Abstract
Cochlear implants are one of the most successful neuroprosthetic devices that have been developed to date. Profoundly deaf patients can achieve speech perception after complete loss of sensory input. Despite the improvements many patients experience, there is still a large degree of outcome variability. It has been proposed that central plasticity may be a major factor in the different levels of benefit that patients experience. However, the neural mechanisms of how plasticity impacts cochlear implant learning and the degree of plasticity’s influence remain unknown. Here, we review the human and animal research on three of the main ways that central plasticity affects cochlear implant outcomes.
INTRODUCTION
Hearing loss is a disabling condition affecting many tens of millions of people worldwide [1]. Deafness can impact quality of life beyond loss of a sensory system fundamental to social communication, and is now identified as a risk factor for a number of conditions including dementia and Alzheimer’s disease [2,3]. Human subjects with profound deafness can be treated with cochlear implants, which are neuroprosthetic devices that directly electrically stimulate the auditory nerve [4]. However, there is a large amount of variability in learning rates and peak levels of speech perception across individual cochlear implant users [5-7]. Learning to hear with cochlear implants is proposed to require plasticity within the central auditory system [8-12], but the mechanisms by which real-world experience or explicit behavioral training enables plasticity and improves outcomes is poorly understood.
Cochlear implants were developed in human subjects over a period of decades, evolving from a single wire to the multi-electrode devices of today [13]. Thes devices are placed within the cochlear and interface directly with the spiral ganglia of the auditory nerve, bypassing any earlier deficits in the hearing pathway such as hair cell damage or conductive hearing loss. However, our understanding of how cochlear implants interface with the auditory system beyond the auditory nerve, how they activate the higher-level central auditory neuraxis, and how these neural systems adapt over time to reinterpret the signals as meaningful sounds is still limited. Central auditory processing is required for patients to be able to use the auditory information provided through cochlear implants [14], and experience-dependent adaptation to cochlear implant input is important for patient outcomes. Most patients require months or even years to reach maximal perceptual performance [5, 15-18], and targeted training improves both speech and music perception in patients [19], suggesting that neural plasticity is a major determinant of patient outcomes. Additionally, for patients with sub-optimal outcomes, few predictive markers exist, and there are no widely accepted methods to affect those outcomes once patient performance has plateaued.
The neural and perceptual adaptation to cochlear implants can also be studied in animals, to reveal fundamental mechanisms and principles by which training affects auditory processing and perception in a way that cannot easily be examined in human subjects. To identify neurobiological factors contributing to successful implant use and inter-subject variability in learning and performance, animal models of cochlear implants have been developed [20-22]. There is a wide body of literature implicating neuroplasticity in cochlear implant performance in both humans and animals. This plasticity can be broken down into either adaptive or cross-modal. Adaptive plasticity is how the central auditory system changes in response to the cochlear implant. Cross-modal plasticity is the idea that one sensory modality can activate a separate sensory system’s central processing areas, in particular when one type of sensory input has been reduced such as in deafness or blindness. Cross-modal plasticity occurs after a period of auditory deprivation, such that the auditory cortex is is re-organized to support other sensory processes, including visual and somatosensory input. Much of the research on cross-modal plasticity in deaf patients focuses on visual stimuli evoking activity in the central auditory system. Depending on the extent of this reorganization and the capacity of the auditory cortex to revert to primarily auditory processing, this can be detrimental to cochlear implant outcomes. Furthermore, cross-modal plasticity occurs differentially in prelingually deaf patients (who have had no auditory experience) and postlingually deaf patients, whose auditory system development was normal. In contrast to the unclear benefits and even potentially disruptive effects of cross-modal plasticity, training and experience with the cochlear implant is important in adaptation to the new form of auditory stimulation. Speech perception performance improves with time, even when there is a reduction of residual unaided hearing, suggesting an adaptive process in the central auditory system. These types of plasticity that occur and interact surrounding cochlear implant use are the topic of this review.
ADAPTIVE PLASTICITY
Following loss of auditory input and cochlear implantation, the central auditory system and perhaps other parts of the brain need to change post-operatively in order to adapt to the electrical input provided by cochlear implants. This is of particular importance when hearing loss occurs in the mature auditory system, which has experience with previous auditory signals. In addition to the spectral degradation of the information provided by cochlear implants, there is presumably some degree of “frequency mismatch” in stimulation. This mismatch refers to the fact that most cochlear implants only cover the most basal turn of the cochlea and thus may not stimulate neurons with low characteristic frequencies. Instead, low frequency stimuli stimulate cochlear locations that are more basal (higher frequency) than the locations that were stimulated by the same sound prior to hearing loss [23]. Over time, the perception of this frequency shift can normalize. Studies using normal hearing subjects listening to acoustic models of cochlear implants suggest that gradual introduction of the shift over several training sessions can result in faster adaptation and perhaps better generalization to new talkers [24-26]. The adaptation of the auditory system over time with cochlear implants has been observed in a longitudinal positron emission tomography (PET) study in both pre- and postlingually-deaf patients. Postlingually-deaf patients. Postlingually-deaf patients showed an increase in PET activation of Broca’s area during speech perception post-implantation, but this was not observed in prelingually-deaf patients. The observation was coupled with increasing speech perception capabilities in the postlingually-deaf group, but not the prelingually deaf [27]. These findings are supported by an earlier study that Broca’s area activation by speech-reading in cochlear implant patients increases with time post-implantation [28]. In a unique case study, intracranial electrocorticographic recordings were conducted in a bilateral cochlear implant user with refractory epilepsy. Results showed activation of the auditory cortex comparable to that of normal hearing patients in this experienced cochlear implant user, but lacking in tonotopicity (or “electrodopicity”) [29].
