Abstract
Knowledge of head direction cell function has progressed remarkably in recent years. The predominant theory that they form an attractor has been confirmed by several experiments. Candidate pathways that may convey visual input have been identified. The pre-subicular circuitry that conveys head direction signals to the medial entorhinal cortex, potentially sustaining path integration by grid cells, has been resolved. Although the neuronal substrate of the attractor remains unknown in mammals, a simple head direction network, whose structure is astoundingly similar to neuronal models theorized decades earlier, has been identified in insects. Finally, recent experiments have revealed that these cells do not encode head direction in the horizontal plane only, but also in vertical planes, thus providing a 3D orientation signal.
Introduction
Head Direction Cells (HDC) found throughout the navigation circuit (Fig. 1A; see Bubb et al. 2017, 2018) form a neuronal compass that tracks allocentric head orientation. Here we summarize recent progress in uncovering the neuronal pathways that convey visual landmark information to the HDC network (Yoder et al. 2015; Jacob et al. 2017; Page and Jeffery, 2018; Korienko et al. 2018). We also summarize recent studies (Tukker et al. 2015; Winter et al. 2015; Preston-Ferrer et al. 2016; Weiss et al. 2017; Simonnet et al, 2017; Simonnet et Fricker, 2017; Nassar et al., 2018) that explore the functional and anatomical relationship between the HDC network and grid cell networks in the MEC.
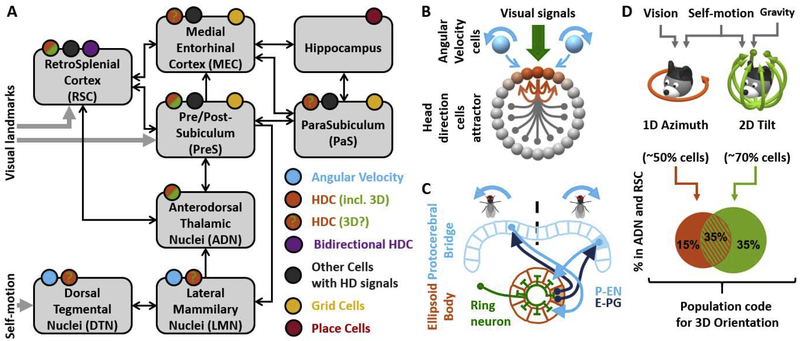
Figure 1: Overview of the HDC system and recent progress.
A: HD circuitry in rodents. Colored circles indicate the presence of angular velocity cells (blue), HDC, including 3D-tuned HDC (orange/green in regions where 3D HDC have been identified; orange with green question marks where 3D responses have not been tested), bidirectional cells (violet; Jacob et al. 2017), other cells with HD responses (dark grey; Peyrache et al. 2017; Laurens et al. 2019; Kornienko et al., 2018), grid cells (yellow) and place cells (red). Arrows represent connections between regions. Self-motion signals enter the HD network through the DTN, and visual landmark signals through the RSC and/or the PreS. After Bubb et al. 2017, 2018; Clark and Taube 2012, Weiss and Derdikman, 2018; Yoder and Taube, 2011; Yoder et al. 2011, 2015.
B: Schematic structure of a HDC attractor. Active (orange) and inhibited (light grey) HDCs are represented along a ring. Excitatory (orange) and inhibitory (grey) connections originating from one HDC are shown inside the ring. Visual landmark signals (green) anchor the packet of active HDC to a given location. Angular velocity cells (blue) may encode clockwise (right) or counterclockwise (left) rotation and cause the packet of active HDC to shift.
C: HDC attractor in Drosophila (see text for details).
D: Representation of 3D head orientation in the HD system. 3D orientation is decomposed into azimuth (head orientation in the head horizontal plane, orange) and head tilt relative to vertical (green). Azimuth signals are anchored to visual landmarks, and tilt to gravity signals. Both can be updated in response to self-motion. Bottom: HDC may represent azimuth only, tilt only or both conjunctively. The corresponding proportions of cells in the HD system are shown as a Venn diagram (data from mice ADN and RSC; Angelaki et al. 2019; proportions in bat PreS are comparable; Finkelstein et al. 2015). 50% of cells encode azimuth, and about 70% represent tilt. The probability of representing azimuth and tilt are independent, such that about 35% cells encode tilt and azimuth conjunctively.
A prominent theory that HDC form an attractor network (Fig. 1B; Redish et al., 1996; Skaggs et al., 1995; Zhang, 1996) emerged shortly after their discovery (Ranck 1984; Taube et al. 1990a,b). Yet, it received little experimental evidence until recently. We review recent studies supporting this hypothesis in mammals (Peyrache et al. 2015; Butler et al. 2017; Bassett et al. 2018; Park et al. 2019; Gardner et al. 2019; Chaudhuri et al., 2019; Rubin et al., 2019) and insects, where the physical structure of the circuit is astoundingly similar to a functional ring attractor (Fig. 1C; Seelig and Jayaraman 2015; Kim et al. 2017; Green et al. 2017, 2019; Turner-Evans et al. 2017).
We end this review by considering the three-dimensional (3D) organization and the coordinate frame of the head direction system (Fig. 1D; Finkelstein et al. 2015; Laurens et al. 2016; Page et al. 2017; Angelaki et al. 2019).
Head direction cells form a ring attractor that integrates multisensory signals
A prominent theoretical framework of HD function is the attractor model (Fig. 1B), where HDC are represented along a conceptual ring (no topological organization is known in the mammalian HDC circuit), each cell’s position representing its allocentric preferred direction (PD). Visual input cells that fire when the head faces a specific direction in the visual environment activate the corresponding HDC. Recurrent connections within the population create ‘winner takes all’ dynamics where a packet of cells with similar PD, i.e. adjacent on the conceptual ring, activate each other while inhibiting other cells. Through mutual excitation, this cell packet may remain active even if visual inputs are withheld, allowing the attractor to ‘memorize’ HD in darkness. Angular velocity cells (Fig. 1B, blue) provide self-motion inputs that encode rotation (angular) velocity and can cause the activity packet to slide, thus updating the HD signal. This model accounts for the HDC’s ability to anchor to visual landmarks, memorize head orientation in darkness, and integrate rotation signals (Redish et al., 1996; Skaggs et al., 1995; Zhang, 1996; Clark and Taube 2012; Sun et al. 2018). As described in the next section, a neuronal circuit that closely matches the attractor model has been described in insects (Fig. 1C).
