Abstract
Background
Oral malodor is a very discomforting condition deriving from the presence of volatile sulfur compounds in the expired air. In halitosis of intraoral etiology, the volatile sulfur compounds are metabolic products of the oral microorganisms within the biofilm coating the tongue dorsum as well as other tissues in the oral cavity. The aim of this study was to characterize and compare the microbial composition of tongue biofilm in volunteers suffering from halitosis and healthy volunteers by means of both the culture method and culture‐independent cloning technique.
Results
A high bacterial variety (more than 80 different species) was detected using the combination of both methods. A distinct bacterial composition was revealed in the halitosis‐associated biofilms compared with the health‐associated biofilms. Actinomyces graevenitzii was shown to be significantly associated with the halitosis condition. The culture method identified 47 species, included Veillonella rogosae, never isolated from the tongue biofilm of halitosis patients so far. In the healthy condition, the culture‐dependent method showed that the most frequent species were Streptococcus parasanguinis among the aerobes and Veillonella spp. among the anaerobes. The culture‐independent cloning method detected more than 50 species. Streptococci, in particular S. mitis/oralis, S. pseudopneumoniae, and S. infantis as well as Prevotella spp., were found most frequently in halitosis patients. Streptococcus salivarius and Rothia mucilaginosa were found more frequently in the healthy condition.
Conclusions
The combination of the culture‐dependent and culture‐independent cloning techniques allowed for a widespread analysis of the tongue biofilm in halitosis patients. The results can support further pharmacological research for new antimicrobial agents and halitosis therapy strategies.
Keywords: culture‐independent cloning technique, halitosis, microbial culture, tongue biofilm
Oral malodor is a discomforting condition deriving from the presence of volatile sulfur compounds in the expired air. The study characterized and compared the microbial composition of tongue biofilm in volunteers suffering from halitosis and healthy volunteers by means of both the culture method and culture‐independent cloning technique, revealing a distinct bacterial composition of halitosis‐associated biofilms compared with the health‐associated biofilms and a high bacterial diversity within the halitosis‐associated biofilms.

1. INTRODUCTION
Halitosis is widely known as malodor deriving from exhaled breath due to the presence of volatile sulfur compounds (VSCs) arising from the oral cavity or from the upper airways (Scully & Greenman, 2008). The VSCs include hydrogen sulfide, methyl mercaptan, and dimethyl sulfide (Scully & Porter, 2008). The volatile products causing intraoral halitosis derive from the interaction of oral microbiota with specific substrates, such as the amino acid cysteine, methionine, tryptophan, arginine, and lysine that are metabolized into the different VSCs (Dzink & Socransky, 1990).
Clinical halitosis is classified according to the primary source. We can therefore distinguish between intraoral halitosis, with the oral cavity as etiological source, and extraoral halitosis, usually a symptom of a pathological disease (Tangerman & Winkel, 2010), such as an organ dysfunction or systemic disease. In that context, respiratory disorders or respiratory tract inflammations, as well as diseases of the gastrointestinal system, can result in the release of smelly gases within the oral cavity and the nose. Concerning the gastrointestinal apparatus, gastroesophageal reflux disease (GERD) and Helicobacter pylori‐related diseases are also associated with bad breath. Systemic diseases such as diabetes, renal failure, liver disease, trimethylaminuria, hypermethioninemia, and cystinosis can also have a specific malodor as a clinical manifestation (Madhushankari et al., 2015; Scully & Porter, 2008; Tangerman & Winkel, 2010).
The organoleptic difference between the intraoral and extraoral halitosis consists in the composition of the VSCs. Indeed, hydrogen sulfide and methyl mercaptan have been found to be the main contributors to intraoral halitosis, whereas dimethyl sulfide is more associated with extraoral, “blood‐borne” halitosis (Tangerman & Winkel, 2010). Intraoral halitosis is associated with periodontal diseases, poor oral hygiene, salivary flow alterations, cancerous lesions, and bone necrosis (Dzink & Socransky, 1990). It is etiologically related to the microbiota of the dorsal tongue biofilm (Roldán, Herrera, & Sanz, 2003; Yaegaki & Coil, 2000), and in particular to the presence of anaerobic microorganisms responsible for the production of VSCs, such as Centipeda periodontii, Eikenella corrodens, Fusobacterium nucleatum, F. periodonticum, Porphyromonas gingivalis, Prevotella melaninogenica, P. intermedia, Solobacterium moorei, Tannerella forsythia, and Treponema denticola. Due to its papillary structure that creates an ecological niche for microorganisms, the tongue biofilm represents an oral microenvironment which is well distinguished from the supragingival biofilm, also known as dental plaque, and the subgingival biofilm (Amou, Hinode, Yoshioka, & Grenier, 2014; Bernardi et al., 2018; Bernardi, Marzo, & Continenza, 2016; Bernardi, Zeka, Mummolo, Marzo, & Continenza, 2013).
To date, the halitosis‐relevant literature comprises many studies on the microbial characterization of the biofilm using in vitro models, culture technique, species‐specific PCR (Brunner, Kurmann, & Filippi, 2010; Mashima, Kamaguchi, & Nakazawa, 2011), confocal laser scanning microscopy study (Bernardi et al., 2019), and quantitative PCR assays (Vancauwenberghe et al., 2013), allowing for the study of the targeted species, as well as a few studies applying high‐throughput sequencing to tongue biofilm (Hall et al., 2017; Ren et al., 2016; Seerangaiyan et al., 2017).
Up to now, over 300 bacterial species have been found inhabiting the tongue (Yang et al., 2013), revealing a high bacterial diversity within this biofilm (Mashima et al., 2011; Mashima & Nakazawa, 2013; Vancauwenberghe et al., 2013).
The aim of this study was to characterize the in vivo biofilm on the dorsal tongue surface combining molecular and culture techniques in healthy volunteers and halitosis patients, in order to understand which microbial taxa contribute to the halitosis‐associated tongue biofilm. So far, this combination of methods has not been used to study this particular biofilm. The open‐end approach of the molecular cloning technique in addition to the culture method represents a valid contribution to the research in this field.
2. METHODS
2.1. Subjects and samples
According to the study protocol six patients affected by oral malodor and six healthy volunteers were recruited. The presence of halitosis was assessed by the instrumental measurement of exhaled air, using a sulfide monitor (Halimeter, manufactured by Interscan Corporation). Furthermore, the medical history and dental history were comprehensively checked as well as periodontal clinical investigations performed: Periodontal probing and gingival bleeding were assessed. Subsequently, the tongue dorsum biofilm was collected using 0.1 ml sterile inoculating loops. The sampling was performed with two loops. The pooled samples were divided and stored in two vials containing 0.75 ml Reduced Transfer Fluid (RTF) (Syed & Loesche, 1972) and kept at −80°C prior to use.