However, neuroimaging approaches pose some limitations, such as slow temporal dynamics and poor spatial resolution on human studies of adaptation to hearing loss and cochlear implantation. Therefore, several models of hearing loss and cochlear implantation in animals have been developed. These include established procedures for cochlear implantation in the cat, ferret, guinea pig, and marmoset, as well as newer studies of cochlear implants in rodents. These rodent systems present the added utility of transgenic animals for examining mechanisms of plasticity [30,31*].
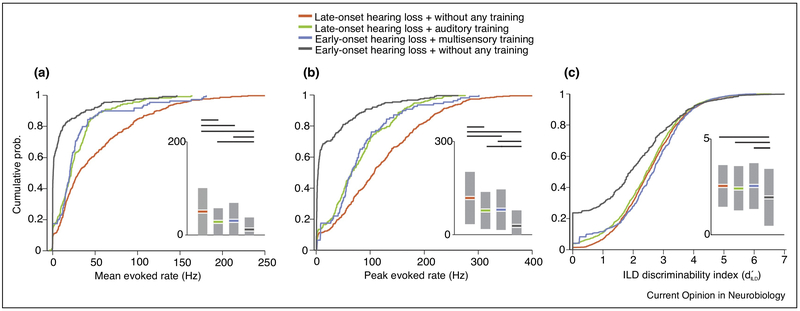
Auditory studies conducted in ferrets have shown that following monaural deprivation, plasticity is engaged in order to adapt to the new and altered cues for sound localization. Extracellular recordings in the ferret auditory cortex showed that there were distinct neural processes underlying reweighing and remapping localization cues, which mirrored the different types of behavioral adaptations observed in intermittently monaurally deprived humans [32]. Furthermore, the ferret has been used to study sound localization after bilateral cochlear-implantation [33]. Bilaterally implanted animals with early onset deafness had more difficulty with sound localization than those with lateonset hearing loss. However, multisensory training improved performance in implanted ferrets with early-onset hearing loss, and this improvement was correlated with increased responsiveness of the auditory cortex (Figure 1) [34**]. The benefits of training with the implant are further highlighted by studies in congenitally deaf cats, where behavioral training combined with cochlear stimulation induces more improvement in temporal processing than stimulation alone in the primary auditory cortex, but not in the inferior colliculus [35*].
Figure 1.
Cumulative probability functions showing the relative magnitude of the stimulus-evoked responses of A1 neurons, grouped by age of onset of hearing loss and training history in animals with BiCIs. A, Mean sound-evoked firing rates. B, Peak sound-evoked firing rates. C, ILD discriminability index computed from rate-level functions. Insets, Modified box-plots showing the means and 95% confidence intervals of each spike rate measure, grouped in the same fashion as the probability functions. The probability functions and bars indicating that the means have been color-coded to identify the different groups. The horizontal lines indicate significant intergroup differences, as revealed by Tukey HSD tests for post-ANOVA pairwise comparisons. Figure is from Ref 34, with permission.
