Abstract
In recent years, advancement in genetics has profoundly helped to gain a more comprehensive molecular, pathogenic, and prognostic picture of pheochromocytomas and paragangliomas (PPGLs). Newly discovered molecular targets, particularly that targets cell membranes or signaling pathways have helped move nuclear medicine in the forefront of PPGL precision medicine. This is mainly based on the introduction and increasing experience of various PET radiopharmaceuticals across PPGL genotypes quickly followed by implementation of novel radiotherapies and revised imaging algorithms. Particularly, 68Ga-labeled-SSAs have shown excellent results in the diagnosis and staging of PPGLs and in selecting patients for PRRT as a potential alternative to 123/131I-MIBG theranostics. PRRT using 90Y/177Lu-DOTA-SSAs has shown promise for treatment of PPGLs with improvement of clinical symptoms and/or disease control. However, more well-designed prospective studies are required to confirm these findings, in order to fully exploit PRRT’s antitumoral properties to obtain the final FDA approval. Such an approval has recently been obtained for high‐specific-activity 131I-MIBG for inoperable/metastatic PPGL.
The increasing experience and encouraging preliminary results of these radiotherapeutic approaches in PPGLs now raises an important question of how to further integrate them into PPGL management (e.g. monotherapy or in combination with other systemic therapies), carefully taking into account the PPGLs locations, genotypes, and growth rate. Thus, targeted radionuclide therapy (TRT) should preferably be performed at specialized centers with an experienced interdisciplinary team. The future perspectives include the introduction of dosimetry and biomarkers for therapeutic responses for more individualized treatment plans, α-emitting isotopes, combination of TRT with other systemic therapies.
Keywords: pheochromocytoma, paraganglioma, 18F-FDOPA, 68Ga-DOTATATE, 18F-FDG, 131I-MIBG, 123I-MIBG PET/CT, peptide receptor radionuclide therapy, theranostics, somatostatin receptor
Current classification of PPGL
Pheochromocytomas (PHEOs) and paragangliomas (PGLs, together called PPGLs) are neuroendocrine tumors (NETs) arising from neural crest derived adrenal or extra-adrenal paraganglia, respectively. Around 5–10% of solitary pheochromocytomas are hereditary, whereas tumor multiplicity or extra-adrenal tumors are related to currently known germline mutations in 40–70% of patients. These are now recognized to be caused by at least 20 susceptibility genes (Crona, et al. 2017; Dahia 2014; Fishbein, et al. 2017; Jochmanova and Pacak 2018). A majority of PPGLs can be described as carrying either germline, or somatic mutations. Depending on their transcription profile, recent data obtained from The Cancer Genome Atlas (TCGA) has divided PPGL into 3 clinically relevant clusters:
Pseudohypoxia group (cluster 1) is divided into at least two subgroups: the first, tricarboxylic acid (TCA) cycle-related germline mutations mainly in succinate dehydrogenase subunits (SDHx) and fumarate hydratase (FH). The second subgroup includes, von Hippel-Lindau tumor suppressor (VHL)- and endothelial PAS domain protein 1 (EPAS1)-related somatic and germline mutations.
Kinase signaling group (cluster 2) consists of germline or somatic mutations in ret proto-oncogene (RET), neurofibromin 1 (NF1), transmembrane protein 127 (TMEM127), MYC-associated factor X (MAX), and HRas proto-oncogene (HRAS).
Wnt signaling group (cluster 3) includes newly recognized somatic mutations in cold shock domain containing E1 (CSDE1), as well as somatic gene fusions affecting mastermind like transcriptional coactivator 3 (MAML3).
Most importantly from a clinical standpoint, some correlations exist between the gene(s) involved and tumor’s anatomic location:
PHEO: RET, VHL, SDHx, NF1, MAX, TMEM127, HRAS.
Extra-adrenal retroperitoneal PGLs: SDHx, FH, VHL (rare), prolyl hydroxylase 1 and 2 (PHD1/2) and EPAS1/ hypoxia-inducible factor 2 alpha (HIF2A).
Head and neck PGL (HNPGL): SDHx.
Major predictors for hereditary PPGL include a family and personal history of PPGL, inherited in an autosomal dominant manner but with varying penetrance, characteristic syndromic presentation, young age at diagnosis, multifocality, unusual location/s (e.g. heart, urinary bladder), and/or tumor recurrence, particularly in the adrenal gland (Benn et al. 2015, Favier et al. 2015, Jafri, et al. 2013; Neumann, et al. 2009, Neumann et al, 2018, Welander et al. 2011). Renal cell carcinoma (RCC), gastrointestinal stromal tumor (GIST), pituitary adenoma, and rarely, pulmonary chondroma, neuroblastoma, and neuroendocrine neoplasms (usually carcinoid) can also be related to SDHx and rarely to other PPGL susceptibility gene mutations (Casey, et al. 2017; Papathomas, et al. 2014; Pasini, et al. 2008). Other clinical manifestations can also be suggestive of particular gene mutations. For example, prior medullary thyroid cancer (MTC) for RET, café-au-lait spots/neurofibromas for NF1, RCC/hemangioblastomas/pancreatic tumors for VHL, congenital polycythemia and duodenal somatostatinoma for EPAS1/HIF2A (Zhuang, et al. 2012), and RCC/leiomyomas for FH (Castro-Vega, et al. 2014).
Furthermore, PPGL with an underlying SDHB mutation are associated with a higher risk of aggressive behavior than other hereditary PPGLs leading ultimately to death, particularly due to development of metastatic disease. The risk of metastasis in SDHB-related tumors has been estimated to range from 30% to 80% (Amar et al. 2005, Amar et al. 2007, Benn et al. 2006, Brouwers et al. 2006, Gimenez-Roqueplo et al. 2003, Hamidi et al. 2017, King et al. 2011, Neumann et al. 2004, Turkova, et al. 2016). In addition to impacting the distribution of disease, the genomically-distinct subgroups of PPGLs have different patterns of catecholamine secretion and the expression of cell membrane receptors and transporters, which impact their imaging phenotype, particularly the uptake of catecholamines or their precursors (Eisenhofer et al. 1999, Eisenhofer, et al. 2011, Fonte et al. 2012).
68Ga-DOTA-SSAs in PPGL and imaging phenotypes across PPGL subgroups
There are diverse radionuclide imaging techniques available for the diagnosis, staging and follow-up of PPGLs. Beyond their ability to specifically detect and localize PPGLs, these imaging approaches variably characterize these tumors at cellular and molecular levels. More recently, gallium-68 (68Ga)-labeled somatostatin receptor analogs (SSAs) positron emission tomography-computed tomography (PET/CT) is being increasingly performed to detect PPGLs and assess their somatostatin receptor (SSTR) expression which is paramount for PRRT in inoperable or locally aggressive/metastatic disease (Nolting, et al. 2018, Taieb et al. 2019).
In a recent systematic review and meta-analysis, the pooled PPGL detection rate of 68Ga-DOTA-SSA PET/CT in patients with unknown genetic status was 93% (95% confidence interval [CI] 91–95%), which was significantly higher than that of 18F-fluorodihydroxyphenylalanine (18F-FDOPA) PET/CT (80% [95% CI 69–88%]), 18F-fluorodeoxyglucose (18F-FDG) PET/CT (74% [95% CI 46–91%]), and 123/131I-MIBG scintigraphy (38% [95% CI 20–59%], p<0.001 for all) (Han, et al. 2018). This meta-analysis demonstrated the intrinsic value of 68Ga-DOTA-SSA PET/CT in the detection of PPGL even when a physician is not aided by the knowledge of genetic status of the patient. On the contrary, in another meta-analysis (Kan, et al. 2018), 68Ga-DOTA-SSA PET/CT detected more PPGL lesions with germline mutations (did not specify the exact mutation) than 18F-FDG PET/CT (97% vs. 79%), however only 4 studies (pooled patients = 79) included in this meta-analysis used both radiopharmaceuticals (Chang et al. 2016, Janssen et al. 2015, Janssen et al. 2016a, Tan et al. 2015).
