Abstract
Bacteria express multiple diverse capsular polysaccharides (CPSs) for protection against environmental and host factors, including the host immune system. Using a mouse TCR transgenic CD4+ T cell, BθOM, that is specific for Bacteroides thetaiotaomicron (B. theta) and a complete set of single CPS-expressing B. theta strains, we ask whether CPSs can modify the immune responses to specific bacterial antigens. Acapsular (Acap) B. theta, which lacks all B. theta CPSs, stimulated BθOM T cells more strongly than wild-type B. theta. Despite similar levels of BθOM antigen expression, many single CPS-expressing B. theta strains were anti-stimulatory and weakly activated BθOM T cells, but a few strains were pro-stimulatory and strongly activated BθOM T cells just as well or better than Acap. B. theta strains that expressed an anti-stimulatory CPS blocked antigen delivery to the immune system, which could be rescued by Fc receptor-dependent antibody opsonization. All single CPS-expressing B. theta strains stimulated the innate immune system to skew towards M1 macrophages and release inflammatory cytokines in a MyD88-dependent manner, with anti-stimulatory CPS activating the innate immune system more weakly than pro-stimulatory CPS. The expression of anti-stimulatory versus pro-stimulatory CPSs on B. theta outer membrane vesicles (OMVs) also regulated immune responses. Moreover, anti-stimulatory and pro-stimulatory single CPS-expressing B. theta strains regulated the activation of antigen-specific and polyclonal T cells as well as clearance of dominant antigen in vivo. These studies establish that the immune responses to specific bacterial antigens can be modulated by a diverse set of CPSs.
INTRODUCTION
Many human gut bacteria synthesize phase variant capsular polysaccharides (CPS) on their surface (1). Although many functions of CPSs remain poorly understood, CPSs are known to be immunomodulatory (2). The best studied immunoregulatory CPS is the polysaccharide A capsule (PSA), which is one of eight CPS expressed in Bacteroides fragilis (3). PSA is zwitterionic and purified PSA has been shown to modulate host cytokine levels and induce CD4+ FoxP3+ regulatory T cells (Tregs) that protect against a variety of models of autoimmune inflammatory diseases (4–7). In support of these findings, other zwitterionic polysaccharide (ZPS)-encoding bacteria from diverse taxa induced more IL-10 and Tregs in human mononuclear cells than those without the ZPS motif (8). Non-zwitterionic capsules have also been shown to exhibit anti-inflammatory properties. For example, neutrally charged cell surface β-glucan/galactan (CSGG) polysaccharides that were purified from Bifidobacterium bifidum induced Treg cells in the intestine and suppressed inflammation in a T cell transfer model of colitis (9). Bacterial capsules also enable bacteria to evade adaptive immune responses as a Bifidobacterium breve (B. breve) strain that expressed exopolysaccharide (EPS) persisted longer than an acapsular strain in wild-type (WT) mice but not in B cell-deficient mice (10). Specifically, capsules can mask bacterial surface antigens from exposure to antibody responses, leading to weaker antibody responses against an EPS-coated versus acapsular B. breve strain (10). In addition, capsules can promote a mutually beneficial state of tolerance with the innate immune system in the intestine. Both exopolysaccharide from Bacillus subtilis and a large soluble polysaccharide released by Helicobacter hepaticus have been shown to induce anti-inflammatory M2 macrophages (11, 12). CPSs from other pathogens such as Streptococcus pneumoniae can also subvert innate immune recognition by impeding complement deposition and bacteria phagocytosis (13, 14). Collectively, these studies reveal the importance of CPSs in microbiota-immune interactions.
A limitation of current studies is that they examined a small subset of capsules expressed by a given bacterium. One recent study interrogated the roles of a complete set of bacterial capsules in a single species by individually expressing all eight capsules in Bacteroides thetaiotaomicron (B. theta, Bθ) (15). The Gram-negative anaerobe B. theta is a model gut symbiont that is a prominent member of the adult human gut microbiota and degrades a variety of diet, host, and microbial glycans (16). B. theta dedicates a substantial portion of its genome—182 genes in 8 distinct genomic loci—to CPS production and expresses eight distinct CPSs (CPS1-CPS8), which can be several hundred nanometers thick (17). The WT B. theta strain outcompeted all of the single CPS-expressing strains in C57BL/6 germ-free mice, demonstrating that the ability to express multiple CPSs is advantageous in vivo (15). Higher levels of IgA production correlated with increased abundance of the CPS5-expressing strain, suggesting that CPS5 may promote evasion of adaptive immune responses (15). This study provided key insights into the roles of CPSs in competitive intestinal colonization, but only examined how the presence of an immune system influences colonization among single CPS-expressing B. theta. The mechanisms by which a complete set of CPSs affects immune responses remain poorly understood.
Another limitation of current studies is that these studies only investigated how the CPS itself interacts with the immune system. It is unknown if CPSs on bacteria can modulate the immune response to dominant bacterial antigens. Progress in this area has been hampered by the lack of a model system in which a CD4+ T cell response can be examined for a specific gut symbiont. With our BθOM T cell system, which consists of a T cell receptor (TCR) transgenic CD4+ T cell that is specific for an outer membrane (OM) protein in B. theta, we are able to control expression of a dominant B. theta antigen. We have previously characterized the BθOM T cell system and shown that BθOM T cells are specific for BT4295, a SusE/SusF OM lipoprotein contained in one of B. theta’s many polysaccharide utilization loci (PULs) (18). We have also demonstrated that BθOM T cells are functional in vivo as adoptively transferred BθOM T cells proliferate and differentiate into regulatory T cells (Tregs) and effector T cells (Teff) in the colon, colon-draining lymph node (cdLN), and spleen in healthy mice colonized with B. theta (18).
Using our BθOM T cell system (18) and a complete set of single CPS-expressing B. theta (15), we investigated if all CPSs on B. theta modulate the immune responses to a dominant B. theta antigen. An acapsular B. theta strain (Acap) that lacks all B. theta CPSs stimulated BθOM T cells more strongly than wild-type (WT) B. theta, suggesting that CPSs may regulate the interactions between specific bacterial antigens and the immune system. We then performed the first unbiased examination of how a complete set of CPSs modulate the host’s immune responses to dominant antigen using a defined symbiont-specific T cell model system. Although many single CPS-expressing B. theta strains were anti-stimulatory and suppressed the immune response to a dominant antigen, we identified a few pro-stimulatory strains that activated the immune system just as well or better than Acap. We reveal that by altering CPS expression, even at the level of outer membrane vesicles (OMVs), bacteria can control the delivery of dominant antigens and the innate and adaptive immune responses. We show that by directing antigen accessibility, CPSs also enable bacteria to regulate immune responses in vivo including activation of antigen-specific and polyclonal T cells as well as clearance of dominant antigen. These results demonstrate that the ability to dynamically express diverse CPSs equips bacteria to modulate immune responses to dominant antigens.
MATERIALS AND METHODS
Strains and Culture Conditions
The single CPS-expressing B. theta strains used in this study have been generated and characterized previously (15, 19). Strains were routinely grown in TYG medium (10g/L tryptone, 5g/L yeast extract, 4g/L D-glucose, 100mM KH2PO4, 8.5mM (NH2)4SO4, 15mM NaCl, 10μM Vitamin K3, 2.63μM FeSO4•7H2O, 0.1mM MgCl2, 1.9μM hematin, 0.2mM L-histidine, 3.69nM Vitamin B12, 413μM L-cysteine, and 7.2μM CaCl2•2H2O), minimal medium (20g/L tryptone, 10g/L yeast extract, 5g/L D-glucose, 8.25mM L-cysteine, 78μM MgSO4•7H2O, 294μM KH2PO4, 230μM K2HPO4, 1`.4mM NaCl, 7.9μM Hemin (hematin), 4μM Resazurin, 24μM NaHCO3, 68μM CaCl2•2H2O), or on brain heart infusion agar plates containing 10% horse blood (Quad Five, Rygate, Montana) with gentamicin (200 μg/mL) (BHI-blood-gent plates). Bacteria were routinely grown at 37 °C in a Bactron IV Anaerobic Chamber (Sheldon Manufacturing) or in a BD GasPak™ EZ small Incubation Container with BD GasPak™ EZ Anaerobe Container System Sachets with Indicator. B theta has a different core oligosaccharide in place of the O antigen repeating unit found in LPS, and makes lipooligosaccharide (LOS), which can also stimulate innate immunity. Each of the single CPS-expressing strains have been examined for LOS expression, and they have been shown to express the same levels (20).
For GFP labeling of the CPS-producing strains, a plasmid encoding for GFP expression was integrated into the chromosome of each strain as previously described (21, 22). Briefly, E. coli S17–1 λ pir containing the pWW3452 vector (22) was grown to mid-log phase in LB medium containing ampicillin (300 μg/ml), and CPS-producing strains were grown anaerobically to mid-log phase in TYG medium. Cells were washed and combined at an approximately 1:1 ratio before plating on a BHI agar plate containing 10% horse blood (BHI-blood plate). After 1 day aerobic incubation at 37 °C, cell mass was scraped off the plate and dilutions were plated on BHI-blood plates containing 200 μg/ml gentamicin and 25 μg/ml erythromycin. After 2 days of anaerobic growth at 37 °C, colonies were restreaked on the same medium and grown anaerobically for an additional 2 days. Isolated colonies were inoculated into TYG medium for overnight growth, and resulting cultures were confirmed to fluoresce when exposed to UV light.