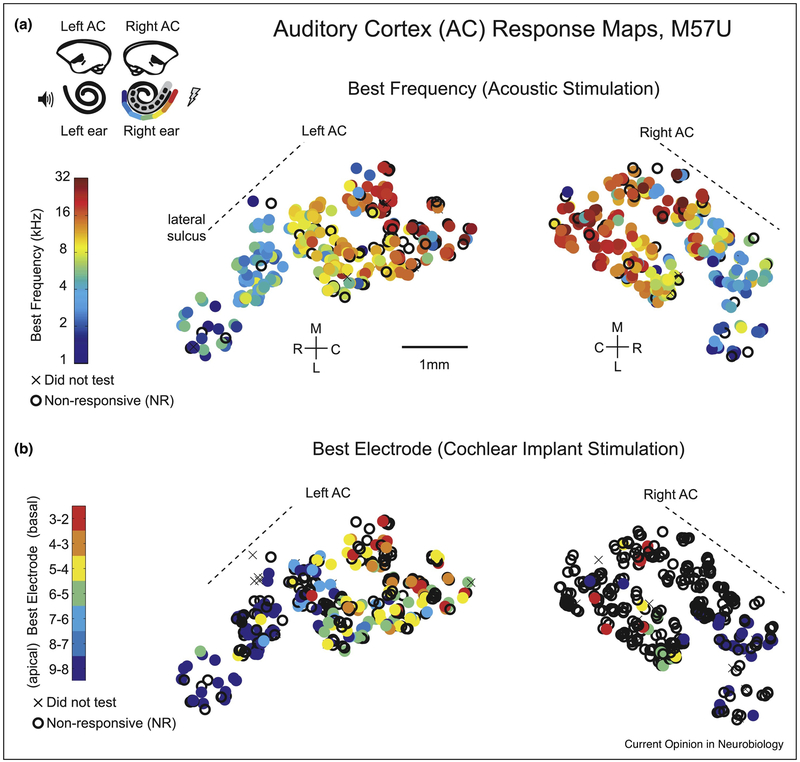
Marmosets have been useful for studying the neural mechanisms of cochlear implant use, as they are non-human primates with sophisticated vocal communication abilities [36]. Furthermore, technical advances enable chronic recordings in behaving animals and possible transgene expression [37,38]. In unilaterally deafened cochlear implanted marmosets, tone-evoked and implant-evoked auditory cortical responsiveness were monitored. A large proportion of neurons that responded to acoustic stimuli did not respond to cochlear implant stimulation (Figure 2) [39**]. Notably, primates in this study received no training with the implant, which might in part account for the limited cortical activation. This was also the case for temporally modulated electrical and acoustic stimuli. Single-unit recordings revealed that cochlear implant electrical stimulation produced both stimulus-synchronized and nonsynchronized firing, with individual units responding similarly to acoustic and electrical stimulation [40].
Figure 2.
Topographic maps of auditory cortex responses to acoustic and CI stimulation. A, Acoustic BF maps of left and right auditory cortex (AC) of a marmoset implanted unilaterally in the right cochlea with a CI electrode array (inset plot). Dashed lines indicate approximate positions of the lateral sulcus. Each circle represents a single neuron recorded at that cortical surface location, color-coded by its BF. Black open circles represent neurons nonresponsive (NR) to acoustic tones and bandpass noise. Crosses indicate neurons only tested with CI. B, CI best electrode maps show the same neurons as in A, with color corresponding to CI best electrode. Stimulation was between adjacent contacts in the electrode array, indicated by the pair of numbers on the y-axis. Symbols are the same as in A. Crosses indicate neurons only tested with acoustic stimulation. Figure is adapted from Ref 37, with permission.
PRELINGUAL CROSS-MODAL PLASTICITY
Cross-modal plasticity can be categorized based on the cochlear implant users experience with sound. Early evidence of cross-modal plasticity came from studies on congenitally deaf patients, whose auditory system had no acoustic input prior to cochlear implantation. A link between the degree of cross-modal plasticity and speech perception outcomes after cochlear implantation has been suggested. Lee et al. showed early evidence of cross-modal plasticity using PET in a small study of congenitally deaf children [41,42]. Hypometabolism of the pre-operative auditory cortex (measured bilaterally) was positively correlated with cochlear implant outcomes, suggesting that activity in the auditory cortex by other sensory inputs is maladaptive for ultimate auditory processing. In follow-up studies, the degree of auditory cortical PET activation by visual stimuli was negatively correlated with cochlear implant outcomes, but only in the right hemisphere. Decreased visual responsiveness of the auditory cortex post-implantation was positively correlated with behavioral outcomes [42,43]. Separate EEG studies conducted post-implantation showed that visually-evoked potentials, measured as the negative-going evoked potential N1, in auditory cortical areas of cochlear implant patients were correlated with speech perception [44-46]. However, the effect was observed only in the right hemisphere, and sometimes in terms of N1 amplitude (but not latency) or in other cases N1 latency (but not amplitude). These studies suggested a link between cross-modal plasticity and performance with cochlear implants in the pre-lingually deaf population. A recent study examined differences in visually evoked potentials and auditory evoked potentials between normal hearing and congenitally deaf children. Auditory N1 latency and amplitude, as well as visual N1 latency were reduced, but these changes were not associated across sensory modalities, indicating intra-modal rather than cross-modal plasticity [47].
To understand the changes that are occurring in congenitally-deaf patients, animal studies have been conducted to assess the changes in the auditory system due to sensory deprivation. Developmental hearing loss results in hyperexcitability and decreases in inhibitory synaptic strength in the auditory cortex, changes which persist into adulthood, as shown by studies of conductive hearing loss in gerbils [48]. In neonatally deafened cats with no sensory restoration, there was a loss of tonotopy in the auditory cortex, but if cochlear implant intervention was used after an extended period of deafness (6 months), this tonotopy could be rescued [8,49]. However, even if auditory input was fully restored, there were deficits in performance on auditory tasks [50].