2.2. Clinical halitosis assessment
A total of 12 patients and volunteers were recruited at the Dental Clinic of the University of Basel, Switzerland. The patients included in the study suffered from intraoral halitosis. The exclusion criteria were (a) presence of extraoral halitosis, (b) diagnosis of a mental illness, (c) patients aged under 18 years, (d) the intake of antibiotics in the previous 3 months before the start of the study and/or the use of antiseptics one month before study start, and (e) poor general health with reference to American Society of Anesthesiologists Physical Classification System. Prior to the sampling procedure, a general medical history questionnaire was submitted to the participants of the study (Table 1). The periodontal status of each participant was then assessed and documented, using the Periodontal Screening and Recording (PSR) Index, recommended by the American Dental Association as an established stage of oral diagnostic examinations for all dental patients (Periodontology, 1993). The presence of VSCs was determined by means of a Halimeter (Brunner et al., 2010), and the results were recorded. Lastly, the tongue dorsum biofilm samples were collected as described above.
Table 1.
Anamnestic questionnaire
| Patient number |
| Anamnestic questionnaire |
| Age |
| Gender |
| Current health Status |
| Do You suffer from chronic gastroesophageal reflux? |
| Do You suffer from diabetes? |
| Do You suffer from renal disease (chronic kidney failure)? |
| Did You undergo antibiotic treatment during the last 3 months? |
| If so, do You remember the medication? |
| Habits |
| Do You drink alcohol regularly? (more than three times a week) |
| Do You smoke? |
| Do You brush Your tongue? If yes, with what frequency? |
| Periodontal health status |
| Does the patient wear a removable prosthetic device? |
| Number of present teeth |
| Number of missing teeth |
| PSR INDEX |
| PSR™ |
| Code 0 indicated periodontal health (neither bleeding on probing nor defective restoration margins and gingival sulcus depths <3.5 mm) |
| Code 1 indicated bleeding on probing, no defective restoration margins and a gingival sulcus depth <3.5 mm at a minimum of one site within the sextant |
| Code 2 indicated bleeding on probing, the presence of supra‐ or subgingival calculus, defective restoration margins, and a gingival sulcus depth <3.5 mm at a minimum of one site within the sextant |
| Code 3 indicated bleeding on probing and a pocket depth of 3.5–5.5 mm at a minimum of one site within the sextant |
|
Code 4 indicated that a pocket depth >5.5 mm was present at a minimum of one site within the sextant (American Dental Association and American Academy of Periodontology, 1992) |
| VSCs Analysis result |
2.3. Culture method
The culture method was performed as described in detail previously (Schirrmeister et al., 2009). The vials containing the samples in RTF were thawed at 36°C in a water bath and vortexed for 30–45 s. For the isolation and identification of the microorganisms, 100 µl of the undiluted sample and serial dilutions thereof were cultivated. The serial dilutions (10−1–10−7) were prepared in peptone yeast medium (PY). Each dilution was plated on yeast cysteine blood agar plates (HCB) to cultivate anaerobic bacteria at 37°C for 10 days and on Columbia blood agar plates (CBA), incubated at 37°C and 5%–10% CO2 atmosphere for 5 days to cultivate aerobic species. The resulting colony types were phenotypically evaluated and counted to calculate the number of colony‐forming units (CFUs) per ml in the original sample. All colony types were subcultivated to obtain pure cultures which were analyzed by MALDI‐TOF (MALDI Biotyper, Bruker Daltonik GmbH), as described in detail by our own group (Anderson et al., 2014).
2.4. DNA Isolation
The biofilm samples were centrifuged at 16.000 g for 10 min, and the supernatant was discarded. Lysis of microbial cells was then performed using a Precellys 24 bead mill homogenizer (PEQLab Biotechnologie GmbH) in ATL buffer (QiaAMP Micro Kit; Qiagen, Hilden). The vials were shaken twice at 3,500 rpm for 30 s. The DNA was subsequently purified by means of QiaAMP Micro Kit (Qiagen, Hilden) according to the manufacturer's protocol for tissue samples. The total microbial DNA was eluted twice with 50 µl AE buffer (Qiagen) and then stored at −20°C.
2.5. PCR Amplification of 16S rRNA Genes
Bacterial 16S rRNA genes were amplified using the following universal primers: 27F‐YM (5′‐AGAGTTTGATYMTGGCTCAG‐3′) and 1492R (5′ TACGGYTACCTTGTTACGACTT‐3′) (Frank et al., 2008). The PCR amplification was performed in a total volume of 50 µl. The reaction mixture contained 1 × PCR buffer (Qiagen), 0.2 mM each of the four deoxyribonucleoside triphosphates (dNTPs; PEQLab Biotechnologie), 0.5 µM of forward and reverse primers, 2 µl UTaq‐Polymerase (Qiagen), and 5 µl of the isolated sample DNA. The PCR cycling conditions consisted of a denaturation step at 94°C for 2 min, followed by 35 cycles with denaturation at 94°C for 1 min; annealing at 55°C for 1 min; extension at 72°C for 1.5 min; and a final extension step at 72°C for 10 min.
A no‐template control and a positive control were included in each set of PCRs. PCR reaction products were analyzed by electrophoresis in a 1.5% agarose gel and positive reactions were used to prepare clone libraries.
2.6. Cloning of PCR products and analysis of clone libraries
The 16S rDNA amplification products were ligated into the PCR®2.1‐TOPO® plasmid vector using the TOPO TA Cloning® Kit (Invitrogen, Life Technologies) according to the manufacturer's protocol and as described in detail earlier (Anderson et al., 2012). Fifty white clones from each library were picked, and the presence of inserts was confirmed by PCR amplification with their respective primers, followed by gel electrophoresis. PCR products of all recombinants were subjected to a restriction enzyme digestion with Hha I, Rsa I, and Hinf I (New England Biolabs GmbH). Fragment length patterns were compared and grouped if they were similar. One representative clone was selected from each group and used for sequencing. Sequencing was performed on an automated ABI 3,730 × l DNA Analyzer (Applied Biosystems, Life Technologies GmbH).
2.7. Sequence analysis
The sequence data obtained from the ABI sequencer were visually proofread and edited using the Ridom TraceEdit software (Ridom GmbH). The partial and almost full‐length 16S rDNA sequences were compared with those from public sequence databases, GenBank, EMBL, and DDBJ using the BLAST program, which was run through the server hosted by the National Center for Biotechnology Information (http://www.ncbi.nigh.gov/BLAST) (Altschul, Gish, Miller, Myers, & Lipman, 1990).
The sequences that showed 98% similarity or less with public database sequences were checked for chimeras with the Pintail software (version 1.0) (Ashelford, Chuzhanova, Fry, Jones, & Weightman, 2005). The chimeric sequences were excluded from further analysis. The sequences with a 99%–100% match to a database sequence were considered to belong to the same species as the one with the highest similarity and score bits. In addition, all 16S rDNA sequences were compared with the database sequences of the Ribosomal Database Project (http://rdp.cme.msu.edu/) (Cole et al., 2009).
2.8. Statistical analysis
The concentration and the abundance of the species were analyzed with descriptive and associative statistical test (Wilcoxon rank sum and Fisher's exact tests). All calculations were done by the statistical software STATA 14.1.
