Comparative planetary evolution for Earth, Mars, and exoplanets holds the key to uncovering the chemistry-to-life transition.
Abstract
We advocate an integrative approach between laboratory experiments in prebiotic chemistry and geologic, geochemical, and astrophysical observations to help assemble a robust chemical pathway to life that can be reproduced in the laboratory. The cyanosulfidic chemistry scenario described here was developed by such an integrative iterative process. We discuss how it maps onto evolving planetary surface environments on early Earth and Mars and the value of comparative planetary evolution. The results indicate that Mars can offer direct evidence for geochemical conditions similar to prebiotic Earth, whose early record has been erased. The Jezero crater is now the chosen landing site for NASA’s Mars 2020 rover, making this an extraordinary opportunity for a breakthrough in understanding life’s origins.
INTRODUCTION
We begin with the premise that life emerged on Earth from chemistry that led to the synthesis of molecular building blocks, which, in turn, self-assembled to form cells. This prebiotic chemistry must have been a natural and robust extension of the geochemical and environmental conditions readily available somewhere on the planet.
Here, we focus on the prebiotic synthesis of the nucleotides, amino acids, and lipids needed for life as we know it and the planetary environmental context that makes that synthesis possible. We will not address the steps to self-assembly into cells—see the review by Szostak (1) for that—but neither do we intend to draw a line of any scientific importance between the two stages. We simply envisage chemistry morphing smoothly into biology. However, there is a point further down the path that does have significance in which RNA and peptides exceed a certain length so that exploration of sequence space by the system can no longer be exhaustive, and nascent biology thus proceeds along a pathway dictated by contingency. Up to that point, prebiotic chemistry and early biology most likely followed a deterministic trajectory, and if similar sequences of conditions prevailed upon other planets, we might expect the same chemistry and early biology to play out.
Substantial recent progress in both prebiotic chemistry and exploration of early planetary surface environments motivates us to outline the current state of the field as we see it. We advocate an integrative approach between prebiotic chemistry and paleoenvironmental context, constrained by geologic and geochemical observations to help resolve many of the remaining challenges. New insights into Earth’s early environment have now been joined by markedly improved understanding of early Mars, making it possible to make chronologically constrained comparisons of planetary history and environmental evolution. In the coming decades, atmospheric spectra of Earth-like exoplanets will also be obtained, ultimately allowing for a radical expansion of the breadth of geochemistries we can study. How can we leverage these new insights to understand life’s origins?
APPROACH AND ASSUMPTIONS
Our underlying assumption is that the chemical pathway to life is robust yet linked to specific environmental conditions that can be understood through comparative planetary evolution. We use this as a guiding principle in defining the environmental processes important in directing the origin of life. We also assume that extant life, despite billions of years of evolution, has retained some direct vestiges of its prebiotic chemistry, as well as those planetary conditions in its core biochemistry. If so, these data can be used to constrain early geochemical processes. In our basic heuristic approach, we see prebiotic chemistry and environmental geochemistry as mutually informative, and the discovery process as essentially iterative.
Even if there are multiple pathways to life, there are some basic constraints from chemistry and planetary evolution that would render the early steps deterministic and within well-defined limits. An example of constraints from chemistry is the need for specific chemical elements, which cannot be substituted. An example of constraints from planetary evolution is the availability of those same elements; i.e., we understand how they can be incorporated and/or used by an emerging biochemistry. We will elaborate on those basic constraints, as they are encountered in the specific scenario for life’s origins based on life emerging from the geochemical and environmental evolution of a terrestrial planet, and allowing for some chemical components or energy sources delivered from interplanetary space. However, we do not consider here panspermia, i.e., the delivery of life or its essential components, in preassembled form, from space.
Before life can start, key building blocks and other molecules containing C, H, N, O, P, and S must be formed from simple feedstocks under environmental conditions, and the building blocks must then undergo assembly into higher-order structures. We note that life has never been observed to originate from inanimate materials on modern Earth despite the abundance of potential feedstocks and the myriad conditions that now pertain. This suggests that different feedstocks and conditions are required. Focusing on C and N, initial considerations probe the relationship between the oxidation state of plausible atmospheric and surficial feedstocks and the average oxidation state of C and N in (extant) biological molecules (Fig. 1). Hydrogen cyanide (HCN) emerges from this analysis as an ideal feedstock, although environmental reduction of carbon is required. Cyanosulfidic chemistry, as described, in part, in the next section, effects this reduction using protons and photochemically generated electrons and initiates a reaction network that leads to high yields of a large number of the building blocks thought necessary to initiate life and not much else besides (2).
Fig. 1. Carbon and nitrogen oxidation states and biology.
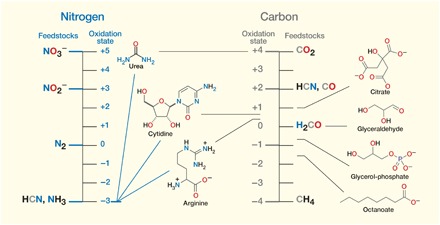
Nitrogen in biological molecules is predominantly in the −3 oxidation state, whereas carbon is in a broader range roughly centering on 0. Thus, if methane (CH4) were to have been used as an initial carbon feedstock, oxidation chemistry would have been required. Similarly, carbon dioxide (CO2) would have required reduction. In light of just the foregoing, hydrogen cyanide (HCN) can be seen as a near-ideal feedstock, as it provides both nitrogen in approximately the right oxidation state and carbon only in need of some reduction. This partial reduction actually turns out to be necessary for the C─C bond construction chemistry required to link carbon atoms into the linear and branched chains so often found in biological molecules. During cyanosulfidic chemistry, these bonds can be made highly efficiently by the addition of HCN to aldehydic reduction products of itself, or related nitriles, to give cyanohydrins [R2C(OH)CN], which can be further reduced and homologated (i.e., add a constant unit, often −CH2−) by the addition of more HCN. Hydrogen cyanide, along with derivatives produced through environmental processing, can be converted by cyanosulfidic chemistry into all the compounds shown (for those which are chiral, in racemic form).
Many previous approaches seek pathways to the synthesis of life’s building blocks based on chemistry that is largely unconstrained by likely planetary conditions at the time of synthesis. One example is the low-yield, unselective synthesis of nucleosides via reaction of products of the formose reaction (unselective oligomerization of formaldehyde, H2CO, as carbon feedstock, induced by heating with calcium hydroxide in concentrated solution) with products of nitrogenous chemistry (unselective polymerization of concentrated solutions of HCN). On the other hand, invoking a particular geochemical scenario without preliminary guidance from experimental chemistry tends to lead to low-yield syntheses of only a few of the relevant molecules often along with numerous by-products. Well-known examples are the Miller-Urey experiment to simulate lightning in a highly reducing atmosphere [using methane (CH4) and ammonia (NH3) as carbon and nitrogen feedstocks] and the postulated chemistry of deep sea hydrothermal vents [using carbon dioxide (CO2) as a carbon feedstock and invoking serpentinization followed by Fischer-Tropsch reactions to facilitate reductive transformation into biomolecules]. In contrast, the hypothesis of cyanosulfidic chemistry was developed through an iterative process in which chemistry clues were used to constrain geochemical scenarios, which then provided clues for further chemistry (3). This latter scenario is elaborated below, mapping its chemical pathways to evolving surface environments.
