Abstract
Purpose
Intraoperative optical coherence tomography (iOCT) may facilitate successful transition to descemet membrane endothelial keratoplasty (DMEK) surgery via improved efficiency of tissue orientation. The purpose of this study is to report a large consecutive series of iOCT-assisted DMEK, inclusive of all learning curve cases.
Design
Prospective consecutive case series.
Methods
The Determination of Feasibility of Intraoperative Spectral Domain Microscope Combined/Integrated OCT Visualization During En Face Retinal and Ophthalmic Surgery (DISCOVER) study is a single-site, multi-surgeon, IRB-approved investigational device prospective study. The first 100 consecutive iOCT-assisted DMEK surgeries performed by one attending corneal surgeon (JMG) and six novice surgeons (cornea fellows under supervision) were reviewed. iOCT was utilized for tissue orientation. Patient demographics, tissue characteristics, intraoperative parameters and postoperative complications are reported.
Outcomes
1. Utility of iOCT based on surgeon reporting during surgery. 2. Intraoperative graft unscrolling efficiency. 3. Frequency of post-operative complications.
Results
One hundred eyes of 76 patients were enrolled. Forty-three cases were performed by one staff physician and 57 cases were performed by six cornea fellows. Concurrent phacoemulsifcation with lens implantation was performed in 52 cases (52%). Nine eyes (9%) required rebubbling. Two eyes (2.0%) experienced primary graft failure. One graft failure resulted from surgeon error in interpreting the iOCT. Average unscrolling time was 4.4 ± 4.1 minutes (range: 0.7–27.6 minutes).
Conclusions
iOCT facilitates DMEK orientation without the need for external markings. For novice DMEK surgeons, complication rates and unscrolling times compare favorably with alternative tissue orientation methods.
Introduction
Descemet membrane endothelial keratoplasty (DMEK) provides superior results compared to Descemet stripping automated endothelial keratoplasty (DSAEK) for the treatment of corneal endothelial diseases. Specifically, DMEK has been shown to provide faster visual rehabilitation, better visual acuity, and a lower rate of corneal rejection [1,2,3,4]. Despite its advantages, DMEK requires a surgeon to acquire new skills and may result in in a steep learning curve [3,5,6]. As such, a number of strategies have been developed to improve surgeons’ confidence and success with DMEK surgery.
Intraoperative optical coherence tomography (iOCT) has been previously shown to facilitate DMEK surgery [7,8,9]. iOCT allows real-time visualization and identification of DMEK scroll orientation. iOCT achieves this without potentially harmful external markings (e.g. s-stamp or peripheral notches) and may be helpful in instances of corneal opacity or advanced corneal edema when visualization is compromised [7,8,9].
Although its use has been previously described, there have been no large studies to date reporting the clinical outcomes of intraoperative OCT-assisted DMEK. The purpose of this study is to report the results of a single consecutive series of DMEK surgeries, including all “learning curve” cases. We previously reported our initial experience with eight eyes undergoing DMEK surgery with iOCT that suggested a potential role for iOCT in identification of graft orientation and confirmation of graft placement [9]. Herein, we examine our first 100 cases of iOCT-assisted DMEK surgery performed by either a single attending surgeon (JMG) or cornea fellows under supervision.
Methods
All cases were performed between August 2014 and February 2018 as part of the Determination of feasibility of Intraoperative Spectral domain microscope Combined/integrated OCT Visualization during En face Retinal and ophthalmic surgery (DISCOVER) study, a single-site, prospective multi-surgeon investigational device interventional case series assessing the utility intraoperative OCT for ophthalmic surgery [10]. The study was approved by the Cleveland Clinic Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients prior to enrollment.
Donor Tissue
All donor tissue was provided by Eversight eye bank (Ann Arbor, Michigan, USA). Corneas was “pre-stripped” by a certified eye bank technician, employing a 9.5 mm partial trephination and then stripping Descemet membrane with non-toothed forceps, leaving a small (<20%) undisturbed peripheral “hinge.” For the first twenty cases, “fold-over” micro S-stamping was performed during eye bank preparation as an aid for tissue orientation employing a previously described technique [11]. iOCT was employed as the sole method for tissue orientation for the subsequent eighty cases. All tissue was stored in Optisol GS (Bausch & Lomb, Rochester, New York, USA) prior to surgical use.