3. RESULTS
3.1. Clinical assessment
The six recruited halitosis patients, four female and two male subjects, were between 25 and 65 years old. Two patients claimed to suffer from gastroesophageal disorder within the limit of the physiological disturbance, and one of them was a smoker. Tongue brushing was not performed by any of them as part of normal oral hygiene procedure. The PSR Index was between 0 and 3, indicating a certain degree of periodontal disease and the Halimeter values ranged from 122 to 226 parts per billion (Table 2).
Table 2.
Overview of the outcomes of the anamnestic and clinical assessments
| Age | Gender | Gastroesophageal reflux | Diabetes | Renal disease | Alcohol | Smoke | Brush tongue | Rem. Prost | PSR Index | HALIMETER | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Halitosis Group | 62 | F | YES | NO | NO | NO | NO | NO | NO | 1 | 122 ppb |
| 38 | M | NO | NO | NO | NO | NO | NO | NO | 1 | 133 ppb | |
| 51 | M | NO | NO | NO | NO | NO | NO | NO | 3 | 152 ppb | |
| 65 | F | NO | NO | NO | NO | NO | NO | NO | 1 | 123 ppb | |
| 43 | F | YES | NO | NO | NO | NO | NO | NO | 0 | 125 ppb | |
| 29 | F | NO | NO | NO | NO | YES | NO | NO | 1 | 226 ppb | |
| Control Group | 33 | F | NO | NO | NO | NO | NO | NO | NO | 0 | 0 |
| 26 | F | NO | NO | NO | NO | NO | NO | NO | 0 | 0 | |
| 25 | M | NO | NO | NO | YES | NO | NO | NO | 0 | 0 | |
| 23 | F | NO | NO | NO | NO | NO | ONCE A WEEK | NO | 0 | 0 | |
| 28 | F | NO | NO | NO | NO | NO | ONCE A DAY | NO | 0 | 0 | |
| 22 | M | NO | NO | NO | NO | NO | NO | NO | 0 | 0 |
The ages of the six healthy volunteers ranged between 22 and 33 years. The tongue plaque was sampled from four females and two males. One volunteer consumed alcohol on a regular basis, and two subjects brushed the dorsal tongue surface regularly. The PSR Index and the Halimeter values were 0 for all healthy volunteers (Table 2).
3.2. Microbiological analysis
The combination of the culture‐dependent methods and the molecular cloning technique revealed a high abundance and diversity of bacterial species in both the halitosis and control groups. A high bacterial variety (more than 80 different species) resulted from the combination of the two methods. While the culture method identified almost 47, the culture‐independent cloning method detected 55 species.
3.3. Culture analysis revealed a distinct bacterial composition of halitosis‐associated biofilms compared to the health‐associated biofilms
By means of MALDI‐TOF analysis, it was possible to identify 47 different microbial species overall. A total of 36 different species were identified in the halitosis condition, and 36 different species were identified in the samples derived from the healthy condition. The culture analysis of the microflora disclosed distinguishable differences in the abundance distribution of the aerobic and anaerobic species within the tongue dorsum biofilm of healthy volunteers and halitosis patients (Figures 1, 2). In particular, in the halitosis condition 18 aerobic and 18 anaerobic species were identified; similarly, in the healthy group 19 aerobic species and 17 anaerobic species were detected. The highest percentage of CFUs among aerobic species (1.9 × 108 CFU/ml) in the halitosis volunteers was found for Streptococcus mitis (Figures 3,4); in the healthy volunteers, the highest percentage of CFUs among aerobic species was found for Streptococcus parasanguinis (1.11 × 108 CFU/ml). Among the anaerobic species, the highest percentage was found for Veillonella atypica (7.6 × 107 CFU/ml) in the halitosis group and for Veillonella spp. (9 × 106 CFU/ml) in the healthy group (Figures 3,4). A statistically significant association was found between the presence of Actinomyces graevenitzii and the halitosis condition (p < .05) (Figure 3). In addition, the culture analysis allowed the identification of V. rogosae in the tongue biofilm also of halitosis patients.
Figure 1.
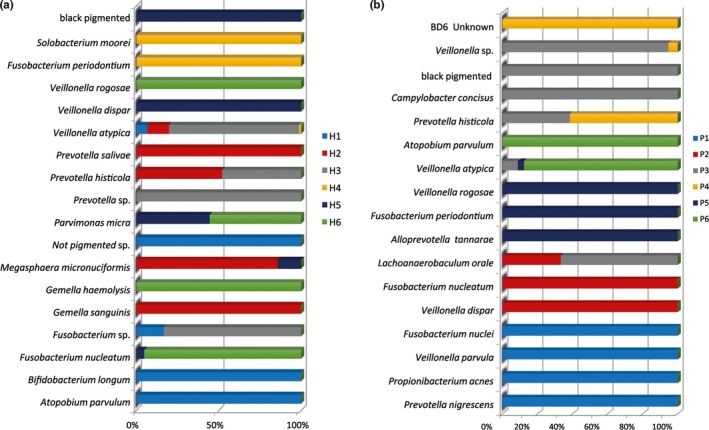
Culture technique: (a) Relative distribution (in % CFU) of anaerobic bacteria among the halitosis patients and (b) relative distribution (in % CFU) of anaerobic bacteria among the healthy volunteers
Figure 2.
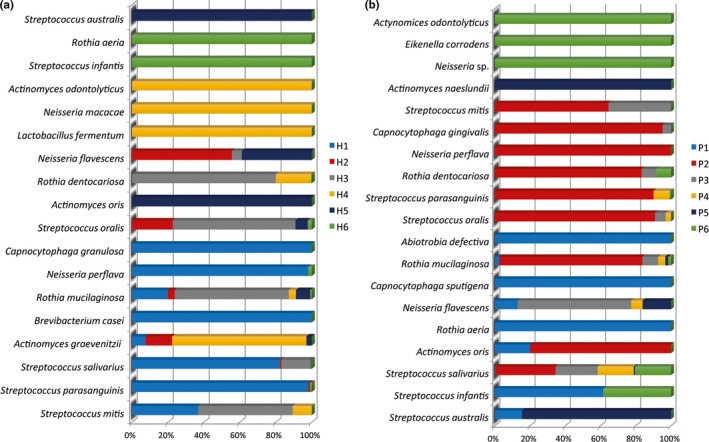
Culture technique: (a) Relative distribution (in % CFU) of aerobic bacteria among the halitosis patients and (b) relative distribution (in % CFU) of aerobic bacteria among the healthy volunteers
Figure 3.