In a recent non-comparative study from the Royal North Shore Hospital in Australia, 68Ga-DOTATATE PET/CT was evaluated for initial staging (n=28) and restaging (n=18, including 8 metastatic cases) for PHEOs and for initial staging (n=8) and restaging (n=18, including 4 metastatic cases) for PGLs. Overall, 68Ga-DOTATATE PET/CT had a sensitivity of 84% for PHEO (21/24) and 100% (7/7) for PGL (Gild, et al. 2018). Further, 68Ga-DOTATATE PET/CT resulted in change of management in 50% PHEOs (23/46) and 44% (12/27) PGLs.
In a retrospective mixed cohort study in 12 patients of neural crest tumors (n=11, PPGL; n=1, medullary thyroid carcinoma), per patient and per lesion detection rates of 68Ga-DOTATATE PET/CT was found to be superior than iodine-123 (123I)-labeled metaiodobenzylguanidine (123I-MIBG) SPECT/CT [83% (10/12) vs. 42% (5/12) and 96.7% (29/30) vs. 23.3% (7/30)] (Naji et al. 2011). Further, three of 4 SDHB patients were detected by 68Ga-DOTATATE PET/CT whereas 123I-MIBG SPECT/CT was negative in all SDHB patients (Naji et al. 2011). 68Ga-DOTATATE performed superior to 18F-FDG PET/CT (96.2% vs. 91.4%) in a mixed cohort of 23 (n=10 SDHx mutated) patients and 123/124I-MIBG detected very few lesions (30.4% in 7 patients) (Chang et al. 2016). Also, per lesion detection rate of 68Ga-DOTATATE PET/CT was superior to 123I-MIBG scintigraphy (100 %, 29/27 vs. 6.9%, 2/27) in 10 extra-adrenal PGL patients (Kroiss et al. 2015).
The use of 68Ga-DOTA-SSA, has shown excellent results in localizing PHEOs or PGLs, including primary and/or metastatic PPGLs (Hofman, et al. 2015a, Kroiss, et al. 2015, Naji, et al. 2011, Sharma, et al. 2013, Sharma, et al. 2014, Sharma, et al. 2015).
Head-to-head comparison between 68Ga-DOTA-SSAs and 18F-FDOPA PET/CT has been performed in only 7 studies: one retrospective study from Innsbruck Medical University (68Ga-DOTATOC in 20 patients with unknown genetic background) (Kroiss, et al. 2013), 5 prospective studies from the National Institutes of Health (NIH) (68Ga-DOTATATE in 17 patients with metastatic SDHB-, 22 patients with metastatic sporadic, 20 patients with HNPGLs, 14 patients with polycythemia and PGL syndromes, and 23 patients with SDHD-related PPGL, respectively) (Janssen et al. 2015, Janssen et al. 2016a, Janssen et al. 2016b, Janssen et al. 2017, Jha et al. 2018a) and one prospective study from La Timone University Hospital (68Ga-DOTATATE in 30 patients) (Archier et al. 2016) (Table 1). The separate data for apparently sporadic PHEO could be extracted from one study (Archier et al. 2016) for 10 patients in whom 18F-FDOPA PET/CT showed better patient-based and lesion-based detection rates than 68Ga-DOTA-SSA PET/CT (100% vs. 90% and 94% vs. 81%, respectively). 68Ga-DOTA-SSA PET/CT might be inferior to 18F-FDOPA PET/CT in the detection of small PHEOs (Archier et al. 2016). However, the preliminary study from NIH in apparently sporadic PHEOs shows that 68Ga-DOTA-SSA performs similar to 18F-FDOPA PET/CT (Jha et al. 2019b).
Table 1.
Per lesion and per patient detection rates of 68Ga-DOTA-SSA PET/CT with 18F-FDOPA PET/CT and/or 18F-FDG PET/CT and/or anatomic imaging in various cohorts of PPGL
| Cohort | Authors/Year | Country | Type of study | Gold Standard | |||||
|---|---|---|---|---|---|---|---|---|---|
| (n) | Per patient | Per lesion | Per patient | Per lesion | Per patient | Per lesion | Per patient | Per lesion | |
| Metastatic SDHB-related PPGL | Janssen, et al. 2015 | USA | Prospective single center | Composite of anatomic and functional imaging | 17 | ||||
| n=17 | n=17 | n=17 | n=16 | n=17 | n=17 | n=17 | n=17 | ||
| Sporadic metastatic PPGL | Janssen, et al.2016 | USA | Prospective single center | Composite of anatomic and functional imaging | 22 | ||||
| n=22 | n=22 | n=22 | n=12 | n=22 | n=22 | n=22 | n=22 | ||
| Pediatric SDHx-related PPGL | Jha, et al.2018 | USA | Retrospective single center | Composite of anatomic and functional imaging | 9 | N.A. | |||
| n=8 | n=8 | n=8 | n=8 | n=8 | n=8 | ||||
| Head and neck PGL | Janssen, et al.2016 | USA | Prospective single center | 18F-FDOPA or CT/MRI | 20 | N.A. | N.A. | N.A. | N.A. |
| n=20 | n=20 | n=20 | n=20 | ||||||
| Polycythemia-PGL syndrome | Janssen, et al. 2017 | USA | Prospective single center | Composite of anatomic and functional imaging | 14 | N.A. | N.A. | N.A. | N.A. |
| n=13 | n=14 | n=13 | n=14 | ||||||
| MAX-related PPGL | Taieb, et al. 2018 | USA, France | Histology | 6 | |||||
| n=3 | n=3 | n=3 | n=6 | n=4 | n=4 | n=6 | n=6 | ||
| Mixed cohort | Archier et al. 2016 | France | Histology and composite of anatomic and functional imaging | 30 | N.A. | ||||
| n=30 | n=29 | n=30 | n=29 | n=30 | n=29 | ||||
| Extraadrenal PGL | Kroiss et al. 2013 | Austria | Retrospective single center | Combined cross-sectional imaging | 20 | N.A. | N.A. | ||
| n=20 | n=20 | n=20 | n=20 | ||||||
The study reports mean number of 90Y-DOTATATE PRRT cycles per patient and does not provide the range of cycles. CT/MRI: anatomic imaging with computed tomography and/or magnetic resonance imaging; 18F-FDG: 18F-fluorodeoxyglucose; 18F-FDOPA: 18F-fluorodihydroxyphenylalanine; 68Ga-DOTA-SSA: gallium-68 (68Ga)-labeled somatostatin receptor analogs; MAX: germline mutation of MYC-associated factor X gene; N.A.: not available; PGL: paraganglioma; PPGL: pheochromocytoma/paraganglioma; PET/CT: positron emission tomography-computed tomography; SDHB: germline mutation of succinate dehydrogenase subunit B gene; SDHx: SDHx: germline mutation in one of the succinate dehydrogenase complex genes.
Note: Includes the studies where head-to-head comparison between 68Ga-DOTA-SSA PET/CT and 18F-FDOPA PET/CT was performed. The data from or 18F-FDG PET/CT and/or anatomic imaging was also included if they were available. The reference standard or denominator was heterogeneous among various studies and ranged from histology to composite of anatomical and functional imaging. Even though pathology is considered as gold standard, in patients with metastatic disease biopsy of multiple sites is neither feasible nor ethical. Therefore, adoption of a composite of imaging and/or pathologic findings should be considered a robust alternative (Hofman and Hicks et al. 2015).