PLANETARY CONDITIONS AND PREBIOTIC CHEMISTRY: ITERATING TO CONVERGENCE
High-energy reprocessing—by meteors, lightning, and solar flares—of planetary atmospheres containing C, H, O, and N compounds initially produces a small collection of diatomic species, principally CN•, CO, and NO•. These species are resilient at high temperatures by virtue of strong bonding and favorable Franck-Condon factors. Cooling leaves the neutral CO and ground-state radical NO• unchanged, but CN• acquires a hydrogen atom becoming HCN in the process. The extreme energies required for the initial atomization of the atmospheric components can come from impacts and electrical discharges. In the case of impacts, carbonaceous material in the impactor will oftentimes also be atomized and so contribute to the production of HCN, CO, and NO•. In what follows, our focus is mainly on the fate of HCN, but the fate of CO and NO• must also be considered when the full picture is painted. If the atmosphere was originally composed of CO2, N2, and water vapor, the presence of carbonaceous impactor material, which is more reduced than CO2, can significantly increase the efficiency and yield of HCN synthesis. If the atmosphere was more reduced, due for example to impact of a large iron-rich object, then HCN synthesis would also be more efficient. The triple bond of HCN produced in such a way effectively stores some of the energy of this molecule’s violent creation, and this energy is partly responsible for thermodynamically driving cyanosulfidic chemistry down the line.
Both Earth and Mars had N2-CO2 atmospheres, perhaps as early as 4.3 billion years (Ga) ago; the same is expected for many of Earth-like exoplanets of similar mean density and bulk composition. High-energy reprocessing of their atmospheres (by impacts, volcanogenic and thunderstorm lightning, and solar flares) was also more active than current rates on Earth. For this initial step, the required environments seem to be present on both Mars and Earth as early as ~4.3 Ga and as late as ~3.1 Ga for Mars, and possibly even later (4). Basins filled with shallow water, and possibly with redox stratification driven by photooxidation of iron, can accumulate sediments with minerals formed in the presence of water, in exchange with the atmosphere (Fig. 2A). For HCN to be a feedstock for prebiotic chemistry, it must be delivered to the surface and concentrated in some way. Simple rain-in is not enough, as it would not give a sufficient steady-state concentration in water for efficient synthetic chemistry. The formation of ferrocyanide is an extremely favorable process and is to be expected in a body of water containing Fe2+ and exposed to an atmosphere containing HCN. The step of stockpiling ferrocyanide salts requires a planetary surface supporting lakes, small shallow seas, and lagoons, permanently or intermittently, over a long period. Surface areas need to be sufficiently large and have depths of at least 1 to 10 m for the chemistry and accumulation to proceed, as in the case of the Gale crater lake on Mars (5–7) and other analogous sites where subaqueous sedimentary rocks accumulated. The mid-range ultraviolet (UV) light capable of depleting ferrocyanide in water is removed by deep water (>10 m) or by episodes of large sulfur presence in the upper atmosphere due to enhanced volcanic activity. The latter episodes might be important on Mars but are very short-lived (8). Also short-lived and less likely after about 4.3 Ga are UV-blocking organic hazes produced by strongly reducing atmospheric conditions, e.g., due to giant iron-rich impactors or unusually high H2 outgassing rates. Therefore, the most plausible way to stockpile ferrocyanide salts is in lakes of at least a few meters depth, which should be common (Fig. 2A).
Fig. 2. Creating stockpiles of initial compounds for prebiotic chemistry.
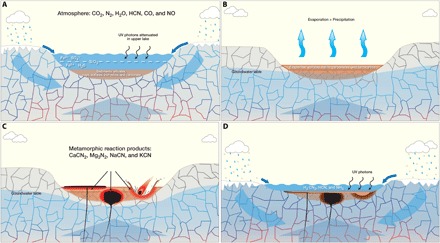
Conceptual model for accumulation of strata in shallow subaqueous basins and their interaction with water and the atmosphere is shown. The model is based on early Mars, but we expect it to be applicable to prebiotic Earth. (A) Lake or shallow sea in contact with bedrock and the atmosphere. Water is supplied by surface runoff from precipitation and/or melting ice, groundwater infiltration from adjacent highlands, and thermally buoyant deep basinal waters warmed by the ambient geothermal gradient and flowing upward through permeable fractures in the crust. HCN in the atmosphere (due to impact delivery and processing) interacts with dissolved iron in the water body and below the depth of UV penetration allows ferrocyanide to form. (B) Variations in climate result in episodic drying of the shallow water body, creating a variety of salts including ferrocyanide. Preservation of ferrocyanide is enhanced by burial beneath the reach of UV energy. (C) Thermal pulses created by igneous intrusions, volcanic activity, or large impacts cause contact metamorphism of cyanide salt deposits to a variety of reaction products including CaCN2, KCN, Mg3N2, and NaCN. (D) Exposure of these metamorphic reaction products to neutral pH water yields H2CN2, HCN, and NH3 in solution. In turn, if these species are exposed to SO2 and surface water is shallow enough to be affected by mid-range UV radiation, cyanosulfidic chemistry can then produce a diverse set of products, which correspond to the nucleotide, amino acid, and lipid precursor molecules of extant biochemistry.
Mixed ferrocyanide salts, such as CaK2[Fe(CN)6] and MgNa2[Fe(CN)6], are relatively insoluble and so would settle to the bottom of the body of water and mix in with other sediments (Fig. 2A) where they would be shielded from UV irradiation, which would otherwise deplete ferrocyanide by photoaquation and photooxidation to ferricyanide. This phase of HCN production, sequestration, and concentration could extend for a long period, the upper limit being dictated by the (hydrolytic) stability of ferrocyanide. Significantly, enough feedstock can likely be accumulated in a matter of 102 to 103 years. During periods of desiccation (Fig. 2B), ferrocyanide salts are very stable as surface deposits under mid-range UV light or as buried deposits [see detailed modeling by Toner and Catling (9)]. Last, sediment accumulation rates at the Gale crater suggest that the lake basin environment could have lasted for millions to tens of millions of years (6) ensuring sufficient time for concentration of salts through repeated drying cycles. Even if ferrocyanides that precipitated earlier were degraded by hydrolysis, this cycling would have meant that fresh deposits could have been laid down. Evidence for intermittent desiccation within the Gale lake strata has been identified (10), and higher stratigraphic positions show greater concentrations of salts (11). To be clear, the point here is not that the Gale lake was a specific repository for cyanide salts, rather it serves to illustrate that lakes on Mars could have had the potential to sequester these salts generally; it remains to be seen what the diversity of redox states is represented by the global inventory of salts to be explored by future landed missions. If Earth is a guide, then this diversity may be very broad, and it seems reasonable to infer that Martian lakes of older geologic age may be most prone to reducing conditions favorable for cyanide salt precipitation.
Heating of dry ferrocyanide salts distributed within sedimentary terrains to temperatures up to 700°C results in thermal metamorphosis to reaction products (Fig. 2C), the composition of which depends on the nature of the salts’ cations. This heating could be the result of igneous intrusive or volcanic activity or large impacts. Focusing only on those products directly relevant to synthesis, CaK2[Fe(CN)6] gives CaCN2 and KCN, and MgNa2[Fe(CN)6] ought to give Mg3N2 and NaCN based on the known products of thermal metamorphosis of Mg2[Fe(CN)6] and Na4[Fe(CN)6].
The next step is the onset of the prebiotic synthesis, which happens quickly in planetary context. Given the availability of stockpiles of initial compounds, the process can be triggered by episodes of volcanic eruptions delivering high concentrations of SO2 while retaining access to mid-range UV light in shallow water reservoirs. These environments can be available on early Earth and Mars (12). Given the speed of this step, its chemistry can proceed simultaneously with the previous third step of the thermal metamorphosis of the dry ferrocyanides, as soon as liquid water becomes available. Upon the addition of water buffered to near-neutral pH, these otherwise stable products give the reactive species cyanamide (H2CN2), HCN, and NH3 in solution (Fig. 2D). If acetylene (C2H2) also turns out to be crucial, it can be made by hydration of CaC2, which, in turn, results from the heating of CaCN2 to 1000°C. H2CN2, HCN, and NH3 in solution undergo a variety of chemistries, but cyanosulfidic chemistry converts them into a remarkable set of products, which correspond to the nucleotide, amino acid, and lipid precursor molecules of extant biochemistry, and not much else (2).