Recent studies have provided strong support for this framework. These studies rely on a rationale illustrated in Fig. 2A–D. When foraging in light (Fig. 2A), HDC remain stable over minutes (Fig. 2B). In darkness, HDC can “path-integrate” rotation signals but may accumulate errors, resulting in a drift of the direction at which HDC fire (Fig. 2C). Importantly, the attractor network theory predicts that individual HDCs should drift coherently, i.e. by a same amount and in the same direction, such that the relationship between PDs are preserved (Fig. 2D), since their firing reflects the drift of a single HD signal encoded by the entire network.
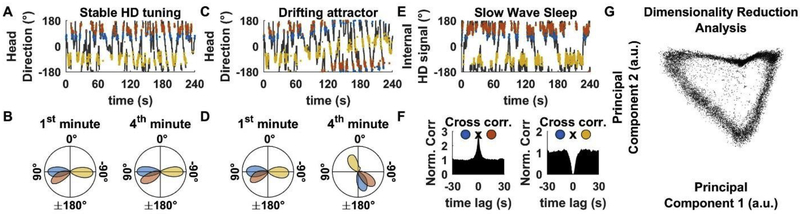
Figure 2: Experimental support for the Head Direction Cell attractor.
Panels show the simulated responses of 3 HDC (tuning curves modelled as Von Mises distribution with σ=18° and peak firing = 30 spk/s) during random trajectories.
A: Firing of three HDC when the HD attractor is consistently anchored to allocentric HD, e.g. in light. Grey curve: animal’s trajectory; blue, orange and yellow: spikes from the three cells. Each cell fires at a fixed head direction.
B: Tuning curves during the first (left) and fourth (right) minute in panel A, shown as a polar plot, where the angle represents HD and the radius represents firing rate (in arbitrary units).
C: Firing of three HDC when the attractor drifts, e.g. following experimental disturbances. Directionality tuning drifts coherently across cells.
D: Tuning curves during the first (left) and fourth (right) minutes in panel C, demonstrating a coherent drift of all cells.
E: Firing of three HDC during slow wave sleep. The cells’ firing is anchored to an internally generated HD signal that drifts randomly (grey).
F: Temporal cross-correlation analysis of HDC during slow-wave sleep (from simulated data in panel E). Since the internal HD signal drifts slowly, HDC with similar spatial properties will also fire together over time and therefore exhibit positive temporal cross-correlations (left: cross-correlation between blue and orange cells). Reciprocally, cells with opposite spatial properties will exhibit negative temporal cross-correlations (right: cross-correlation between blue and yellow cells).
G: Illustration of dimensionality reduction analysis (based on the simulated responses of 100 HDC; tuning curves: Von Mises distribution with σ=18° and peak firing = 30 spk/s; during random trajectories). When plotted on a reduced dimensionality space, population responses adopt a circular topology that reflects the HDC attractor.
This prediction was verified by Butler et al. (2017), who inactivated optogenetically DTN-projecting cells in the Nucleus Prepositus Hypoglossi. These cells provide angular velocity signals to the HD attractor; and accordingly their inactivation caused HDC to drift when recorded in darkness. As predicted, simultaneously recorded ADN HDC drifted coherently. In another study, Bassett et al. (2018) demonstrated that attractor activity exists in the ADN of rat pups even before their eyes open. Path integration in the HD attractor was poorly calibrated, resulting in a drift of HDC that was coherent across cells.
The HDC attractor thus provides an internal sense of orientation in the absence of visual reference. Even in light, it doesn’t necessarily anchor to visual landmarks. This has been illustrated by Park et al. (2019), who placed rats on a rotating platform and trained them to avoid two zones, one referenced to the platform and another referenced to allocentric landmarks. HDC in the MEC anchored to neither the platform nor allocentric landmarks; instead, kept re-anchoring to various frames over short time scales (~10s). Yet, individual HDCs remained anchored one to the other, supporting the attractor model again. This experiment stresses that the HDC attractor doesn’t encode HD in a unique reference frame (i.e. like a magnetic compass) but can instead anchor dynamically, depending on yet undefined behavioral and/or cognitive goals.
It is notable that Peyrache and colleagues (2015) observed attractor dynamics during slow-wave sleep (SWS) and rapid eye movements sleep (REM). The firing properties of ADN and PreS HDC resembled those in awake animals, suggesting the attractor encoded a drifting HD signal (Fig. 2E). HDC with nearby PDs exhibited positive temporal correlations (Fig. 2F, left), since they tend to fire at similar times, whereas pairs with opposite PDs were anti-correlated (Fig. 2F, right), as they never fired simultaneously. These correlations were conserved during SWS and REM as well as when awake, both within and across regions, demonstrating that internal attractor activity persists during sleep. This result has been recently reproduced in MEC (Gardner et al. 2019). Remarkably relevant to the next section, neural data from Peyrache et al. 2015 re-analyzed (Chaudhuri et al., 2019; Rubin et al., 2019) through un-supervised dimensionality reduction techniques showed that the population response of HDC cluster along a 1D circular manifold that reflects the topology of the ring attractor (Fig. 2F).