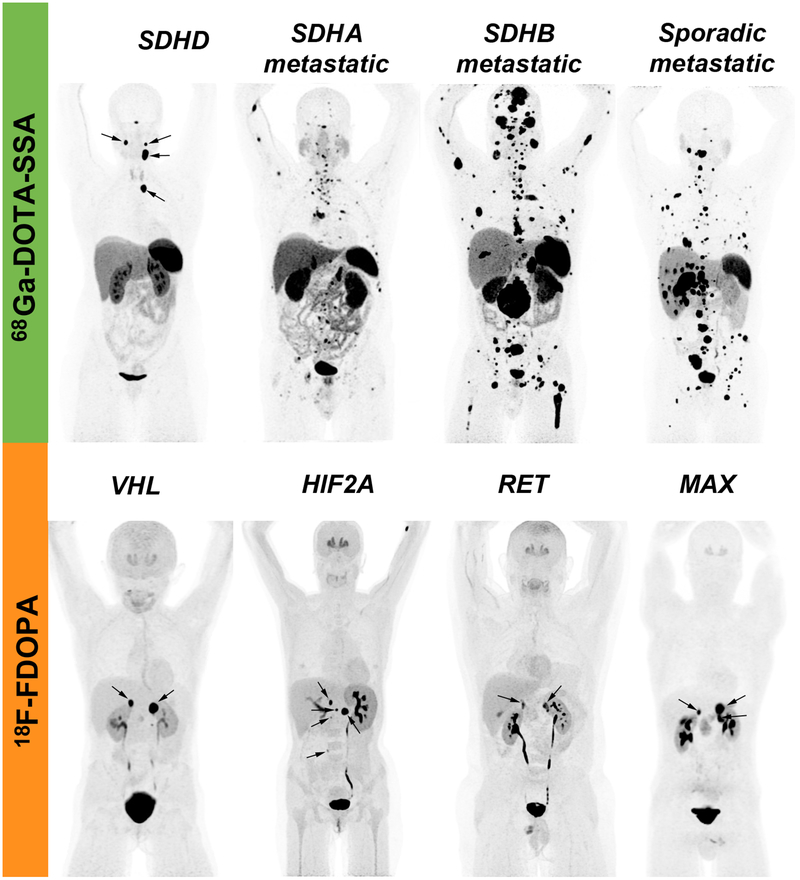
The elevated clinical value of 68Ga-DOTATATE was also observed in the pediatric population with SDHx mutation (Jha, et al. 2018b). 68Ga-DOTA-SSA PET/CT can therefore, be recommended for diagnosis, staging, and follow-up imaging of PPGL with underlying SDHx mutations (Janssen et al. 2015; Jha, et al. 2019a; Jha et al. 2018a; Jha et al. 2018b). Further, it is suggestive that 68Ga-DOTA-SSA PET/CT is the most sensitive tool in the detection of HNPGLs, especially SDHD-related tumors, which may be very small in size and/or fail to sufficiently concentrate 18F-FDOPA (Figure 1). One would consider that detection of additional sites in patients with diffuse metastatic disease is of limited interest. However, many of these lesions are not well-characterized (small nodes) or even detected (bone marrow metastases) by anatomical imaging. The suboptimal value of conventional imaging poses a diagnostic challenge in the evaluation of metastatic PPGL patients. Although assessment of RECIST criteria by cross sectional imaging is currently the referential method, incorporation of PET using SSA as part of a surveillance program seems prudent for accurate evaluation (Kong, et al. 2019). Also, functional imaging can localize subcentimetric new lesions which takes time to appear on cross sectional imaging and hence, can aid in change of treatment earlier in case of progression. Further, in biochemically negative patients, the tumor load can be adequately determined by the functional imaging.
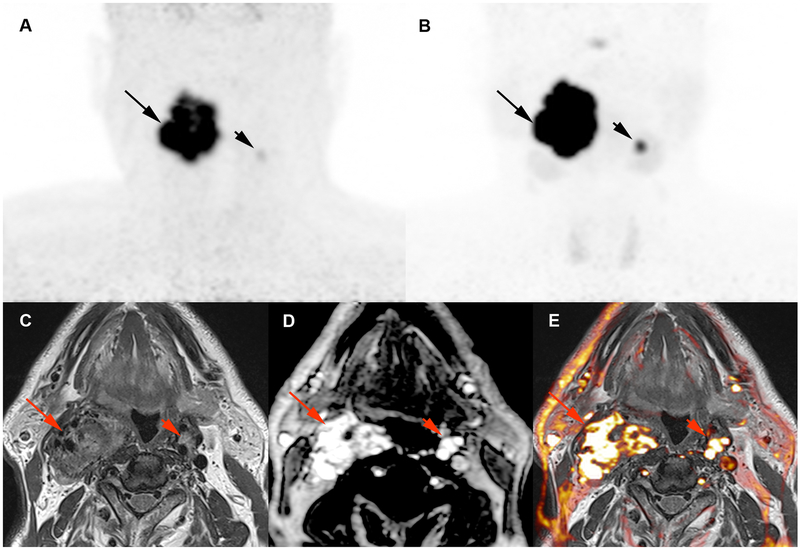
Figure 1. Multifocal SDHD-related vagal PGLs (VPGLs). A.
Craniocervical 18F-FDOPA PET image (maximum intensity projection: MIP) showing the 2 VPGLs (arrows). B. Craniocervical 68Ga-DOTATATE image (MIP) showing 2 VPs (arrows) with higher tumor uptake compared to 18F-FDOPA PET. C-E: magnetic resonance (MR) images (C: T2 weighted image; D: T1-weighted dynamic contrast enhanced (DCE) image; E: T1-weighted DCE image/T2 weighted image fusion image) showing both VPGLs with arterial enhancement pattern on magnetic resonance angiogram images.
Follow-up imaging varies from patient to patient. The more intensive follow-up imaging is required in metastatic patients or patients who are at high-risk to develop metastases. Such high risk-factors have been identified as: SDHB, SDHA germline mutations, ATRX mutation, male sex, patient with large primary tumors (various studies have identified that from >4.5–5 cm to >10 cm), noradrenergic or dopaminergic biochemical phenotype, older age at primary tumor diagnosis (>40–50 years), and high proliferative index (King et al. 2011, Schovanek et al. 2014, Jha et al. 2018, Plouin et al. 2016, Hamidi et al. 2017, Crona et al. 2019, Hescot et al. 2019, Mei et al. 2019). These patients should be followed up indefinitely involving either whole-body CT/MRI and/or functional imaging. All of these patients should receive whole-body CT/MRI at baseline along with whole-body functional PET/CT imaging per algorithm for the staging. Depending upon the severity of disease and therapy (chemotherapy, cold SSA, PRRT), the optimal length of follow-up imaging in these patients should be decided. The patients with high metastatic load (lungs and liver metastases) and/or progressive disease should be imaged every 6–8 months whereas the metastatic patients who are stable or are under wait-and-watch protocol should be imaged every 12-months. At our institution, every patient gets whole-body anatomic and functional follow-up imaging. The information from anatomic and functional imaging along with biochemical results and clinical information is considered during evaluation regarding disease progression. The patients with only primary tumors, follow-up anatomic imaging should occur after 3 months of surgical resection to evaluate residual and/or recurrent tumor. Following no evidence of recurrence/residual disease, repeat follow-up imaging should take every year for first 5-years and then if the patient is free of disease, it can be increased to every 3-years, however, patients should receive yearly biochemical evaluation. The response evaluation using RECIST, PERCIST, and EORTC has been described in solid tumors. However, they have not been validated in PPGL especially PERCIST or EORTC using SSA PET/CT. Further studies in this regard would be warranted. Moreover, metabolic tissue burden can have a significant effect on SUV measurements for PET imaging. So, when using PET response, this effect should be mitigated by normalizing uptake values to a reference tissue (blood pool or liver) (Viglianti et al. 2018). Further, when SSA PET/CT is used to evaluate long-acting SSA therapy, it must be kept in mind that in most cases long-acting SSA therapy increases intensity of uptake (both SUVmax and normalized to liver) within metastases and hence, the results should be interpreted accordingly (Cherk et al. 2018).
The rare mutations: EPAS1 (HIF2A), PHDs, MAX, and FH remain an exception among susceptibility genes since they lead to PPGLs that concentrate less 68Ga-DOTA-SSA in contrast to SDHx-related and other PPGLs, however findings need to be confirmed in a larger group of patients with FH mutation (Darr, et al. 2016; Janssen et al. 2017; Nambuba, et al. 2015; Taieb, et al. 2018). The mechanism for this phenotype is currently largely unexplained. Von Hippel-Lindau syndrome, which is another pseudohypoxia-related disorder but with normal succinate levels, presents with a high 18F-FDOPA uptake in contrast to 18F-FDG uptake that varies from low to high. In the kinase signaling group, 18F-FDOPA is probably the best radiopharmaceutical due to its high tumor uptake together with a limited uptake in the remaining (unaffected) adrenal gland (Taieb et al. 2018). However, data is still limited regarding the value of 68Ga-DOTA-SSA PET/CT in these patients including MEN2 syndrome patients where head-to-head comparison with 18F-FDOPA has to be performed to provide any firm clinical conclusion and recommendation (Figure 2–3). However, for MEN2 patients with positive biochemistry and CT/MRI showing unilateral or bilateral adrenal tumors, functional imaging is not needed.