Mouse Experiments
All experimental procedures were performed under approval by Washington University’s Animal Studies Committee. All non-germ-free mice were housed in an enhanced specific pathogen-free facility. BθOM transgenic mice on the Rag1−/− background were maintained by breeding to a nontransgenic Rag1−/− mouse (18). Mice were randomly assigned into groups. Animal numbers for each experiment were chosen based on the minimum numbers required in previous studies to observe significant changes in the readouts measured in each experiment. Mice aged 6–12 weeks old of varying genders were used, dependent on availability, and none of these were involved in any previous experiments. Groups within an experiment were age and gender matched to the greatest extent possible.
Functional in vitro T cell stimulation assay
The BθOM T cell stimulation assay was performed as previously described (18). Bone marrow cells isolated from the tibia and femurs of C57BL/6, MyD88−/−, or FcRγ−/− mice were cultured in M-CSF for 7 days to differentiate into BMDM. BMDM were stimulated with IFN-γ at 2,000 U/mL in I-10 media (Iscove’s Modified Dulbecco’s Medium (IMDM), 10% FBS, glutamine, and gentamicin) and plated on a 96-well plate at 1×105 cells per well. The cells were washed with PBS 24 hours later and kept in 100 μL of fresh I-10 medium without IFN-γ. For BMDCs, bone marrow cells were cultured in 10% FMS tyrosine kinase 3 (Flt3L) ligand supernatant and 5 ng/mL GM-CSF for 7 days and plated on a 96-well plate at 1×105 cells per well. When splenic CD11c+ DCs were used, splenocytes were enriched for CD11c+ DCs using a MACS CD11c microbeads and plated on a 96-well plate at 1×105 cells per well. 5×105 cells isolated from the spleen and lymph nodes of BθOM transgenic mice or 1×105 BθOM T cell hybridomas or BθOM T cell blasts with or without 10 μg/mL of B. theta antibody were then added per well in 50 μL with 50 μL of half log dilutions of B. theta strains or OMVs. Single CPS-expressing B. theta strains were grown in a 5 mL TYG culture at 37 °C overnight and then re-inoculated at 1:50 and grown to mid-log phase. Cultures were washed once and resuspended in PBS prior to adding to the assay. 24 hours later, the supernatant containing the T cells was transferred to a fresh 96-well plate and spun down at 1200 rpm. The cells were washed with FACS buffer and stained for CD4, CD45.1, and CD69. All samples were run on a BD FACSCanto and analyzed using FlowJo.
Generation of BθOM T cell hybridomas
BθOM T cell hybridomas were generated as previously described (18). A 2 mL B. theta (BT5482 strain) overnight culture was spun down and pelleted, washed 1X in PBS, and resuspended in 1 ml PBS and used as an antigen source for immunization. An equal volume of B. theta/PBS mixture was emulsified with Incomplete Freund’s Adjuvant (IFA) and 50 μL was injected into the rear footpads of C57BL/6 mice (male). Ten days later the draining popliteal lymph nodes were removed and a single cell suspension of 2×107 cells was cultured in 7 ml of RPMI 1640 supplemented with 10% FCS, 2 mM glutamine, 1 mM HEPES, 1 mM Sodium Pyruvate, 5×10−5 M 2-mercaptoethanol, 50 ug/mL gentamicin, and 14 μL of the B. theta/PBS antigen source (1:500 dilution) for 3 days. Blasting BθOM T cells were harvested by centrifugation and fused to the BW 5147 a- b- T cell hybridoma fusion partner using a standard protocol (23).
Generation of BθOM T cell blasts
BMDM were stimulated with IFN-γ at 2,000 U/ml in I-10 media (see above) and plated on a 24-well plate at 5×105 cells per well. The cells were washed with PBS 24 hours later and kept in 1mL I-10 media. 1 μM BθOM peptide and 2×105 isolated CD4+ BθOM T cells were added per well in 0.5 mL with 50U IL-2 and incubated for six days at 37 °C. BθOM T cell blasts were then harvested from the supernatant and added to the in vitro macrophage cell assay.
Quantitative ELISA for BT4295
BT4295 protein levels in the single CPS-expressing B. theta strains were measured by quantitative ELISA as previously described (18). Single CPS-expressing B. theta strains were grown in a 5 mL TYG culture at 37 °C overnight and then re-inoculated at 1:50 and grown to mid-log phase. Samples were obtained from equivalent numbers of B. theta from OD600-measured cultures. Bacteria were lysed in 100 mM CHAPS detergent and incubated with agitation for 1 hour at room temperature (RT) and stored at 4 °C overnight. An Immulon 2 ELISA plate was coated overnight with purified anti-BT4295 antibody ERC11 in carbonate coating buffer [5 μg/mL (pH value of 9.6)] at 4 °C, washed, blocked with buffer (PBS with 0.5% bovine serum albumin and 0.1% Tween 20) for 1 hour at RT. Plates were washed and samples were added for 2 hours at RT, washed again, and then the anti-BT4295 antibody biotin-4E9 (5 μg/mL) was added for 1 hour at RT. Plates were washed again, and a 1:5000 dilution for streptavidin horseradish peroxidase was added for 30 min at RT. Plates were washed and 100 μL of 1-Step Ultra TMB substrate was added to each well and A450 was determined. Unknown sample concentrations were quantified by comparison to a standard curve of recombinant BT4295 protein performed in the same ELISA using GraphPad Prism software.
Live Cell Phagocytosis Assay
BMDM were plated on a 96 well glass bottom plate with #1.5 cover glass and a black frame (CellVis) at 1×105 cells per well in I-10 media overnight. The cells were stained with 5 μM CellTracker™ Orange CMTMR Dye for 37 °C x 30 min then washed. Cells were washed and incubated in 150 μL I-10 media. 50 μL of a 1:31 dilution of single CPS-expressing B. theta strains that endogenously expressed GFP with or without 10 μg/mL of B. theta antibody was added to each well. B. theta strains expressing a single CPS and GFP were grown in a 2mL TYG culture at 37 °C overnight to mid log phase. Cultures were washed once and resuspended in PBS prior to adding to the assay. Four hours later, images were acquired in the same z-plane using an Olympus IX70 microscope with a 100x/1.4NA oil objective, a Yokogawa spinning-disk confocal scanning unit, and a Hamamatsu Orca Flash4 CMOS camera. Cells were maintained at 37 °C with 5.0% CO2 in a Tokai Hit humidified chamber. Images were acquired with NIS-Elements AR software and the number of bacteria uptaken per BMDM was measured manually using Imaris software. A total of 150 individual cells were measured for each bacterial strain analyzed with 50 cells measured per experiment in 3 experiments.
Microscopic imaging of Capsules by India Ink Staining
B. theta capsule thickness was measured by India ink staining as described previously (17). Samples were taken from fresh cultures of B. theta strains grown to late exponential phase in TYG media. A 10 μL aliquot of the fresh culture was mixed on a slide with an equivalent amount of India ink and a cover glass was added. Cells were imaged with a Leica SP8 confocal laser scanning fluorescent microscope and capsule thickness was measured using Imaris and FIJI software. A total of 200 individual cells were measured for each bacterial strain analyzed. Four measurements were made per cell and averaged to generate a single average capsule width.
Microscopic imaging of Capsules by Scanning Electron Microscopy
Samples for quick-freeze, deep-etch scanning electron microscopy (SEM) were obtained as described previously (17). Briefly, 5 mL cultures of B. theta strains grown to late exponential phase in TYG media were gently centrifuged. A 3 microliter droplet of the resulting bacterial pellet was layered onto a thin slice of aldehyde-fixed boiled egg white (used for support during freezing) and frozen by abrupt application of the sample against a liquid helium cooled copper block with a Cryopress freezing machine. Frozen samples were transferred to a liquid nitrogen cooled Leica Ace 900 freeze etching unit, fractured, and “deep-etched” for 2 min at −104 °C. Specimens were then rotary-replicated with ~3.5 nm of platinum applied from an angle of 20° above the horizontal, and ‘backed’ with a ~8.5 nm film of pure carbon deposited from an 85° angle. Replicas were picked up on hexagonal 100-mesh Formvar-carbon coated microscope grids and imaged in a JEOL 1400 microscope with attached AMT digital camera.
Generation of B. theta-specific antibodies
C57BL/6 mice were immunized with killed CPS1 and boosted, and then splenic B cells were fused with P3Ag8.6.5.3 myeloma cells to create hybridomas (24).
In vitro macrophage skewing and cytokine release assay
BMDM or BMDCs were plated on a 96-well plate at 1×105 cells per well in I-10 media for 1 hour at 37 °C. 50 μL of half log dilutions of B. theta strains or OMVs were added. Single CPS-expressing B. theta strains were grown in a 5 mL TYG culture at 37 °C overnight and then re-inoculated at 1:50 and grown to mid-log phase. Cultures were washed once and resuspended in PBS prior to adding to the assay. 20 hours later, the supernatant was transferred to a fresh 96-well plate and stained for IL-2, IL-4, IL-6, IL-10, TNF, IFN-γ, and IL-17A using a BD Cytometric Bead Array Mouse TH1/TH2/TH17 cytokine kit. The BMDM were washed once with PBS, treated with trypsin, transferred to a fresh 96-well plate, surface stained with CD11b, CD86, PD-L1 for 20 min at 4 °C, fixed and permeabilized with a FoxP3 transcription factor staining buffer set for 1 hour at 4 °C, and intracellularly stained with iNOS for 30 min 4 °C. All samples were run on a BD FACSCanto and analyzed using FlowJo.