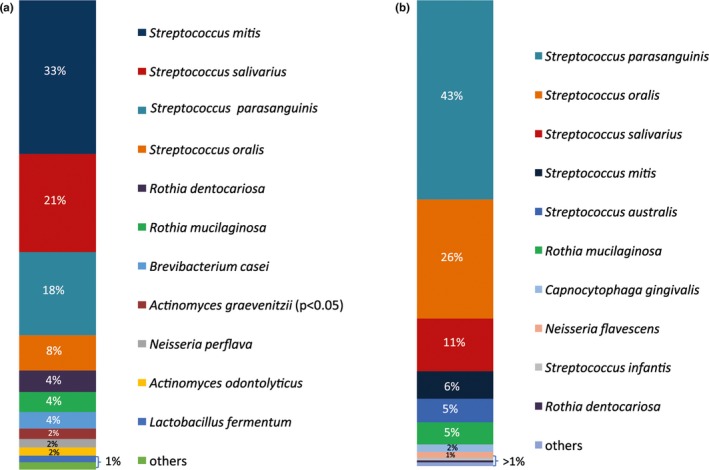
Culture technique: (a) Microbial composition (in % CFU) of aerobic bacteria in biofilm samples of halitosis patients and (b) bacterial concentration composition (in % CFU) of aerobic species in biofilm samples of healthy volunteers. The significantly associated species (p value <.05) are marked
Figure 4.
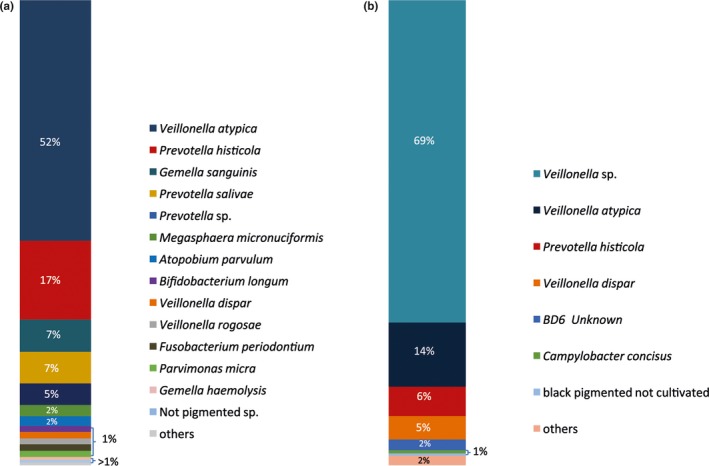
Culture technique: (a) Microbial composition (in % CFU) of anaerobic bacteria in biofilm samples of halitosis patients and (b) microbial composition (in % CFU) of anaerobic bacteria in biofilm samples of healthy volunteers. The significantly associated species (p value <.05) are depicted
3.4. Analysis of the 16S rDNA clone libraries disclosed a high bacterial diversity within the halitosis‐associated biofilms
The molecular identification confirmed the presence of the bacterial species detected by the culture method, and it allowed us to detect even more species including various Streptococcus and other taxa including Haemophilus parainfluenzae, Okadaella gastrococcus, and Tannerella forsythia (Figures 5 and 6).
Figure 5.
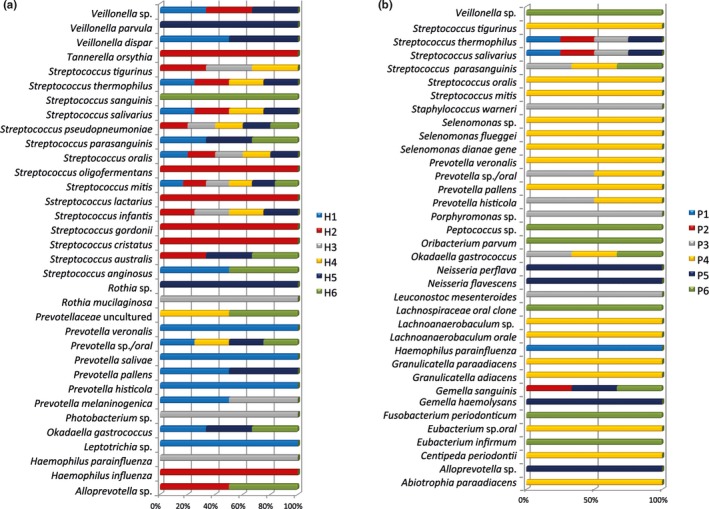
Cloning technique: (a) Relative distribution of all bacteria among the halitosis patients (in %). (b) Relative distribution of all bacteria among the healthy volunteers (in %)
Figure 6.
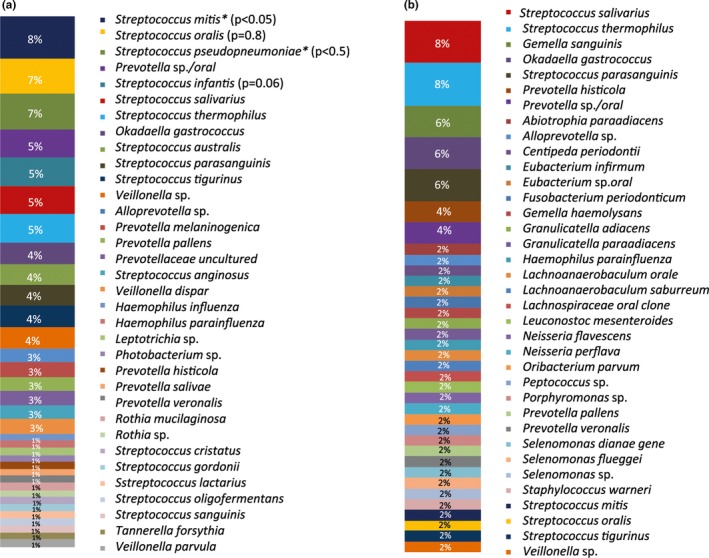
Cloning technique: (a) Relative abundance (in %) of all bacteria in biofilm samples of halitosis patients. The significantly associated species (p value <.05) are marked. (b) Relative abundance (in %) of all bacteria in biofilm samples of healthy volunteers
More specifically, the other species detected in halitosis samples were Streptococcus anginosus, S. cristatus, S. gordonii, S. lactarius, S. oligofermentans, S. thermophilus, S. tigurinus, S. pseudopneumoniae, S. australis, Okadaella gastrococcus, Prevotella sp., P. histicola, P. pallens, P. melaninogenica, P. veroralis, and Veillonella parvula (Figure 5).
The adjunctive taxa detected in the samples derived from the healthy volunteers were Gemella sanguinis, Streptococcus thermophilus, Porphyromonas sp., Prevotella pallens, Haemophilus parainfluenzae, Abiotrophia para‐adiacens, and Selenomonas sp.
The most abundant species found in the halitosis condition was Streptococcus mitis (Figure 6). The most abundant species among the samples derived from the healthy condition was Streptococcus salivarius (Figure 6). The statistical analysis revealed a significant association (p value < .05) of S. mitis and S. pseudopneumoniae with the halitosis condition (Figure 6). Some taxa were only found in the halitosis patients, but not in the healthy controls, for example, Okadaella gastrococcus (4% abundance), Leptotrichia sp. (1% abundance), and Tannerella forsythia (1% abundance).
4. DISCUSSION
Intraoral halitosis is predominantly caused by bacteria. According to literature, it is widely accepted that the microbial composition of the dorsal tongue surface correlates with the VSCs' production as stated in different studies (Amou et al., 2014; Aylıkcı & Colak, 2013; Bosy, Kulkarni, Rosenberg, & McCulloch, 1994; De Boever & Loesche, 1995; Hess, Greenman, & Duffield, 2008; Kazor et al., 2003; Yang et al., 2013). The VSCs produced by the dorsal tongue microbiota are the molecules directly responsible for the oral malodor.
