide-derivatized hyaluronic acid and gelatin decreased adhesions, improved digital function, and decreased the gliding resistance of the grafts. In contrast, flexor tendon reconstruction with intrasynovial allograft tendon has been infrequently reported in either the clinical literature34 or animal studies52. A recent study demonstrated that the biomechanical advantages of tendon reconstruction with autografts instead of devitalized allografts were minimal58, although the clinical validity of a small-animal model with small tendon lesions outside of zone II can be debated. A comparison of the data from the current study of allograft intrasynovial tendons with those in our previous report on autograft extrasynovial tendons25 suggests that digital function after use of an intrasynovial allograft modified with carbodiimide-derivatized hyaluronic acid and gelatin is superior to that after use of an extrasynovial autograft modified in the same way. Clinical comparisons between autograft and allograft tendons have also been well documented in ACL reconstructions, albeit with contradictory outcomes26-28,59. Because of differing clinical circumstances related to the lack of graft motion in ligament reconstruction, though, it is not clear that studies of tendon and ligament graft adhesions are comparable.
Pring et al. tested the normal flexor digitorum profundus tendon insertion in human cadavers60. The breaking strength at the tendon insertion was 558 N, which was less than half of the flexor digitorum profundus tendon breaking strength (reported as 1175 N). Although canine flexor digitorum profundus tendons are smaller than human flexor digitorum profundus tendons, the failure strength at the canine flexor digitorum profundus insertion is similar, 564 N61. In contrast to the normal values, the initial failure strength after use of the most common technique for tendon-bone repair—i.e., use of a pullout suture and a bone tunnel—is 70 N in both the canine model62,63 and the human cadaveric model64. This insertion repair strength decreases at three weeks after the repair to <50% of the initial repair strength62,63. Even at six weeks after repair, the strength is no greater than the initial strength65. The strength of the tendon-bone attachment may be even lower for a tendon graft than for a direct repair.
In the current study, six of twenty-eight allografts ruptured at the distal tendon-bone junction. The rupture rate was higher than that reported for autografts25,66. Although no difference in failure strength at the tendon-bone insertion site was found between the CHG and saline-solution groups, there were more ruptures in the CHG group than in the saline-solution group (four versus two). This might be related to the effect of the surface modification of the distal part of the tendon within the osseous tunnel, which may have affected tendon-bone integration. In future studies, we will shield the distal part of the tendon from the surface treatment, in the hope of improving tendon-bone healing. Fewer adhesions in the CHG group may also have played a role, since dense adhesions would have added to the effective strength of the tendon-bone junction and also reduced the amount of tensile force transmitted to the distal juncture in vivo. Finally, the acellular nature of the allograft may have played a role, since cellular ingrowth was required for healing to occur51. Delayed healing is a common feature of allografts clinically26,27.
This study had several limitations. First, the data were obtained only at six weeks postoperatively. Mechanical and biological behaviors of the allograft were not assessed at time points earlier or later than six weeks. Second, we did not analyze residual hyaluronic acid on the tendon surfaces. However, a previous report demonstrated no difference in surface hyaluronic acid concentration after six weeks in an in vivo autograft model25. Third, cell viability was observed in only two samples in each group and only with non-quantitative methods. Fourth, we did not specifically label host and graft cells and therefore cannot definitively identify the source of the cells in the grafts noted after the animals were killed. However, since the freeze-thaw technique has been shown to produce necrosis of >97% of all viable tenocytes56, there would have been no, or very few, viable cells located within the allograft tendon at the time of implantation, so the cells noted in the retrieved grafts are the result of migration of host cells. Finally, some biological analyses were not performed such as analysis of collagen and cytokine synthesis. Thus, we are unable to comment on cellular function in our model.