A neuronal attractor for representing head direction in insects
Complex brains follow complex organization. In our opinion, the simplicity of the neuronal circuit for head direction in insects, where anatomy directly meets function, is a most remarkable finding of the past decade (Fig. 1C; see Franconville et al. 2018 for a detailed review of this circuitry). Using 2-photon imaging, Seelig and Jayaraman (2015) showed that in flies the circuit matches the ring attractor model strikingly. Neurons in the ellipsoid body (EB; Fig. 1C, orange) form an attractor that encodes head direction (Seelig and Jayaraman 2015; Kim et al. 2017; Green et al. 2017; Green and Maimon 2018; Turner-Evans et al. 2019). Remarkably, these neurons are anatomically organized along a ring with an inverse topological organization (i.e. clockwise rotations of the animal are represented by counterclockwise rotation of the activity packet). Cells in the left and right protocerebral bridge (PB; Fig. 1C, blue) are responsive to counterclockwise/clockwise rotations of the animal. Recurrent connections between the EB and the PB (P-EN: protocerebral bridge-ellipsoid body noduli neurons; E-PG: ellipsoid body-protocerebral bridge gall neurons) integrate rotation signals (Green et al. 2017, Turner-Evans et al. 2017). For instance, if the fly faces a certain direction (represented by the orange-filled tile in the EB in Fig. 1C) and the animal rotates clockwise, the activity of E-PG and the right PB responsiveness to clockwise rotation will activate one tile in the PB (Fig. 1C, blue-filled tile). In turn, the corresponding PEN neurons will activate the EB’s neurons located counterclockwise relative to the orange filled tile, thus displacing neuronal activity in the EB to integrate the animal’s rotation. Other insect species possess analogous neural circuits (Varga and Ritzmann 2016; Stone et al. 2017; see Turner-Evans and Jayaraman, 2016 for a review), and attractor dynamics has been demonstrated in the EB of cockroaches (Varga and Ritzmann 2016). Several mathematical models of this circuit have been developed (Cope et al. 2017; Kakaria and de Bivort 2017; Stone et al. 2017).
Recent studies (Fisher et al. 2019, Kim et al. 2019) have identified ‘ring neurons’ (Fig. 1C, green) that convey visual landmark information to the EB. Remarkably, their synapses to the EB can dynamically reorganize in an experience-dependent manner to map visual landmarks to the head direction signal in the EB (Fisher et al. 2019, Kim et al. 2019).
In an elegant experiment where flies walked spontaneously in a straight line in darkness, Green et al. (2019) used focal stimulation to reposition the EB activity packet. They observed that flies responded by performing a turn, which restored the activity packet to its initial position. This suggests a causal link, where behavior (walking in a straight line) is guided by EB activity.
Visual landmark control of the HDC network
Visual landmarks represent a major stimulus to anchor HDC. Based on lesion studies, Yoder et al. (2011, 2015) proposed that visual landmark signals travel from cortical areas, including RSC, to the PreS, that projects to the LMN. The RSC may play a key role in this function: it is thought to transform visual information from an egocentric to allocentric reference frame (see Clark et al. 2018, Mitchell et al. 2018 for reviews) and a fMRI study in humans (Auger et al. 2017) has implicated it in identifying stable visual landmarks to support spatial navigation. In this section, we review a recent study (Jacob et al. 2017) that identified a potential neuronal substrate of landmark processing in RSC. We also discuss additional reports of visual HD signals in the MEC and PaS (Kornienko et al. 2018).
We illustrate the findings of Jacobs et al. (2018) in Fig. 3A–F. Rats explored two interconnected, symmetrical, compartments (Fig. 3A–E) that were visually identical but discriminable based on odor cues. HDC in RSC responded at the same allocentric HD in both compartments (Fig. 3, orange). In contrast, some cells responded at opposite allocentric directions (Fig. 3A,B, purple), suggesting that they encoded visually-driven head orientation signals. These cells are termed ‘bidirectional’ (BD) cells since, when averaged across both compartments, their tuning appears bidirectional. They are further separated into ‘between-compartments’ (BC) cells that exhibit opposite (unidirectional) tuning curves between compartments (Fig. 3A) and ‘within-compartments’ (WC) cells that exhibited a limited degree of bimodality within each compartment (Fig. 3B). Importantly, the response of BD cells persists in darkness (Fig. 3C), demonstrating that they are not exclusively visually driven but instead register to the HDC attractor that sustains their response in darkness. When the arena was rotated by 90°, the responses of both HDC and BDC rotated to follow the environment in ~78% of trials (Fig. 3D). Interestingly, in the remaining 22%, the rotation of HDC responses didn’t follow the environment, whereas BDC did (Fig. 3E). These are likely instances where, following the environment’s rotation, local cues were recognized as non-stationary and were registered to a new head direction. BD cells identified in the symmetrical arena (Fig. 3A–E) didn’t exhibit any directional tuning in a square arena (Fig. 3F), probably because of the different structure of the visual environment.
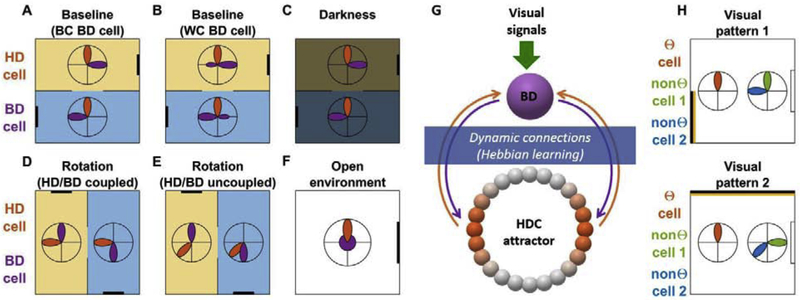
Figure 3: “Bidirectional” (BD) cells in the retrosplenial cortex (Jacob et al. 2017) and visually-driven HDC in the MEC and PaS (Kornienko et al. 2018).
A-E: Recordings in a two-compartment arena. Each compartment is identified by an odor cue (symbolized in yellow and blue) and possesses one visual cue (black bar), placed symmetrically across compartments. The tuning curves of a HD cell (orange) and BD cell (violet) are shown as polar plots. A: BD cells with “Between Compartment” (BC) response, where the cell is tuned to a unique direction in each compartment. B: BD cells with “Within Compartment” (WC) response, where the cell exhibits a limited degree of bidirectionality in each compartment. D,E: response where the arena is rotated by 90° and BD cells remained anchored to HDC (D) or uncoupled from HDC (E). All panels are schematic illustrations drawn by the authors of the present review.