Cyanosulfidic chemistry needs a source of hydrated electrons—reduction of HCN being needed to make molecules at the average oxidation level of extant biological molecules (Fig. 1)—but is adversely affected by hydroxyl radicals, so the hydrated electrons probably should not be made by radiolysis of water (by <190-nm UV light). Instead, mid-range UV irradiation (by >200-nm UV light) of a variety of anions generates hydrated electrons and products of oxidation of the anion. Most recently, it has been shown (13) that a combination of ferrocyanide and sulfite is an extremely efficient source of hydrated electrons upon UV irradiation. Sulfite (SO32−) is derived from dissolution of atmospheric SO2 and would be largely available on early Earth (12) and has also been suggested for Mars. Reduction of ferricyanide to ferrocyanide by sulfite outcompetes the back reaction of electrons with ferricyanide and renders the process catalytic in ferrocyanide. Furthermore, S(IV) is oxidized to S(VI) in the latter mixed system, giving twice the reducing capacity per sulfite and producing the geochemically more relevant oxidized sulfur species, sulfate.
Phosphate is required to make the nucleotide and lipid precursor products of cyanosulfidic chemistry. Phosphate can be present at any preceding stage of the timeline, as it is stable. But we still know little about the P cycle on early Mars and early Earth. Phosphate could derive from anoxic corrosion of meteoritic schreibersite or from weathering of apatite from igneous rocks—known to be present on Mars (14, 15). It is also possible that lower–oxidation state oxyacid salts, such as phosphite and hypophosphite, were present at earlier stages and then somehow became photooxidized to phosphate. This would solve certain issues that arise because of the insolubility of phosphate. It is also possible that the electrons liberated by this photooxidation could contribute to cyanosulfidic reductive homologation chemistry.
Last, nitrite derived from NO• could add to the mix and bring about further chemistries, although the simultaneous presence of bisulfite and nitrite is unlikely to give productive chemistry other than the synthesis of hydroxylamine by a Raschig process. Further chemistry that is needed to progress to the stage at which the building blocks are assembled into higher-order structures is principally activation chemistry, and nitrite has recently been shown to participate in a systems chemistry synthesis of the potent activating agent, methyl isonitrile.
It is not possible to constrain the time associated with cyanosulfidic building block synthesis and activated building block assembly. However, the time scales for degradation of species such as RNA are quite short, so synthesis has to be at least as fast to allow accumulation of material [see Rimmer et al. (16)]. It is certainly conceivable that the organic synthesis phase of the timeline could occur on a time scale of weeks, even hundreds of years seems too slow.
EARTH-MARS COMPARISON: LESSONS FOR PREBIOTIC CHEMISTRY
A meaningful Earth-Mars comparison is now possible thanks to a vastly improved understanding of Mars. Comparison to Mars is very valuable for our approach to prebiotic chemistry, because evidence for the epoch of prebiotic chemistry on Earth is not well preserved in the terrestrial rock record (see “Surface age” in Fig. 3). This is due to the impact of recycling of rocks by plate tectonics on Earth over the course of billions of years; in contrast, Mars is dominated by very old rocks due to the specific absence of plate tectonics. Rocks older than 3.5 to 4 Ga are very rare on Earth but quite common on Mars. The past decade of rover- and orbiter- based exploration of Mars has demonstrated compositionally diverse and unexpectedly thick and extensive deposits of sedimentary rocks, extending in age to well before 3.8 Ga (Noachian time) (6, 7, 14, 17–19). These rocks form sequences hundreds to thousands of meters thick, are spread across regions hundreds to thousands of kilometers, and often include authigenic and diagenetic minerals formed in aqueous solutions, in some cases representing evaporite deposits (11, 18, 20–23). Authigenic minerals precipitate from chemical concentration in the water column, often because of evaporation, but also during shallow burial when water interacts with sediment particles. Diagenetic minerals precipitate in sediment pore spaces during deeper burial and conversion of sediment into rock and by replacement of the minerals composing sediment particles. Mineral assemblages, of potentially evaporitic origin, include a range of clay minerals, sulfates, iron oxides, amorphous and crystalline silica, chlorides, and rare carbonates. This is significant because these rocks are widespread and span a broad cross section of Martian time including some of the oldest terrains observed. Thus, the potential for these to be involved in postdeposition thermal metamorphic reactions is high, which is supported by some observations of the Martian thermal metamorphism (24).
Fig. 3. Schematic timeline for Mars and Earth.

Comparison of the key events in the environmental histories of Earth and Mars. For example, Mars has lost the bulk of its early atmosphere, followed by loss of its hydrosphere, whereas Earth has always retained both. Therefore, the formation of hydrated minerals continues on Earth but ceased early in Mars’ history. On the other hand, Mars can offer evidence for geochemical conditions similar to Hadean and Archaean Earth during the epoch of Earth’s prebiotic chemistry. This evidence has been mostly erased from Earth’s rock record: This is illustrated by the panels marked “Surface age,” referring to crater retention age for Mars, and preserved rock record for Earth.
On the basis of detailed analysis of the sedimentary succession deposited in the Gale crater, Curiosity rover data and orbiter data both support accumulation of hundreds to thousands of meters of strata. The rover data indicate diverse authigenic and diagenetic mineralogies, some of which are likely evaporitic in composition (7, 14). The orbiter data show that these possible evaporites occur at higher stratigraphic positions (11). Given that the Gale crater is representative of sedimentary fill across a broad part of Mars (17, 18), it is likely that chemically precipitated mineral deposits concentrating other soluble materials, such as sulfates, chlorides, carbonates, clay minerals, a broad range of iron, manganese, boron, phosphorous, and nitrogen-bearing species, were available on Mars for transformation via thermal metamorphic processes. With the exception of carbonates, these mineral and elemental enrichments have all been observed by the Curiosity rover at the Gale crater (7, 14, 25–30). It is possible that carbonates are present in small amounts (31), below the detection of limit of x-ray diffraction (~2%), or present as amorphous solids (32).
The important point here is that the geologic context of these thick, chemically enriched strata shows that they could be spread across significant areal expanses and across a broad expanse of geologic time and therefore subject to a variety of thermal events including intrusive and extrusive igneous events and shock heating mechanisms. Heating mechanisms, as explained in the previous section (and in Fig. 2C), are important to the prebiotic cyanosulfidic synthesis. At least low- to moderate-grade metamorphic rocks are observed locally on Mars (33, 34) and in Martian meteorites (35), although specific geologic environments of metamorphism are hard to identify. We suggest that igneous intrusions rising up through sedimentary layers to cause contact metamorphism might have occurred in many places on Mars where surface volcanism is observed [e.g., (36–40)]. In one particularly relevant example, igneous dikes in Valles Marineris are seen crosscutting light-toned bedrock, with the possible expression of thermal alteration in adjacent contact zones [see Brustel et al. (41) and their Fig. 4a]. It is not known what the nature of the intruded light-toned bedrock is, but sedimentary deposits, including potential evaporites, are widespread in the Valles Marineris system (42). In other places, volcanic lava flows may have caused contact metamorphism of potential evaporite deposits such as at Northeast Syrtis (43–45). The cone fields of southwestern Elysium Planitia have been interpreted to record significant release of volatiles during contact metamorphism of sedimentary materials, possibly including evaporites (46).