Generation of OMVs
Two-liter B. theta TYG cultures were grown overnight at 37°C in an anaerobic chamber and spun down at 6000 rpm for 15 min in a Beckman floor centrifuge (10.1 rotor) (25). The supernatant was filtered through a 0.45 μm filter. The filtered supernatant was and then spun down in a Beckman centrifuge (25.5 rotor) at 38,500 g for 1 hour in polypropylene 40 mL tubes. The supernatant was poured off and the pellet washed once with PBS by spinning in a Beckman centrifuge (25.5 rotor) at 38,500 g for 1 hour. The OMV pellets were suspended in PBS and pooled. The OMV concentration of OMVs was determined using the Bradford protein assay.
Extraction of purified CPS
Purified CPS1 was extracted by the hot water phenol method (17). 5 mL overnight cultures grown in TYG medium were inoculated into 250 mL of minimal medium with 0.5% glucose for 2 days, centrifuged, flash frozen in liquid nitrogen, and stored at −80 °C. Cells were resuspended in 100 mL hot water and then an equal volume of phenol was added. The mixture was stirred for 1 hour at 65 °C, cooled at 4 °C overnight, and centrifuged. The aqueous phase was dialyzed against deionized distilled water (12–14 kDa cutoff) and then lyophilized to dryness. Preparations were resuspended in buffer containing 20 mM Tris HCl (pH 7.4), 2.5 mM MgCl2, and 0.5 mM CaCl2, RNaseA and DNase I were added before samples were rotated gently overnight at 37 °C. Proteinase K was then added and samples were incubated overnight at 65 °C. After the addition of phenol, samples were vortexed and centrifuged. The aqueous layer was again dialyzed and then lyophilized to dryness.
BθOM and polyclonal T cell stimulation in vivo
Rag1−/− mice were placed on 0.66 mg/mL ciprofloxacin, 2.5 mg/mL metronidazole, and 20 mg/mL sugar-sweetened grape Kool-Aid Mix in the drinking water at 3 weeks of age for 2–3 weeks. Mice were then taken off antibiotic and given regular water for four days before gavage with 100 μL of B. theta strains at 1 × 108 colony-forming units (CFU) per mL. B. theta strains had been grown anaerobically from single isolates in TYG medium at 37 °C for 24 hours (26). Each culture was mixed with sterile PBS and glycerol to a final concentration of 20% glycerol and frozen at −80 °C in single-use aliquots. Three days after gavage, Rag1−/− mice were injected retro-orbitally with 2 × 105 BθOM T cells isolated from the peripheral lymph nodes (axially, brachial, inguinal, superficial and deep cervical, lumbar, and caudal), mLNs, and spleen. Cells were enriched for CD4+ T cells by negative selection using a homemade cocktail of antibodies (anti-mouse Ter-119, CD11c, CD11b, CD8α, CD19, CD45R/B220, CD49b, and CD24) and anti-biotin microbeads. Fecal pellets were obtained on days 0 and 8–9 to determine colonization. Seven days after T cell transfer, mice were euthanized and leukocytes were isolated from the lamina propria. For germ-free experiments, C57BL/6 germ-free mice were gavaged with 100 μL of B. theta strains at 1 × 108 colony-forming units (CFU) per mL and leukocytes were also isolated from the lamina propria ten days after gavage. Colons and cecums were flushed with PBS to remove colonic contents and fat tissue was pulled off. Intestines were cut longitudinally and laterally into freshly made 20 mL of predigestion solution (1X HBSS without Ca2+ and Mg2+, 10mM HEPES, 5mM EDTA, 5% fetal bovine serum (FBS), and 1mM DTT). Samples were incubated for 20 min at 37°C while shaking at 220 rpm and then vortexed for 10 sec. Tissue was transferred to 20 mL of fresh predigestion solution and incubated while shaking for another 20 minutes. After vortexing for 10 sec, tissue was transferred to a fresh tube containing 20 mL of 1X HBSS without Ca2+ and Mg2+ and 10 mM HEPES and incubated while shaking for 20 min. Tissue was cut into small pieces using fine scissors and digested for 30 min in freshly made digestion solution (1× HBSS with Ca2+ and Mg2+, 10mM HEPES, 5% FBS, 10mg/ml of DNase, and 5mg/ml of Collagenase) while shaking at 200 rpm. Tissue was mechanically disrupted using a gentleMACS™ Octo Dissociator (Miltenyi) using the program: m_intestine_01. Samples were filtered through a 100 μm filter. The cdLN and spleen were removed and processed into single cell suspensions using Eppendorf tube lids and filtered through a 70 μm filter. All samples were counted, stained with anti-mouse CD4, CD45.1, CD44, CD62L, FoxP3, IFN-γ, and/or IL-17A, and run on a BD FACSCanto.
Bacterial clearance in vivo
1 × 106 CPS1 or CPS8 was injected i.p into C57BL/6 mice. Peritoneal fluid was collected 0, 1, 2, 4, 6, or 8 hours after injection. Peritoneal fluid was plated on BHI-blood plates with 200 μg/mL gentamicin at 37 °C in an anaerobic chamber for 2 days to determine the CFU.
Quantification and Statistical Analysis
None of the investigators were blinded to sample identity during any of the described experiments and no samples or measurements were excluded from analysis. For each experiment, the total number of biological replicates “n” is indicated in the corresponding legend along with a description of what is considered a replicate (mice, replicate cultures, antibody titers, etc.) as well as the number of experiments conducted. FlowJo software v10.4.2 (FlowJo, LLC, Ashland, OR) was used to analyze all flow cytometry experiments. Statistical analyses were performed in Prism software v7 (GraphPad Software, Inc., La Jolla, CA). In the live cell imaging experiments, NIS-Elements AR software (Nikon, Tokyo, Japan) was used to capture images and uptake B. theta was counted manually in Imaris software v8.4 (Oxford Instruments, Zurich, Switzerland). In the confocal imaging experiments, Leica Application Suite X software (Leica Microsystems, Wetzlar, Germany) was used to capture images and the thickness of the B. theta capsule was measured in FIJI (Image J). When data did not generally follow a normal distribution, nonparametric tests (Mann-Whitney) were used to determine statistical significant. Unless otherwise indicated, statistical significance is indicated as follows: * p < 0.05; ** p < 0.01; *** p < 0.001; and **** p < 0.0001. Number of animals used (n) and statistical tests used are indicated in each figure legend.