F: Recording in an open square arena. The directional tuning of HDC is maintained, but the tuning of BDC disappears.
G: Conceptual model (after Jacob et al. 2017; Page and Jeffery 2018). BD cells integrate HD, visual and contextual information to identify stationary landmarks and in turn provide landmark signals to the HDC attractor. In a visually symmetrical environment, BD cells connect to two opposite parts of the HDC attractor (orange), resulting in their bidirectional response.
H: Recording of visually-driven HDC (Kornienko et al. 2018). Recordings are performed in a square arena with a stable orienting cue (shown in white on the right side) and an unstable cue (yellow/black stipe) alternating between two configurations (top versus bottom panel). The tuning curves of three example cells are shown as polar plots. Θ-modulated cells behave as traditional HDC and exhibit stable tuning; non-Θ-modulated cells are visually driven and remap independently one from the other when the visual pattern changes. All panels are schematic illustrations drawn by the authors of the present review.
A conceptual model of BDC, based on (Jacob et al. 2017, Page and Jeffery, 2018) is shown in Fig. 3G. In essence, BDCs associate a given visual signal with the corresponding allocentric HD (or two corresponding HD in a visually symmetrical environment) through synaptic plasticity. In turn, BDCs are assumed to activate the corresponding HDC. By this process, these cells memorize the allocentric bearing of stationary visual landmarks, and anchor HDC to these stable landmarks. Some components of this model, such as the projection from BDC to HDC, remain hypothetical. Despite this limitation, by dissociating traditional HDC from visually driven cells, Jacob et al. (2017) have introduced a thought-provoking paradigm to study visual control of the HDC network.
Another type of visually driven HDC has been identified in the Parasubiculum and MEC (Korienko et al. 2018). Mice walked in an arena equipped with a stable visual cue (Fig. 3H, white) and an additional visual cue that alternated between two configurations (Fig. 3H, yellow/black strip). Part of HDC (Fig. 3H, orange; left polar plots) behaved like traditional HDC. Their PD were generally stable between visual patterns, indicating that mice maintained a stable sense of allocentric orientation. If these cells’ PD shifted, they remained anchored one to another. Other cells (Fig. 3H, green/blue; right polar plots), had dissimilar PD between visual patterns, and the changes in PD were incoherent between cells. Thus, these cells appeared to be primarily visually-driven and weren’t anchored to the HDC attractor. Interestingly, the firing of traditional HDC was phase-locked to the local theta rhythm, whereas the firing of visually-driven HDC wasn’t, indicating a physiological difference between these neuronal types. Visually-driven HDC identified by Kornienko et al. (2018) are distinct from BD cells identified by Jacob et al. (2017) since they are not anchored to the HDC attractor. They may be part of another pathway through which vision contributes to navigation, that remains to be explored.
Neural pathways in the PreS
The PreS is involved in at least two functional pathways within the HDC circuit. First, the grid cell system uses HD signals (Winter et al. 2015, Weiss et al. 2017), likely originating from the ADN and relayed to the MEC by the PreS (Tukker et al. 2015, Preston-Ferrer et al. 2016). Second, the PreS receives visual signals from the RSC (and other visual areas; see Yoder et al. 2011, Yoder and Taube 2011) and projects to the LMN to anchor the HD attractor to the PreS and therefrom to the LMN (Yoder et al. 2011, 2015). Here we review recent studies of the neural circuitry within the PreS that pave the way towards understanding how it participates to these functions.
The first pathway, where ADN projects to PreS which, in turn, projects to MEC, is now well understood. Most PreS HDC are layer 3 pyramidal neurons that receive ADN inputs and project predominantly to the MEC (Tukker et al. 2015, Preston-Ferrer et al. 2016). The response of these HDC is also shaped by two types of PreS interneurons that receive ADN inputs: fast-spiking interneurons activated monosynaptically by ADN HDC and Martinotti cells activated by layer 3 pyramidal cells (Simonnet et al, 2017; Simonnet et Fricker, 2018; Nassar et al., 2018).
Less is known about the second pathway that carry landmark signals from RSC to PreS to LMN. Layer 3 neurons receive RSC and striate/extrastriate visual cortex afferents (Yoder and Taube, 2011; Simonnet and Fricker, 2018), and may thus integrate visual signals. However, these neurons don’t project to the LMN. Instead, pyramidal neurons in Layer 4 (or other deep layers), that also contain HDC, project to the LMN (Huang et al. 2017; Simonnet and Fricker, 2018). However, the afferent connectivity of these layers is still unknown.
These studies have mapped part of the PreS circuitry with remarkable detail (see also Peyrache et al. 2019), while leaving some questions open, in particular whether HDC signals from the ADN to the MEC and visual signals from the RSC to the LNM merely cross each other or interact; and the nature of visual landmark signals projected by the PreS to the LMN.
HDC encode head orientation in three dimensions
Until recently, most HDC studies were conducted on horizontal surfaces. What do HDC encode when animals behave in 3D? Recent works have shown that HDC use a 3D compass where one degree of freedom, azimuth, is affixed to the head-horizontal plane, and the remaining two degrees of freedom encode head tilt relative to vertical.
When animals walk on a horizontal plane, HDC encode head orientation in this plane (i.e. azimuth). How is azimuth defined when the head tilts? Early studies (Stackman et al. 2000, Calton and Taube 2005) showed that, when rats walk on vertical surfaces, HDC encode head direction in the locomotion (or head-horizontal) plane (Fig. 4A, orange). However, how azimuth updates during 3D motion wasn’t understood until recently. Page et al. (2017) demonstrated that HDC in the ADN of rats maintain allocentric invariance (Fig. 4A, see legend) by using a “dual-axis rule” where azimuth is updated by rotation in the locomotion plane (Fig. 4A, red arrows) or when this plane rotates in space (Fig. 4A, blue). Laurens and Angelaki (2018) developed a mathematically equivalent model called “tilted azimuth”. This model is now supported by HDC recordings in the ADN of mice (Angelaki et al. 2019), HDC recordings in the ADN of rats by Shinder and Taube (2018), re-analyzed by Laurens and Angelaki 2019, and explains HDC responses in the PreS of bats (Finkelstein et al. 2015).