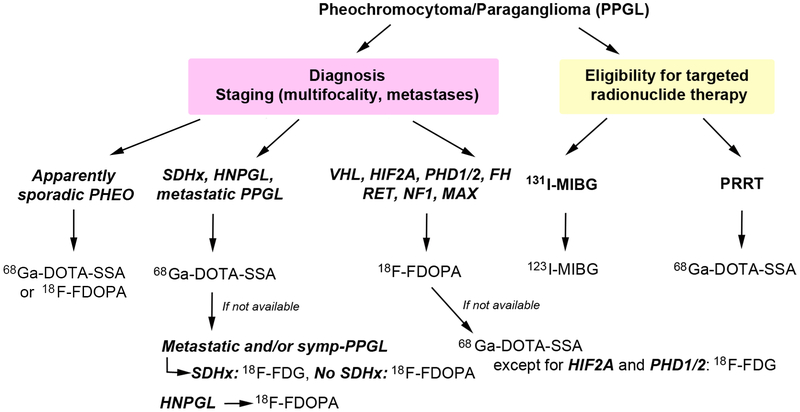
Figure 2. Proposed clinical algorithm for nuclear imaging investigations of pheochromocytoma/paraganglioma patients according to genotype.
The algorithm has been proposed for patients with suspected or confirmed PPGL in order to help towards the appropriate use of PET radiopharmaceuticals across PPGL subtypes. It may suffer from potential limitation of reported small number of cases. The optimal follow-up algorithm for non-proband SDHx-associated PPGLs remains to be determined but mostly relies on annual biochemical screening and MRI at regular intervals.
CT/MRI: anatomic imaging with computed tomography and/or magnetic resonance imaging; 18F-FDG: 18F-fluorodeoxyglucose; 18F-FDOPA: 18F-fluorodihydroxyphenylalanine; FH: fumarate hydratase gene, 68Ga-DOTA-SSA: gallium-68 (68Ga)-labeled somatostatin receptor analogs; HNPGL: head and neck paraganglioma; HIF2A: hypoxia-inducible factor 2 alpha, MAX: germline mutation of MYC-associated factor X gene; NF1: neurofibromatosis 1 gene; N.A.: not available; PHEO: pheochromocytoma; PPGL: pheochromocytoma/paraganglioma; PET/CT: positron emission tomography-computed tomography; PHD1/2: prolyl hydroxylase 1 and 2 gene; RET: rearranged during transfection gene; SDHB: germline mutation of succinate dehydrogenase subunit B gene; SSA: somatostatin analog; SDHx: germline mutation in one of the succinate dehydrogenase complex genes; symp-PPGL: sympathetic PPGL; PRRT: peptide receptor radionuclide therapy.
Figure 3. First-choice PET radiopharmaceuticals across genotypes.
As proposed in Figure 2, when genetic status is unknown and in absence of tumor multifocality or familial trait, 68Ga-DOTA-SSA PET/CT should be proposed as first-line imaging modality in HNPGL, sporadic PHEO, extra-adrenal PGL, and metastatic cases (Janssen et al. 2016a, Janssen et al. 2016b, Jha et al. 2018a, Jha et al. 2018b, Jha et al. 2019a, Jha et al. 2019b, Kroiss et al. 2013, Han et al. 2019). For those associated with mutations in NF1/RET/MAX, 18F-FDOPA PET/CT should be recommended first due to the optimal tumor-to-adrenal uptake ratio that facilitate their detection. However, since 18F-FDOPA is not widely available, this could be replaced by 68Ga-DOTA-SSA or 123I-MIBG depending upon specific technical or legislative limitations across countries. 123I-MIBG is also needed to select potential patients with 131I-MIBG therapy (Figure 2). Many of these reports are based on extremely rare cohorts (HIF2A, PHD1/2, FH, MAX, pediatric SDHx), therefore these studies have extremely small number of patients. However, due to the lack of information on functional imaging modalities available in the scientific literature, it becomes important to report findings obtained from even small study cohort. Also, the reference standard or denominator was heterogeneous among various studies and ranged from histology to composite of anatomical and functional imaging. Even though pathology is considered as gold standard, in patients with metastatic disease biopsy of multiple sites is neither feasible nor ethical. Therefore, adoption of a composite of imaging and/or pathologic findings should be considered a robust alternative (Hofman and Hicks et al. 2015).
More recently, new radiopharmaceuticals have been proposed (124I-MIBG, 18F-MFBG) for targeting norepinephrine transporter and appear superior to 123I-MIBG (Hartung-Knemeyer et al. 2012, Cistaro et al. 2015, Pandit-Taskar et al. 2018).
Theranostics
Personalized (precision) medicine has already made its mark and has the potential to enhance patient management. It consists in adapting healthcare strategies tailored to individual and disease characteristics. Beyond the expected clinical benefits of personalized medicine, theranostics could also have a significant positive economic effect. Nuclear medicine has a central role in personalized medicine via theranostic approaches. The term “theranostics” was coined by John Funkhouser which encapsulates the integration of diagnostic and therapeutic functions within the same pharmaceutical platform (a theranostics pair) (Kelkar and Reineke. 2011). Therefore, results derived from an imaging study based on a compound labeled with a diagnostic radionuclide can precisely determine whether an individual patient is likely to benefit from a specific treatment using the same related compound labeled with a therapeutic radionuclide. Initially, 123/131I-MIBG targeting norepinephrine transporter system and more recently 68Ga/90Y/177Lu-DOTA-SSA analogs targeting peptide (somatostatin) receptors have been introduced as theranostic agents for PPGLs (Nolting, et al. 2018).
131I-MIBG theranostics
In advanced PPGLs, 131I-MIBG treatment is still recommended for 123I-MIBG positive patients. Conventional 123I-MIBG scintigraphy is first used to assess these tumors for the presence of cell membrane norepinephrine transporter system. However, the majority of studies on 131I-MIBG theranostics are retrospective, heterogeneous in terms of baseline patient characteristics, and use of treatment protocols. A large systematic review and meta-analysis of 17 studies comprising 243 patients on 131I-MIBG published between 1984 and 2012 (follow-up duration, 24 to 62 months) showed a complete response, partial response, and stable disease in 3% (95% CI, 6–15%), 27% (95% CI, 19–37%), and 52% (95% CI, 41–62%) of pooled patients, respectively (van Hulsteijn, et al. 2014).