RESULTS
Most, but not all, single CPS-expressing B. theta strains are anti-stimulatory and suppress antigen-specific adaptive immune responses
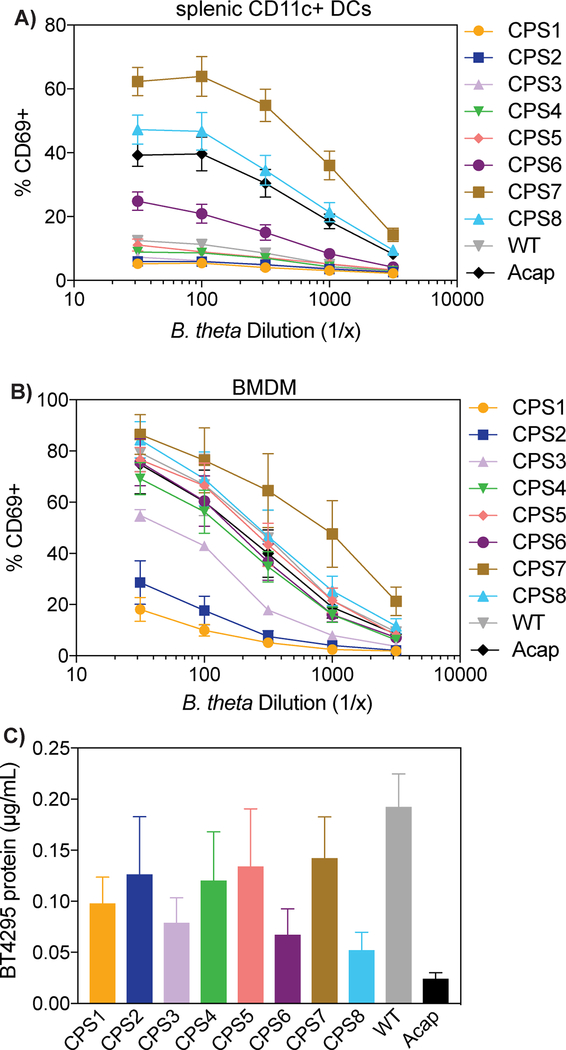
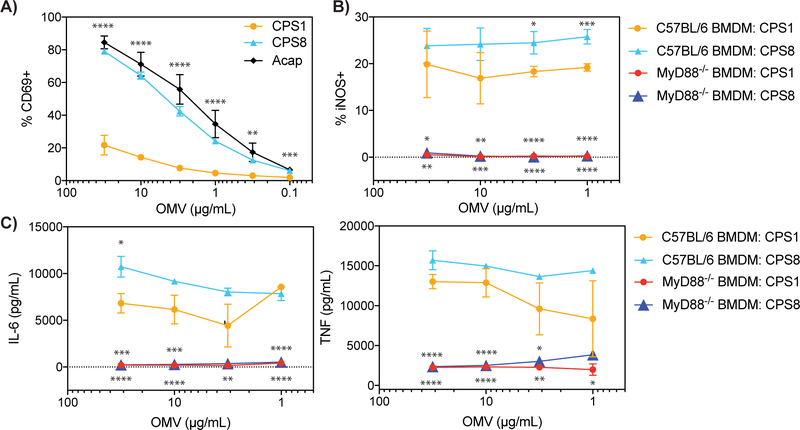
To determine if CPSs can regulate the immune response to dominant antigens, we first compared the ability of B. theta WT and Acap, which lacks all B. theta CPSs, to stimulate BθOM CD4+ T cells. Using primary CD11c+ splenic dendritic cells as the antigen presenting cells (APCs), we found that Acap stimulated BθOM T cells more strongly than WT (Fig. 1A). We hypothesized `that all CPSs on B. theta suppress the immune response to a dominant antigen and examined the ability of a complete set of 8 single CPS-expressing B. theta strain to stimulate BθOM T cells. Most single CPS-expressing B. theta strains were anti-stimulatory and weakly activated BθOM T cells, but one single CPS-expressing B. theta strain, CPS8, was pro-stimulatory and activated BθOM T cells similarly or even better than Acap in the presence of primary CD11c+ splenic DCs (Fig. 1A). The CPS7 strain also strongly stimulated, but previously it has been shown to very weakly express a capsule, and thus acted essentially as an Acap strain (15). To interrogate further the roles of CPSs on B. theta to modulate the immune response to specific bacterial antigens, we chose to focus on one anti-stimulatory CPS, CPS1, and one pro-stimulatory CPS, CPS8. Using these two model single CPS-expressing B. theta strains, we found that these differences in BθOM T cell stimulation were also present when bone marrow-derived macrophages (BMDM) were used as the APC (Fig. 1B), extending our finding that CPSs on B. theta differentially alter T cell responses to dominant antigens. Protein levels of the BθOM T cell antigen BT4295 were not statistically different between all of the single CPS-expressing B. theta strains, ruling out the possibility that the differences in BθOM T cell stimulation were due to differences in antigen expression (Fig. 1C). These findings demonstrate that bacteria can modulate adaptive immune responses to their dominant antigens by altering CPS expression.
FIGURE 1. Most B. theta CPSs are anti-stimulatory and prevent BθOM T cell recognition of a dominant antigen in B. theta.
(A) Percentage of CD69 expressing BθOM T cells after a 24 hour culture with CD11c+ splenic DCs loaded with B. theta CPS1-8, WT, or Acap (n = 6, three experiments).
(B) Percentage of CD69 expressing BθOM T cells after a 24 hour culture with CPS1-8, WT, or Acap loaded onto BMDM (n = 6, three experiments).
(C) Concentration in microgram per milliliter of BT4295 protein expressed in CPS-8, WT, and Acap (n = 4, four experiments).
Anti-stimulatory CPSs on B. theta block the immune system’s access to a dominant antigen
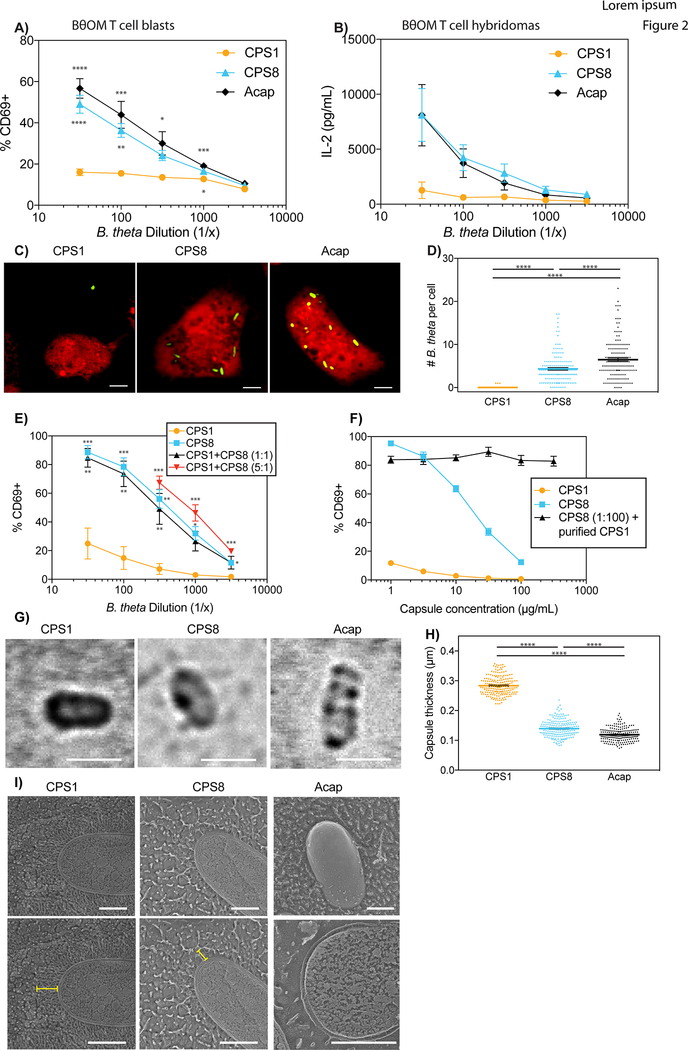
We hypothesized that anti-stimulatory single CPS-expressing B. theta strains suppress antigen-specific CD4+ T cell responses because the CPSs either block antigen uptake, antigen processing, or antigen presentation. We first used BθOM T cell blasts, which are BθOM T cells that have been previously activated in vitro, to examine if anti-stimulatory CPSs on B. theta impede antigen presentation. BθOM T cell blasts have already upregulated co-stimulatory molecules, enabling us to test if CPSs on B. theta block antigen presentation by preventing critical co-stimulatory molecules from being expressed on the APC surface. T stimulation assays with BθOM T cell blasts recapitulated our results with primary BθOM T cells. Pro-stimulatory single CPS-expressing B. theta, including CPS8 and Acap, strongly activated BθOM T cell blasts. Anti-stimulatory single CPS-expressing B. theta, including CPS1, weakly activated BθOM T cell blasts, suggesting that anti-stimulatory CPSs on B. theta do not inhibit the antigen presentation pathway (Figs. 2A and S1A). Using BθOM T cell hybridomas, which only require TCR stimulation to be activated, we next investigated if B. theta expressing anti-stimulatory CPSs block antigen processing. BθOM T cell hybridomas only recognize major histocompatibility complex (MHC) class II loaded with peptide and do not need any co-stimulation, enabling us to test if CPSs on B. theta prevent peptide-MHC II complexes from being processed and expressed on the APC surface. BθOM T cell hybridomas were also strongly activated by B. theta expressing pro-stimulatory CPSs but weakly activated by B. theta with anti-stimulatory CPSs (Figs. 2B and S1B), demonstrating that anti-stimulatory CPSs on B. theta do not block the antigen processing pathway. To determine if anti-stimulatory single CPS-expressing B. theta strains inhibit the uptake of dominant antigens by APCs, we cultured labeled BMDMs with B. theta strains that expressed a single CPS and GFP and performed live cell imaging. B. theta strains with pro-stimulatory CPSs such as CPS8 and Acap were highly phagocytosed whereas B. theta strains with anti-stimulatory CPSs such as CPS1 were poorly phagocytosed (Figs. 2C, 2D, S1C, and S1D), revealing that B. theta can control the delivery of dominant antigen to the immune system.
FIGURE 2. Anti-stimulatory CPSs block uptake of a dominant antigen in B. theta, partly due to capsule thickness.
(A) Percentage of CD69 expressing BθOM T blasts after a 24 hour culture with BMDM loaded with CPS1, CPS8, or Acap (n = 6, three experiments).
(B) IL-2 levels in picogram per milliliter produced by BθOM T cell hybridomas after a 24 hour culture with BMDM loaded with CPS1, CPS8, or Acap (n = 6, three experiments).
(C and D) (C) Representative live cell images and (D) Quantification of uptaken GFP-labeled CPS1, CPS8, or Acap per BMDM after a 4 hour culture (n = 150, three experiments). Scale bar = 5 μm. (E) Percentage of CD69 expressing BθOM T cells after a 24 hour culture with BMDM loaded with CPS1, CPS8, or CPS1 and CPS8 in a 1:1 or 5:1 ratio (n = 6, three experiments)
(F) Percentage of CD69 expressing BθOM T cells after a 24 hour culture with BMDM loaded with CPS1, CPS8, or a 1:100 dilution of CPS8 and varying concentrations of purified CPS1 (n = 3, three experiments). (G and H) (G) Representative images and (H) Quantification of capsule thickness of CPS1, CPS8, and Acap stained with India ink (n = 200, three experiments). Scale bar = 2 μm. (I) Quick-freeze, deep-etch scanning electron micrographs of CPS1, CPS8, and Acap B. theta. The yellow line indicates the width of each capsule. Scale bar = 0.5 μm.