In summary, we have successfully developed a canine model of flexor tendon reconstruction following failed primary repair. This model is reproducible, uniform, reliable, and highly clinically relevant for the study of flexor tendon grafts. We also demonstrated that the surface modification of allografts with carbodiimide-derivatized hyaluronic acid and gelatin improved digital function, reduced adhesions, and decreased gliding resistance compared with those characteristics following use of allografts without such modification. These results are similar to the outcomes when carbodiimide-derivatized hyaluronic acid and gelatin was used in autografts in a previous report25. Compared with the autografts in that previous published report, the allografts had fewer adhesions and provided better digital function. However, tendon-bone healing of the allograft repairs was delayed, leading to more ruptures and weaker distal attachments. If this adverse effect could be overcome, intrasynovial allografts treated with carbodiimide-derivatized hyaluronic acid and gelatin could become a clinically useful alternative to the current benchmark of extrasynovial autografts for reconstruction of failed tendon repairs in the hand.
Appendix
A figure showing SEM images of CHG-treated, saline-solution-treated, and normal flexor tendons is available with the electronic version of this article on our web site at jbjs.org (go to the article citation and click on “Supporting Data”).
Footnotes
Investigation performed at the Biomechanics Laboratory, Department of Orthopedic Surgery, Mayo Clinic, Rochester, Minnesota
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the Musculoskeletal Transplant Foundation (MTF). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Rosberg HE Dahlin LB. Epidemiology of hand injuries in a middle-sized city in southern Sweden: a retrospective comparison of 1989 and 1997. Scand J Plast Reconstr Surg Hand Surg. 2004;38:347-55. [DOI] [PubMed] [Google Scholar]
- 2.Costilla V Bishai DM. Lawnmower injuries in the United States: 1996 to 2004. Ann Emerg Med. 2006;47:567-73. [DOI] [PubMed] [Google Scholar]
- 3.Fetter-Zarzeka A Joseph MM. Hand and fingertip injuries in children. Pediatr Emerg Care. 2002;18:341-5. [DOI] [PubMed] [Google Scholar]
- 4.Simon A Kazár G. [Epidemiology of hand injuries based on surveys in Vas County]. Magy Traumatol Ortop Kezseb Plasztikai Seb. 1993;36:141-6. Hungarian. [PubMed] [Google Scholar]
- 5.Strickland JW. Flexor tenolysis. Hand Clin. 1985;1:121-32. [PubMed] [Google Scholar]
- 6.Silfverskiöld KL May EJ. Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion. J Hand Surg Am. 1994;19:53-60. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez JD Stern PJ. Complex injuries including flexor tendon disruption. Hand Clin. 2005;21:187-97. [DOI] [PubMed] [Google Scholar]
- 8.McClinton MA Curtis RM Wilgis EF. One hundred tendon grafts for isolated flexor digitorum profundus injuries. J Hand Surg Am. 1982;7:224-9. [DOI] [PubMed] [Google Scholar]
- 9.Amadio PC Wood MB Cooney WP 3rd Bogard SD. Staged flexor tendon reconstruction in the fingers and hand. J Hand Surg Am. 1988;13:559-62. [DOI] [PubMed] [Google Scholar]
- 10.Liu TK Yang RS. Flexor tendon graft for late management of isolated rupture of the profundus tendon. J Trauma. 1997;43:103-6. [DOI] [PubMed] [Google Scholar]
- 11.Smith P Jones M Grobbelaar A. Two-stage grafting of flexor tendons: results after mobilisation by controlled early active movement. Scand J Plast Reconstr Surg Hand Surg. 2004;38:220-7. [DOI] [PubMed] [Google Scholar]
- 12.Abrahamsson SO Gelberman RH Lohmander SL. Variations in cellular proliferation and matrix synthesis in intrasynovial and extrasynovial tendons: an in vitro study in dogs. J Hand Surg Am. 1994;19:259-65. [DOI] [PubMed] [Google Scholar]