Animal studies have confirmed the presence of cross-modal plasticity after developmental hearing loss. Congenitally deaf cats exhibited enhanced performance on a range of visual psychophysical tasks. This improvement in visual behavior in deafened animals seemed to depend on activity in the auditory cortex [51]. However, the degree to which cross-modal plasticity is detrimental to cochlear implant outcomes is not clear. In congenitally deaf cats with cochlear implants, there was an increase in visual responsiveness in higher order auditory cortical areas However, the activation of auditory cortex by cochlear implants was similar between congenitally deaf and normal hearing cats [52]. Furthermore, auditory evoked potentials in visual areas could be evoked in congenitally deafened cats both implanted late in deafness [53**]. While this does not rule out the possibility that speech comprehension could be negatively impacted by cross-modal plasticity it does question the interpretation that increased visual activation of the auditory cortex reduces the ability of the auditory cortex to respond to future auditory stimuli.
POSTLINGUAL CROSS-MODAL PLASTICITY
Cross-modal plasticity likely occurs in postlingually deafened patients as well, but the degree to which it occurs and the effects on performance are less understood. Limitations of neuroimaging techniques restrict the necessary longitudinal studies to fully characterize these effects. Magnetic resonance imaging (MRI) can be used only with significant limitations in cochlear implant users, as the implanted magnet introduces imaging artifacts. The invasiveness of PET limits its utility. The recent development of functional near-infrared spectroscopy (fNIRS) has helped advance imaging in cochlear implant users, overcoming these invasive/electrical interference issues. This may help clarify issues surrounding cross-modal plasticity in postlingually deaf patients, as it will allow more intra-subject tests to compare cortical activity pre and post implantation [54-56]. The spatial resolution of fNIRS is quite limited, however, more so than that of MRI. In order to address the limitations of individual imaging modalities, more recent studies simultaneously used different techniques, such as EEG and fNIRS to increase resolution [57*].
A series of EEG studies in human subjects showed evidence that cross-modal activation (visually evoked potentials in the auditory cortex) negatively correlated with speech outcomes in post-lingually deafened cochlear implant users, as was observed in pre-lingually deaf cochlear implant users [58-60]. Additionally, increased coupling between the occipital lobe and the temporal lobe as measured by fMRI may predict poor cochlear implant outcomes [61*]. Conversely, studies using alternative neuroimaging techniques proposed that this cross-modal plasticity was not maladaptive, but instead can be beneficial post-implantation by optimizing audiovisual integration [28,62,63**]. Another potential hypothesis is that the type of cross-modal plasticity is important. Visual activation of the auditory cortex may be maladaptive, but auditory activation of the visual cortex promotes performance [64]. Perhaps this distinction reflects that visual inputs to auditory cortex are abnormal, whereas auditory cortical inputs to the visual cortex are normal and serve some adaptive function in healthy subjects.
Over the past several decades, various animal models of adult onset deafness to investigate the cortical changes that occur after sensory deprivation, overcoming some of the limitations of human imaging studies. Cortical reorganization occurs even when sensory input is deprived after normal auditory development, across model organisms. Changes in tonotopy, best frequency, and spontaneous and evoked activity have been observed [65]. For instance, in cochlear ablations and noise-induced trauma in cats, a tonotopic reorganization of the auditory cortex towards intact frequencies was observed [66,67]. In ferrets with adult onset deafness, extensive cross-modal reorganization was observed, with the auditory cortex becoming responsive to somatosensory input [68]. It seems that changes in cortical inhibition underlies many of the functional changes observed. Scholl and Wehr found that acoustic trauma led to complex changes in cortical inhibitory and excitatory responses across frequency tuning curves [69]. A recent study in mice investigated the effects of reduced inhibitory signaling and hyper excitability of the auditory cortex after peripheral auditory nerve damage on recovery of sound processing, revealing that excitability returned to baseline, but the reduction in inhibitory signaling was sustained [70**]. However, in the same study that showed negative impacts on inhibitory synapses in developmental hearing loss, adult onset hearing loss showed no long-term impacts on inhibitory function [48].
CONCLUSIONS
The success of the cochlear implant is evident through the restoration of hearing and speech perception across patient populations. However, an increased ability to predict outcomes is needed to help understand and address the persistent variability in outcomes [6]. The central auditory system seems to first adapt to the loss of sensory input and then to the restoration of auditory input. It is clear that early intervention in prelingually-deaf patients improves the possibility for functional use of cochlear implants, and is related to enabling normal auditory development and reducing development of potentially maladaptive cross-modal activation of the auditory cortex. In a normally-developed auditory system, the adaptive or maladaptive nature of cross-modal plasticity is poorly understood, and further longitudinal studies that include pre- and post-implant time points are necessary. Human studies are complemented by emerging animal models of cochlear implant use that reveal mechanisms of adaptation and plasticity. Knowledge of the optimal or suboptimal conditions for engaging plastic mechanisms can be potentially harnessed to predict and improve outcomes in cochlear implant patients.