Data represent mean ± SEM. One-way ANOVA analysis (A) ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, (D) ∗∗∗∗p < 0.0001, (E) p = 0.0207, (F) ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, (H) ∗∗∗∗p < 0.0001.
Given that CPSs are phase variant and can be shed into the environment (2), we wondered if single CPS-expressing B. theta strains can also act in trans. Mixing the pro-stimulatory CPS8-expressing B. theta strain with either the anti-stimulatory CPS1-expressing B. theta strain or purified CPS1 capsule failed to decrease BθOM T cell stimulation (Figs. 2E and 2F), suggesting that CPS1 cannot inhibit CPS8 on B. theta in trans. Live cell imaging of BMDM treated with unlabeled CPS1-expressing B. theta as well as B. theta that expressed CPS8 and GFP revealed that CPS8-expressing B. theta was still readily uptaken by BMDM in the presence of CPS1-expressing B. theta (data not shown). To rule out the possibility that CPS8-expressing B. theta could be enhancing the uptake and BθOM T cell stimulation of CPS1-expressing B. theta, we also incubated BMDMs with unlabeled CPS8-expressing B. theta and B. theta that expressed CPS1 and GFP and found no increase in uptake of CPS1-expressing B. theta (data not shown). These findings definitively demonstrate that the anti-stimulatory CPS1 and pro-stimulatory CPS8 do not function in trans to alter B. theta uptake or BθOM T cell stimulation.
We then used India ink staining and quick-freeze, deep-etch scanning electron microscopy (SEM) to examine if the thicknesses of the CPSs contribute to their anti- and pro-stimulatory properties. Both India ink staining and SEM showed that CPS1 is thicker than CPS8, suggesting that thicker CPSs, along with known differences in sugar composition (15), may play a role in impeding APC phagocytosis (Figs. 2G–2I, S1E–S1G). Taken together, these studies demonstrate that by changing the CPS expressed, bacteria can control the ability of the innate and adaptive system to access dominant antigens.
Fc receptor-dependent antibody optimization can rescue immune access to dominant antigen on B. theta with anti-stimulatory CPSs
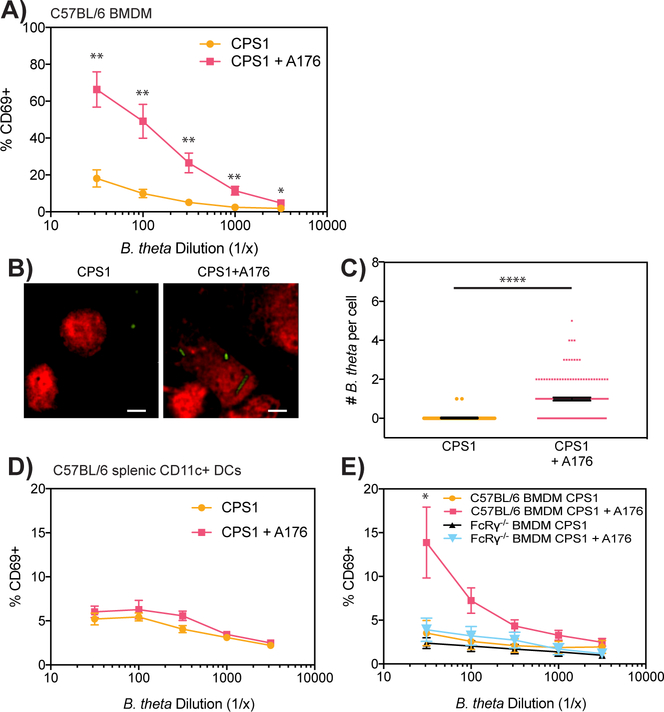
Since antibody opsonization has been shown to increase bacterial uptake (13), we hypothesized that antibody opsonization could overcome the ability of anti-stimulatory CPSs to block access to dominant antigens. We generated the A176 antibody, which recognize B. theta and is IgG2a, κ (Fig. S2A). Since A176 binds to all single CPS-expressing B. theta, A176 recognizes an antigen that is present in all B. theta strains and is not unique to the CPS1 capsule. Adding A176 to our BθOM T cell stimulation assay allowed CPS1-expressing B. theta to now strongly activate BθOM T cells (Figs. 3A and S2B). Live cell imaging demonstrated that CPS1-expressing B. theta’s enhanced BθOM T cell activation in the presence of A176 was due to A176’s ability to increase the uptake of CPS1-expressing B. theta in BMDMs (Figs. 3B and 3C). The addition of A176 had no effect on BθOM T cell activation by CPS1-expressing B. theta when splenic CD11c+ DCs were used as the APCs, suggesting that A176 likely increased CPS1 uptake through a receptor present on BMDM but not splenic CD11c+ DCs (Fig. 3D). Since antibodies have been shown to increase bacterial uptake through Fc receptors, we tested the mechanism of A176’s enhanced bacterial uptake with FcRγ−/− BMDM, which lack the Fc receptor common γ chain and do not express any of the activating mouse FcγRs (FcγRI, FcγRIII, and FcγRIV). A176 no longer increased CPS1-expressing B. theta’s BθOM T cell stimulation in the presence of FcRγ−/− BMDM, demonstrating that A176 increases CPS1 uptake and BθOM T cell stimulation by antibody opsonization through Fc receptors (Fig. 3E). Thus, the immune system can overcome anti-stimulatory CPSs and restore access to dominant antigens through Fc receptor-dependent antibody opsonization.
FIGURE 3. Blockade of dominant antigen delivery by anti-stimulatory CPSs can be overcome by antibody opsonization through FcRγ.
(A) Percentage of CD69 expressing BθOM T cells after a 24 hour culture with CPS1 only or CPS1 + A176 antibody loaded onto BMDM (n = 6, three experiments).
(B and C) (B) Representative live cell images and (C) Quantification of uptaken GFP-labeled CPS1 per BMDM after a 4 hour culture with or without A176 antibody (n = 150, three experiments). Scale bar = 5 μm.
(D and E) Percentage of CD69 expressing BθOM T cells after a 24 hour culture with CPS1 and A176 antibody loaded onto (D) CD11c+ splenic DCs (n = 6, three experiments) or (E) C57BL/6 or FcRγ−/− BMDM (n = 6, three experiments).
Data represent mean ± SEM. Student’s t test: (A) ∗∗p < 0.01, ∗p < 0.05, (C) ∗∗∗∗p < 0.0001.
All single CPS-expressing B. theta stimulate the innate immune system through MyD88, but anti-stimulatory CPSs on B. theta decrease innate immune system activation
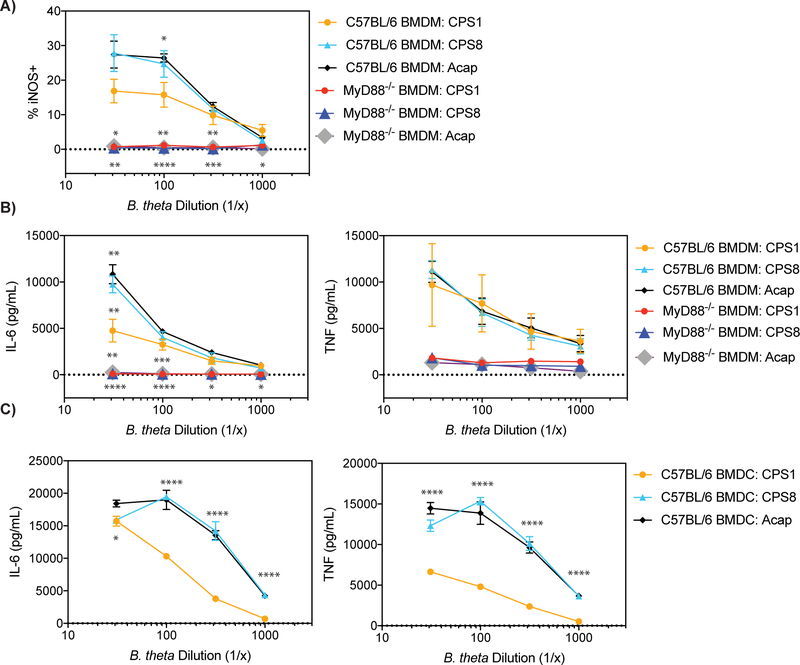
We next sought to determine if the single CPS-expressing B. theta strains activate the innate immune system. None of the single CPS-expressing B. theta upregulated expression of the co-stimulatory molecules PD-L1 and CD86 in BMDM (Fig. S3A). Despite differences in APC uptake, all single CPS-expressing B. theta upregulated the expression of inducible NO synthase (iNOS) in BMDM, which is an inflammatory M1 marker (Figs. 4A and S3B) (27). All single CPS-expressing B. theta also induced both BMDMs and BMDCs to release the inflammatory cytokines IL-6 and TNF (Figs. 4B, 4C, S3C, and S3D). Activation of the innate immune system by single CPS-expressing B. theta was dependent on the MyD88 pathway as MyD88−/− BMDMs did not skew to an M1 phenotype or release IL-6 and TNF (Figs. 4B, 4C, S3B, and S3C). Although B. theta strains with anti-stimulatory CPSs activated the innate immune system, they stimulated the innate immune system more weakly than B. theta strains with pro-stimulatory CPSs and induced fewer M1 macrophages and inflammatory cytokines (Figs. 4A–4C). Adding the A176 antibody had no effect on BMDM skewing to M1 macrophages or IL-6 and TNF cytokine release in the presence of CPS1-expressing B. theta (Figs. S2C and S2D). To explore what innate receptor may be involved through the MyD88 pathway, we tested TLR4-deficient BMDMs. The TLR4-deficient BMDM responded identically to the wt BMDM (data not shown), indicating that TLR4 was not involved. These results demonstrate that although all single CPS-expressing B. theta strains stimulate the innate immune system through MyD88, anti-stimulatory CPSs may help shield B. theta from fully activating the innate immune system.