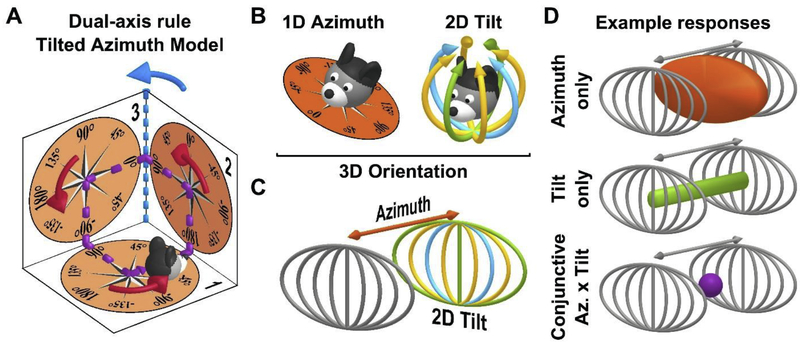
Figure 4: HDC encode head orientation in 3D.
A: Dual-axis rule and tilted azimuth model. Azimuth is measured on a compass (orange; tilted azimuth compass) affixed to the locomotion plane. Based on the dual-axis rule, azimuth is updated (1) when rotating on the locomotion plane (red arrows) and (2) when the plane rotates about an earth-vertical axis (blue; e.g. azimuth changes from 90° to 180° when moving from surface n°2 to surface n°3 along the violet trajectory). This rule ensures that azimuth maintains spatial invariance. For instance, when completing the trajectory shown in violet, the dual-axis rule registers a total rotation of 360° (three red turns and one blue turn). If only red turns updated the attractor, there would be a discrepancy with the allocentric environment.
B: Decomposition of 3D head orientation into 1D azimuth (left) and 2D tilt (right). Head tilt is two-dimensional, e.g. it can occur along the pitch (green), roll (blue) or intermediate (yellow) axes, and has a spherical topology since 180° tilt in any direction results in the same final position (upside-down).
C: Three-dimensional head orientation space, represented as the cartesian product of 1D azimuth and 2D tilt. The spherical 2D tilt space is projected onto a plane (using an equal-area Mollweide projection). An azimuth axis is added, orthogonal to this plane, to create a 3D space.
D: Representation of the response space of HDC responses with various degrees of dimensionality, in the 3D orientation space. Top: a HDC that encodes only azimuth will respond when the head faces a certain azimuth, and at any head orientation. The corresponding volume (orange) forms a vertical slice in the 3D space. Middle: a HDC that encodes only tilt will discharge around a certain tilt position (e.g. 90° pitch here) but at all possible azimuth angles. The corresponding volume (green) forms a line parallel to the azimuth axis. Bottom: a conjunctive HDC will discharge when the head reaches a specific range of tilt and azimuth, i.e. in the neighborhood of a single point (violet).
The mammalian’s navigation system is adapted to 3D navigation, as shown by the finding of 3D place cells in bats (Yartsev and Ulanovski 2013) and rats (Grieves et al. 2019). Tilted azimuth provides a 1D signal to navigate on inclined surfaces, but do HDC encode the remaining two degrees of freedom (2D tilt relative to vertical) to form a full 3D compass? Research in rodents (Stackman et al. 2000; Calton and Taube 2005; Taube and Shinder, 2019) long surmised that HDC encode only 1D azimuth. More recent studies focused on flying and arboreal mammals: Finkelstein et al (2015) found that HDC in the PreS of bats encode 3D head orientation, and Laurens et al. (2016) found that cells in the ADN of Macaque encode tilt (but couldn’t test whether they also encode azimuth). Finally, Angelaki et al. (2019) identified 3D HDC in the ADN, RSC and cingulum fiber bundle of mice, demonstrating that 3D tuning exists in rodents. They developed a framework to express HDC responses: 3D head orientation is decomposed in 1D tilted azimuth and 2D tilt, Fig. 4B, which together form a 3D volume (Fig. 4C). Within this volume, individual HDC may encode azimuth only (Fig. 4D, top), tilt only (Fig. 4D, middle) or azimuth and tilt conjunctively (Fig. 4D, bottom). Such a population of HDC with mixed levels of dimensionality may optimize the representation of head orientation across time scales (Finkelstein et al. 2018). Laurens et al (2016) and Angelaki et al. (2019) also demonstrated that tilt responses are anchored to Earth’s gravity, likely sensed by the vestibular system. Thus, HDC encode 3D orientation by using visual landmarks (for azimuth tuning), gravity sensing (for tilt tuning) and by integrating rotation signal (to derive an azimuth signal, and possibly a tilt signal) (Fig. 1D).
A recent fMRI study (Kim and Maguire 2018) identified azimuth and tilt responses in the RSC of humans engaged in a virtual reality task. However, only azimuth responses were found in the ADN. This task, being purely visual, may have failed to evoke tilt response in ADN, but may have recruited the RSC which is more visually oriented. Together, these results suggest that 3D tuning may be a ubiquitous property of the mammalian HD system.
Conclusions
Recent years have seen considerable innovations in the field of HDC research. The attractor network model, that had long been confined to theoretical studies, is now well supported by experimental studies in mammals, and demonstrated, to a spectacular degree, in insects. Functional and anatomical understanding of the HDC network progresses (see also Dudchenko et al. 2019 for a review of how HDC correlate with behavior in mammals). Finally, several studies have uncovered the principles of how HDC encode 3D head orientation.