HIGHLIGHTS.
There is a period of adaptation after cochlear implantation that impacts ultimate utility of the implant.
In prelingually deaf patients, there is visual activation of central auditory areas, but there is limited evidence that this has negative effects on cochlear implant performance.
Cross-modal activation is present in postlingually deaf patients, but this may provide benefits in cochlear implant use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pienkowski M, Kunov H: Suppression of distortion product otoacoustic emissions and hearing threshold. J Acoust Soc Am 2001, 109:1496–1502. [DOI] [PubMed] [Google Scholar]
- 2.Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner E, Satterfield S, Ayonayon HN, Ferrucci L, Simonsick EM, et al. : Hearing Loss and Cognitive Decline in Older Adults. Jama Internal Medicine 2013, 173:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson RS, Auduong P, Miller AT, Gurgel RK: Hearing Loss as a Risk Factor for Dementia: A Systematic Review. Laryngoscope Investigative Otolaryngology 2017, 2:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merzenich MM, Michelson RP, Pettit CR, Schindler RA, Reid M: Neural encoding of sound sensation evoked by electrical stimulation of the acoustic nerve. Ann Otol Rhinol Laryngol 1973, 82:486–503. [DOI] [PubMed] [Google Scholar]
- 5.Chang S-A, Tyler R, Dunn C, Haihong J, Witt S, Gantz B, Hansen MR: Performance overtime on adults with simultaneous bilateral cochlear implants. J Am Acad Audiol 2010, 21:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blamey: Factors Affecting Auditory Performance of Post linguistically Deaf Adults Using Cochlear Implants: An Update with 2251 Patients. 2013. [DOI] [PubMed]
- 7.Tang L, Thompson CB, Clark JH, Ceh KM, Yeagle JD, Francis HW: Rehabilitation and Psychosocial Determinants of Cochlear Implant Outcomes in Older Adults. Ear Hear 2017. [DOI] [PubMed] [Google Scholar]
- 8.Fallon JB, Irvine DR, Shepherd RK: Neural prostheses and brain plasticity. J Neural Eng 2009, 6:065008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiss LA, Turner CW, Erenberg SR, Gantz BJ: Changes in pitch with a cochlear implant over time. J Assoc Res Otolaryngol 2007, 8:241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiss LA, Turner CW, Karsten SA, Gantz BJ: Plasticity in human pitch perception induced by tonotopically mismatched electro-acoustic stimulation. Neuroscience 2014, 256:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svirsky MA, Silveira A, Suarez H, Neuburger H, Lai TT, Simmons PM: Auditory learning and adaptation after cochlear implantation: a preliminary study of discrimination and labeling of vowel sounds by cochlear implant users. Acta Otolaryngol 2001, 121:262–265. [DOI] [PubMed] [Google Scholar]
- 12.Svirsky MA, Silveira A, Neuburger H, Teoh SW, Suarez H: Long-term auditory adaptation to a modified peripheral frequency map. Acta Otolaryngol 2004, 124:381–386. [PubMed] [Google Scholar]
- 13.Mudry A, Mills M: The early history of the cochlear implant: a retrospective. JAMA Otolaryngol Head Neck Surg 2013, 139:446–453. [DOI] [PubMed] [Google Scholar]
- 14.Moore DR, Shannon RV: Beyond cochlear implants: awakening the deafened brain. Nat Neurosci 2009, 12:686–691. [DOI] [PubMed] [Google Scholar]
- 15.Hamzavi J, Baumgartner WD, Pok SM, Franz P, Gstoettner W: Variables affecting speech perception in postlingually deaf adults following cochlear implantation. Acta Otolaryngol 2003, 123:493–498. [DOI] [PubMed] [Google Scholar]
- 16.Ruffin CV, Tyler RS, Witt SA, Dunn CC, Gantz BJ, Rubinstein JT: Long-term performance of Clarion 1.0 cochlear implant users. Laryngoscope 2007, 117:1183–1190. [DOI] [PubMed] [Google Scholar]
- 17.Krueger B, Joseph G, Rost U, Strauss-Schier A, Lenarz T, Buechner A: Performance groups in adult cochlear implant users: speech perception results from 1984 until today. Otol Neurotol 2008, 29:509–512. [DOI] [PubMed] [Google Scholar]
- 18.Fu QJ, Galvin J, Wang X, Nogaki G: Effects of auditory training on adult cochlear implant patients: a preliminary report. Cochlear Implants Int 2004, 5 Suppl 1:84–90. [DOI] [PubMed] [Google Scholar]
- 19.Fu QJ, Galvin JJ 3rd: Perceptual learning and auditory training in cochlear implant recipients. Trends Amplif 2007, 11:193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leake PA, Hradek GT, Rebscher SJ, Snyder RL: Chronic intracochlear electrical stimulation induces selective survival of spiral ganglion neurons in neonatally deafened cats. Hear Res 1991, 54:251–271. [DOI] [PubMed] [Google Scholar]