FIGURE 4. All B. theta CPSs stimulate the innate immune system in a MyD88-dependent manner, but anti-stimulatory CPSs activate the innate immune system more weakly than pro-stimulatory CPSs.
(A) Percentage of iNOS expressing C57BL/6 or MyD88−/− BMDM after a 24 hour culture with CPS1, CPS8, or Acap (n = 4, three experiments).
(B and C) IL-6 and TNF levels in picogram per milliliter produced by (B) C57BL/6 or MyD88−/− BMDM and (C) C57BL/6 BMDC after a 24 hour culture with CPS1, CPS8, or Acap (n = 4–6, three experiments).
Data represent mean ± SEM. One-way ANOVA analysis (A and B) ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, (C) ∗p < 0.05, ∗∗∗∗p < 0.0001.
Single CPS-expressing OMVs also regulate immune responses to dominant antigens
Although our above findings utilized whole B. theta, B. theta also releases OMVS, which can deliver B. theta antigens to immune cells in the host intestine (19). OMVs also contain a CPS outer layer, but the roles of CPSs on OMVs remain poorly understood. The most well-studied CPS on OMVs is PSA. OMVs containing PSA have been shown to be immunomodulatory because WT OMVs, but not OMVs that lack PSA (ΔPSA OMVs), induce CD4+CD25+FoxP3+ Tregs and prevent experimental colitis (28). A limitation of this study is that only ΔPSA OMVs that lack PSA but still express other CPSs were tested. Since our BθOM T cell antigen is also present on OMVs, we examined if single CPS-expressing OMVs can regulate the immune response to dominant antigens. In accordance with our findings using single CPS-expressing B. theta, OMVs with anti-stimulatory CPSs such as CPS1 weakly activated BθOM T cells, while OMVs with pro-stimulatory CPSs such as CPS8 and Acap strongly activated BθOM T cells (Fig. 5A). Although OMVs with anti-stimulatory CPS1 or pro-stimulatory CPS8 did not upregulate the co-stimulatory molecules CD86 and PD-L1 in BMDM (data not shown), they both activated the innate immune system to skew to M1 macrophages and release IL-6 and TNF inflammatory cytokines in a MyD88-dependent manner (Figs. 5B and 5C). Similar to whole B. theta expressing CPS1, OMVs with anti-stimulatory CPS1 activated the innate immune system more weakly than OMVs with pro-stimulatory CPS8 (Figs. 5B and 5C). Since A176 also binds to CPS1 OMVs, we added A176 to our BθOM T cell stimulation assay with OMVs containing anti-stimulatory CPS1 but saw no increase in BθOM T cell stimulation. These studies are the first time the adaptive and innate immune responses of a complete set of CPS on OMVs have been reported and demonstrate that CPSs at the level of OMVs can also modulate innate and adaptive immune responses to dominant antigen.
FIGURE 5. OMV CPSs have similar immunomodulatory effects on a B. theta dominant antigen as CPSs on whole bacteria.
(A) Percentage of CD69 expressing BθOM T cells with CPS1, CPS8, and Acap OMVs loaded onto BMDM (CPS1 and CPS8: n = 7, three experiments; Acap: n =3, two experiments).
(B) Percentage of iNOS expressing C57BL/6 or MyD88−/− BMDM after a 24 hour culture with CPS1 and CPS8 OMVs (n = 6, three experiments).
(C) IL-6 and TNF levels in picogram per milliliter produced by C57BL/6 or MyD88−/− BMDM and after a 24 hour culture with CPS1 and CPS8 OMVs (n = 6, three experiments).
Data represent mean ± SEM. One-way ANOVA analysis: (A-C) ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.000. 5B and 5C * above the CPS8 line are comparing CPS1 vs CPS8, * above red/blue triangles are comparing CPS1 vs MyD88−/− CPS1, and CPS8 vs MyDD8−/− CPS8.
Single CPS-expressing B. theta modulate antigen-specific and polyclonal immune responses in vivo
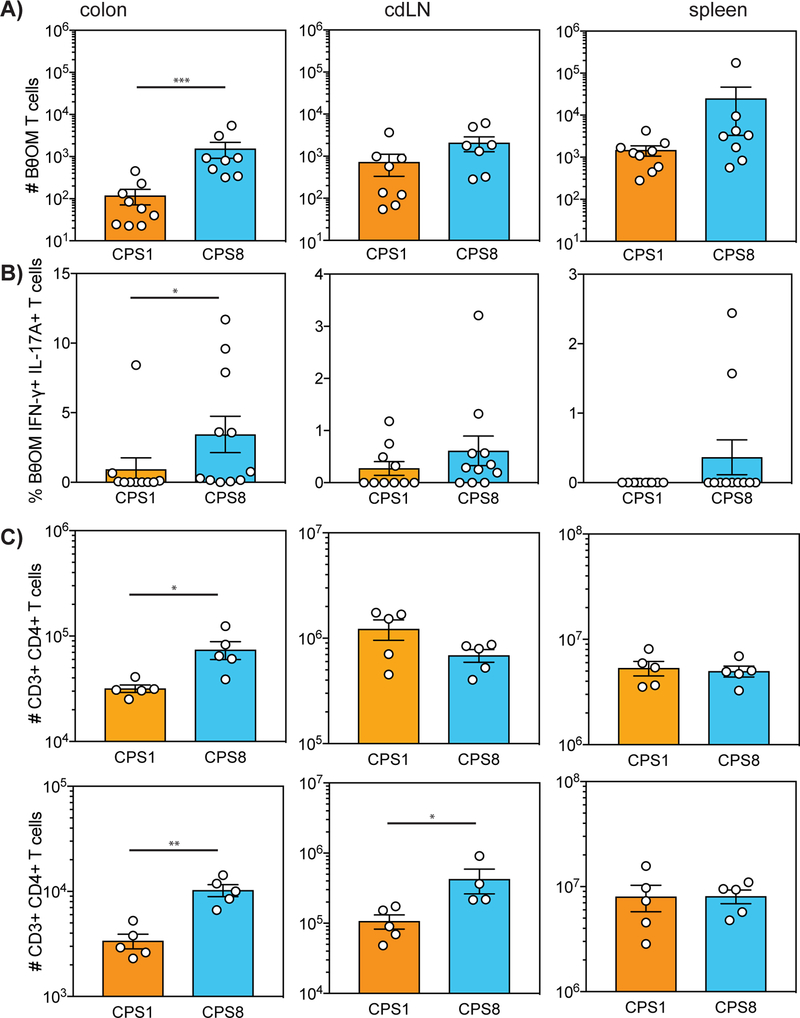
We then evaluated the ability of CPSs on B. theta to regulate the immune responses to dominant antigens in vivo using our previously published BθOM T cell transfer model (18). Rag1−/− mice were pretreated with antibiotics for two weeks, gavaged with anti-stimulatory CPS1-expressing B. theta or pro-stimulatory CPS8-expressing B. theta on day 0, adoptively transferred with BθOM T cells on day 3, and then harvested on day 10 (Fig. S4A). Although B. theta expressing CPS1 or CPS8 colonized mice equally (Fig. S4B), we recovered fewer BθOM T cells in the colon lamina propria of mice colonized with CPS1-expressing B. theta than with CPS8-expressing B. theta (Fig. 6A). These findings are consistent with our in vitro results and further demonstrate that anti-stimulatory CPS1-expressing B. theta weakly activate BθOM T cells whereas pro-stimulatory CPS8-expressing B. theta strongly activate BθOM T cells in vivo. Although there were no differences in the percentage of naive CD44+ CD62L- or activated CD44+ CD62L- BθOM T cells (Figs. S4C and S4D), CPSs on B. theta also directed the differentiation of antigen-specific T cells as BθOM T cells in the colon differentiated into more IFN-γ+ IL-17A+ T cells in the presence of B. theta containing pro-stimulatory CPS8 (Fig. 6B). In addition, CPS8-expressing B. theta induced more effector IFN-γ+ IL-17A- TH1 and IFN-γ- L-17A+ TH17 BθOM T cells, while CPS1-expressing B. theta induced more FoxP3+ BθOM Tregs cells in the colon, although these trends did not reach statistical significance (Figs. S4E–S4G). These studies demonstrate that CPSs on B. theta can control the delivery of specific bacterial antigens and manipulate antigen-specific immune responses in vivo.
FIGURE 6. CPSs modulate antigen-specific and polyclonal T cell responses in vivo.
(A and B) (A) Number of live CD4+ CD45.1+ BθOM T cells and (B) % of BθOM IFN-γ+ IL-17A+ T cells in the colon, colon-draining lymph node (cdLN), and spleen of SPF Rag1−/− mice gavaged with CPS1 or CPS8 and adoptively transferred with BθOM T cells three days later (n = 24 mice, three experiments).