Surgical Technique
All surgery was performed under retrobulbar anesthesia based on surgeon preference for patient comfort and akinesia. For cases with concurrent phacoemulsification, dilation was achieved via preoperative topical administration of a compounded solution consisting of 0.5% tropicamide, 0.5% cyclopentolate and 2.5% phenylephrine (our institution’s standard regimen for cataract surgical patients). No intracameral pharmacologic dilating agents were employed. When pupillary dilation was deemed insufficient for cataract surgery, mechanical dilation was achieved with the use of a disposable pupil expansion device.
Healon (Santa Ana, Calif. Johnson & Johnson Surgical Vision, Inc., USA) was the only viscoelastic employed for all cases, owing to its highly cohesive property. After marking the desired graft position on the corneal surface, a reverse Terry-Sinskey hook (Bausch and Lomb, St. Louis, Missouri, USA) was used to strip the host Descemet membrane in a circular diameter approximately 0.25 mm larger than the intended graft diameter. Viscoelastic was removed using irrigation and aspiration and Miochol (Bausch & Lomb, Rochester, New York, USA) was instilled to constrict the pupil. For all cases, a small peripheral iridectomy was created by excising a small piece of iris with Vannas scissors via a 1mm inferior limbal incision.
The pre-stripped tissue was cut by the surgeon using a disposable corneal punch (Moria SA) and then lifted from the stromal bed with tying forceps. The tissue was stained with trypan blue (0.06%, VisionBlue; D.O.R.C. International B.V., Netherlands) for three minutes, rinsed with balanced salt solution, and then drawn into an insertion apparatus consisting of a Straiko Modified Jones Tube (Gunther Weiss Scientific, Portland, Oregon, USA) connected to a 3 cc syringe. The scroll was injected into anterior chamber through a 3.0 mm temporal clear corneal incision which was sutured prior to graft manipulation. Grafts were unscrolled using a “no-touch” technique in which the anterior chamber was kept shallow and the graft is manipulated by external tapping or sweeping gestures.
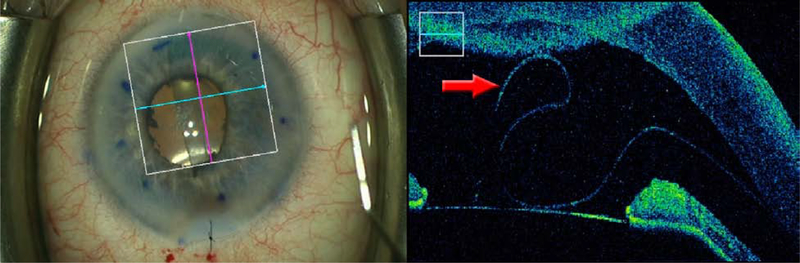
Using a microscope-integrated system (RESCAN 700; Carl Zeiss Meditec, Oberkochen, Germany), iOCT was used during the unscrolling process to verify tissue orientation. iOCT images are viewable via a “heads-up” display in the surgeon’s right ocular or with an external video monitor on the display panel. The iOCT can be controlled with the microscope foot pedal or via an assistant on the video monitor. Tissue orientation was determined by orienting the raster perpendicular to the long axis of the scroll and observing the curling behavior of the tissue margins. Clear evidence of curling toward anterior aspect of the graft (e.g. “scrolls on top”) was considered evidence of correct tissue orientation (Figure 1).
Figure 1.
Representative images demonstrating scrolling behavior of properly oriented grafts. iOCT images are cropped to show only the horizontal raster (blue line). Top Left: Surgeon’s view of a “tight” scroll. Top Right: Classic “double scroll on top” appearance confirming proper graft orientation. Middle Left: Surgeon’s view of a “loose” scroll. Middle Right: iOCT of the unscrolled edge demonstrate s inward scrolling toward the anterior surface of the graft margin, confirming proper graft orientation. Bottom Left: Surgeon’s view of a partially unscrolled graft. The red arrow on the enface view (showing the faint edge of a scroll) corresponds to the overfolded edge of the anterior scroll visible on iOCT (Bottom Right). Proper graft orientation was confirmed during subsequent unscrolling by observing the overfolded edge moving to the surgeon’s right.
After unscrolling, 20% sulfur hexafluoride was injected to attach the graft to the host cornea. Ten minutes were allowed to pass with a complete gas fill with intraocular pressure in the range of 30–40 mm Hg before a gas/fluid exchange was performed. The anterior chamber was left with an approximately 80–90% gas fill to allow passage of aqueous through the peripheral iridectomy to avoid pupillary block. The patient was instructed to remain supine as much as possible during the first 24 hours after the surgery and for four hours daily for the subsequent week. The typical postoperative visits occurred on day one, week one, month one, then every 3 months for the first year.