- 21.Ryugo DK, Kretzmer EA, Niparko JK: Restoration of auditory nerve synapses in cats by cochlear implants. Science 2005, 310:1490–1492. [DOI] [PubMed] [Google Scholar]
- 22.Pinilla M, Ramirez-Camacho R, Jorge E, Trinidad A, Vergara J: Ventral approach to the rat middle ear for otologic research. Otolaryngol Head Neck Surg 2001, 124:515–517. [DOI] [PubMed] [Google Scholar]
- 23.McDermott H, Sucher C, Simpson A: Electro-acoustic stimulation. Acoustic and electric pitch comparisons. Audiol Neurootol 2009, 14 Suppl 1:2–7. [DOI] [PubMed] [Google Scholar]
- 24.Knudsen EI, Knudsen PF: Visuomotor adaptation to displacing prisms by adult and baby barn owls. J Neurosci 1989, 9:3297–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linkenhoker BA, Knudsen EI: Incremental training increases the plasticity of the auditory space map in adult barn owls. Nature 2002, 419:293–296. [DOI] [PubMed] [Google Scholar]
- 26.Svirsky MA, Talavage TM, Sinha S, Neuburger H, Azadpour M: Gradual adaptation to auditory frequency mismatch. Hear Res 2015, 322:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen B, Gjedde A, Wallentin M, Vuust P: Cortical plasticity after cochlear implantation. Neural Plast 2013, 2013:318521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rouger J, Lagleyre S, Demonet JF, Fraysse B, Deguine O, Barone P: Evolution of crossmodal reorganization of the voice area in cochlear-implanted deaf patients. Hum Brain Mapp 2012, 33:1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nourski KV, Etler CP, Brugge JF, Oya H, Kawasaki H, Reale RA, Abbas PJ, Brown CJ, Howard MA 3rd: Direct recordings from the auditory cortex in a cochlear implant user. J Assoc Res Otolaryngol 2013, 14:435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King J, Shehu I, Roland JT Jr., Svirsky MA, Froemke RC: A physiological and behavioral system for hearing restoration with cochlear implants. J Neurophysiol 2016, 116:844–858.The authors developed a physiologically and behaviorally validated cochlear implant surgery in rats. In this approach, an 8-channel array can be inserted up to one full turn in the cochlea and rats can be trained to perform auditory tasks using the implant. This system will enable further studies on the effects of central neuroplasticity in cochlear implant performance.
- 31.Claussen AD, Vielman Quevedo R, Mostaert B, Kirk JR, Dueck WF, Hansen MR: A mouse model of cochlear implantation with chronic electric stimulation. PLoS One 2019, 14:e0215407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keating P, Rosenior-Patten O, Dahmen JC, Bell O, King AJ: Behavioral training promotes multiple adaptive processes following acute hearing loss. Elife 2016, 5:e12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartley DE, Vongpaisal T, Xu J, Shepherd RK, King AJ, Isaiah A: Bilateral cochlear implantation in the ferret: a novel animal model for behavioral studies. J Neurosci Methods 2010, 190:214–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isaiah A, Vongpaisal T, King AJ, Hartley DE: Multisensory training improves auditory spatial processing following bilateral cochlear implantation. J Neurosci 2014, 34:11119–11130.Ferrets were deafened either at the onset of hearing or as adults, cochlear implanted as adults either unilaterally or bilaterally, and then tested on an auditory localization task. Unilaterally implanted animals could not perform the task, and bilaterally implanted animals with early onset hearing loss performed poorly. However, multisensory training improved the performance of bilaterally implanted animals with early hearing loss.
- 35.Vollmer M, Beitel RE, Schreiner CE, Leake PA: Passive stimulation and behavioral training differentially transform temporal processing in the inferior colliculus and primary auditory cortex. J Neurophysiol 2017, 117:47–64.Profoundly deaf cats were exposed to either passive intracochlear electrical stimulation, behavioral training with intracochlear electrical stimulation, or no intracochlear electrical stimulation. Electrophysiology was conducted in the inferior colliculus and auditory cortex. While passive stimulation improved temporal coding in both the auditory cortex and inferior colliculus, behavioral training only showed effects in the auditory cortex.