In clinical practice, patients affected by this health issue address their dentist or dental hygienist in order to solve it (Thoppay et al., 2019). The first steps for a correct diagnosis are to obtain data using a general medical history questionnaire and to clinically evaluate the oral health status and the detection of the VSCs (Seemann et al., 2014). The detection of VSCs is a crucial step and topic of debate. Indeed, as reported by Scully C et al. the clinical assessment can be performed using portable gas chromatography or a sulfide monitor or organoleptic assessment, performed by the nose of the clinicians (Scully & Greenman, 2012). The last method is considered the gold standard in the clinical practice, but the clinician sniff can present many side effects such as the transmission of diseases or subjectivity level (Falcão et al., 2017). The portable gas chromatography can be preferred if the clinical situation requires a differentiation of the VSCs. The sulfide monitor instead can be sufficient for an initial objective assessment of halitosis (Scully & Greenman, 2012). In our clinical assessment, the general medical history questionnaire revealed the absence of mechanical tongue scraping among the adopted oral hygiene habits. The clinical examination allowed for the documentation of the periodontal status, and the objective assessment of VSCs by means of the sulfide monitor enabled the diagnosis of halitosis associated with the tongue coating. However giving the limit of the sulfide monitor, we were not able to assess the degree of the halitosis condition. The periodontal status was found to be in good condition in the healthy volunteers' group and with signs of disease in the halitosis group. Two patients belonging to the halitosis group also showed GERD, which can be a primary cause of oral malodor. Indeed, the GERD lowers the pH in the oral cavity and therefore influences the microbial composition of the oral biofilm of teeth, mucosa, and tongue dorsum. However, the microbial composition of the tongue biofilm belonging to these two particular patients did not show any taxa significantly predominant. Among the aerobes, the most abundant species were Streptococcus parasanguinis and Okadella gastrococcus, while among the anaerobes the most abundant species were Veillonella atypica, Prevotella histicola, and Veillonella Rogosae. Interestingly, the patient suffering from GERD presented as most abundant species the V. Rogosae. As stated before, the source of the oral malodor is found in the microbial metabolism. Many studies have reported that the composition of the microflora is characterized by a great diversity and accompanied by the presence of high proportions of anaerobic bacteria (Anesti et al., 2005; Loesche & Kazor, 2003; Mantilla Gómez et al., 2001; Roldán et al., 2003).
The combination of culture and culture‐independent methods applied in the present study confirmed this trend, showing a high variability of the microbial population of the biofilm, and a higher proportion of the aerobic taxa in the halitosis group.
In particular, we were able to detect the main species associated with oral malodor so far, including Prevotella melaninogenica, Fusobacterium periodonticum, Tannerella forsythia, and Solobacterium moorei.
Previous studies profiled the microbiota in halitosis patients and healthy individuals by means of culture‐dependent and culture‐independent techniques in order to understand the microflora dominating this pathological biofilm microenvironment (De Boever & Loesche, 1995; Hess et al., 2008; Kato et al., 2005; Kazor et al., 2003; Mantilla Gómez et al., 2001; Seerangaiyan et al., 2017). In 1966, Gordon and Gibbons were the first to report the prevalence of bacterial species on the tongue surface using culture methods (Gordon & Gibbons, 1966). They found streptococci, Veillonella spp., micrococci, staphylococci, Bacteroides spp., Neisseria spp., Fusobacterium spp., and unidentified Gram‐negative rods and cocci. Later, De Boever and Loesche made a first effort to determine which of the bacterial species colonizing the tongue surface correlated with oral malodor (De Boever & Loesche, 1995). In that context, they isolated cultivable bacteria from tongue plaque from halitosis patients and found that the prevalent Gram‐positive halitosis‐associated bacterial species were Actinomyces spp., Streptococcus salivarius, S. sanguinis, and Rothia dentocariosa, whereas the prevalent Gram‐negative halitosis‐associated bacterial species were Prevotella intermedia, Capnocytophaga spp., and Fusobacterium spp. Our study confirmed the presence of these aerobic species associated with halitosis condition,
Since the detection of uncultivable bacteria is not possible using solely culture‐dependent methods, the available information on the microbiota situated on the tongue surface was limited. After applying culture‐independent methods, namely the amplification, cloning, and sequencing of 16S rRNA cistrons, Kazor et al. managed to determine the bacterial composition on the tongue surface in halitosis patients more comprehensively (Kazor et al., 2003). Interestingly, the author found the most prevalent bacterial species were Atopobium parvulum and Solobacterium moorei. In contrast, other bacterial species such as Streptococcus salivarius and Rothia mucilaginosa were predominant in healthy subjects (Kazor et al., 2003). This finding was confirmed in the present study, in which S. salivarius and R. mucilaginosa were also found in healthy. In healthy subjects R. mucilaginosa comprise 5% CFU, in halitosis 4%. In another study, Haraszthy et al. applied the combination of the anaerobic culture and direct amplification of 16S ribosomal DNA using an open‐ended method similar to the one in the present study, in an attempt to overcome the limits of the culture technique (Haraszthy et al., 2007). They found Streptococcus salivarius and Campylobacter concisus as the most prevalent species in the control group. These species were found in the control group of our study, too. In addition, Actinomyces graevenitzii, statistically associated with the halitosis condition in the present study, was also one of the most prevalent species in halitosis group in the Haraszthy et al. study (Haraszthy et al., 2007).
Moreover, the present results revealed, in accordance with these earlier findings, the presence of Actinomyces odontolyticus, Solobacterium moorei, Streptococcus oralis, and S. sanguinis in halitosis patients. These bacterial species were often detected in halitosis biofilm in literature (Haraszthy et al., 2007). Riggio et al. profiled and compared the microbiota on the tongue dorsum by means of culture‐independent techniques, using PCR amplification, cloning, and sequencing of 16S rRNA genes (Riggio et al., 2008). The authors concluded that the tongue dorsum presents a higher microbial diversity in halitosis samples compared with the controls. According to the authors' findings, S. salivarius was present in high concentrations both in the halitosis and control groups (Riggio et al., 2008). The present study confirmed these findings. Consequently, it can be assumed that this microorganism does not play an etiological role in the development of oral malodor.
Recently, Yang et al. used pyrosequencing in a cross‐sectional and longitudinal study for a comparison of the microbial communities in halitosis patients and in healthy volunteers (Yang et al., 2013). They found that Prevotella spp. and Leptotrichia spp. were positively linked to hydrogen sulfide (Yang et al., 2013). Similarly, Ren et al. found members of the genera Prevotella and Leptotrichia (and Actinomyces, Selenomonas etc.) in halitosis with pyrosequencing (Ren et al., 2016). Seerangaiyan et al. using Illumina MiSeq high‐throughput sequencing found Leptotrichia, Prevotella, Selenomonas, and Tannerella taxa abundant in halitosis, whereas several Streptococcus species were more abundant in the control (Seerangaiyan et al., 2017).