- 13.Duffy FJ Seiler JG Hergrueter CA Kandel J Gelberman RH. Intrinsic mitogenic potential of canine flexor tendons. J Hand Surg Br. 1992;17:275-7. [DOI] [PubMed] [Google Scholar]
- 14.Abrahamsson SO Gelberman RH Amiel D Winterton P Harwood F. Autogenous flexor tendon grafts: fibroblast activity and matrix remodeling in dogs. J Orthop Res. 1995;13:58-66. [DOI] [PubMed] [Google Scholar]
- 15.Leversedge FJ Zelouf D Williams C Gelberman RH Seiler JG 3rd. Flexor tendon grafting to the hand: an assessment of the intrasynovial donor tendon-A preliminary single-cohort study. J Hand Surg Am. 2000;25:721-30. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi M Seiler JG 3rd Boardman ND 3rd Tramaglini DM Gelberman RH Woo SL. Tensile properties of canine intrasynovial and extrasynovial flexor tendon autografts. J Hand Surg Am. 1997;22:457-63. [DOI] [PubMed] [Google Scholar]
- 17.Tzianabos AO Cisneros RL Gershkovich J Johnson J Miller RJ Burns JW Onderdonk AB. Effect of surgical adhesion reduction devices on the propagation of experimental intra-abdominal infection. Arch Surg. 1999;134:1254-9. [DOI] [PubMed] [Google Scholar]
- 18.Shih HN Fang JF Chen JH Yang CL Chen YH Sung TH Shih LY. Reduction in experimental peridural adhesion with the use of a crosslinked hyaluronate/collagen membrane. J Biomed Mater Res B Appl Biomater. 2004;71:421-8. [DOI] [PubMed] [Google Scholar]
- 19.Mao JS Liu HF Yin YJ Yao KD. The properties of chitosan-gelatin membranes and scaffolds modified with hyaluronic acid by different methods. Biomaterials. 2003;24:1621-9. [DOI] [PubMed] [Google Scholar]
- 20.Park SN Park JC Kim HO Song MJ Suh H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide cross-linking. Biomaterials. 2002;23:1205-12. [DOI] [PubMed] [Google Scholar]
- 21.Park SN Kim JK Suh H. Evaluation of antibiotic-loaded collagen-hyaluronic acid matrix as a skin substitute. Biomaterials. 2004;25:3689-98. [DOI] [PubMed] [Google Scholar]
- 22.Cashman J Burt HM Springate C Gleave J Jackson JK. Camptothecin-loaded films for the prevention of postsurgical adhesions. Inflamm Res. 2004;53:355-62. [DOI] [PubMed] [Google Scholar]
- 23.Sun YL Yang C Amadio PC Zhao C Zobitz ME An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22:984-9. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka T Sun YL Zhao C Zobitz ME An KN Amadio PC. Optimization of surface modifications of extrasynovial tendon to improve its gliding ability in a canine model in vitro. J Orthop Res. 2006;24:1555-61. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C Sun YL Amadio PC Tanaka T Ettema AM An KN. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid. An in vivo canine model. J Bone Joint Surg Am. 2006;88:2181-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dustmann M Schmidt T Gangey I Unterhauser FN Weiler A Scheffler SU. The extracellular remodeling of free-soft-tissue autografts and allografts for reconstruction of the anterior cruciate ligament: a comparison study in a sheep model. Knee Surg Sports Traumatol Arthrosc. 2008;16:360-9. [DOI] [PubMed] [Google Scholar]
- 27.Jackson DW Grood ES Goldstein JD Rosen MA Kurzweil PR Cummings JF Simon TM. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176-85. [DOI] [PubMed] [Google Scholar]
- 28.Kustos T Bálint L Than P Bárdos T. Comparative study of autograft or allograft in primary anterior cruciate ligament reconstruction. Int Orthop. 2004;28:290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole DW Ginn TA Chen GJ Smith BP Curl WW Martin DF Poehling GG. Cost comparison of anterior cruciate ligament reconstruction: autograft versus allograft. Arthroscopy. 2005;21:786-90. [DOI] [PubMed] [Google Scholar]