One of the main drawbacks of conventional MIBG preparations is that more than 99% of the MIBG molecules are not labeled with 131I, therefore, possibly competing for norepinephrine transporter binding sites and disrupting norepinephrine reuptake mechanism. This may lead to decreased absorbed dose and possibly increased side effects especially with high-dose administrations (>12mCi/kg) (Gonias et al. 2009, Carrasquillo et al. 2016). Hematotoxicity was the most significant toxicity especially thrombocytopenia (Carrasquillo et al. 2016). Low-dose administrations (149 mCi) were well-tolerated, however, following high-dose administrations (>12mCi/kg), grade 3 or 4 thrombocytopenia and leukopenia was observed in 83–87% of patients, requiring platelet transfusion and growth factors (>12mCi/kg) (Gonias et al. 2009, Carrasquillo et al. 2016). Myelodysplastic syndrome (MDS) and acute leukemias (ALs) have been reported in patients treated with large amounts of 131I-MIBG and chemotherapy (Carrasquillo et al. 2016) with a 4% of MDS incidence with high-dose administrations (Gonias et al. 2009). Other reported toxicities are hypothyroidism (11–20%) even with low-dose administrations, hypogonadism with both low-dose and high-dose administrations, acute respiratory distress syndrome and bronchiolitis obliterans and hypertension with high-dose administrations only (Carrasquillo et al. 2016). Hypertensive crises (despite blocking reuptake of norepinephrine), renal failure, and hepatotoxicity was rarely reported (Carrasquillo et al. 2016). More recently, high‐specific-activity (HSA) 131I-MIBG that consists almost entirely of 131I-MIBG (HSA, ~2,500 mCi/mg, 92,500 MBq/mg) has been developed (Pryma, et al. 2018). In a recent multicentric phase II study by Pryma et al, 68 and 50 PPGL patients received one or two therapeutic doses of HSA 131I-MIBG, respectively (Pryma et al. 2018). All treated patients had disease progression on prior therapy or were ineligible for chemotherapy or other therapy. HSA 131I-MIBG was administrated at 0.3 GBq/kg. Of the 68 patients who received at least one therapeutic dose of HSA 131I-MIBG, 17 (25%; 95% CI, 16–37%) had a durable reduction in baseline antihypertensive medication use. Most patients (92% among the 64 patients) with evaluable disease experienced partial response (23.4%, 15/64) or stable disease (68.7%, 44/64). The median overall survival (OS) was 37 months (95% CI, 31–49 months), however it was 44 months (95% CI, 32–60 months) for patients who received two therapeutic doses of HSA 131I-MIBG versus 18 months (95% CI, 4–31 months) for those who received one dose. Prolonged myelosuppression was the most common cause for patients not receiving the second therapeutic dose. Interestingly, median survival rates for patients with or without lung and/or liver metastases was similar (43 vs. 41 months). No patients had drug‐related acute hypertensive events. Sixty-one patients (90%) experienced hematologic adverse events, which were grade 3 or 4 adverse events or other severe adverse events in 49 (72%) of these patients. Seventeen patients (25%) required hematological supportive care. Treatment‐related serious adverse events included hematologic toxicities (13 patients, 19%), pulmonary embolism in 2 patients (3%), myelodysplastic syndrome in 3 patients (4%). Secondary malignancies included acute myeloid leukemia and acute lymphocytic leukemia in one patient each. HSA 131I-MIBG has recently received FDA approval. Although effective, the use of HSA 131I-MIBG is associated with higher rate of hematologic toxicity which possibly got amplified due to previous cytotoxic therapies together with some individual susceptibilities. However, these side effects should be well considered before recommending it to patients and they need to be informed about the risks when being consented for such a therapeutic option. 123I-MIBG scintigraphy should be used as a theranostic radiopharmaceutical for determining if a patient is eligible for 131I-MIBG therapy and patient-specific dosimetry should be performed to optimize therapy. Although not widely available, the positron emission tomography (PET) equivalent, 124I-MIBG has significant advantages in terms of spatial resolution and its ability to quantify uptake for more reliable dosimetry calculations that would be preferable when HSA 131I-MIBG is considered as a therapeutic option.
PRRT using radiolabeled-somatostatin analogs
PPRT in NET (PPGL excluded)
PRRT is now a well-established treatment option for well-differentiated advanced NETs. The principle of SSTR-directed PRRT is to selectively deliver radiation dose to tumors that overexpress SSTRs with preservation of healthy surrounding tissues. PRRT is based on the administration of SSA labeled with a therapeutic isotope [e.g. 177Lutetium (177Lu), 90Yttrium (90Y)]. Selection of good candidates is based on the use of a companion diagnostic agent corresponding to the same SSA labeled with 111Indium or 68Ga, which is now preferred for molecular imaging of PPGL. PRRT with 177Lu was pioneered by the Erasmus group who demonstrated highly favorable results in a large cohort of NET patients. Despite the impressive results achieved using these approaches, this therapy was initially performed only in academic centers and used on a compassionate basis. This limitation led the results to be scarce, prohibiting the establishment of an evidence base that typically accompanies registration and approval of cancer therapies. This drawback was recently addressed by studies done on midgut NETs following the recent release of NETTER-1 results. In NETTER-1, 177Lu-DOTATATE (4 administrations of 7.4 GBq every 8 weeks) with an augmented dose of 30 mg octreotide long-acting release (LAR) formulation every 4 weeks was compared to 60 mg octreotide LAR only in patients with progressive somatostatin-receptor expressing midgut NETs (ileal in 75% of patients) (Strosberg, et al. 2017). The interim analysis was encouraging for 177Lu-DOTATATE, with an objective response of 18% vs 3% compared to octreotide alone, with median progression-free survival (PFS) not yet reached in 177Lu-DOTATATE group vs 8.4 months using an augmented-dose of octreotide LAR). There was a reduced mortality in the PRRT arm (14 vs. 26 deaths in control arm) which represented a 60% lower estimated risk of death in PRRT arm than in control arm and hazard ratio for death with PRRT vs. control arm being 0.4 (p=0.004). Importantly, for an essentially palliative therapy, the toxicity profile of PRRT was acceptable and quality of life of patients was increased (Strosberg, et al. 2018). In a retrospective study, survival and response rates of addition of cold SSA to PRRT as a combination and/or maintenance therapy were evaluated and compared with PRRT alone in 168 patients of advanced gastroenteropancreatic NETs. The patients were divided into 2 groups: group 1 received PRRT alone (81 patients) and group 2 (87 patients) received PRRT combined with SSA followed by SSA as maintenance therapy or PRRT followed by SSA as maintenance therapy. The median PFS and OS was found to be significantly higher in group 2 (48 and 91 months, respectively) compared with group 1 (27 and 47 months, respectively). This showed the potential role of SSA as a combination and/or maintenance therapy in tumor control of gastroenteropancreatic NETs who underwent PRRT (Yordanova, et al. 2018). These data have provided a valuable impetus for studying the wider application of the 68Ga/177Lu-DOTATATE strategy in the management of NETs and various SSTR expressing tumors.
Prognostic markers for PRRT in NET
The main predictors of poor OS outcome after PRRT are non-specific and include high Ki-67 index (greater than 10%), high uptake on 18F-FDG, involvement of more than two organ systems, local vs. distant metastases, low Karnofsky performance score (≤ 70%), high tumor burden, identified progressive disease after first PRRT, and when PRRT is delivered as salvage therapy after chemotherapy (Bodei et al. 2015b, Delpassand et al. 2014, Ezziddin et al. 2014, Gabriel et al. 2018, Kesavan and Turner 2016, Wolf et al. 2019). In another study, highly elevated maximum standardized uptake values (SUVmax) on pre-therapeutic 68Ga-DOTATOC were associated with tumor shrinkage, reduction of tumor markers, and improved overall clinical condition after PRRT with 90Y-DOTATOC (Oksuz, et al. 2014). Elevated baseline inflammation-based index (IBI) score (derived from serum C-reactive protein and albumin levels) and persistently elevated IBI between PRRT cycles were also associated with inferior PFS and OS in one small retrospective study in metastatic NETs (Black, et al. 2018).
Toxicity profiles of PRRT
Toxicity is limited, especially when using 177Lu due to its lower tissue penetration range compared with 90Y (Table 3).
Table 3.