Last, large impacts directly striking sedimentary rocks would have generated thermal anomalies extending to significant depths (47–49); sequences of sedimentary rocks may have been broadly affected by this mechanism (50), including metamorphism (24). The thermal anomalies generated by very large impacts can exceed the melting point of rock (~1000°C), even to the point of vaporization (~3000°C); therefore, smaller impacts, or postshock thermal anomalies associated with larger impacts, are expected to thermally metamorphose rock in the range between 200° and 1000°C (51) and bring about reactions of interest in the origin of life. The contact metamorphic zone adjacent to extrusive and intrusive igneous rocks can vary markedly based on the volume of the igneous rock, extending from millimeters to kilometers in thickness; greater thickness is also influenced by the presence of water that can circulate and advect heat through permeable rock across broader zones than what thermal conduction alone can do. Temperatures are lower than the melting point of rock and, thus, appropriate for driving reactions involving prebiotic materials.
Curiosity rover data demonstrate multiple lake environments characterized by neutrally to mildly acidic pH, low to high salinity, and variable redox states of both iron and sulfur species. C, H, O, S, N, P, Fe, Mn, and B were measured directly in minerals and amorphous compounds as key prebiotic elements (25–30, 32). Nitrogen-bearing compounds are present in both reduced and oxidized forms (27), and NO in pyrolysis experiments has an abundance that is substantially above background, pointing to a likely source within the mudstone lake sediments. Soluble N conceivably could have been derived from the atmosphere via fixation by sulfide minerals during reactions similar to that seen in pyrrhotite, pyrite, and magnetite assemblages on Earth (52). Neutral-to-alkaline pH conditions were most likely to have promoted the formation of clay minerals (25). Potential authigenic magnetite formation pathways include UV-promoted reactions with dissolved Fe2+ in the water column (53) or with carbonate minerals in the sediment (54), and partial oxidation of dissolved Fe2+ by dissolved O2 (55). Perhaps the most likely scenario for magnetite formation is via clay formation and “saponitization” (25) or precipitation from a redox-stratified lake (7). Tosca et al. (56) have shown that magnetite formation can accompany clay mineral formation in lakes, also associated with H2 release to the atmosphere. H2 is an important feedstock in the synthesis of atmospheric CH4 and prebiotic organic compounds (57, 58). Phosphorous is enriched in Gale crater lake sediments (30), and manganese (28) and boron (29) are enriched in veins that crosscut lake sediments, all suggesting elemental mobility of these biologically important elements within aqueous environments on ancient Mars.
PLANETARY CONDITIONS IN GENERAL: CASTING A WIDER NET
Our Solar System contains three large rocky planets—Earth, Venus, and Mars, which appear to be good representatives of the rocky planets seen to orbit other stars. Many rocky exoplanets exhibit an unexpectedly tight compositional similarity (in bulk Si/Fe interior) to Earth and Venus for a wide mass range of up to 10 times Earth’s (59). The evidence is provided by accurate mean densities measured for a dozen such exoplanets and implies commonalities in the formation of rocky planets, especially when it comes to chemical composition. Rocky planets in this mass range (1 to 10 ME) are known as super-Earths. Measurements of thousands of exoplanets in the same range of sizes indicate that rocky planets are quite common around Sun-like stars.
When we discuss surface conditions here, we want to think in terms of access: (i) to long-lived liquid H2O; (ii) to C, N, S, P, and Fe; (iii) to mid-range UV light; (iv) to thermal and chemical gradients (e.g., redox gradients, vents, and volcanoes); and (v) to stable climate provided by a stable orbit and rotation axis, as well as functioning geochemical cycles. Local environments with shallow lakes (tens of meters depth) that are susceptible to dry-wet cycles and have close-to-neutral pH would be important, and perhaps also would be coastal marine environments of shallow seas.
Atmospheres
Metal-silicate partitioning affects the chemistry of a planet’s mantle and may fundamentally alter atmospheric formation. In particular, for Earth and Mars, the composition of the early outgassed atmosphere will be chemically linked to the oxidation state of the planet’s mantle (60) (see Fig. 4). For super-Earths, the degree to which Si and O are partitioned into the core has a strong influence on the mantle composition, as shown by Schaefer et al. (61) with the help of new high-pressure experiments. The process depends on planet mass, as is observed in the Mars-Earth comparison, but the dependence is not strong enough to alter the overall outcome. One reason is that highly reducing atmospheres (e.g., dominated by CH4, NH3, and H2) are photochemically unstable and short-lived even for quite reduced mantles, as long as the planet has surface oceans and vapor H2O in the atmosphere. This is due to the fast escape of H to space after photolysis of CH4 and NH3 (62, 63). In general, outgassed carbon would be converted into CO2, and an N2-CO2 atmosphere would be generated on a time scale of about 105 years (64–66). Therefore, Earth-size and super-Earth exoplanets with Si, Fe, and Mg ratios and bulk compositions similar to Earth are commonly expected to have secondary atmospheres of N2 and CO2 from general considerations. All these do not exclude short-lived reduced states triggered by large iron-rich impactors, allowing some of the CO2 to be reduced to CO. Despite being short-lived, they enhance the efficiency of chemical stockpiling (see Fig. 2). In addition, it should be noted that the effect of minor outgassing of H2, CO, and other reducing gases is to create a “weakly reducing” prebiotic atmosphere that is essentially devoid of molecular oxygen at a planet’s surface and, thus, conducive to prebiotic chemistry.
Fig. 4. Planet core formation and atmospheres.
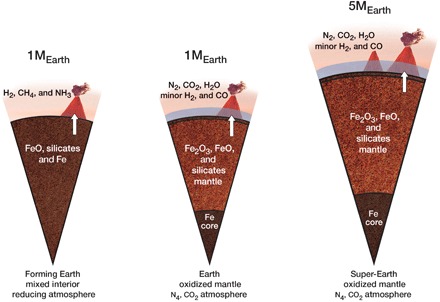
Rocky planets in a wide range of masses are commonly expected to usually have atmospheres dominated by N2 and CO2 when their orbits fall within the broader habitable zone (aka liquid-water belt) of their host stars and after some iron has been removed from the mantle by forming a core—the full transition takes 107 to 108 years and is illustrated by the first and second cutouts for the case of Earth (60, 61). A highly reducing atmosphere (as shown in the first cutout) is photochemically unstable on rocky planets with surface oceans and vapor H2O in the atmosphere because of the fast escape of H to space after photolysis of CH4 and NH3. In general, outgassed carbon would be converted into CO2, and an N2-CO2 atmosphere would be generated on a time scale of about 105 years (64–66).
Hydrospheres
For rocky planets with modest bulk amounts of water that orbit within their star’s liquid water belt, commonly referred to as the “habitable zone,” the deep-water cycle operates for a wide range of planet masses and ensures long-term persistence of liquid surface reservoirs to form seas and lakes of at least tens of meters depth (67). The Earth’s deep-water cycle controls the exchange of water between the mantle, crust, and surface. In the case of Earth, this process is tied to plate tectonics and is likely also for super-Earths that contain even small amounts of water (68, 69). For planets without active plate tectonics, such as lower-mass Mars, the persistence of liquid surface reservoirs cannot be guaranteed. However, the past decade of research on Mars shows that water was still amply abundant in the early evolution of that planet, and so we infer the same of Mars-like exoplanets. In our comparison of early Mars to Earth, we are interested to know whether Mars’ hydrosphere lasted long enough to allow prebiotic chemistry similar to Earth’s, as well as an imprint of prebiotic chemistry markers into the geological record preserved on Mars.