Surgeon Feedback Assessment and Statistical Analysis
A surgeon feedback questionnaire was completed intraoperatively by the attending surgeon that reviewed overall impact of iOCT on the surgical procedure and perceived value of the imaging to the surgical procedure. Statistical analysis was performed using independent samples t-tests for continuous variables and Fisher’s exact tests for proportions where appropriate with GraphPad InStat Software (GraphPad Software, La Jolla, CA; www.graphpad.com).
Results
One-hundred consecutive eyes of 76 patients, (28 male, 48 female) were enrolled and included in the analysis. Median follow-up duration was 2.0 years (range: 0.9 – 4.5 years). The mean patient age was 67.6 years (range: 49–90). The indications for DMEK in this series were Fuchs endothelial dystrophy (n = 87), failed prior graft (n = 6), pseudophakic bullous keratopathy (n = 5), posterior polymorphous corneal dystrophy (n = 1), and endothelial decompensation from previous refractive surgery (n = 1). No eyes with anterior chamber intraocular lenses, a history of pars plana vitrectomy, or glaucoma drainage devices underwent DMEK surgery in the study.
All surgeries were performed by either a single attending corneal surgeon (n = 43) or by novice surgeons (six cornea fellows under the supervision of the attending surgeon, n = 57). The median number of DMEK cases performed by individual fellows was ten (range 1–15). DMEK was performed in combination with phacoemulsifcation and posterior chamber intraocular lens implantation in 52 cases (52%). Forty six (46%) eyes were pseudophakic prior to DMEK surgery. Two (2%) eyes remained phakic at the time of DMEK surgery.
The mean donor age was 64.8 years (range: 55–75 years). The mean preoperative donor endothelial cell density was 2693 cells/mm2 (range: 2278–3484 cells/mm2). The average implanted graft diameter was 7.7 ± 0.1 mm (range: 7.0–7.75 mm). Twenty (20%) donor grafts (representing the first twenty cases in the series) also received a micro “S-stamp” at the time of preparation. For three (15%) of the cases in which an S-stamp was employed, the visibility of the S-stamp was poor enough (either due to reduced host corneal clarity or poor S-stamp quality) such that iOCT was used exclusively to determine tissue orientation.
In all (100%) cases, the surgeon reported that iOCT provided useful real-time feedback and did not interfere with the surgical procedure in any way. For determining graft orientation, the iOCT image on the attached external video monitor (rather than “heads-up” overlay in the surgeon’s right ocular) was used preferentially in all cases. The external monitor was preferred because the smaller, lower resolution image in the ocular was often insufficient to readily visualize the graft and required dimming the microscope lights to enhance visibility.
Nine eyes (9%) required rebubbling due to postoperative graft maladherence as judged by the attending surgeon (>25% graft detachment or any detachment involving the visual axis). The frequency of rebubbling was 9.5% (4/42) for attending cases and 8.9% (5/56) for fellow cases (Fisher’s exact test, p = 1.00).* The rebubbling rates for S-stamp cases compared to non S–stamp cases were 20.0% (4/20) and 6.4% (5/78), respectively (Fisher’s exact test, p = 0.08). For 3 of the 4 cases in which an S-stamped tissue required rebubbling, the area of detachment was centered around the S-stamp (Figure 2). In the remaining case, the stamp could not be visualized and its location was indeterminate.
Figure 2.
Slit lamp photos one week following uncomplicated DMEK surgery. Left: The graft is detached in the temporal quadrant with focal overlying edema. The area of graft detachment is centered directly over the S-stamp (white arrow) which is faintly visible in the red circle (Right). Inset highlights outline of S-stamp within the center of the focal edema.
Two eyes (2.0%) experienced primary graft failure. One graft failure was attributable to poor tissue quality. In this case, the initial surgery was straightforward including a relatively rapid (<3 minute), atraumatic tissue unscrolling and definitive evidence of proper graft orientation by scrolling behavior on iOCT (confirmed on postoperative video review). After presenting with an attached but edematous graft on postoperative day one, the patient returned with a completely detached free-floating scroll at postoperative week one. The mate of this donor cornea was also used for DMEK surgery and developed large peripheral detachments despite a similarly uncomplicated surgery.