A diversity of cell types across the navigation system exhibit head directionality. However, their anchoring to the canonical HD attractor may be dynamic (e.g. BD cells, Jacobs et al. 2017) or inexistent (e.g. Kornienko et al. 2018). This has raised the question of whether all HDC participate to an attractor network (Dudchenko et al. 2019); some even questioning whether the attractor theory is pertinent. In our opinion, the existence of the HD attractor is largely supported, but not all cells that exhibit HD tuning participate to it – at least, not always. This points to the need of establishing which cells participate to the attractor and under what conditions; and perhaps developing distinct nomenclature for canonical attractor-anchored HDC and other cell types with directional responses.
Highlights.
Head Direction Cells form an attractor that stores head orientation and integrates self-motion.
The neuronal substrate of the Head Direction attractor has been identified in insects.
Recently discovered “Bidirectional” cells may carry visual landmark signals.
The pre-subiculum conveys Head Direction signals to the entorhinal cortex.
Head Direction Cells encode head orientation in 3D.
Acknowledgements
This work was supported by Simons Collaboration on the Global Brain, grants 542949 and R01-AT010459, and NIH grant DC004260.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
Rules:
• Most references from the last 5 years (that means 2014 to 2019). I cite a few earlier references because they are of particular interest; they are shown in italic.
• At least 10% of references should be marked as paper of special or outstanding interest. They must be from the last two years. I mark them in bold and include 2017 to 2019 in the “last two years”. I made an exception for a 2016 paper.
- 1. *.Angelaki DE, Ng J, Abrego AM, Cham HX, Dickman JD, & Laurens J (2019). A gravity-based three-dimensional compass in the mouse brain. bioRxiv, 570382. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Angelaki et al. identify HDC tuned to 3D orientation in mice. They characterize their responses comprehensively and derive a model of 3D HDC tuning across mammalian species.
- 2.Auger SD, Zeidman P, & Maguire EA (2017). Efficacy of navigation may be influenced by retrosplenial cortex-mediated learning of landmark stability. Neuropsychologia, 104, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. *.Bassett JP, Wills TJ, & Cacucci F (2018). Self-organized attractor dynamics in the developing head direction circuit. Current Biology, 28(4), 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Basset et al. demonstrate HDC exist in rat pups before they open their eyes. These HDC track the motion of the rat and are anchored one to another, indicating that attractor dynamics already exists.
- 4.Bicanski A, & Burgess N (2016). Environmental anchoring of head direction in a computational model of retrosplenial cortex. Journal of Neuroscience, 36(46), 11601–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. *.Bubb EJ, Kinnavane L, & Aggleton JP (2017). Hippocampal–diencephalic–cingulate networks for memory and emotion: An anatomical guide. Brain and neuroscience advances, 1, 2398212817723443. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Bubb et al. author a comprehensive review the anatomy of the navigation circuit in rodents and primates.
- 6.Bubb EJ, Metzler-Baddeley C, & Aggleton JP (2018). The cingulum bundle: Anatomy, function, and dysfunction. Neuroscience & Biobehavioral Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. *.Butler WN, Smith KS, van der Meer MA, & Taube JS (2017). The head-direction signal plays a functional role as a neural compass during navigation. Current Biology, 27(9), 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Butler et al. perform an otogenetic inhibition of the nucleus prepositus, which presumably conveys self-motion signal to the HDC network. This causes HDC drift without altering attractor properties, and affects behavioral performance. This is one of the first studies to causally link HDC to behavior.
- 8.Calton JL, & Taube JS (2005). Degradation of head direction cell activity during inverted locomotion. Journal of Neuroscience, 25(9), 2420–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhuri R, Gercek B, Pandey B, Peyrache A, & Fiete I (2019). The intrinsic attractor manifold and population dynamics of a canonical cognitive circuit across waking and sleep. Nature neuroscience, 22(9), 1512–1520. [DOI] [PubMed] [Google Scholar]
- 10.Clark BJ, & Taube JS (2012). Vestibular and attractor network basis of the head direction cell signal in subcortical circuits. Frontiers in neural circuits, 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark BJ, Simmons CM, Berkowitz LE, & Wilber AA (2018). The retrosplenial-parietal network and reference frame coordination for spatial navigation. Behavioral neuroscience, 132(5), 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cope AJ, Sabo C, Vasilaki E, Barron AB, & Marshall JA (2017). A computational model of the integration of landmarks and motion in the insect central complex. PloS one, 12(2), e0172325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudchenko PA, Wood ER, & Smith A (2019). A new perspective on the head direction cell system and spatial behavior. Neuroscience & Biobehavioral Reviews. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein A, Derdikman D, Rubin A, Foerster JN, Las L, & Ulanovsky N (2015). Three-dimensional head-direction coding in the bat brain. Nature, 517(7533), 159. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein A, Ulanovsky N, Tsodyks M, & Aljadeff J (2018). Optimal dynamic coding by mixed-dimensionality neurons in the head-direction system of bats. Nature communications, 9(1), 3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. **.Fisher YE, Lu J, D’Alessandro I, & Wilson RI (2019). Sensorimotor experience remaps visual input to a heading-direction network. Nature, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Fisher et al. describe ‘ring’ neurons that convey visual landmark information to the HD compass system of Drosophila, as well as their functional connectivity. They demonstrate that this connectivity remaps in an experience-dependent manner when flies explore new environments.
- 17. *.Franconville R, Beron C, & Jayaraman V (2018). Building a functional connectome of the Drosophila central complex. Elife, 7, e37017. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Franconville et al. author a detailed review of the neural circuitry within and surrounding the central complex of the Drosophila.