- 36.Agamaite JA, Chang CJ, Osmanski MS, Wang X: A quantitative acoustic analysis of the vocal repertoire of the common marmoset (Callithrix jacchus). J Acoust Soc Am 2015, 138:2906–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson LA, Della Santina CC, Wang X: Temporal bone characterization and cochlear implant feasibility in the common marmoset (Callithrix jacchus). Hear Res 2012, 290:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, et al. : Generation of transgenic non-human primates with germline transmission. Nature 2009, 459:523–527. [DOI] [PubMed] [Google Scholar]
- 39.Johnson LA, Della Santina CC, Wang X: Selective Neuronal Activation by Cochlear Implant Stimulation in Auditory Cortex of Awake Primate. J Neurosci 2016, 36:12468–12484.Marmosets were unilaterally deafened and cochlear implanted, then single unit accoustic and implant evoked responses were recorded from both auditory cortices. The authors found that the cochlear implant stimulation activated fewer neurons than accoustic stimuli in both the contralateral and ipsilateral hemispheres. Implant non-responsive neurons tended to be tuned to frequency and sound level, providing a potential explanation for the deficits in cochlear implant users’ performance on task utilizing these modalities.
- 40.Johnson LA, Della Santina CC, Wang X: Representations of Time-Varying Cochlear Implant Stimulation in Auditory Cortex of Awake Marmosets (Callithrix jacchus). J Neurosci 2017, 37:7008–7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, Lee MC, Kim CS: Cross-modal plasticity and cochlear implants. Nature 2001, 409:149–150. [DOI] [PubMed] [Google Scholar]
- 42.Lee H, Giraud A, Kang E, Oh S, Kang H, Kim C, Lee D: Cortical activity at rest predicts cochlear implantation outcome. Cereb Cortex 2007. [DOI] [PubMed] [Google Scholar]
- 43.Giraud AL, Lee HJ: Predicting cochlear implant outcome from brain organisation in the deaf. Restor Neurol Neurosci 2007, 25:381–390. [PubMed] [Google Scholar]
- 44.Liang M, Zhang J, Liu J, Chen Y, Cai Y, Wang X, Wang J, Zhang X, Chen S, Li X, et al. : Visually Evoked Visual-Auditory Changes Associated with Auditory Performance in Children with Cochlear Implants. Front Hum Neurosci 2017, 11:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell J, Sharma A: Visual Cross-Modal Re-Organization in Children with Cochlear Implants. PLoS One 2016, 11:e0147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckley KA, Tobey EA: Cross-modal plasticity and speech perception in pre- and postlingually deaf cochlear implant users. Ear Hear 2011, 32:2–15. [DOI] [PubMed] [Google Scholar]
- 47.Corina DP, Blau S, LaMarr T, Lawyer LA, Coffey-Corina S: Auditory and Visual Electrophysiology of Deaf Children with Cochlear Implants: Implications for Cross-modal Plasticity. Front Psychol 2017, 8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takesian AE, Kotak VC, Sanes DH: Age-dependent effect of hearing loss on cortical inhibitory synapse function. J Neurophysiol 2012, 107:937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fallon JB, Shepherd RK, Irvine DR: Effects of chronic cochlear electrical stimulation after an extended period of profound deafness on primary auditory cortex organization in cats. Eur J Neurosci 2014, 39:811–820. [DOI] [PubMed] [Google Scholar]
- 50.von Trapp G, Aloni I, Young S, Semple MN, Sanes DH: Developmental hearing loss impedes auditory task learning and performance in gerbils. Hear Res 2017, 347:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lomber SG, Meredith MA, Kral A: Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci 2010, 13:1421–1427. [DOI] [PubMed] [Google Scholar]
- 52.Land R, Baumhoff P, Tillein J, Lomber SG, Hubka P, Kral A: Cross-Modal Plasticity in Higher-Order Auditory Cortex of Congenitally Deaf Cats Does Not Limit Auditory Responsiveness to Cochlear Implants. J Neurosci 2016, 36:6175–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Land R, Radecke JO, Kral A: Congenital Deafness Reduces, But Does Not Eliminate Auditory Responsiveness in Cat Extrastriate Visual Cortex. Neuroscience 2018, 375:149–157.The authors examined how auditory evoked potentials in the auditory cortex differ in congenitally deafened cats versus normal hearing cats. Adult congenitally deaf cats received cochlear implants, and intracochlear electrical stimulation showed slightly reduced, but similar evoked activity in visual areas compared to normal hearing animals.