The results deriving from our culture‐independent “open‐ended” technique in combination with the culture technique confirmed the presence of the taxa found in these high‐throughput sequencing studies, specifically the detection of several Prevotella species with both methods, for example, P. histicola, which was found in high concentrations in samples from the halitosis patients with culture technique. Moreover, our methods revealed the significant presence of S. mitis and S. pseudopneumoniae in the halitosis samples which might indicate their role in the adhesion to the tongue surface during the biofilm formation. In contrast to the high‐throughput sequencing studies, with our methodological approach by means of sequencing full‐length 16S rDNA fragments, we were able to differentiate the many Streptococcus species that were detected. Both Seerangayian K et al. and Yang et al. found certain OTUs (operational taxonomic units) of the genus Streptococcus associated with healthy study participants, yet they were not able to achieve a clear species‐level analysis (Seerangayian K et al., 2017; Yang et al., 2013).
The low number of participants in our study is an obvious limitation; however, other reports draw conclusions regarding the etiological flora for halitosis using similar study populations, for example, the study by Kazor CE et al. using a culture‐independent approach on six halitosis patients and five healthy controls, or the study by Ren W et al. comparing five halitosis patients with five controls (Kazor et al., 2003; Ren et al., 2016). In our study, the results of the combination of culture‐dependent and culture‐independent “open‐ended” cloning techniques highlighted the most prevalent bacterial species within the halitosis biofilms and the bacterial species influencing the healthy biofilms. This had not been performed yet. The aerobic and anaerobic cultivable species from the halitosis group corresponded to the taxa reported by many authors: All of those species except for Veillonella rogosae were previously found on the tongue dorsum of halitosis subjects. This species had previously been isolated from supragingival dental plaque and from the tongue biofilm of healthy individuals (Arif et al., 2008; Mashima et al., 2011; Mashima & Nakazawa, 2013). Veillonella rogosae is a Gram‐negative, nonmotile, nonsporulating coccoid and appears as a single cell or in short chains. It is strictly anaerobic and oxidase‐negative. It exhibits pyroglutamic acid arylamidase and variable alkaline phosphatase activity. Major acid end products are acetic and propionic acids (Arif et al., 2008). Veillonella genus has always been connected with the production of VSCs and is therefore responsible for malodor (Mashima et al., 2011), but to our knowledge, V. rogosae was never associated with halitosis so far.
The cloning method, exploiting a “hypothesis‐free” approach to achieve a greater overview of the total microbial diversity, showed a high variability among the detected species between the two groups. Particularly in the halitosis group, it allowed for the detection of different Streptococcus spp, Haemophilus parainfluenzae, Prevotella pallens, P. veroralis, Photobacterium spp. Leptotrichia wadei, and Tannerella forsythia, in line with the results obtained by (Riggio et al., 2008; Yang et al., 2013). In the control group, using the cloning method, we were able to detect Abiotrophia para‐adiacens, Granulicatella spp., Lachnoanaerobaculum saburreum, Selenomonas spp., and Staphylococcus warneri, which were not detected by means of culture‐dependent methods. Particularly in the control group, two interesting species were noted: Selenomonas is a genus which is generally taken to be a volatile sulfur compounds producer (Persson et al., 1990). In general, S. mitis, S. oralis, and S. pseudopneumoniae are rather seen as belonging to the healthy physiological flora than associated with any oral disease. However, the 16S rRNA gene of S. mitis and S. pseudopneumoniae, as shown by the recent study of Tze et al., has a 98% correspondence with a new isolated species from the tongue dorsum in a halitosis patient: the S. halitosis. Hence, it might be possible that these taxa would provide favorable conditions in the microenvironment of the tongue biofilm for other, halitosis‐associated taxa to thrive.
5. CONCLUSION
In conclusion, in combining the culture method and culture‐independent cloning technique this study confirmed the wide variety of the tongue microbiota in halitosis patients, including new species that had not been detected so far. A combination of different microbial techniques is recommended to analyze the etiological microflora associated with halitosis. Increased knowledge of the microbiota of the tongue biofilm is essential for further research to develop new antimicrobial agents for halitosis therapy strategies.
CONFLICT OF INTERESTS
None declared.
AUTHORS' CONTRIBUTIONS
Sara Bernardi and Annette Anderson contributed to conceptualization; Kirstin Vach contributed to formal analysis; Sara Bernardi contributed to investigation; Lamprini Karygianni, Ali Al‐Ahmad, Elmar Hellwig, and Guido Macchiarelli contributed to supervision; Andreas Filippi, Andrea Zurcher, and Ali Al‐Ahmad facilitated the resources; Sara Bernardi, Annette Anderson, Kristin Vach, Lamprini Karygianni, Andreas Filippi, Andrea Zurcher, and Ali Al‐Ahmad contributed to writing–original draft preparation; Elmar Hellwig, Guido Macchiarelli, Lamprini Karygianni, Ali Al‐Ahmad, Annette Anderson, Andreas Filippi, Andrea Zurcher, and Kristin Vach contributed to writing–review and editing; all authors read and approved the final manuscript.
6. Ethics statement
The study design was reviewed and approved by the Ethics Committee of the Albert‐Ludwigs‐University of Freiburg (74/15).
ACKNOWLEDGEMENTS
The authors are grateful to Bettina Spitzmüller, Kristina Kollmar, Annette Wittmer, Georg Heuzeroth, and Grant Anderson for their help in sampling, laboratory procedures, and English revision of the paper. This study was partly supported by the German Research foundation (DFG, AL‐1179/2‐1).