UV irradiation
For atmospheric CO2 levels that exceed 10 mbar, the high-energy stellar UV light (wavelengths below about 200 nm) that is harmful to prebiotic chemistry (e.g., by hydroxyl radical production) is always attenuated. Liquid water provides a similar shield even at millimeter depths. At the same time, for the young Sun and similar stars, mid-range UV light (wavelengths between 200 and 300 nm), which is helpful to synthetic photochemistry, is always available on the surface in dose rates sufficient for prebiotic chemistry (70, 71). Under these conditions, the only abundant trace gases that could block the needed mid-range UV are H2S and SO2, but they seem unlikely to attain high-enough concentrations in the atmosphere, based on our current understanding of even extraordinary outgassing events on Mars or Earth (5). The geochemical conditions for forming UV-blocking hazes are even more extreme than for H2S. Our UV requirement is stringent because mid-range UV light plays multiple roles. It has the right energy to both make and break chemical bonds and can, thus, play two crucial roles: as a source of energy and as a very specific selection agent in chemical evolution. The latter role is essential in avoiding the concomitant accumulation of a multitude of harmful by-products, and there is growing evidence of the remarkable UV photostability of life’s building blocks when compared to their isomers and tautomers (70, 72, 73). As a source of energy, mid-range UV irradiation can generate hydrated electrons, e.g., from ferrocyanide in the presence of sulfite, which is both very efficient and catalytic (13). This photoredox cycling is very sensitive to wavelength, and the only known anions that are relevant geochemically also require mid-range UV light.
A general consensus is emerging that exoplanets with bulk compositions and overall conditions resembling the Hadean and Archaean Earth, and Noachian to Hesperian Mars, might not be exceptional in our galaxy. Earth and Mars appear as widely representative planets rather than unique outliers. Access to liquid H2O of different salinity and pH (in lakes and oceans), as well as an N2-CO2 atmosphere that allows direct sunlight and precipitation (e.g., for dry-wet cycles), might be common features. Effective attenuation of x-rays, cosmic rays, and UV light below 200 nm coexists naturally with direct sunlight in these atmospheres. The planet surfaces will provide access to metals (and nonmetals), namely, Fe and P, as well as trace amounts of HCN, SO2, H2S, and NO• in sufficient concentrations for prebiotic chemistry (12, 60, 74).
OUTLOOK
We advocate an integrative iterative approach between laboratory experiments and observations of planetary systems to help assemble a robust chemical pathway to life. The cyanosulfidic chemistry scenario described here is one such approach; there could be others, but they should be built on the notion that any viable pathway should invoke planetary processes that can be observed and compared to laboratory experiments. There are many challenges and open questions that remain before such a robust pathway can be put together, even before the consequent steps of polymerization, vesicle formation and encapsulation, nonenzymatic replication, etc. are considered. However, much work has been completed now, and the goal appears within reach. The direct geochemical exploration of Mars enabled by sample return gives us an extraordinary opportunity to complete the project described here, by allowing experimental access to environments and initial conditions that have been erased from Earth’s record due to recycling by plate tectonics.
Looking further into the general ubiquity of prebiotic chemistry and life elsewhere, the challenge of understanding the early Earth-Mars atmosphere can be addressed with the help of spectroscopic exploration of the atmospheres of multiple rocky exoplanets at different evolutionary stages. The theoretical prediction that rocky planets in a wide range of masses will have oxidized mantles and, therefore, anoxic and weakly reducing N2-CO2 atmospheres needs to be confirmed observationally, and the finer details understood. The theory behind this hypothesis is strong and makes specific predictions that can be scrutinized by the upcoming astronomical instruments (both spaceborne and ground based), because the atmospheric gas signatures are robust and unambiguous.
The history of H2O acquisition and distribution on planetary surfaces is another general open question. That alone might be a crucial difference between Mars and Earth, although it is not clear how severely it would affect prebiotic chemistry, as the latter requires a relatively short time window. This is one more example where access to the early Mars record is invaluable and can help us generalize to the broader field of exoplanets and life as a planetary phenomenon that extends beyond Earth.
As future steps, once the main open questions are satisfactorily addressed (even with small details that need more work), we could probably agree that the synthesis of the essential monomers for life is understood. Then, we can turn immediately to the next step of self-assembly, because that should occur under similar or related environmental conditions, which will be very strongly constrained by the demands—now well-understood—of prebiotic synthesis. That will bode well for solving self-assembly as well.
Acknowledgments
We thank our colleagues A. Knoll, D. Catling, E. Boyd, J. Szostak, J. Hurowitz, and S. Ranjan for reading the manuscript and providing numerous helpful comments and suggestions, which improved our paper. Our reviewers J. Kasting and N. Tosca made excellent suggestions for which we are very thankful. Funding: We thank the Simons Foundation for funding (D.D.S.: grant no. 290360; J.D.S.: grant no. 290362; J.P.G.: grant no. 477125). D.D.S. acknowledges the Harvard Origins of Life Initiative. Author contributions: All three authors participated in coming up with the original idea for the paper, in writing portions of the main text, and in editing the entire paper for accuracy and clarity. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the materials cited here. Additional data related to this paper may be requested from the authors.
REFERENCES AND NOTES
- 1.Szostak J., The narrow road to the deep past: In search of the chemistry of the origin of life. Angew. Chem. Int. Ed. 56, 11037–11043 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Patel B. H., Percivalle C., Ritson D. J., Duffy C. D., Sutherland J. D., Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 7, 301–307 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritson D. J., Battilocchio C., Ley S. V., Sutherland J. D., Mimicking the surface and prebiotic chemistry of early Earth using flow chemistry. Nat. Commun. 9, 1821 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson S. A., Howard A. D., Moore J. M., Grant J. A., A cold-wet middle-latitude environment on Mars during the Hesperian-Amazonian transition. J. Geophys. Res. 121, 1667–1694 (2016). [Google Scholar]