- 30.Brown JA Brophy RH Franco J Marquand A Solomon TC Watanabe D Mandelbaum BR. Avoiding allograft length mismatch during anterior cruciate ligament reconstruction: patient height as an indicator of appropriate graft length. Am J Sports Med. 2007;35:986-9. [DOI] [PubMed] [Google Scholar]
- 31.Ramesh R Kumar N Sharma AK Maiti SK Singh GR. Acellular and glutaraldehyde-preserved tendon allografts for reconstruction of superficial digital flexor tendon in bovines: Part I—Clinical, radiological and angiographical observations. J Vet Med A Physiol Pathol Clin Med. 2003;50:511-9. [DOI] [PubMed] [Google Scholar]
- 32.Gulati AK Cole GP. Nerve graft immunogenicity as a factor determining axonal regeneration in the rat. J Neurosurg. 1990;72:114-22. [DOI] [PubMed] [Google Scholar]
- 33.Goertzen M Dellmann A Gruber J Clahsen H Bürrig KF. Anterior cruciate ligament allograft transplantation for intraarticular ligamentous reconstruction. Arch Orthop Trauma Surg. 1992;111:273-9. [DOI] [PubMed] [Google Scholar]
- 34.Liu TK. Clinical use of refrigerated flexor tendon allografts to replace a silicone rubber rod. J Hand Surg Am. 1983;8:881-7. [DOI] [PubMed] [Google Scholar]
- 35.Tejwani SG Shen W Fu FH. Soft tissue allograft and double-bundle reconstruction. Clin Sports Med. 2007;26:639-60. [DOI] [PubMed] [Google Scholar]
- 36.Tohyama H Yasuda K. Extrinsic cell infiltration and revascularization accelerate mechanical deterioration of the patellar tendon after fibroblast necrosis. J Biomech Eng. 2000;122:594-9. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka T Sun YL Zhao C Zobitz ME An KN Amadio PC. Effect of curing time and concentration for a chemical treatment that improves surface gliding for extrasynovial tendon grafts in vitro. J Biomed Mater Res. 2006;79:451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bishop AT Cooney WP 3rd Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma Injury. 1986;26:301-12. [DOI] [PubMed] [Google Scholar]
- 39.Zhao C Amadio PC Momose T Couvreur P Zobitz ME An KN. Effect of synergistic wrist motion on adhesion formation after repair of partial flexor digitorum profundus tendon lacerations in a canine model in vivo. J Bone Joint Surg Am. 2002;84:78-84. [PubMed] [Google Scholar]
- 40.Zhao C Amadio PC Paillard P Tanaka T Zobitz ME Larson DR An KN. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2004;86:320-7. [DOI] [PubMed] [Google Scholar]
- 41.Zhao CF Amadio PC Berglund L An KN. The A3 pulley. J Hand Surg Am. 2000;25:270-6. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka T Zhao C Sun YL Zobitz ME An KN Amadio PC. The effect of carbodiimide-derivatized hyaluronic acid and gelatin surface modification on peroneus longus tendon graft in a short-term canine model in vivo. J Hand Surg Am. 2007;32:876-81. [DOI] [PubMed] [Google Scholar]
- 43.Chen MY Sun YL Zhao C Zobitz ME An KN Moran SL Amadio PC. Substrate adhesion affects contraction and mechanical properties of fibroblast populated collagen lattices. J Biomed Mater Res B Appl Biomater. 2008;84:218-23. [DOI] [PubMed] [Google Scholar]
- 44.Zhao C Amadio PC Momose T Zobitz ME Couvreur P An KN. Remodeling of the gliding surface after flexor tendon repair in a canine model in vivo. J Orthop Res. 2002;20:857-62. [DOI] [PubMed] [Google Scholar]
- 45.Honner R Meares A. A review of 100 flexor tendon reconstructions with prosthesis. Hand. 1977;9:226-31. [DOI] [PubMed] [Google Scholar]
- 46.Strickland JW. Flexor tendon injuries. Part 3. Free tendon grafts. Orthop Rev. 1987;16:18-26. [PubMed] [Google Scholar]
- 47.Beris AE Darlis NA Korompilias AV Vekris MD Mitsionis GI Soucacos PN. Two-stage flexor tendon reconstruction in zone II using a silicone rod and a pedicled intrasynovial graft. J Hand Surg Am. 2003;28:652-60. [DOI] [PubMed] [Google Scholar]
- 48.Strickland JW. Delayed treatment of flexor tendon injuries including grafting. Hand Clin. 2005;21:219-43. [DOI] [PubMed] [Google Scholar]