Bernardi S, Karygianni L, Filippi A, et al. Combining culture and culture‐independent methods reveals new microbial composition of halitosis patients' tongue biofilm. MicrobiologyOpen. 2020;9:e958 10.1002/mbo3.958
The present study was carried out at the Department of Operative Dentistry and Periodontology, Faculty of Medicine, Center for Dental Medicine, University of Freiburg, and at the Department of Oral Surgery, Oral Radiology and Oral Medicine and Centre of Dental Traumatology, University of Basel.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- Altschul, S. F. , Gish, W. , Miller, W. , Myers, E. W. , & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215(3), 403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- Amou, T. , Hinode, D. , Yoshioka, M. , & Grenier, D. (2014). Relationship between halitosis and periodontal disease ‐ associated oral bacteria in tongue coatings. International Journal of Dental Hygiene, 12(2), 145–151. 10.1111/idh.12046 [DOI] [PubMed] [Google Scholar]
- Anderson, A. C. , Hellwig, E. , Vespermann, R. , Wittmer, A. , Schmid, M. , Karygianni, L. , & Al‐Ahmad, A. (2012). Comprehensive analysis of secondary dental root canal infections: A combination of culture and culture‐independent approaches reveals new insights. PLoS One, 7(11), e49576 10.1371/journal.pone.0049576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, A. C. , Sanunu, M. , Schneider, C. , Clad, A. , Karygianni, L. , Hellwig, E. , & Al‐Ahmad, A. (2014). Rapid species‐level identification of vaginal and oral lactobacilli using MALDI‐TOF MS analysis and 16S rDNA sequencing. BMC Microbiology, 14, 312 10.1186/s12866-014-0312-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anesti, V. , McDonald, I. R. , Ramaswamy, M. , Wade, W. G. , Kelly, D. P. , & Wood, A. P. (2005). Isolation and molecular detection of methylotrophic bacteria occurring in the human mouth. Environmental Microbiology, 7(8), 1227–1238. 10.1111/j.1462-2920.2005.00805.x [DOI] [PubMed] [Google Scholar]
- Arif, N. , Do, T. , Byun, R. , Sheehy, E. , Clark, D. , Gilbert, S. C. , & Beighton, D. (2008). Veillonella rogosae sp. nov., an anaerobic, gram‐negative coccus isolated from dental plaque. International Journal of Systematic and Evolutionary Microbiology, 58, 581–584. 10.1099/ijs.0.65093-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashelford, K. E. , Chuzhanova, N. A. , Fry, J. C. , Jones, A. J. , & Weightman, A. J. (2005). At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Applied and Environmental Microbiology, 71(12), 7724–7736. 10.1128/AEM.71.12.7724-7736.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylıkcı, B. U. , & Colak, H. (2013). Halitosis: From diagnosis to management. Journal of Natural Science, Biology, and Medicine, 4(1), 14–23. 10.4103/0976-9668.107255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi, S. , Bianchi, S. , Botticelli, G. , Rastelli, E. , Tomei, A. R. , Palmerini, M. G. , … Macchiarelli, G. (2018). Scanning electron microscopy and microbiological approaches for the evaluation of salivary microorganisms behaviour on anatase titanium surfaces: In vitro study. Morphologie, 102(336), 1–6. 10.1016/j.morpho.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Bernardi, S. , Continenza, M. A. , Al‐Ahmad, A. , Karygianni, L. , Follo, M. , Filippi, A. , … Macchiarelli, G. (2019). Streptococcus spp. and Fusobacterium nucleatum in tongue dorsum biofilm from halitosis patients : A fluorescence in situ hybridization ( FISH ) and confocal laser scanning microscopy (CLSM ) study. The New Microbiologica, 42(2), 108–113. [PubMed] [Google Scholar]
- Bernardi, S. , Marzo, G. , & Continenza, M. A. (2016). Dorzalna površina jezika i halitoza: morfološki aspekti dorsal lingual surface and halitosis: A morphological point of view. Acta Stomatol Croat, 50(1), 151–157. 10.1564/asc50/2/8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi, S. , Zeka, K. , Mummolo, S. , Marzo, G. , & Continenza, M. A. (2013). Development of a new protocol: A macroscopic study of the tongue dorsal surface. Italian Journal of Anatomy and Embryology, 118(2 supplement), 24 10.13128/IJAE-13925 [DOI] [Google Scholar]
- Bosy, A. , Kulkarni, G. V. , Rosenberg, M. , & McCulloch, C. (1994). ‘Relationship of oral malodor to periodontitis: Evidence of independence in discrete subpopulations’. The Journal of Periodontology, 65(1), 37–46. 10.1902/jop.1994.65.1.37 [DOI] [PubMed] [Google Scholar]
- Brunner, F. , Kurmann, M. , & Filippi, A. (2010). ‘The correlation of organoleptic and instrumental halitosis measurements’. Schweizer Monatsschrift Fur Zahnmedizin = Revue Mensuelle Suisse d’odonto‐stomatologie = Rivista Mensile Svizzera Di Odontologia E stomatologia/SSO, 120(5), 402–408. [PubMed] [Google Scholar]
- Cole, J. R. , Wang, Q. , Cardenas, E. , Fish, J. , Chai, B. , Farris, R. J. , … Tiedje, J. M. (2009). The ribosomal database project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Research, 37(SUPPL. 1), 141–145. 10.1093/nar/gkn879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boever, E. H. , & Loesche, W. J. (1995) ‘Assessing the contribution of anaerobic microflora of the tongue to oral malodor’. Journal of the American Dental Association (1939), 126(10), 1384–1393. 10.14219/jada.archive.1995.0049 [DOI] [PubMed] [Google Scholar]
- Dzink, J. L. , & Socransky, S. S. (1990). Amino acid utilization by Fusobacterium nucleatum grown in a chemically defined medium. Oral Microbiology and Immunology, 5(3), 172–174. 10.1111/j.1399-302X.1990.tb00418.x [DOI] [PubMed] [Google Scholar]
- Falcão, D. P. , Miranda, P. C. , Almeida, T. F. G. , Scalco, M. G. D. S. , Fregni, F. , & Amorim, R. F. B. D. (2017). Assessment of the accuracy of portable monitors for halitosis evaluation in subjects without malodor complaint. Are they reliable for clinical practice ? Abstract. Journal of Applied Oral Science, 25(5), 559–565. 10.1590/1678-7757-2016-0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, J. A. , Reich, C. I. , Sharma, S. , Weisbaum, J. S. , Wilson, B. A. , & Olsen, G. J. (2008). Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Applied and Environmental Microbiology, 74(8), 2461–2470. 10.1128/AEM.02272-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, D. F. , & Gibbons, R. J. (1966). Studies of the predominant cultivable micro‐organisms from the human tongue. Archives of Oral Biology, 11(6), 627–632. 10.1016/0003-9969(66)90229-9 [DOI] [PubMed] [Google Scholar]
- Hall, M. W. , Singh, N. , Ng, K. F. , Lam, D. K. , Goldberg, M. B. , Tenenbaum, H. C. , … Senadheera, D. B. (2017). ‘Inter‐personal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity’. Npj Biofilms and Microbiomes, 3(2), 10.1038/s41522-016-0011-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszthy, V. I. , Zambon, J. J. , Sreenivasan, P. K. , Zambon, M. M. , Gerber, D. , Rego, R. , … Parker, C. (2007). ‘Identification of oral bacterial species associated with halitosis’. Journal of the American Dental Association (1939), 138(8), 1113–1120. 10.1111/mono.12075. [DOI] [PubMed] [Google Scholar]