- 18.Gardner RJ, Lu L, Wernle T, Moser MB, & Moser EI (2019). Correlation structure of grid cells is preserved during sleep. Nature neuroscience, 22(4), 598. [DOI] [PubMed] [Google Scholar]
- 19.Green J, Adachi A, Shah KK, Hirokawa JD, Magani PS, & Maimon G (2017). A neural circuit architecture for angular integration in Drosophila. Nature, 546(7656), 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green J, & Maimon G (2018). Building a heading signal from anatomically defined neuron types in the Drosophila central complex. Current opinion in neurobiology, 52, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. **.Green J, Vijayan V, Pires PM, Adachi A, & Maimon G (2019). A neural heading estimate is compared with an internal goal to guide oriented navigation. Nature Neuroscience, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Green et al. manipulate the HD signal stored in the central complex of the Drosophila, while animals attempt to walk in a straight line. They demonstrate that flies respond to disruption of this signal by altering their trajectories, indicating that the HD signal in the central complex is used to guide locomotor behavior.
- 22.Grieves RM, Jedidi-Ayoub S, Mishchanchuk K, Liu A, Renaudineau S, & Jeffery KJ (2019). The place-cell representation of volumetric space in rats. bioRxiv, 698175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. *.Hardcastle K, Maheswaranathan N, Ganguli S, & Giocomo LM (2017). A multiplexed, heterogeneous, and adaptive code for navigation in medial entorhinal cortex. Neuron, 94(2), 375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Hardcastle et al. develop a linear-nonlinear model to characterize multimodal responses in the navigation circuit, and use it to describe the population response of the medial entorhinal cortex.
- 24.Harvey RE, Thompson SM, Sanchez LM, Yoder RM, & Clark BJ (2017). Post-training inactivation of the anterior thalamic nuclei impairs spatial performance on the radial arm maze. Frontiers in neuroscience, 11, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang LW, Simonnet J, Nassar M, Richevaux L, Lofredi R, & Fricker D (2017). Laminar localization and projection-specific properties of presubicular neurons targeting the lateral mammillary nucleus, thalamus, or medial entorhinal cortex. Eneuro, 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. **.Jacob PY, Casali G, Spieser L, Page H, Overington D, & Jeffery K (2017). An independent, landmark-dominated head-direction signal in dysgranular retrosplenial cortex. Nature neuroscience, 20(2), 173. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Jacob et al. identify cells that encode HD but are primarily anchored to vision. They use a visually symmetrical arena to identify these cells; as the result these cells exhibit bidirectional tuning curves and are names “bidirectional” cells. These cells may be at the interface between the HD network and visual pathways.
- 27.Kakaria KS, & de Bivort BL (2017). Ring attractor dynamics emerge from a spiking model of the entire protocerebral bridge. Frontiers in behavioral neuroscience, 11, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SS, Rouault H, Druckmann S, & Jayaraman V (2017). Ring attractor dynamics in the Drosophila central brain. Science, 356(6340), 849–853. [DOI] [PubMed] [Google Scholar]
- 29.Kim M, & Maguire EA (2018). Encoding of 3D head direction information in the human brain. Hippocampus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. **.Kim SS, Hermundstad AM, Romani S, Abbott LF, & Jayaraman V (2019). Generation of stable heading representations in diverse visual scenes. Nature, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Kim et al. describe ‘ring’ neurons that convey visual landmark information to the HD compass system of Drosophila. They use optogenetics activations to induce plasticity between ring neurons and compass neurons. This plasticity likely represents how flies learn to map novel visual setting onto head orientation.
- 31.Kornienko O, Latuske P, Bassler M, Kohler L, & Allen K (2018). Non-rhythmic head-direction cells in the parahippocampal region are not constrained by attractor network dynamics. eLife, 7, e35949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurens J, Kim B, Dickman JD, & Angelaki DE (2016). Gravity orientation tuning in macaque anterior thalamus. Nature neuroscience, 19(12), 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurens J, & Angelaki DE (2018). The brain compass: a perspective on how self-motion updates the head direction cell attractor. Neuron, 97(2), 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurens J, & Angelaki DE (2019). A Model-Based Reassessment of the Three-Dimensional Tuning of Head Direction Cells in Rats. Journal of neurophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurens J, Abrego A, Cham H, Popeney B, Yu Y, Rotem N, … & Angelaki D (2019). Multiplexed code of navigation variables in the anterior limbic system. bioRxiv, 684464. [Google Scholar]
- 36.Mitchell AS, Czajkowski R, Zhang N, Jeffery K, & Nelson AJ (2018). Retrosplenial cortex and its role in spatial cognition. Brain and neuroscience advances, 2, 2398212818757098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nassar M, Simonnet J, Huang LW, Mathon B, Cohen I, Bendels MH, … & Fricker D (2018). Anterior Thalamic Excitation and Feedforward Inhibition of Presubicular Neurons Projecting to Medial Entorhinal Cortex. Journal of Neuroscience, 38(28), 6411–6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page HJ, & Jeffery KJ (2018). Landmark-based updating of the head direction system by retrosplenial cortex: A computational model. Frontiers in Cellular Neuroscience, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Page HJ, Wilson JJ, & Jeffery KJ (2017). A dual-axis rotation rule for updating the head direction cell reference frame during movement in three dimensions. Journal of neurophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park EH, Keeley S, Savin C, Ranck JB Jr, & Fenton AA (2019). How the Internally Organized Direction Sense Is Used to Navigate. Neuron, 101(2), 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peyrache A, Lacroix MM, Petersen PC, & Buzsáki G (2015). Internally organized mechanisms of the head direction sense. Nature neuroscience, 18(4), 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peyrache A, Schieferstein N, & Buzsaki G (2017). Transformation of the head-direction signal into a spatial code. Nature communications, 8(1), 1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peyrache A, Duszkiewicz AJ, Viejo G, & Angeles-Duran S (2019). Thalamocortical processing of the head-direction sense. Progress in neurobiology, 101693. [DOI] [PubMed] [Google Scholar]
- 44. *.Preston-Ferrer P, Coletta S, Frey M, & Burgalossi A (2016). Anatomical organization of presubicular head-direction circuits. Elife, 5, e14592. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Preston-Ferrer et al. study the prominent HDC cell type in the PreS, which are layer III pyramidal neurons. They track their efferent connectivity towards MEC.