The second graft failure occurred due to an inverted graft as the result of surgeon error in interpreting the iOCT (Figure 3). The inverted graft occurred during a case with a prolonged unscrolling time (>15 minutes) using tissue that not been S-stamped. Although the surgery was initially fellow-performed, worsening visualization prompted the attending surgeon to assume the role of primary surgeon midway through the unscrolling process. While the attending physician was positioned at the microscope oculars, the novice surgeon (viewing the OCT image on the external monitor) incorrectly interpreted the scroll as being properly oriented and the attending physician failed to independently confirm the result. The error was discovered upon review of the surgical video after the graft was noted to be detached at one week postoperatively.
Figure 3:
Left: Surgeon’s view of a faintly visible DMEK scroll. Right: iOCT showing a “scroll on top” however the distal scroll is curling away from the anterior surface (reverse “S-configuration”). This graft was erroneously interpreted as properly oriented leading to primary graft failure.
Surgical videos were available and analyzed for 92 cases (92%).† The average “unscrolling time” (defined as the time from onset of intraocular graft manipulation until full gas fill) was 4.4 ± 4.1 minutes (median: 3.2 minutes, range: 0.5–27.6 minutes). The average unscrolling times for cases which required rebubbling versus those that did not require rebubbling were 5.7 (median = 3.5) ± 8.3 minutes and 4.2 (median = 3.2) ± 4.2 minutes, respectively (two-tailed independent t test, p=0.42).
Discussion
Intraoperative OCT in ophthalmic surgery has gained popularity with the increased availability of microscope-integrated iOCT systems. To date, however, there have been only three case series specifically evaluating the utility of iOCT in DMEK surgery. These reports demonstrated that iOCT was helpful in identifying remnants of Descemet membrane left on the host cornea, localizing the endothelial side of the donor graft, and facilitating tissue orientation when visualization is poor [7,8,9]. These studies, however, were limited by small sample size (26, 14 and 8 patients) and did not report tissue unscrolling times. Additionally, in two of these studies the surgeons had previous DMEK experience [7,8]. Our study represents a single surgeon’s entire longitudinal experience with DMEK surgery, both as primary surgeon and while supervising cornea fellows, and includes all “learning curve” cases (i.e. the attending surgeon’s only previous DMEK experience as primary surgeon was a single successful DMEK surgery performed under supervision via a different technique three years previously).
Overall, iOCT was extremely reliable (99% successful) for DMEK tissue orientation. iOCT is not dependent on direct visualization and therefore less prone to error in cases of reduced anterior chamber visibility (e.g. dense arcus senilis or corneal edema). The graft was easily visualized in all cases, including three cases in which an S-stamp was present but could not be readily identified. The single case of an inverted graft occurred due to surgeon error in iOCT interpretation rather failure of the iOCT itself. The deceptive configuration (Figure 2), an S-shaped folding of the distal scroll occurs infrequently and may confuse the novice surgeon if they are not observing the distal-most aspect of the scroll when assessing orientation. This configuration may also explain anecdotal cases in which surgeons have reported an inconsistency with the “scroll on top” contradicting the unmistakable configuration of a pre-placed orientation mark such as an S-stamp.
Several previous studies have reported the results of techniques to improve DMEK graft orientation. Bachman et al described a marking technique in which three small semicircular trephinations were created in an asymmetric pattern at the graft margin [12]. They reported a 100% success rate (n=25) for achieving correct graft orientation with the marks preventing an inverted graft in 16% of cases. The authors noted that three (12%) of the grafts failed prematurely, likely attributable to excessive tissue manipulation during preparation. Veldman et al reported a 100% success rate (n=133) for S-stamp cases compared to a 90.6% success rate for cases relying on observation of scrolling behavior alone [13]. Of note, however, this study did not include the “learning curve” cases of the primary surgeons and the same institution subsequently reported three cases (out of 371 cases, or 0.8%) in which S-stamped grafts were mistakenly attached upside-down. Although these cases were identified and corrected at the time of initial surgery, each graft demonstrated significantly reduced postoperative endothelial cell counts (51–67% loss) six months postoperatively.
iOCT also facilitated efficient surgery via rapid identification tissue orientation. Sales et al reported an average unscrolling time of 5.7 +/− 3.9 minutes for S-stamped grafts and 6.4 +/− 4.4 minutes for non S-stamped grafts [14]. Our average unscrolling time of 4.4 +/− 4.1 minutes compares favorably, especially when viewed in the context of our data including all “learning curve” cases for the primary surgeon as well as six cornea fellows in training. Similar to Sales et al, our study did not detect a correlation between unscrolling time and the need for postoperative rebubbling.