(C) Number of T cells among live leukocytes that are CD3+ CD4+ in the colon, cdLN, and spleen of germ free mice gavaged with CPS1 or CPS8. (n = 20 mice, two experiments)
Data represent mean ± SEM. Mann-Whitney test for non-normally distributed data: (A) ∗∗∗p < 0.001, (B) ∗p < 0.05, (C) ∗p < 0.05, ∗∗p < 0.01.
Since BθOM T cells are specific for a single antigen in B. theta, we next sought to determine if single CPS-expressing B. theta could also modulate polyclonal immune responses. We colonized germ-free C57BL/6 mice with B. theta expressing CPS1 or CPS8. Ten days later we harvested T cells from the colon lamina propria, colon draining lymph node (cdLN), and spleen. Although there were no differences in T cell differentiation (Figs. S4H–S4M), anti-stimulatory CPS1-expressing B. theta weakly activated polyclonal T cells, while pro-stimulatory CPS8-expressing B. theta strongly activated polyclonal T cells in vivo in the colon lamina propria (Fig. 6C). These findings reveal that by altering CPS expression, bacteria can also regulate polyclonal T cell responses in vivo.
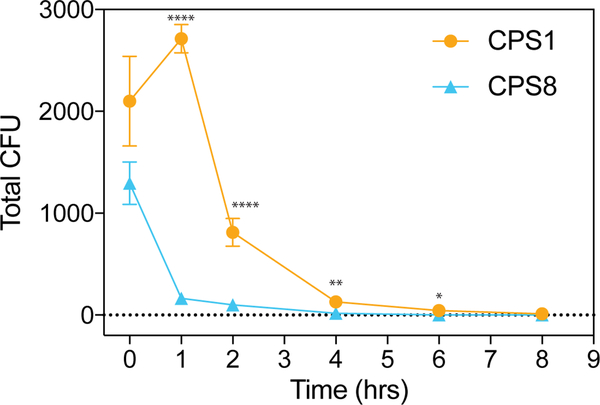
CPSs on B. theta control clearance of B. theta in vivo
When the intestine is perforated, intestinal bacteria can migrate into the peritoneal cavity and disseminate in the bloodstream (29). Since B. theta has been reported to cause sepsis and bacteremia (30, 31), we next examined the functional roles of CPSs on B. theta in vivo in the peritoneum. We injected B. theta expressing CPS1 or CPS8 intraperitoneally, harvested the peritoneal fluid 4 hours later, and measured the B. theta remaining in the peritoneal fluid. CPS8-expressing B. theta exhibited enhanced bacterial clearance from the peritoneal fluid compared to anti-stimulatory CPS1-expressing B. theta, likely because anti-stimulatory CPS1-expressing B. theta is poorly uptaken by immune cells (Fig. 7A). We then performed a time course of bacterial clearance in the peritoneal fluid and found substantial differences in B. theta clearance as early as 1 hour post-injection. These results confirmed that readily phagocytosed CPS8-expressing B. theta is cleared much more rapidly than the poorly uptaken CPS1-expressing B. theta (Fig. 7A). Thus, CPSs on B. theta also modulate the immune responses to dominant antigen by controlling clearance of specific bacterial antigens in vivo.
FIGURE 7. CPSs control clearance of a dominant B. theta antigen in vivo.
Total CFU in the peritoneal interstitial fluid in C57BL/6 mice injected with 1e6 CFU of CPS1 or CPS8 i.p. and lavaged (A) 0, 1, 2, 4, 6, or 8 hours later. n = 90 mice, three experiments.
Data represent mean ± SEM. Student’s t test: ∗p < 0.05, *∗∗p < 0.001, *∗∗*p < 0.0001.
DISCUSSION
Bacteria express a variety of different surface CPSs, but the functional consequences of bacteria phase variance remain poorly understood. CPSs are known to be immunomodulatory, but current studies have only investigated the immune effects of a handful of CPSs such as the zwitterionic PSA produced by B. fragilis (32). Our recent study explored the roles of a complete set of CPSs in competitive intestinal colonization, but we did not study how CPSs on B. theta influence the immune response (15). In addition, current studies have focused on how the CPS itself interacts with the immune system (5, 28, 33). It remains unknown if CPSs on bacteria can regulate the immune responses to specific bacterial antigens.
Our symbiont-specific T cell system combined with a complete set of single CPS-expressing B. theta strains provide an opportunity to interrogate if the immune responses to dominant antigens can be modulated by CPSs on bacteria. Our findings reveal a critical role for CPSs on B. theta to regulate antigen accessibility and the innate and adaptive immune responses to specific bacterial antigens. While many single CPS-expressing B. theta strains were anti-stimulatory, a few strains were pro-stimulatory and activated BθOM T cells just as well or better than an acapsular B. theta strain that lacked all B. theta CPSs. B. theta strains expressing anti-stimulatory CPSs suppressed BθOM T cell stimulation by blocking uptake of dominant antigen. Poor uptake of B. theta with anti-stimulatory CPSs may be partly explained by their thicker capsules, but could also be due to differences in CPS composition. Pro-stimulatory CPS8 consists of 19% N-acetyl-glucosamine, 2% galactose, 63% glucose, 14% mannose, and 1% glucuronic acid (15). Anti-stimulatory CPS1 consists of 22% N-acetyl-glucosamine, 33% glucose, 9% mannose, and 36% galacturonic acid (15). Although CPS1 may be more negatively charged due to the presence of galacturonic acid, which could play a role in CPS1’s poor uptake, this compositional analysis is limited to the monosaccharides present in the purified B. theta capsules. CPSs contain many complex carbohydrates and more studies will be needed to decipher why some CPSs are poorly phagocytosed. Taken together, these findings demonstrate that CPSs on bacteria control the delivery of dominant antigens to the immune system.
Poor uptake and BθOM T cell stimulation with CPS1-expressing B. theta could be rescued by Fc receptor-dependent antibody opsonization through the A176 antibody. The A176 antibody binds to B. theta but is not specific to CPS1, suggesting that it recognizes an epitope that is either conserved throughout all eight B. theta capsules or is not present on the capsule surface. A176 may increase immune recognition of CPS1-expressing B. theta by several mechanisms. For example, A176 could mask a surface molecule that inhibits phagocytosis or stimulate a surface molecule to trigger uptake. Given the literature showing that antibody conformation is more important for antibody neutralization in viruses than the antibody epitope or binding affinity (34), we hypothesize that A176 may increase phagocytosis by binding to CPS1-expressing B. theta in a particular geometrical configuration that allows CPS1-expressing B. theta to be amenable to APC uptake. This may also explain the inability of A176 to upregulate BθOM T cell stimulation in the presence of OMVs expressing CPS1. OMVs are 20–250 nm in diameter and are much smaller than whole bacteria (35), so A176 may not be able to form the optimal antibody configuration to opsonize CPS1-expressing OMVs. These results show that the immune system can overcome the ability of CPSs on B. theta to block phagocytosis by generating antibodies that restore immune access to dominant antigens through Fc receptor-dependent antibody opsonization.
In addition to regulating adaptive immune responses, all single CPS-expressing B. theta activated the innate immune system in a MyD88-dependent manner. Since MyD88 is downstream of many Toll-like receptors (TLRs) and B. theta expressing anti-stimulatory CPSs are poorly uptaken, we suggest that these innate immune responses are primarily due to interactions between B. theta and surface pattern recognition receptors and may represent immune adaptations to overcome CPS phase variance, especially to detect anti-phagocytic bacteria. Although all single CPS-expressing B. theta activated the innate immune system, B. theta expressing anti-stimulatory CPSs activated the innate immune system more weakly than B. theta expressing pro-stimulatory CPSs. These differences in innate immune activation may be due to variations in CPS composition, especially in the immunomodulatory molecules present, or the ability of anti-stimulatory CPSs to shield immunostimulatory molecules from activating the innate immune system.
Many Gram-negative bacteria release OMVs, which can deliver bacterial antigens to host immune cells in the intestine (19). CPSs on OMVs such as PSA on B. fragilis OMVs have been shown to regulate the immune system (28), but the roles of a complete set of CPSs on OMVs have not yet been interrogated. We show that a complete set of CPSs on B. theta OMVs have similar immunomodulatory roles as CPSs on whole B. theta. All single CPS-expressing OMVs activated the innate immune system to skew to M1 macrophages and release inflammatory cytokines. OMVs with anti-stimulatory CPSs more weakly activated BθOM T cells and APCs compared to OMVs with pro-stimulatory CPSs. These findings establish that a complete set of CPSs on OMVs can also regulate adaptive and innate immune responses to dominant antigens.