- 5.Ranjan S., Sasselov D. D., Constraints on the early terrestrial UV environment relevant to prebiotic chemistry. Astrobiology 17, 169–204 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Grotzinger J. P., Gupta S., Malin M. C., Rubin D. M., Schieber J., Siebach K., Sumner D. Y., Stack K. M., Vasavada A. R., Arvidson R. E., Calef F. III, Edgar L., Fischer W. F., Grant J. A., Griffes J., Kah L. C., Lamb M. P., Lewis K. W., Mangold N., Minitti M. E., Palucis M., Rice M., Williams R. M. E., Yingst R. A., Blake D., Blaney D., Conrad P., Crisp J., Dietrich W. E., Dromart G., Edgett K. S., Ewing R. C., Gellert R., Hurowitz J. A., Kocurek G., Mahaffy P., McBride M. J., McLennan S. M., Mischna M., Ming D., Milliken R., Newsom H., Oehler D., Parker T. J., Vaniman D., Wiens R. C., Wilson S. A., Deposition, exhumation, and paeloclimate of an Ancient Lake Deposit, Gale Crater, Mars. Science 350, eaac7575 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Hurowitz J. A., Grotzinger J. P., Fischer W. W., McLennan S. M., Milliken R. E., Stein N., Vasavada A. R., Blake D. F., Dehouck E., Eigenbrode J. L., Fairén A. G., Frydenvang J., Gellert R., Grant J. A., Gupta S., Herkenhoff K. E., Ming D. W., Rampe E. B., Schmidt M. E., Siebach K. L., Stack-Morgan K., Sumner D. Y., Wiens R. C., Redox stratification of an Ancient Lake in Gale Crater, Mars. Science 356, eaah6849 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Ranjan S., Wordsworth R., Sasselov D. D., Atmospheric constraints on the surface UV environment of Mars at 3.9 Ga relevant to prebiotic chemistry. Astrobiology 17, 687–708 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Toner J. D., Catling D. C., Alkaline lake settings for concentrated prebiotic cyanide and the origin of life. Geochim. Cosmochim. Acta 260, 124–132 (2019). [Google Scholar]
- 10.Stein N. T., Arvidson R. E., O’Sullivan J. A., Catalano J. G., Guinness E. A., Politte D. V., Gellert R., Van Bommel S. J., Retrieval of compositional end-members from Mars exploration rover opportunity observations in a soil-filled fracture in Marathon Valley, Endeavour Crater Rim. J. Geophys. Res. 123, 278–290 (2018). [Google Scholar]
- 11.Milliken R. E., Grotzinger J. P., Thomson B. J., Paleoclimate of Mars as captured by the stratigraphic record in Gale crater. Geophys. Res. Lett. 37, L04201 (2010). [Google Scholar]
- 12.Ranjan S., Todd Z. R., Sutherland J. D., Sasselov D. D., Sulfidic anion concentrations on early Earth for surficial origins of life chemistry. Astrobiology 18, 1023–1040 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J., Ritson D. J., Ranjan S., Todd Z. R., Sasselov D. D., Sutherland J. D., Photochemical reductive homologation of hydrogen cyanide using sulfite and ferrocyanide. Chem. Commun. 54, 5566–5569 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rampe E., Ming D., Blake D., Bristow T., Chipera S., Grotzinger J., Morris R. V., Morrisone S. M., Vaniman D. T., Yen A. S., Achilles C. N., Craig P. I., Marais D. J. D., Downs R. T., Farmer J. D., Fendrich K. V., Gellert R., Hazen R. M., Thompson L. M., Mineralogy of an ancient lacustrine mudstone succession from the Murray Formation, Gale Crater, Mars. Earth Planet. Sci. Lett. 471, 172–185 (2017). [Google Scholar]
- 15.Treiman A. H., Bish D. L., Vaniman D. T., Chipera S. J., Blake D. F., Ming D. W., Morris R. V., Bristow T. F., Morrison S. M., Baker M. B., Rampe E. B., Downs R. T., Filiberto J., Glazner A. F., Gellert R., Thompson L. M., Schmidt M. E., Deit L. L., Wiens R. C., Mc Adam A. C., Achilles C. N., Edgett K. S., Farmer J. D., Fendrich K. V., Grotzinger J. P., Gupta S., Morookian J. M., Newcombe M. E., Rice M. S., Spray J. G., Stolper E. M., Sumner D. Y., Vasavada A. R., Yen A. S., Mineralogy, provenance, and diagenesis of a potassic basaltic sandstone on Mars: CheMin x-ray diffraction of the Windjana sample (Kimberley area, Gale Crater). J. Geophys. Res. 121, 75–106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rimmer P. B., Xu J., Thompson S., Gillen E., Sutherland J., Queloz D., The origin of RNA precursors on exoplanets. Sci. Adv. 4, eaar3302 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malin M. C., Edgett K. S., 2000, evidence for recent groundwater seepage and surface runoff on Mars. Science 288, 2330–2335 (2000). [DOI] [PubMed] [Google Scholar]
- 18.J. P. Grotzinger, R. E. Milliken, The sedimentary rock record of Mars: Distribution, origins, and global stratigraphy, in Sedimentary Geology of Mars (SEPM Society for Sedimentary Geology, 2012), vol. 102, pp. 1–48. [Google Scholar]
- 19.Grotzinger J. P., Sumner D. Y., Kah L. C., Stack K., Gupta S., Edgar L., Rubin D., Lewis K., Schieber J., Mangold N., Milliken R., Conrad P. G., Marais D. D., Farmer J., Siebach K., Calef F. III, Hurowitz J., McLennan S. M., Ming D., Vaniman D., Crisp J., Vasavada A., Edgett K. S., Malin M., Blake D., Gellert R., Mahaffy P., Wiens R. C., Maurice S., Grant J. A., Wilson S., Anderson R. C., Beegle L., Arvidson R., Hallet B., Sletten R. S., Rice M., Bell J. III, Griffes J., Ehlmann B., Anderson R. B., Bristow T. F., Dietrich W. E., Dromart G., Eigenbrode J., Fraeman A., Hardgrove C., Herkenhoff K., Jandura L., Kocurek G., Lee S., Leshin L. A., Leveille R., Limonadi D., Maki J., McCloskey S., Meyer M., Minitti M., Newsom H., Oehler D., Okon A., Palucis M., Parker T., Rowland S., Schmidt M., Squyres S., Steele A., Stolper E., Summons R., Treiman A., Williams R., Yingst A.; MSL Science Team , A habitable Fluvio-Lacustrine environment at Yellowknife Bay, Gale Crater, Mars. Science 343, 1242777 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Murchie S. L., Mustard J. F., Ehlmann B. L., Milliken R. E., Bishop J. L., McKeown N. K., Noe Dobrea E. Z., Seelos F. P., Buczkowski D. L., Wiseman S. M., Arvidson R. E., Wray J. J., Swayze G., Clark R. N., Des Marais D. J., McEwen A. S., Bibring J.-P., A synthesis of Martian aqueous mineralogy after 1 Mars year of observations from the Mars Reconnaissance Orbiter. J. Geophys. Res. 114, E00D06 (2009). [Google Scholar]
- 21.McLennan S. M., Bell J., Calvin W. M., Christensen P., Clark B. C., de Souza P. A., Farmer J., Farrand W. H., Fike D. A., Gellert R., Ghosh A., Glotch T. D., Grotzinger J. P., Hahn B., Herkenhoff K. E., Hurowitz J. A., Johnson J. R., Johnson S. S., Jolliff B., Klingelhöfer G., Knoll A. H., Learner Z., Malin M. C., McSween H. Y., Pocock J., Ruff S., Soderblom L. A., Squyres S. W., Tosca N. J., Watters W. A., Wyatt M. B., Yen A., Provenance and diagenesis of the evaporate-bearing Burns formation, Meridiani Planum, Mars. Earth Planet. Sci. Lett. 240, 95–121 (2005). [Google Scholar]
- 22.Jensen H. B., Glotch T. D., Investigation of the near-infrared spectral character of putative Martian chloride deposits. J. Geophys. Res. 116, E00J03 (2011). [Google Scholar]
- 23.Michalski J. R., Noe Dobrea E. Z., Niles P. B., Cuadros J., Ancient hydrothermal seafloor deposits in Eridania Basin on Mars. Nat. Commun. 8, 15978 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McSween H., Petrology of Mars. Am. Mineral. 100, 2380–2395 (2015). [Google Scholar]