Beta-emitting radionuclides for targeted radionuclide therapy
| Radionuclide | Half-life (d) | Max energy of emitted particle (Mev) | Path length (mm) Max-Mean |
Useful γ for post-therapy imaging |
|---|---|---|---|---|
| 177Lu | 6.7 | 0.5 | 2.2–0.3 | Yes |
| 90Y | 2.7 | 2.3 | 11.9–2.5 | No |
| 131I | 8 | 0.81 | 2.4–0.3 | Yes |
d: days; 131I: Iodine-131; 177Lu: Lutetium-177; Mev: mega electron volt; mm: millimeter; 90Y: Yttrium-90
There is an extensive experience on safety of PRRT in NET patients and generally it’s found to be well-tolerated. Usually nausea and vomiting are the commonly seen acute side-effects which could primarily be attributed to amino acid infusions concurrently administered with 177Lu-DOTATATE (Strosberg, et al. 2017). Fatigue and abdominal pain can also be accompanied; however, they are usually mild and self-limiting (Strosberg et al. 2017). Hematotoxicity and nephrotoxicity have been commonly identified AEs in patients undergoing PRRT. There have been large patient series that reported grade 3/4 nephrotoxicity ranging from 0% (0/343) with 177Lu (Bergsma, et al. 2016b); 0.3% (20/581) with 177Lu (Brabander, et al. 2017); 1.3% (1/74) with 177Lu (Sabet, et al. 2014); and to 1.5% (12/807) with 90Y, 177Lu, or 90Y+177Lu (Bodei, et al. 2015). Further in 6 patients with a single functional kidney, no acute renal toxicity was reported, however, 2 patients showed reduction of glomerular filtration rate (5.3% and 13.8%, respectively) with 3–5 cycles of 177Lu demonstrating that it is feasible to treat NET patients having single functioning kidney with 177Lu. Moreover, Bergsma et al. also observed that none of the risk factors (hypertension, diabetes, high cumulative injected activity, radiation dose to the kidneys and CTCAE grade) at baseline had a significant effect on renal function over time (Bergsma et al. 2016b), whereas another study recommended clinical screening of patients undergoing PRRT in regards to pre-existing risk factors such as hypertension and diabetes and suggested patients to undergo a thorough dosimetric study and should not receive a BED higher than 28 Gy (Bodei, et al. 2008). The grade 3/4 hematotoxicty reported in large patient series ranged from 9.5% (77/807) with 90Y, 177Lu, or 90Y+177Lu (Bodei et al. 2015); 10% (61/582) with 177Lu (Brabander et al. 2017); 11% (34/320) with 177Lu (Bergsma, et al. 2016a); and to 11.3% (23/208) with 177Lu (Sabet, et al. 2013a). In a meta-analysis comprising 16 studies, short-term myelotoxicity was observed in 10% (221/2104) with PRRT monotherapy. At the same time the grade 3/4 hematotoxicity was observed in 10.2% (7/68) of NET patients treated with 177Lu (Sabet, et al. 2013b) having bone metastases and no hematotoxicty apart from grade 1 anemia in 1 out of 6 NET patients treated with 177Lu (Basu, et al. 2016) having extensive bone marrow involvement shows that PRRT is well-tolerated in this group of patients. Furthermore, MDS or AL were reported to range from 1.4% (3/208) with 177Lu (Sabet et al. 2013a); 2.0% (22/148) with 90Y or 177Lu (Baum, et al. 2018); 2.2% (13/582) with 177Lu (Brabander et al. 2017); and to 1.4% (32/2225) in the meta-analysis comprising 16 studies (Kesavan and Turner 2016). The identified risk factors for hematotoxicity were impaired renal function, low WBC count, extensive tumor mass, high tumor uptake on the scans and/or advanced age, cumulative administered activity (>29.6 GBq) and initial cytopenias, prior number of therapies, prior chemotherapy (alkylating agents), and prior radiotherapy (Bergsma et al. 2016b; Kesavan and Turner 2016; Sabet et al. 2013a). At the same time, a clear correlation between dose and occurrence of therapy related myeloid neoplasms could not be established in a cohort of 34 dosimetry assessed patients (Bodei et al. 2015). Neither the administered radioactive dose nor the type of radionuclide was found to have a significant impact on occurrence of marrow neoplasms suggesting that intrinsic, genetically determined factors may potentially play a critical role in the pathobiology of therapy-related myeloid neoplasms (Bodei et al. 2015; Bodei, et al. 2016). Recently, grade 3/4 hepatotoxicity in NET patients with liver metastases was observed in 3% (20/581) with 177Lu (Brabander et al. 2017) and any hepatotoxicity in 10.8% (10/93) with either of 90Y or 177Lu or 90Y+177Lu based PRRT (Riff, et al. 2015). Also, cardiotoxicity was reported for the first time in a patient with cardiac metastases having normal heart function before therapy, however, he developed new-onset cardiomyopathy following 2 cycles of 177Lu with persistent cardiac dysfunction for 3 years after PRRT (Hendifar, et al. 2018). Also, repeated PRRT with 8 or more cycles having median administered activity of 63.8 GBq (range 52–96.6) was found to be safe and well-tolerated without any life-threatening AEs (grade 4) (Yordanova, et al. 2017). It was also observed that the frequency of nephrotoxicity and hematotoxicity was highest with 90Y followed by 90Y+177Lu and least with 177Lu based PRRT (Bodei et al. 2015). Further, in a prospective trial of 65 GEP-NET patients treated with 4 cycles of 7.8 Gbq 177Lu-octreotate in combination with1650 mg/m2 capecitabine (n=28) and 1500 mg/m2 temozolomide (n=37), the addition of radiosensitizing chemotherapy did not significantly increase the modest reversible hematotoxicity of PRRT (Kesavan, et al. 2014).
Several unidentified individual susceptibilities to radiation-associated disease have also been reported (Bodei, et al. 2015a). PRRT may be associated with renal or hematological (bone marrow) toxicities that can be minimized by adequate precautions and proper and safe dosing (Bergsma, et al. 2018; Bodei, et al. 2014a; Bodei, et al. 2014b; Brabander, et al. 2017).
Although not randomized, the Erasmus group experience also reported a good tumor response rate in patients with SSTR expressing metastatic bronchial and pancreatic NETs with limited severe long-term toxicities, leukemia or myelodysplastic syndrome being observed in 2% of patients (Brabander et al. 2017).
PRRT in PPGL
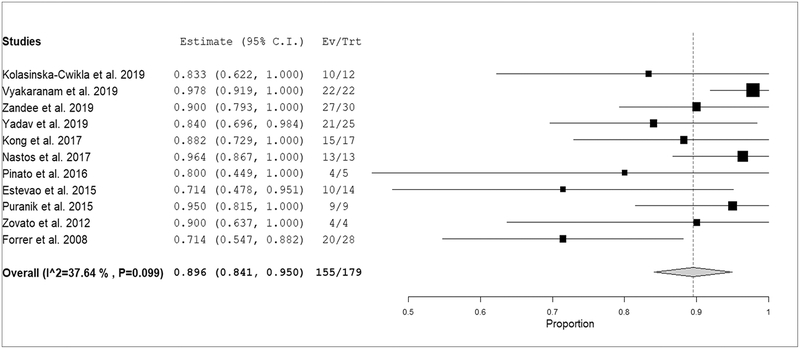
The use of PRRT for patients with PPGLs who had high tumor SSTR expression has mainly been reported in retrospective small case series or case reports (only 3 single center prospective studies) (Table 2). Overall, after PRRT, 89.8% (95% CI: 84.1–95.5%) of pooled patients had achieved disease stabilization/partial response of inoperable/metastatic PPGL with symptomatic improvement in the vast majority of patients (Figure 4–5). No statistically significant heterogeneity among included studies was found (I2 = 37.6%, p=0.099). Importantly, disease status at the time of therapy is not always well described in the studies (Table 2).
Table 2.