Finally, we reveal that CPSs on B. theta can also modulate immune responses to dominant antigens in vivo. In accordance with our in vitro findings, anti-stimulatory CPS1 poorly stimulated BθOM T cells while pro-stimulatory CPS8 strongly stimulated BθOM T cells in our BθOM T cell transfer mouse model. CPS expression on B. theta also directed BθOM T cell differentiation as BθOM T cells in the colon converted to more IFN-γ+ IL-17A+ T cells in the presence of B. theta expressing pro-stimulatory CPS8. The roles of these TH1-like TH17 cells are poorly understood, but they have been shown to play important roles in the pathogenesis of autoimmune diseases, including inflammatory bowel disease (36). CPSs on B. theta also regulated polyclonal immune responses as germ-free mice colonized with anti-stimulatory CPS1-expressing B. theta weakly activated polyclonal T cells, while pro-stimulatory CPS8-expressing B. theta strongly activated polyclonal T cells. Since bacteria can disseminate into the peritoneal cavity when the intestine is perforated, we then examined the roles of CPSs on B. theta in a model of peritoneal infection. Readily phagocytosed B. theta expressing CPS8 was cleared more rapidly from the peritoneal cavity compared to poorly phagocytosed B. theta expressing CPS1, suggesting that CPSs on B. theta may also regulate immune responses during peritoneal infection. Furthermore, anti-stimulatory and pro-stimulatory CPSs have been found to be expressed on B. theta in human gut samples, demonstrating that CPSs on B. theta may also modulate immune responses to specific bacterial antigens in the human intestine (15). Given the vast, unexplored universe of gut bacterial CPS structures, we believe that future studies will expand the known lexicon of bacterial polysaccharide-immune system interactions and may even lead to the discovery of new, bioactive CPS for potential use as therapeutics.
Supplementary Material
KEY POINTS.
B. theta CPSs control the activation of T cells and the clearance of antigen in vivo.
Anti-stimulatory CPSs activate innate immunity, but weaker than pro-stimulatory CPSs.
Anti-stimulatory CPSs block APC access, which can be rescued by opsonization.
ACKNOWLEDGEMENTS
We thank D. Kraemalmeyer for SPF animal care and breeding, M. White and S. Rucknagel for germ-free care and breeding, D. Stewart and T. Tolley for histology, M. Shi for assistance with the Nikon Spinning Disk, R. Brown for insights on the manuscript, M. Diamond for the FcRγ−/−mice, and B. Schreiber for use of the confocal microscope. Quick freeze deep etch experiments and imaging were performed in part through the use of Washington University Center for Cellular Imaging (WUCCI) supported by Washington University School of Medicine, The Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (CDI-CORE-2015-505) and the Foundation for Barnes-Jewish Hospital (3770).
This work was supported by NIH grants F30DK114950 (to S.A.H.) and R21AI142257 (to P.M.A. and E.C.M.).
REFERENCES
- 1.Coyne MJ, and Comstock LE. 2008. Niche-specific features of the intestinal bacteroidales. J. Bacteriol 190: 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter NT, and Martens EC. 2017. The Critical Roles of Polysaccharides in Gut Microbial Ecology and Physiology. Annu. Rev. Microbiol 71: 349–369. [DOI] [PubMed] [Google Scholar]
- 3.Avci FY, and Kasper DL. 2010. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol 28: 107–130. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JL, Jones MB, and Cobb BA. 2018. Polysaccharide-experienced effector T cells induce IL-10 in FoxP3+ regulatory T cells to prevent pulmonary inflammation. Glycobiology 28: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazmanian SK, Round JL, and Kasper DL. 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453: 620–625. [DOI] [PubMed] [Google Scholar]
- 6.Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, and Kasper LH. 2010. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3: 487–495. [DOI] [PubMed] [Google Scholar]
- 7.Ramakrishna C, Kujawski M, Chu H, Li L, Mazmanian SK, and Cantin EM. 2019. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat Commun 10: 2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neff CP, Rhodes ME, Arnolds KL, Collins CB, Donnelly J, Nusbacher N, Jedlicka P, Schneider JM, McCarter MD, Shaffer M, Mazmanian SK, Palmer BE, and Lozupone CA. 2016. Diverse Intestinal Bacteria Contain Putative Zwitterionic Capsular Polysaccharides with Anti-inflammatory Properties. Cell Host Microbe 20: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma R, Lee C, Jeun EJ, Yi J, Kim KS, Ghosh A, Byun S, Lee CG, Kang HJ, Kim GC, Jun CD, Jan G, Suh CH, Jung JY, Sprent J, Rudra D, De Castro C, Molinaro A, Surh CD, and Im SH. 2018. Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of Foxp3(+) regulatory T cells. Sci Immunol 3. [DOI] [PubMed] [Google Scholar]
- 10.Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D, Motherway MO, Shanahan F, Nally K, Dougan G, and van Sinderen D. 2012. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc. Natl. Acad. Sci. U. S. A 109: 2108–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danne C, Ryzhakov G, Martinez-Lopez M, Ilott NE, Franchini F, Cuskin F, Lowe EC, Bullers SJ, Arthur JSC, and Powrie F. 2017. A Large Polysaccharide Produced by Helicobacter hepaticus Induces an Anti-inflammatory Gene Signature in Macrophages. Cell Host Microbe 22: 733–745 e735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paynich ML, Jones-Burrage SE, and Knight KL. 2017. Exopolysaccharide from Bacillus subtilis Induces Anti-Inflammatory M2 Macrophages That Prevent T Cell-Mediated Disease. J. Immunol. 198: 2689–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andre GO, Converso TR, Politano WR, Ferraz LF, Ribeiro ML, Leite LC, and Darrieux M. 2017. Role of Streptococcus pneumoniae Proteins in Evasion of Complement-Mediated Immunity. Front. Microbiol 8: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonsson S, Musher DM, Chapman A, Goree A, and Lawrence EC. 1985. Phagocytosis and killing of common bacterial pathogens of the lung by human alveolar macrophages. J. Infect. Dis 152: 4–13. [DOI] [PubMed] [Google Scholar]
- 15.Porter NT, Canales P, Peterson DA, and Martens EC. 2017. A Subset of Polysaccharide Capsules in the Human Symbiont Bacteroides thetaiotaomicron Promote Increased Competitive Fitness in the Mouse Gut. Cell Host Microbe 22: 494–506 e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, and Gordon JI. 2011. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 9: e1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martens EC, Roth R, Heuser JE, and Gordon JI. 2009. Coordinate regulation of glycan degradation and polysaccharide capsule biosynthesis by a prominent human gut symbiont. J. Biol. Chem 284: 18445–18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wegorzewska MM, Glowacki RWP, Hsieh SA, Donermeyer DL, Hickey CA, Horvath SC, Martens EC, Stappenbeck TS, and Allen PM. 2019. Diet modulates colonic T cell responses by regulating the expression of a Bacteroides thetaiotaomicron antigen. Sci Immunol 4.doi: 10.1126/sciimmunol.aau9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hickey CA, Kuhn KA, Donermeyer DL, Porter NT, Jin C, Cameron EA, Jung H, Kaiko GE, Wegorzewska M, Malvin NP, Glowacki RW, Hansson GC, Allen PM, Martens EC, and Stappenbeck TS. 2015. Colitogenic Bacteroides thetaiotaomicron Antigens Access Host Immune Cells in a Sulfatase-Dependent Manner via Outer Membrane Vesicles. Cell Host Microbe 17: 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson AN, Choudhury BP, and Fischbach MA. 2018. The Biosynthesis of Lipooligosaccharide from Bacteroides thetaiotaomicron. MBio 9.doi: 10.1128/mBio.02289-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koropatkin NM, Martens EC, Gordon JI, and Smith TJ. 2008. Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure 16: 1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitaker WR, Shepherd ES, and Sonnenburg JL. 2017. Tunable Expression Tools Enable Single-Cell Strain Distinction in the Gut Microbiome. Cell 169: 538–546 e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen PM, and Unanue ER. 1984. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J. Immunol 132: 1077–1079. [PubMed] [Google Scholar]
- 24.Kearney JF, Radbruch A, Liesegang B, and Rajewsky K. 1979. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J. Immunol 123: 1548–1550. [PubMed] [Google Scholar]
- 25.Chutkan H, Macdonald I, Manning A, and Kuehn MJ. 2013. Quantitative and qualitative preparations of bacterial outer membrane vesicles. Methods Mol. Biol 966: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjursell MK, Martens EC, and Gordon JI. 2006. Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J. Biol. Chem 281: 36269–36279. [DOI] [PubMed] [Google Scholar]
- 27.Sica A, and Mantovani A. 2012. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, and Mazmanian SK. 2012. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 12: 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wexler HM 2007. Bacteroides: the good, the bad, and the nitty-gritty. Clin. Microbiol. Rev 20: 593–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brook I 1989. Anaerobic bacterial bacteremia: 12-year experience in two military hospitals. J. Infect. Dis 160: 1071–1075. [DOI] [PubMed] [Google Scholar]
- 31.Simmon KE, Mirrett S, Reller LB, and Petti CA. 2008. Genotypic diversity of anaerobic isolates from bloodstream infections. J. Clin. Microbiol 46: 1596–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surana NK, and Kasper DL. 2012. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunol. Rev 245: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, and Mazmanian SK. 2011. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332: 974–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bale S, Dias JM, Fusco ML, Hashiguchi T, Wong AC, Liu T, Keuhne AI, Li S, Woods VL Jr., Chandran K, Dye JM, and Saphire EO. 2012. Structural basis for differential neutralization of ebolaviruses. Viruses 4: 447–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulp A, and Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol 64: 163–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamali AN, Noorbakhsh SM, Hamedifar H, Jadidi-Niaragh F, Yazdani R, Bautista JM, and Azizi G. 2019. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol. Immunol 105: 107–115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.