- Hess, J. , Greenman, J. , & Duffield, J. (2008). Modelling oral malodour from a tongue biofilm. Journal of Breath Research, 2(1), 017003 10.1088/1752-7155/2/1/017003 [DOI] [PubMed] [Google Scholar]
- Kato, H. , Yoshida, A. , Awano, S. , Ansai, T. , & Takehara, T. (2005). ‘Quantitative detection of volatile sulfur compound‐ producing microorganisms in oral specimens using real‐time PCR’. Oral Diseases, 11(s1), 67–71. 10.1111/j.1601-0825.2005.01096.x [DOI] [PubMed] [Google Scholar]
- Kazor, C. E. , Mitchell, P. M. , Lee, A. M. , Stokes, L. N. , Loesche, W. J. , Dewhirst, F. E. , & Paster, B. J. (2003). Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. Journal of Clinical Microbiology, 41(2), 558–563. 10.1128/JCM.41.2.558-563.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche, W. J. , & Kazor, C. (2003). Microbiology and treatment of halitosis. Current Infectious Disease Reports, 5(3), 220–226. 10.1007/s11908-003-0077-8 [DOI] [PubMed] [Google Scholar]
- Madhushankari, G. S. , Yamunadevi, A. , Selvamani, M. , Mohan Kumar, K. P. , Basandi, P. S. , & Madhushankari, G. S. (2015). Halitosis ‐ an overview: Part‐I ‐ classification, etiology, and pathophysiology of halitosis. Journal of Pharmacy & Bioallied Sciences, 7(Suppl 2), S339–S343. 10.4103/0975-7406.163441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla Gomez, S. , Danser, M. M. , Sipos, P. M. , Rowshani, B. , van der Velden, U. , & van der Weijden, G. A. (2001). Tongue coating and salivary bacterial counts in healthy/gingivitis subjects and periodontitis patients. Journal of Clinical Periodontology, 28(10), 970–978. [DOI] [PubMed] [Google Scholar]
- Mashima, I. , Kamaguchi, A. , & Nakazawa, F. (2011). The distribution and frequency of oral veillonella spp. in the tongue biofilm of healthy young adults. Current Microbiology, 63(5), 403–407. 10.1007/s00284-011-9993-2 [DOI] [PubMed] [Google Scholar]
- Mashima, I. , & Nakazawa, F. (2013). Identification of Veillonella tobetsuensis in tongue biofilm by using a species‐specific primer pair. Anaerobe, 22, 77–81. 10.1016/j.anaerobe.2013.04.015 [DOI] [PubMed] [Google Scholar]
- Periodontology, A. D. A. A. A. (1993). Periodontal screening & recording an early detection system. Journal of the New Jersey Dental Association. United States, 64(2), 7–9,11. [PubMed] [Google Scholar]
- Persson, S. , Edlund, M.‐B. , Claesson, R. , & Carlsson, J. (1990). The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiology and Immunology, 5(4), 195–201. 10.1111/j.1399-302X.1990.tb00645.x [DOI] [PubMed] [Google Scholar]
- Ren, W. , Zhang, Q. , Liu, X. , Zheng, S. , Ma, L. , Chen, F. , … Xu, B. (2016). Supragingival plaque microbial community analysis of children with halitosis. Journal of Microbiology and Biotechnology, 26(12), 2141–2147. 10.4014/jmb.1605.05012 [DOI] [PubMed] [Google Scholar]
- Riggio, M. P. , Lennon, A. , Rolph, H. J. , Hodge, P. J. , Donaldson, A. , Maxwell, A. J. , & Bagg, J. (2008). ‘Molecular identification of bacteria on the tongue dorsum of subjects with and without halitosis’. Oral Diseases, 14, 251–258. 10.1111/j.1601-0825.2007.01371.x [DOI] [PubMed] [Google Scholar]
- Roldán, S. , Herrera, D. , & Sanz, M. (2003). Biofilms and the tongue: Therapeutical approaches for the control of halitosis. Clinical Oral Investigations, 7(4), 189–197. 10.1007/s00784-003-0214-7 [DOI] [PubMed] [Google Scholar]
- Roldan, S. , Winkel, E. G. , Herrera, D. , Sanz, M. , & Van Winkelhoff, A. J. (2003). The effects of a new mouthrinse containing chlorhexidine, cetylpyridinium chloride and zinc lactate on the microflora of oral halitosis patients: A dual‐centre, double‐blind placebo‐controlled study. Journal of Clinical Periodontology, 30(5), 427–434. [DOI] [PubMed] [Google Scholar]
- Schirrmeister, J. F. , Liebenow, A.‐L. , Pelz, K. , Wittmer, A. , Serr, A. , Hellwig, E. , & Al‐Ahmad, A. (2009). New bacterial compositions in root‐filled teeth with periradicular lesions. Journal of Endodontics, 35(2), 169–174. 10.1016/j.joen.2008.10.024 [DOI] [PubMed] [Google Scholar]
- Scully, C. , & Greenman, J. (2008). ‘Halitosis (breath odor)’. Periodontology 2000, 48(1), 66–75. 10.1111/j.1600-0757.2008.00266.x [DOI] [PubMed] [Google Scholar]
- Scully, C. , & Greenman, J. (2012). Halitology (breath odour: Aetiopathogenesis and management ). Oral Diseases, 44(18), 333–345. 10.1111/j.1601-0825.2011.01890.x [DOI] [PubMed] [Google Scholar]
- Scully, C. , & Porter, S. (2008). Halitosis. Clinical Evidence, 07, 1–14. [Google Scholar]
- Seemann, R. , Conceicao, M. D. , Filippi, A. , Greenman, J. , Lenton, P. , Nachnani, S. , … Rosenberg, M. (2014). Halitosis management by the general dental practitioner–results of an international consensus workshop. Journal of Breath Research, 8(1), 017101 10.1088/1752-7155/8/1/017101 [DOI] [PubMed] [Google Scholar]
- Seerangaiyan, K. , van Winkelhoff, A. J. , Harmsen, H. J. M. , Rossen, J. W. A. , & Winkel, E. G. (2017). The tongue microbiome in healthy subjects and patients with intra‐oral halitosis. Journal of Breath Research, 11(3), 036010 10.1088/1752-7163/aa7c24 [DOI] [PubMed] [Google Scholar]
- Syed, S. A. , & Loesche, W. J. (1972). Survival of human dental plaque flora in various transport media. Applied Microbiology, 24(4), 638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangerman, A. , & Winkel, E. G. (2010). Extra‐oral halitosis: An overview. Journal of Breath Research, 4(1), 017003 10.1088/1752-7155/4/1/017003 [DOI] [PubMed] [Google Scholar]
- Thoppay, J. R. , Filippi, A. , Ciarrocca, K. , Greenman, J. & De Rossi, S. S. (2019). Halitosis BT In Farah C. S., Balasubramaniam R., & McCullough M. J. (Eds.), Contemporary Oral Medicine: A comprehensive approach to clinical practice, in (pp. 1719–1747). Cham, Switzerland: Springer International Publishing; 10.1007/978-3-319-72303-7_27 [DOI] [Google Scholar]
- Vancauwenberghe, F. , Dadamio, J. , Laleman, I. , Van Tornout, M. , Teughels, W. , Coucke, W. , … Quirynen, M. (2013). The role of Solobacterium moorei in oral malodour. Journal of Breath Research, 7(4), 046006 10.1088/1752-7155/7/4/046006 [DOI] [PubMed] [Google Scholar]
- Yaegaki, K. , & Coil, J. M. (2000). Examination, classification, and treatment of halitosis; clinical perspectives. Journal (Canadian Dental Association), 66, 257–261. [PubMed] [Google Scholar]
- Yang, F. , Huang, S. , He, T. , Catrenich, C. , Teng, F. , Bo, C. , … Xu, J. (2013). Microbial basis of oral malodor development in humans. Journal of Dental Research, 92(12), 1106–1112. 10.1177/0022034513507065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