Main findings from studies performing peptide receptor radionuclide therapy in patients with inoperable/metastatic pheochromocytoma or paraganglioma (case reports were excluded).
| Authors | Year | Country | Type of study | No. of PPGL patients | Progressive disease at baseline | Radiopharmaceutical | No. of cycles | Concomitant treatment | Data used for response assessment | No. of responders (%) | No. of responders or stable disease (%) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kolasinska-Cwikla | 2019 | Poland | Prospective single centre | 13 | 13 (100%) | 90Y-DOTATATE | *2.5 | - | Morphological | 1/12 (8%) |
10/12 (83%) |
35.0 | 68.0 |
| Vyakaranam et al. | 2019 | Sweden | Retrospective single centre | 22 | 9 (41%) | 177Lu-DOTATATE | 3–11 | - | Morphological, biochemical and clinical data | 2/22 (9%) |
22/22 (100%) |
21.6 | 49.6 |
| Zandee et al. | 2019 | Netherlands | Retrospective single centre | 30 | 20 (67%) | 177Lu-DOTATATE | 4 | - | Morphological and clinical data | 7/30 (23%) |
27/30 (90%) |
30.0 | N.A. |
| Yadav et al. | 2019 | India | Retrospective single centre | 25 | 21 (84%) | 177Lu-DOTATATE | 2–8 | Ch (100%) | SSA PET/CT, morphological, biochemical and clinical data | 7/25 (28%) |
21/25 (84%) |
32.0 | N.A. |
| Kong et al. | 2017 | Australia Israel |
Retrospective bicentric | 20 | 6 (30%) | 177Lu-DOTATATE | 1–4 | Ch (45%) | SSA PET/CT, morphological, biochemical and clinical data | 8/17 (47%) |
15/17 (88%) |
39.0 | N.A. |
| Nastos et al. | 2017 | UK | Retrospective single centre | 13 | 13 (100%) | 177Lu-/90Y-DOTATATE | 1–4 | Ch, RT or SSA in some cases | Morphological, biochemical and clinical data | NA | 13/13 (100%) |
38.5 | 60.8 |
| Pinato et al. | 2016 | UK | Case series | 5 | 5 (100%) | 177Lu-DOTATATE | 1–4 | - | SSA PET/CT and morphological data | 1/5 (20%) |
4/5 (80%) |
17.0 | N.A. |
| Estevao et al. | 2015 | Portugal | Retrospective single centre | 14 | 4 (29%) | 177Lu-DOTATATE | 3 | - | SSA PET/CT and clinical data | 10/14 (71%) |
10/14 (71%) |
N.A. | N.A. |
| Puranik et al. | 2015 | Germany | Prospective single centre | 9 | N.A. | 177Lu-/90Y-DOTATATE/ DOTATOC | 2–4 | - | SSA PET/CT, morphological and clinical data | 4/9 (44%) |
9/9 (100%) |
N.A. | N.A. |
| Zovato et al. | 2012 | Italy | Case series | 4 | 4 (100%) | 177Lu-DOTATATE | 3–5 | - | SSA scintigraphy, morphological and clinical data | 2/4 (50%) |
4/4 (100%) |
N.A. | N.A. |
| Imhof et al.** | 2011 | Switzerland | Prospective single centre | 39 | 39 (100%) | 90Y-DOTATOC | 1–10 | - | SSA scintigraphy, morphological, biochemical and clinical data | 7/39 (18%) |
NA | N.A. | N.A. |
| Forrer et al. | 2008 | Switzerland | Retrospective single centre | 28 | 28 (100%) | 177Lu-/90Y-DOTATOC | 1–4 | - | Morphological, biochemical and clinical data | 7/28 (25%) |
20/28 (71%) |
N.A. | N.A. |
| van Essen et al.*** | 2006 | Netherlands | Retrospective single centre | 12 | 4 (33%) | 177Lu-DOTATATE | 4 | - | Morphological, biochemical and clinical data | 2/11 (18%) |
8/11 (73%) |
N.A. | N.A. |
study not included in the meta-analysis for possible patient overlap with the study of Forrer et al.;
study not included in the meta-analysis for possible patient overlap with the study of Zandee et al.; OS: overall survival; N.A.: not available; PFS: progression-free survival; PPGL: pheochromocytoma/paraganglioma; PRRT: peptide receptor radionuclide therapy; Ch: chemotherapy; PET/CT: positron emission tomography-computed tomography; RT: radiation therapy; SSA: somatostatin analog. Note: response assessment criteria usually included various combinations of imaging (morphological and/or 68Ga-DOTA-SSA PET/CT), biochemical, and clinical data as mentioned in the table.
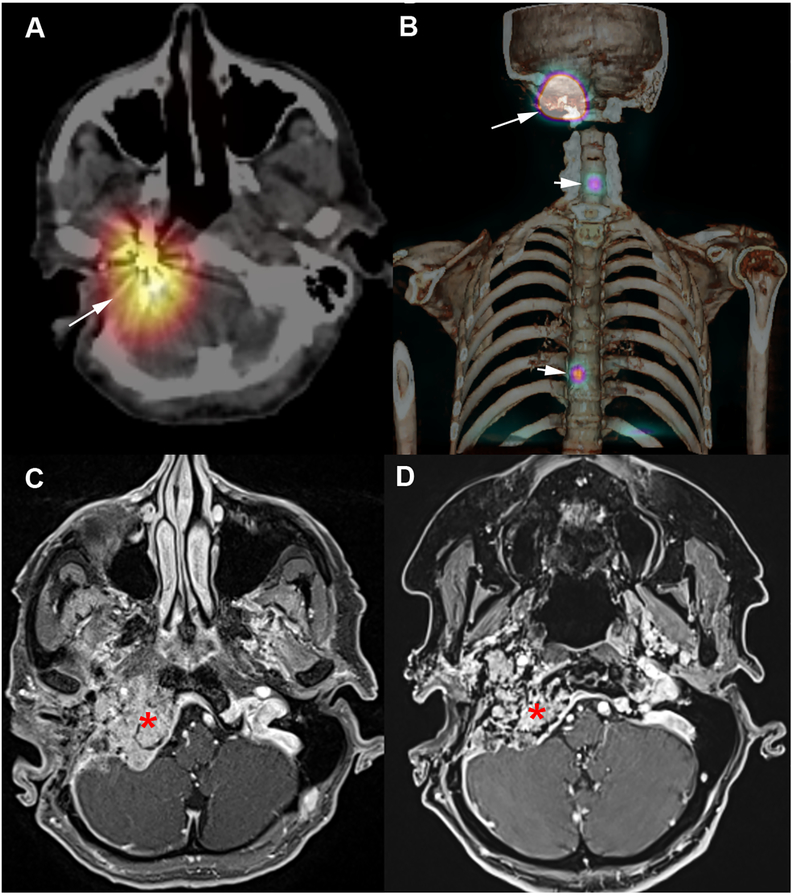
Figure 4. 177Lu-DOTATATE in a SDHB-patient with metastatic jugular paraganglioma.
A-B: Post-therapy SPECT/CT following 1st administration of 177Lu-DOTATATE. A: SPECT/CT fusion images centered over the jugular PGL (long arrow), B: Volume rendering (jugular PGL: long arrow, metastases: short arrows). C: Pre-therapeutic MRI. D: Post-therapeutic MRI (2-months following the 4th cycle of 177Lu-DOTATATE) showing a tumor shrinkage (asterisk).
Figure 5. Proportion of PPGL patients with partial response or stable disease after PRRT.
Plots of individual studies and pooled proportion (with 95% confidence interval) of inoperable/metastatic PPGL patients with response or stable disease after PRRT (random-effects model). The size of the squares indicates the weight of each study. Mild heterogeneity among studies was evident (I-square test = 37.6%, p=0.099).
Treatment response to PRRT has been evaluated in several ways in the included studies as reported in Table 2 (i.e. by using morphological and/or functional and/or clinical data). Nevertheless, we did not find a significant heterogeneity (I2<50%) in the performed meta-analysis about the proportion of response or stable disease after PRRT. Therefore, it is unlikely that the different methods used for evaluating treatment response can influence the pooled results. Even if sometimes combined treatment was performed (as reported in an added column in Table 2) it is unlikely that this has influenced the pooled results on PRRT due to the mild statistical heterogeneity that we have detected among studies.
Van Essen et al., treated 12 patients with metastatic PGLs (5 with head and neck lesions) with 177Lu-DOTATATE. Six patients showed stable disease, 2 patients showed response, and 3 patients showed progressive disease and data was unavailable for 1 patient. However, separate results between tumor locations were not provided (van Essen, et al. 2006). Forrer et al. reported the response rates of PRRT using either 90Y-DOTATOC (25 patients) or 177Lu-DOTATOC (3 patients) in surgically unresectable PPGLs (Forrer, et al. 2008). The authors reported disease stabilization in approximately 50% of the cohort, a response rate which was considered as inferior compared to their own experience in gastroenteropancreatic NETs.
Treatment response may include locoregional control of the primary tumor and complete disappearance of metastases (Gupta, et al. 2014). Zovato et al. had treated 4 inoperable hereditary HNPGLs with 177Lu-DOTATATE (3 to 5 courses) and all had stable disease or partial response documented on SSTR imaging (Zovato, et al. 2012).