A recent comprehensive literature review of DMEK surgery outcomes reported a mean re-bubbling rate of 28.8% (range 2.4–82%) across 17 DMEK case series [15]. iOCT was not employed in any of these studies. Our rate of re-bubbling for cases in which iOCT was used as the sole method for graft orientation (n=78, excluding the two cases of primary graft failure) was comparatively low at 6.4%. Saad et al, who reported iOCT in 14 DMEK eyes found a similar re-bubbling rate of 7% [6]. Our rebubbling rate was higher in cases in which the tissue was S-stamped, although this did not reach statistical significance and may be confounded by the preferential usage of S-stamping in the first twenty cases. However, our observation that the S-stamp was frequently contiguous with the area graft of detachment is suggestive of its impact on graft adherence.
Previous DMEK surgeons reported a similar trend toward increased rebubbling rates in S-stamped grafts vs. non S-stamped grafts (13% vs. 3% p = 0.20) although this did not reach statistical significance, possibly due to insufficient sample size [13]. The authors did not report the frequency with which the S-stamp was involved in the detachment. More recent availability of “pre-loaded” DMEK tissue may also render the effects of tissue stamping more impactful. Early data suggests that cell death may be increased in stamped tissue which has been pre-loaded compared to tissue transported on a corneoscleral rim [16]. It should also be noted that the majority of data evaluating the impact of tissue stamping originates from one of the largest, most experienced eye banks with regard to tissue preparation. This data may not extrapolate to less experienced technicians working in smaller eye banks. Larger, multi-center controlled studies would be beneficial to assess the effect of S-stamping on graft attachment and transplant survival.
Our results demonstrate that iOCT-assisted DMEK can provide similar or improved early outcomes for compared to DMEK performed via alternative methods for tissue orientation. However, several limitations of this study must be addressed. The use of S-stamping during the first twenty cases may have positively impacted the learning curve cases. Our experience, however, was that the graft orientation was more rapidly and easily identifiable by iOCT and the S-stamp was only of ancillary benefit. Moreover, our anecdotal observation that stamped grafts demonstrated more postoperative edema and maladherence triggered our decision to discontinue its usage. The lack of postoperative endothelial cell count data prevents a direct comparison, thus we cannot objectively its effect on long-term graft survival.
The extrapolation of our results is also confounded by the primary surgeon’s prior experience with iOCT during DSAEK surgery. However, we observed that novice surgeons and even surgical technicians could quickly and reliably interpret iOCT images, save for the single instance in which the tissue configuration was misidentified. Moreover, despite including the learning curve cases all of surgeons, our results compare favorably to more experienced DMEK surgeons.
A final limitation of using iOCT involves the cost and availability of the system. As iOCT microscopes are new and relatively expensive, many corneal surgeons may not yet have access to this technology. However, as the devices are further refined and the cost of the technology decreases, we believe that more surgeons will gain access to them in the future. Moreover, the use of eye bank stamped tissue is not without cost, although in the United States this cost is frequently passed onto insurers via current reimbursement models. Future health care models may affect this practice and impact the economics of tissue preparation.
This study confirms the potential value of iOCT during DMEK surgery. This technology may facilitate both novice and experienced surgeons in achieving excellent surgical outcomes and minimizing the DMEK learning curve. A randomized controlled trial comparing iOCT-assisted surgery to S-stamp surgery may help to better assess whether there exists any correlation between S-stamping and the frequency of post-operative rebubbling, particularly for tissue prepared by less experienced eye bank technicians. This effect may be more pronounced with the use of preloaded DMEK tissue. Further studies on the role of iOCT in impacting surgeon learning curves should also be considered.
Acknowledgements/Disclosures
A. Funding/Support: NIH/NEI K23-EY022947-01A1 (JPE); Research to Prevent Blindness (Cole Eye Institutional)
Footnotes
D. Conflict of Interest Statement: JPE and SKS have a license agreement with Leica for an external mount system related to intraoperative OCT. None of these relationships are specifically related to the contents of this report. No other specific conflicts of interest exist related to this study for any of the other authors.
- AP: None
- JG: Zeiss (S);
- SKS: Bausch and Lomb (C, R); Bioptigen (P); Allergan (R); Leica (P), Carl Zeiss Meditec (C);
- JPE: Bioptigen/Leica (C, P), Thrombogenics (C, R), Genentech (C, R), Roche (C), Aerpio (C, R), Alcon (C, R), Novartis (C,R), Allergan (C), Allegro (C), Boehringer Ingelheim (R), Regeneron (C,R);
For the purpose of calculating rebubbling rates, the two cases of primary graft failure were excluded.