- 54.McKay CM, Shah A, Seghouane AK, Zhou X, Cross W, Litovsky R: Connectivity in Language Areas of the Brain in Cochlear Implant Users as Revealed by fNIRS. Physiology, Psychoacoustics and Cognition in Normal and Impaired Hearing 2016, 894:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dewey RS, Hartley DE: Cortical cross-modal plasticity following deafness measured using functional near-infrared spectroscopy. Hear Res 2015, 325:55–63. [DOI] [PubMed] [Google Scholar]
- 56.Lawler CA, Wiggins IM, Dewey RS, Hartley DE: The use of functional near-infrared spectroscopy for measuring cortical reorganisation in cochlear implant users: a possible predictor of variable speech outcomes? Cochlear Implants Int 2015, 16 Suppl 1:S30–32. [DOI] [PubMed] [Google Scholar]
- 57.Chen LC, Stropahl M, Schonwiesner M, Debener S: Enhanced visual adaptation in cochlear implant users revealed by concurrent EEG-fNIRS. Neuroimage 2017, 146:600–608.The authors here conducted simultaneous fNIRS and EEG recordings during visual and auditory stimuli presentation in post-lingually deaf cochlear implant users and normal hearing subjects. By combining these imaging modalities, the authors were able to show results that cochlear implant users adapt more quickly to repeated visual stimuli, but lower amplitude auditory responses as compared to normal hearing users.
- 58.Sandmann P, Dillier N, Eichele T, Meyer M, Kegel A, Pascual-Marqui RD, Marcar VL, Jancke L, Debener S: Visual activation of auditory cortex reflects maladaptive plasticity in cochlear implant users. Brain 2012, 135:555–568. [DOI] [PubMed] [Google Scholar]
- 59.Doucet ME, Bergeron F, Lassonde M, Ferron P, Lepore F: Cross-modal reorganization and speech perception in cochlear implant users. Brain 2006, 129:3376–3383. [DOI] [PubMed] [Google Scholar]
- 60.Kim MB, Shim HY, Jin SH, Kang S, Woo J, Han JC, Lee JY, Kim M, Cho YS, Moon IJ, et al. : Cross-Modal and Intra-Modal Characteristics of Visual Function and Speech Perception Performance in Postlingually Deafened, Cochlear Implant Users. PLoS One 2016, 11:e0148466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lazard DS, Giraud AL: Faster phonological processing and right occipitotemporal coupling in deaf adults signal poor cochlear implant outcome. Nat Commun 2017, 8:14872.In this study, fMRI was conducted in post-lingually deaf cochlear implant users during a visual-based phonological test. Cochlear implant users who had faster reaction times on the test showed altered connectivity in auditory and visual regions and overall poorer cochlear implant performance. Patients who had reaction times more similar to normal hearing subjects showed preserved left lateralization during phonological processing and improved speech perception with cochlear implant.
- 62.Strelnikov K, Rouger J, Demonet JF, Lagleyre S, Fraysse B, Deguine O, Barone P: Visual activity predicts auditory recovery from deafness after adult cochlear implantation. Brain 2013, 136:3682–3695. [DOI] [PubMed] [Google Scholar]
- 63.Anderson CA, Wiggins IM, Kitterick PT, Hartley DEH: Adaptive benefit of crossmodal plasticity following cochlear implantation in deaf adults. Proc Natl Acad Sci U S A 2017, 114:10256–10261.The authors used fNIRS to examine visual speech activation of the superior temporal cortex in post-lingually deaf adults before and after cochlear implantation. They correlated this cross-modal activation with speech perception after 6 months of cochlear implant experience. The results demonstrated an adaptive rather than maladaptive effect of cross-modal plasticity.
- 64.Chen LC, Sandmann P, Thorne JD, Bleichner MG, Debener S: Cross-Modal Functional Reorganization of Visual and Auditory Cortex in Adult Cochlear Implant Users Identified with fNIRS. Neural Plast 2016, 2016:4382656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huetz C, Guedin M, Edeline JM: Neural correlates of moderate hearing loss: time course of response changes in the primary auditory cortex of awake guineapigs. Front Syst Neurosci 2014, 8:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajan R, Irvine DR, Wise LZ, Heil P: Effect of unilateral partial cochlear lesions in adult cats on the representation of lesioned and unlesioned cochleas in primary auditory cortex. J Comp Neurol 1993, 338:17–49. [DOI] [PubMed] [Google Scholar]
- 67.Norena AJ, Tomita M, Eggermont JJ: Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol 2003, 90:2387–2401. [DOI] [PubMed] [Google Scholar]
- 68.Allman BL, Keniston LP, Meredith MA: Adult deafness induces somatosensory conversion of ferret auditory cortex. Proc Natl Acad Sci U S A 2009, 106:5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scholl B, Wehr M: Disruption of balanced cortical excitation and inhibition by acoustic trauma. J Neurophysiol 2008, 100:646–656. [DOI] [PubMed] [Google Scholar]
- 70.Resnik J, Polley DB: Fast-spiking GABA circuit dynamics in the auditory cortex predict recovery of sensory processing following peripheral nerve damage. Elife 2017, 6.This study investigated the dynamics of intracortical inhibition during recovery auditory nerve damage in adult mice. The authors recorded single units in the primary auditory cortex across recovery and found that inhibitory neuron plasticity after damage could predict ultimate recovery.