- 25.Vaniman D. T., Bish D. L., Ming D. W., Bristow T. F., Morris R. V., Blake D. F., Chipera S. J., Morrison S. M., Treiman A. H., Rampe E. B., Rice M., Achilles C. N., Grotzinger J. P., McLennan S. M., Williams J., Bell J. F. III, Newsom H. E., Downs R. T., Maurice S., Sarrazin P., Yen A. S., Morookian J. M., Farmer J. D., Stack K., Milliken R. E., Ehlmann B. L., Sumner D. Y., Berger G., Crisp J. A., Hurowitz J. A., Anderson R., Marais D. J. D., Stolper E. M., Edgett K. S., Gupta S., Spanovich N.; MSL Science Team , Mineralogy of a Mudstone at Yellowknife Bay, Gale Crater, Mars. Science 343, 1243480 (2014). [DOI] [PubMed] [Google Scholar]
- 26.McLennan S. M., Anderson R. B., Bell J. F. III, Bridges J. C., Calef F. III, Campbell J. L., Clark B. C., Clegg S., Conrad P., Cousin A., Marais D. J. D., Dromart G., Dyar M. D., Edgar L. A., Ehlmann B. L., Fabre C., Forni O., Gasnault O., Gellert R., Gordon S., Grant J. A., Grotzinger J. P., Gupta S., Herkenhoff K. E., Hurowitz J. A., King P. L., Le Mouélic S., Leshin L. A., Léveillé R., Lewis K. W., Mangold N., Maurice S., Ming D. W., Morris R. V., Nachon M., Newsom H. E., Ollila A. M., Perrett G. M., Rice M. S., Schmidt M. E., Schwenzer S. P., Stack K., Stolper E. M., Sumner D. Y., Treiman A. H., VanBommel S., Vaniman D. T., Vasavada A., Wiens R. C., Yingst R. A., Team M. S. L. S., Elemental geochemistry of sedimentary rocks in Yellowknife Bay, Gale Crater, Mars. Science 343, 1244734 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Stern J. C., Sutter B., Freissinet C., Navarro-González R., McKay C. P., Douglas Archer P. Jr., Buch A., Brunner A. E., Coll P., Eigenbrode J. L., Fairen A. G., Franz H. B., Glavin D. P., Kashyap S., McAdam A. C., Ming D. W., Steele A., Szopa C., Wray J. J., Martín-Torres F. J., Zorzano M.-P., Conrad P. G., Mahaffy P. R.; MSL Science Team , Evidence for indigenous nitrogen in sedimentary and aeolian deposits from the Curiosity rover investigation of Gale crater, Mars. Proc. Natl. Acad. Sci. U.S.A. 112, 4245–4250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanza N. L., Wiens R. C., Arvidson R. E., Clark B. C., Fischer W. W., Gellert R., Grotzinger J. P., Hurowitz J. A., McLennan S. M., Morris R. V., Rice M. S., Bell J. F. III, Berger J. A., Blaney D. L., Bridges N. T., Calef F. III, Campbell J. L., Clegg S. M., Cousin A., Edgett K. S., Fabre C., Fisk M. R., Forni O., Frydenvang J., Hardy K. R., Hardgrove C., Johnson J. R., Lasue J., Mouélic S. L., Malin M. C., Mangold N., Martìn-Torres J., Maurice S., McBride M. J., Ming D. W., Newsom H. E., Ollila A. M., Sautter V., Schröder S., Thompson L. M., Treiman A. H., Van Bommel S., Vaniman D. T., Zorzano M.-P., Oxidation of manganese in an ancient acquifer, Kimberley formation, Gale crater, Mars. Geophys. Res. Lett. 43, 7398–7407 (2016). [Google Scholar]
- 29.Gasda P. J., Haldeman E. B., Wiens R. C., Rapin W., Bristow T. F., Bridges J. C., Schwenzer S. P., Clark B., Herkenhoff K., Frydenvang J., Lanza N. L., Maurice S., Clegg S., Delapp D. M., Sanford V. L., Bodine M. R., Inroy R. M., In situ detection of boron by ChemCam on Mars. Geophys. Res. Lett. 44, 8739–8748 (2017). [Google Scholar]
- 30.Siebach K. L., Baker M. B., Grotzinger J. P., McLennan S. M., Gellert R., Thompson L. M., Hurowitz J. A., Sorting out compositional trends in sedimentary rocks of the Bradbury group (Aeolis Palus), Gale crater, Mars. J. Geophys. Res. 122, 295–328 (2017). [Google Scholar]
- 31.Morris R. V., Ruff S. W., Gellert R., Ming D. W., Arvidson R. E., Clark B. C., Golden D. C., Siebach K., Klingelhöfer G., Schröder C., Fleischer I., Yen A. S., Squyres S. W., Identification of carbonate-rich outcrops on Mars by the Spirit rover. Science 329, 421–424 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Ming D. W., Archer P. D. Jr., Glavin D. P., Eigenbrode J. L., Franz H. B., Sutter B., Brunner A. E., Stern J. C., Freissinet C., McAdam A. C., Mahaffy P. R., Cabane M., Coll P., Campbell J. L., Atreya S. K., Niles P. B., Bell J. F. III, Bish D. L., Brinckerhoff W. B., Buch A., Conrad P. G., Marais D. J. D., Ehlmann B. L., Fairén A. G., Farley K., Flesch G. J., Francois P., Gellert R., Grant J. A., Grotzinger J. P., Gupta S., Herkenhoff K. E., Hurowitz J. A., Leshin L. A., Lewis K. W., McLennan S. M., Miller K. E., Moersch J., Morris R. V., Navarro-González R., Pavlov A. A., Perrett G. M., Pradler I., Squyres S. W., Summons R. E., Steele A., Stolper E. M., Sumner D. Y., Szopa C., Teinturier S., Trainer M. G., Treiman A. H., Vaniman D. T., Vasavada A. R., Webster C. R., Wray J. J., Yingst R. A.; MSL Science Team , Volatile and organic compositions of sedimentary rocks at Yellowknife Bay, Gale Crater, Mars. Science 343, 1245267 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Ehlmann B. L., Mustard J. F., Clark R. N., Swayze G. A., Murchie S. L., Evidence for low-grade metamorphism, hydrothermal alteration, and diagenesis on Mars from phyllosilicate mineral assemblages. Clay Clay Miner. 59, 359–377 (2011). [Google Scholar]
- 34.Carter J., Poulet F., Bibring J.-P., Mangold N., Murchie S., Hydrous minerals on Mars as seen by the CRISM and OMEGA imaging spectrometers: Updated global view. J. Geophys. Res. 118, 831–858 (2013). [Google Scholar]
- 35.Liu Y., Ma C., Beckett J. R., Chen Y., Guan Y., Rare-earth-element minerals in martian breccia meteorites NWA 7034 and 7533: Implications for fluid-rock interaction in the martian crust. Earth Planet. Sci. Lett. 451, 251–262 (2016). [Google Scholar]
- 36.Wilhelms D. E., Baldwin R. J., The role of igneous sills in shaping the Martian uplands. LPSC 19, 355–365 (1989). [Google Scholar]
- 37.Hiesinger H., Head J. W. III, The Syrtis Major volcanic province, Mars: Synthesis from Mars Global Surveyor data. J. Geophys. Res. 109, E01004 (2004). [Google Scholar]
- 38.Christensen P. R., McSween H. Y. Jr., Bandfield J. L., Ruff S. W., Rogers A. D., Hamilton V. E., Gorelick N., Wyatt M. B., Jakosky B. M., Kieffer H. H., Malin M. C., Moersch J. E., Evidence for magmatic evolution and diversity on Mars from infrared observations. Nature 436, 504–509 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Francis D., Columbia Hills—An exhumed layered igneous intrusion on Mars? Earth Planet. Sci. Lett. 310, 59–64 (2011). [Google Scholar]
- 40.Fawdon P., Skok J. R., Balme M. R., Vye-Brown C. L., Rothery D. A., Jordan C. J., The geological history of Nili Patera, Mars. J. Geophys. Res. 120, 951–977 (2015). [Google Scholar]
- 41.Brustel C., Flahaut J., Hauber E., Fueten F., Quantin C., Stesky R., Davies G. R., Valles Marineris tectonic and volcanic history inferred from dikes in eastern Coprates Chasma. J. Geophys. Res. 122, 1353–1371 (2017). [Google Scholar]
- 42.Fueten F., Flahaut J., Le Deit L., Stesky R., Hauber E., Gwinner K., Interior layered deposits within a perched basin, southern Coprates Chasma, Mars: Evidence for their formation, alteration and erosion. J. Geophys. Res. 116, E02003 (2011). [Google Scholar]
- 43.R. Harvey, J. Griswold, Burial, exhumation, metamorphism and other dastardly deeds exposed at the Hesperian/Noachian boundary in the southern Nili Fossae region, Lunar Planet. Sci. Conf., 41st, Abstract 2045 (2010).