- 49.Pulvertaft RG. Tendon grafting for the isolated injury of flexor digitorum profundus. Bull Hosp Jt Dis Orthop Inst. 1984;44:424-34. [PubMed] [Google Scholar]
- 50.Strickland JW. Flexor tendon surgery. Part 2: free tendon grafts and tenolysis. J Hand Surg Br. 1989;14:368-82. [DOI] [PubMed] [Google Scholar]
- 51.Potenza AD Melone C. Evaluation of freeze-dried flexor tendon grafts in the dog. J Hand Surg Am. 1978;3:157-62. [DOI] [PubMed] [Google Scholar]
- 52.Webster DA Werner FW. Mechanical and functional properties of implanted freeze-dried flexor tendons. Clin Orthop Relat Res. 1983;180:301-9. [PubMed] [Google Scholar]
- 53.Seiler JG 3rd Chu CR Amiel D Woo SL Gelberman RH. The Marshall R. Urist Young Investigator Award. Autogenous flexor tendon grafts. Biologic mechanisms for incorporation. Clin Orthop Relat Res. 1997;345:239-47. [PubMed] [Google Scholar]
- 54.Friedlaender GE. Immune responses to osteochondral allografts. Current knowledge and future directions. Clin Orthop Relat Res. 1983;174:58-68. [PubMed] [Google Scholar]
- 55.Czitrom AA Langer F McKee N Gross AE. Bone and cartilage allotransplantation. A review of 14 years of research and clinical studies. Clin Orthop Relat Res. 1986;208:141-5. [PubMed] [Google Scholar]
- 56.Katsuragi R Yasuda K Tsujino J Keira M Kaneda K. The effect of nonphysiologically high initial tension on the mechanical properties of in situ frozen anterior cruciate ligament in a canine model. Am J Sports Med. 2000;28:47-56. [DOI] [PubMed] [Google Scholar]
- 57.Sun YL Yang C Amadio PC Zhao C Zobitz ME An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22:984-9. [DOI] [PubMed] [Google Scholar]
- 58.Hasslund S Jacobson JA Dadali T Basile P Ulrich-Vinther M Søballe K Schwarz EM O’Keefe RJ Mitten DJ Awad HA. Adhesions in a murine flexor tendon graft model: autograft versus allograft reconstruction. J Orthop Res. 2008;26:824-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheffler SU Schmidt T Gangéy I Dustmann M Unterhauser F Weiler A. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24:448-58. [DOI] [PubMed] [Google Scholar]
- 60.Pring DJ Amis AA Coombs RR. The mechanical properties of human flexor tendons in relation to artificial tendons. J Hand Surg Br. 1985;10:331-6. [DOI] [PubMed] [Google Scholar]
- 61.Yuan F Zhao J Wang Y Huangfu X Lu Q. [Biomechanical research of reconstructing anterior cruciate ligament by implanting various length of autogenous tendon into bone tunnel]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:290-3. Chinese. [PubMed] [Google Scholar]
- 62.Thomopoulos S Matsuzaki H Zaegel M Gelberman RH Silva MJ. Alendronate prevents bone loss and improves tendon-to-bone repair strength in a canine model. J Orthop Res. 2007;25:473-9. [DOI] [PubMed] [Google Scholar]
- 63.Silva MJ Thomopoulos S Kusano N Zaegel MA Harwood FL Matsuzaki H Havlioglu N Dovan TT Amiel D Gelberman RH. Early healing of flexor tendon insertion site injuries: Tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24:990-1000. [DOI] [PubMed] [Google Scholar]
- 64.Matsuzaki H Zaegel MA Gelberman RH Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone I flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33:709-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silva MJ Boyer MI Ditsios K Burns ME Harwood FL Amiel D Gelberman RH. The insertion site of the canine flexor digitorum profundus tendon heals slowly following injury and suture repair. J Orthop Res. 2002;20:447-53. [DOI] [PubMed] [Google Scholar]
- 66.Seiler JG 3rd Gelberman RH Williams CS Woo SL Dickersin GR Sofranko R Chu CR Rosenberg AE. Autogenous flexor-tendon grafts. A biomechanical and morphological study in dogs. J Bone Joint Surg Am. 1993;75:1004-14. [DOI] [PubMed] [Google Scholar]