- 45.Ranck JB Jnr (1984). Head-direction cells in the deep cell layers of dorsal presubiculum in freely moving rats. In Society for Neuroscience Abstracts (Vol. 10, p. 599). [Google Scholar]
- 46.Redish AD, Elga AN, & Touretzky DS (1996). A coupled attractor model of the rodent head direction system. Network: Computation in Neural Systems, 7(4), 671–685. [Google Scholar]
- 47.Rubin A, Sheintuch L, Brande-Eilat N, Pinchasof O, Rechavi Y, Geva N, & Ziv Y (2019). Revealing neural correlates of behavior without behavioral measurements. Nature communications, 10(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanguinetti-Scheck JI, & Brecht M (2019). Home, head direction stability and grid cell distortion. bioRxiv, 602771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seelig JD, & Jayaraman V (2015). Neural dynamics for landmark orientation and angular path integration. Nature, 521(7551), 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinder ME, & Taube JS (2018). Three-dimensional tuning of head direction cells in rats. Journal of neurophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. *.Simonnet J, Nassar M, Stella F, Cohen I, Mathon B, Boccara CN, … & Fricker D (2017). Activity dependent feedback inhibition may maintain head direction signals in mouse presubiculum. Nature communications, 8, 16032. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Simonnet et al. study the microcircuitry of HDC in the PreS. They demonstrate that layer III pyramidal neurons receive ADN afferents. They also identify two types of interneurons that receive ADN projections, either directly or indirectly, and project to layer III pyramidal neurons. They characterize the response properties of this microcircuitry.
- 52.Simonnet J, & Fricker D (2018). Cellular components and circuitry of the presubiculum and its functional role in the head direction system. Cell and tissue research, 373(3), 541–556. [DOI] [PubMed] [Google Scholar]
- 53.Skaggs WE, Knierim JJ, Kudrimoti HS, & McNaughton BL (1995). A model of the neural basis of the rat’s sense of direction. In Advances in neural information processing systems (pp. 173–180). [PubMed] [Google Scholar]
- 54.Stackman RW, Tullman ML, & Taube JS (2000). Maintenance of rat head direction cell firing during locomotion in the vertical plane. Journal of Neurophysiology, 83(1), 393–405. [DOI] [PubMed] [Google Scholar]
- 55.Stone T, Webb B, Adden A, Weddig NB, Honkanen A, Templin R, … & Heinze S (2017). An anatomically constrained model for path integration in the bee brain. Current Biology, 27(20), 3069–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun X, Mangan M, & Yue S (2018). An analysis of a ring attractor model for cue integration In Conference on Biomimetic and Biohybrid Systems (pp. 459–470). Springer, Cham. [Google Scholar]
- 57.Taube JS, Muller RU, & Ranck JB (1990a). Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. Journal of Neuroscience, 10(2), 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taube JS, Muller RU, & Ranck JB (1990b). Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. Journal of Neuroscience, 10(2), 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taube JS (2007). The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci, 30, 181–207. [DOI] [PubMed] [Google Scholar]
- 60.Tukker JJ, Tang Q, Burgalossi A, & Brecht M (2015). Head-directional tuning and theta modulation of anatomically identified neurons in the presubiculum. Journal of Neuroscience, 35(46), 15391–15395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner-Evans DB, & Jayaraman V (2016). The insect central complex. Current Biology, 26(11), R453–R457. [DOI] [PubMed] [Google Scholar]
- 62. **.Turner-Evans D, Wegener S, Rouault H, Franconville R, Wolff T, Seelig JD, … & Jayaraman V (2017). Angular velocity integration in a fly heading circuit. eLife, 6, e23496. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Turner-Evans et al. study the neuronal circuit that implements a HD attractor in the brain of Drosophila. They demonstrate that this circuitry integrates self-motion signals, in a manner which is remarkably similar to theoretical models of the HDC attractor.
- 63.Turner-Evans DB, Jensen K, Ali S, Paterson T, Sheridan A, Ray RP, … & Jayaraman V (2019). The neuroanatomical ultrastructure and function of a biological ring attractor. bioRxiv, 847152. [Google Scholar]
- 64.Valerio S, & Taube JS (2012). Path integration: how the head direction signal maintains and corrects spatial orientation. Nature neuroscience, 15(10), 1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varga AG, & Ritzmann RE (2016). Cellular basis of head direction and contextual cues in the insect brain. Current Biology, 26(14), 1816–1828. [DOI] [PubMed] [Google Scholar]
- 66.Weiss S, Talhami G, Gofman-Regev X, Rapoport S, Eilam D, & Derdikman D (2017). Consistency of spatial representations in rat entorhinal cortex predicts performance in a reorientation task. Current Biology, 27(23), 3658–3665. [DOI] [PubMed] [Google Scholar]
- 67.Winter SS, Clark BJ, & Taube JS (2015). Disruption of the head direction cell network impairs the parahippocampal grid cell signal. Science, 347(6224), 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yartsev MM, & Ulanovsky N (2013). Representation of three-dimensional space in the hippocampus of flying bats. Science, 340(6130), 367–372. [DOI] [PubMed] [Google Scholar]
- 69.Yoder RM, & Taube JS (2011). Projections to the anterodorsal thalamus and lateral mammillary nuclei arise from different cell populations within the postsubiculum: implications for the control of head direction cells. Hippocampus, 21(10), 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoder RM, Clark BJ, & Taube JS (2011). Origins of landmark encoding in the brain. Trends in neurosciences, 34(11), 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoder RM, & Taube JS (2014). The vestibular contribution to the head direction signal and navigation. Frontiers in integrative neuroscience, 8, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoder RM, Peck JR, & Taube JS (2015). Visual landmark information gains control of the head direction signal at the lateral mammillary nuclei. Journal of Neuroscience, 35(4), 1354–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang K (1996). Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory. Journal of Neuroscience, 16(6), 2112–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]