Although our routine practice is to archive all surgical recordings, archived videos were unavailable for eight non-consecutive cases due to either missing or corrupted data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Melles GR, San Ong T, Ververs B, and van der Wees J. Descemet membrane endothelial keratoplasty (DMEK). Cornea. 2006. September 1;25(8):987–90. [DOI] [PubMed] [Google Scholar]
- 2.Tourtas T, Laaser K, Bachmann BO, et al. Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Am J of Ophthalmol. 2012. June 1;153(6):1082–90. [DOI] [PubMed] [Google Scholar]
- 3.Price MO, Giebel AW, Fairchild KM, et al. : Descemet’s membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology 2009; 116: pp. 2361–2368. [DOI] [PubMed] [Google Scholar]
- 4.Anshu A, Price MO, and Price FW: Risk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplasty. Ophthalmology 2012; 119: pp. 536–540. [DOI] [PubMed] [Google Scholar]
- 5.Ham L, Dapena I, van Luijk C, et al. : Descemet membrane endothelial keratoplasty (DMEK) for Fuchs endothelial dystrophy: review of the first 50 consecutive cases. Eye (Lond) 2009; 23: pp. 1990–1998. [DOI] [PubMed] [Google Scholar]
- 6.Terry MA: Endothelial keratoplasty: why aren’t we all doing Descemet membrane endothelial keratoplasty? Cornea 2012; 31: pp. 469–471 [DOI] [PubMed] [Google Scholar]
- 7.Steven P, Le Blanc C, Velten K, et al. Optimizing descemet membrane endothelial keratoplasty using intraoperative optical coherence tomography. JAMA Ophthalmol. 2013. September 1;131(9):1135–42. [DOI] [PubMed] [Google Scholar]
- 8.Saad A, Guilbert E, Grise-Dulac A, et al. Intraoperative OCT-assisted DMEK: 14 consecutive cases. Cornea. 2015. July 1;34(7):802–7. [DOI] [PubMed] [Google Scholar]
- 9.Cost B, Goshe JM, Srivastava S, and Ehlers JP. Intraoperative Optical Coherence Tomography–Assisted Descemet Membrane Endothelial Keratoplasty in the DISCOVER Study. Am J of Ophthalmol. 2015. September 1;160(3):430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers JP, Modi YS, Pecen PE, et al. The Discover Study 3-Year Results: Feasibility and Usefulness of Microscope-Intergrated Intraoperative OCT during Ophthalmic Surgery. Ophthalmology. 2018. July; 125 (7): 1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veldman PB, Dye PK, Holiman JD, et al. Stamping an S on DMEK donor tissue to prevent upside-down grafts: laboratory validation and detailed preparation technique description. Cornea. 2015. September 1;34(9):1175–8. [DOI] [PubMed] [Google Scholar]
- 12.Bachmann BO, Laaser K, Cursiefen C, and Kruse FE. A method to confirm correct orientation of descemet membrane during descemet membrane endothelial keratoplasty. Am J of Ophthalmol. 2010. June;149(6):922–925. [DOI] [PubMed] [Google Scholar]
- 13.Veldman PB, Dye PK, Holiman JD, et al. The S-stamp in Descemet Membrane Endothelial Keratoplasty Safely Eliminates Upside-down Graft Implantation. Ophthalmology. 2016. January;123(1):161–4. [DOI] [PubMed] [Google Scholar]
- 14.Sales CS, Terry MA, Veldman PB, et al. Relationship between tissue unscrolling time and endothelial cell loss. Cornea. 2016. April;35(4):471–6. [DOI] [PubMed] [Google Scholar]
- 15.Deng SX, Lee WB, Hammersmith KM, et al. Descemet Membrane Endothelial Keratoplasty: Safety and Outcomes: A Report by the American Academy of Ophthalmology. Ophthalmology. 2018. February;125(2):295–310. [DOI] [PubMed] [Google Scholar]
- 16.Newman LR, Tran KD, Odell K, et al. Minimizing Endothelial Cell Loss Caused by Orientation Stamps on Preloaded Descemet Membrane Endothelial Keratoplasty Grafts. Cornea. 2019. February;38(2):233–237. [DOI] [PubMed] [Google Scholar]