- 44.Ehlmann B. L., Mustard J. F., An in-situ record of a major environmental transitions on early Mars and northern Syrtis Major. Geophys. Res. Lett. 39, L11202 (2012). [Google Scholar]
- 45.Bramble M. S., Mustard J. F., Salvatore M. R., The history of the northern Syrtis Major region. Icarus 293, 66–93 (2017). [Google Scholar]
- 46.Lanz J. K., Saric M. B., Cone fields in SW Elysium Planitia: Hydrothermal venting on Mars? J. Geophys. Res. 114, E02008 (2009). [Google Scholar]
- 47.Boslough M. B., Venturini E. L., Morosin B., Graham R. A., Williamson D. L., Physical properties of shocked and thermally altered nontronite: Implications for the Martian surface. J. Geophys. Res. 91, E207–E214 (1986). [Google Scholar]
- 48.Kraus R. G., Stewart S. T., Newman M. G., Milliken R. E., Tosca N. J., Uncertainties in the shock devolatilization of hydrated minerals: A case study of nontronite. J. Geophys. Res. 118, 2137–2145 (2013). [Google Scholar]
- 49.Friedlander L. R., Glotch T. D., Bish D. L., Dyar M. D., Sharp T. G., Sklute E. C., Michalski J. R., Structural and spectroscopic changes to natural nontronite induced by experimental impacts between 10 and 40 GPa. J. Geophys. Res. 120, 888–912 (2015). [Google Scholar]
- 50.McCubbin F. M., Shearer C. K., Burger P. V., Hauri E. H., Wang J., Elardo S. M., Papike J. J., Volatile abundances of coexisting merrillite and apatite in the martian meteorite Shergotty. Am. Mineral. 99, 1347–1354 (2014). [Google Scholar]
- 51.H. J. Melosh, Impact Cratering: a Geologic Process (Oxford Univ. Press, New York, 1989). [Google Scholar]
- 52.Summers D. P., Basa R. C. B., Khare B., Rodoni D., Abiotic nitrogen fixation on terrestrial planets: Reduction of NO to amonnia by FeS. Astrobiology 12, 107–114 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Schrauzer G. N., Guth T. D., Hydrogen evolving systems.I. The formation of molecular hydrogen from aqueous suspension of iron(II) hydroxide and reactions with reducible substrates, including molecular nitrogen. J. Am. Chem. Soc. 98, 3508–3513 (1976). [Google Scholar]
- 54.Kim J., Yee N., Nanda V., Falkowski P., Anoxic photochemical oxidation of siderate generates molecular hydrogen and iron oxide. Proc. Natl. Acad. Sci. U.S.A. 110, 10073–10077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.R. Cornell, U. Schwertmann, The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses (Wiley-VCH, Weinheim, Germany, ed. 2, 2003). [Google Scholar]
- 56.Tosca N. J., Ahmed I. A. M., Tutolo B. M., Ashpitel A., Hurowitz J. A., Magnetite authigenesis and the warming of early Mars. Nat. Geosci. 11, 635–639 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller S. L., A production of amino acids under possible primitive Earth conditions. Science 117, 528–529 (1953). [DOI] [PubMed] [Google Scholar]
- 58.Abelson P. H., Chemical events on the primitive Earth. Proc. Natl. Acad. Sci. U.S.A. 55, 1365–1372 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dressing C. D., Charbonneau D., Dumusque X., Gettel S., Pepe F., Cameron A. C., Latham D. W., Molinari E., Udry S., Affer L., Bonomo A. S., Buchhave L. A., Cosentino R., Figueira P., Fiorenzano A. F. M., Harutyunyan A., Haywood R. D., Johnson J. A., Lopez-Morales M., Lovis C., Malavolta L., Mayor M., Micela G., Motalebi F., Nascimbeni V., Phillips D. F., Piotto G., Pollacco D., Queloz D., Rice K., Sasselov D., Ségransan D., Sozzetti A., Szentgyorgyi A., Watson C., The mass of Kepler-93b and the composition of terrestrial planets. Astrophys. J. 800, 135–142 (2015). [Google Scholar]
- 60.D. Catling, J. Kasting, Atmospheric Evolution on Inhabited and Lifeless Worlds (Cambridge Univ. Press, 2017). [Google Scholar]
- 61.Schaefer L., Jacobsen S. B., Remo J. L., Petaev M. I., Sasselov D. D., Metal-silicate partitioning and its role in core formation and composition on super-Earths. Astrophys. J. 835, 234 (2017). [Google Scholar]
- 62.Kasting J. F., Stability of ammonia in the primitive terrestrial atmosphere. J. Geophys. Res. 87, 3091–3098 (1982). [Google Scholar]
- 63.Pavlov A. A., Brown L. L., Kasting J. F., UV shielding of NH3 and O2 by organic hazes in the Archaen atmosphere. J. Geophys. Res. 106, 23267–23287 (2001). [Google Scholar]
- 64.Wetzel D. T., Rutherford M. J., Jacobsen S. D., Hauri E. H., Saal A. E., Degassing of reduced carbon from planetary basalts. Proc. Natl. Acad. Sci. U.S.A. 110, 8010–8013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kasting J. F., What caused the rise of atmospheric O2? Chem. Geol. 362, 13–25 (2013). [Google Scholar]
- 66.Batalha N., Domagal-Goldman S. D., Ramirez R., Kasting J. F., Testing the early Mars H2-CO2 greenhouse hypothesis with 1-D photochemical model. Icarus 258, 337–349 (2015). [Google Scholar]
- 67.Schaefer L., Sasselov D., The persistence of oceans on Earth-like planets: Insights from the deep-water cycle. Astrophys. J. 801, 40–53 (2015). [Google Scholar]
- 68.Valencia D., O’Connell R., Sasselov D., The inevitability of plate tectonics on super-Earths. Astrophys. J. Lett. 670, 45–48 (2007). [Google Scholar]
- 69.Tackley P. J., Ammann M., Brodholt J. P., Dobson D. P., Valencia D., Mantle dynamics in super-Earths: Post-perovskite rheology and self-regulation of viscosity. Icarus 225, 50–61 (2013). [Google Scholar]
- 70.Ranjan S., Sasselov D. D., Influence of the UV environment on the synthesis of prebiotic molecules. Astrobiology 16, 68–88 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Todd Z. R., Fahrenbach A. C., Magnani C. J., Ranjan S., Björkbom A., Szostak J. W., Sasselov D. D., Solvated-electron production using cyanocuprates is compatible with the UV-environment on a Hadean-Archaean Earth. Chem. Commun. 54, 1121–1124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beckstead A. A., Zhang Y., de Vries M. S., Kohler B., Life in the light: Nucleic acid photoproperties as a legacy of chemical evolution. Phys. Chem. Chem. Phys. 18, 24228–24238 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Islam S., Powner M. W., Prebiotic systems chemistry: Complexity overcoming clutter. Chemistry 2, 470–501 (2017). [Google Scholar]
- 74.Ranjan S., Todd Z. R., Rimmer P. B., Sasselov D. D., Babbin A. R., Nitrogen oxide concentrations in natural waters on early Earth. Geochem. Geophys. Geosyst. 20, 2021–2039 (2019). [Google Scholar]