Puranik et al., in Bad Barka included 9 patients with inoperable HNPGLs including 2 patients previously treated by radiotherapy 13 months earlier (Puranik, et al. 2015). All tumors had marked avidity for 68Ga-DOTATOC on PET imaging. Eight patients had jugular PGL with tumor multifocality in 4 patients with coexistence of thoracic PGL in 2 patients and thoraco-abdominal PGLs in 1 patient. One patient with a large vagal PGL (and multifocality) had painful bone metastases. The radiopharmaceutical used was either 90Y-DOTATATE (5 patients) or combination of 90Y-DOTATATE and 177Lu-DOTATATE (4 patients). The number of treatment courses ranged from 2 to 4. Based on 8Ga-DOTATOC PET/CT, 4 patients showed partial metabolic response (15–25 % decrease in SUVmax) and the remaining 5 patients showed stable disease (SUVmax in target lesions - 15 % to 25 %). Patients with partial metabolic response had small reductions in tumor size, but insufficient to achieve morphological partial response criteria. No new lesions were found in any patient but they did not describe the clinical course of the disease and it is probable that these tumors had slow or minimal progression prior to entering the study. Symptomatic improvement was observed in all 6 patients and no patients had worsening quality of life. No renal or haematologic toxicity was observed in this series.
Estêvão et al. also reported that 90% of patients with jugulotympanic PGLs had symptomatic improvement or stabilization after PRRT (Estevao, et al. 2015).
Pinato et al. also reported disease stabilization in 3/5, partial response in 1/5, and progressive disease in 1/5 metastatic PGL (5 with bone metastases and 1 with lung metastases), with all being previously documented as progressive disease (Pinato, et al. 2016).
Kong et al. had treated 20 metastatic PPGLs (7 SDHB, 1 SDHD, 2 without SDHx, 10 unknown status) with 177Lu-DOTATATE in two centers (Kong, et al. 2017). Thirteen patients had sympathetic PPGL, 5 had HNPGL and 2 had both sympathetic PPGL and HNPGL. In 9 patients treated at Peter MacCallum Cancer Centre, the second through fourth cycles were given with radiosensitizing chemotherapy (fluorouracil in 3 patients, oral capecitabine in 1 patient, capecitabine/temozolomide in 1 patient and, temozolomide alone in 4 patients) unless contraindicated. Response was assessed on 68Ga-DOTATATE PET/CT imaging using a Krenning-like semiquantitative scale as follows: stable response = unchanged intensity of previous abnormal uptake, partial response = reduction in intensity by 1 Krenning point in at least one tumor site, complete response (total disappearance of abnormal uptake of previously avid lesions), progressive disease (an increase in intensity or extent of previous abnormal uptake or development of new avid lesions). Minor response was also used to describe smaller size changes not meeting partial response criteria on RECIST 1.1 (10% to 30% decrease in maximum diameter of target lesions). Fourteen of 20 patients were treated for uncontrolled secondary hypertension. Among 13/14 with follow-up clinical information, 8 required reduced doses of anti-hypertensive medications. Eight of 13 patients had symptomatic improvement and 2/13 had complete resolution of symptoms at 3 months after PRRT. In 7/20 patients with plasma metanephrine/normetanephrine data, plasma metanephrine and/or normetanephrine decreased in 6/7 patients whereas plasma chromogranin A (CgA) decreased in 12/14 patients with available plasma CgA data. Among the 17/20 patients evaluable for SSTR response, 8 (47%) had a partial response, 7 (41%) had stable disease, and 2 (12%) had disease progression. Of the 14/20 patients with available and evaluable disease by RECIST 1.1, 4 (29%) had partial RECIST response, 1 (7%) had minor morphologic regression, 7 (50%) had stable disease, and 2 (14%) had progression. Median PFS was 39 months; median OS was not reached (5 deaths; median follow-up, 28 months). Four and 2 patients had grade 3 lymphopenia and thrombocytopenia, respectively. Renal impairment in 2 patients were attributed to underlying impaired renal function (1 secondary to systemic amyloidosis, 1 non described). Hypertension flare was observed in one patient, 3 hours after PRRT administration. No additional toxicity was apparent with its concomitant use of radiosensitizing chemotherapy. Further, 4 patients who progressed or deteriorated with tumor progression or had recurrence of hypertension following first cycle of PRRT were re-treated with additional cycles of PRRT ranging from 1 to 8 cycles. Two patients were provided additional maintenance therapies for persisting disease with further favorable response.
Nastos et al. had described the outcomes of 22 patients with progressive/metastatic PPGLs treated with either 131I-MIBG (11 patients), 90Y-DOTATATE (8 patients), 177Lu-DOTATATE (1 patient) or combination of 131I-MIBG with 90Y/177Lu-DOTATATE (2 patients) (Nastos, et al. 2017). They have shown a response in 11 of 11 (100%) treatments with 90Y-DOTATATE alone or in combination and a mean PFS of 43 months. In the subgroup of 15 patients with extradrenal PGLs (21 treatments, from which 13 were with PRRT and 8 with 131I-MIBG), PRRT performed significantly better than 131I-MIBG in terms of OS, PFS, and response to treatment. Four patients (18.1%) developed renal toxicity related to radionuclide treatment, with grade 3 renal toxicity in a patient treated with 90Y-DOTATATE and 131I-MIBG. Although this study mainly relies on the use of 90Y-DOTATATE for PRRT, it illustrates that SSTR-directed targeted radionuclide therapy should therefore be favored over 131I-MIBG in this subgroup of patients.
In a retrospective study by Zandee et al., 30 inoperable or metastatic PPGL patients underwent PRRT with upto 4 cycles of 177Lu-DOTATATE (7.4 GBq/cycle). Twenty of 30 patients treated were progressive at start. According to RECIST 1.1, 7 (23.3%) patients had partial response and 20 (66.7%) patients had stable disease, whereas 3 (10%) patients had progressive disease. In 20 patients with baseline disease progression, tumor control was observed in 17 (85%). Median PFS was 30 months and median OS was not reached (9 deaths; median follow-up, 52.5 months). Grade 3/4 subacute haematotoxicity occurred in 6 (20%) patients. One patient developed MDS after 45 months following 6 cycles of 177Lu-DOTATATE (including retreatment, cumulative dose 44.4 GBq) and died after 4.5 years of first cycle of PRRT due to complications. This patient was not treated with any prior chemotherapy or 131I-MIBG therapy and lacked bone marrow metastases. A reversible subacute adverse event due to cardiac failure following possible catecholamine release/crisis occurred in two patients (6.7%).
In a recently published retrospective study by Vyakaranam et al., 22 patients received 177Lu-DOTATATE [n=3 with 4 cycles of 7.4 GBq and n=19 with dosimetry-guided PRRT where as many cycles (3 to 11) were administered until 23 Gy to the kidneys or 2 Gy to bone marrow was reached] achieving a median OS of 49.6 (range 8.2–139) months and a median PFS of 21.6 (range 6.7–138) months. Nine of 22 patients treated were progressive at start. According to RECIST 1.1, two (9.1%) patients had partial response, 20 (90.9%) patients had stable disease. Amongst 9 progressive patients at the start of PRRT, 1 achieved partial response and 8 stable disease with a median decrease of 14% (range 0 to 56%) in tumor size. The biochemical response was evaluated with a reduction in catecholamines in 11 of 12 evaluable patients (91.6%) with >50% reduction in 3/12 (25%) of patients whereas increase in catecholamines was observed in 1/12 (8.3%) patients. Further, a reduction in CgA was observed in 13 of 15 evaluable patients (86.7%) with >50% reduction in 6/15 (40%) of patients whereas increase in CgA was observed in 2 (13%) patients. Thus, biochemistry indicated progressive disease in two patients with stable disease on CT/MRI but, in the remaining patients, the biochemical and morphological responses were in agreement. Ten of 22 patients had catecholamine-related symptoms, which in two were aggravated during PRRT, including the development of hypertensive crisis. No renal toxicity and only grade 1 (n=10) or grade 2 (n=6) hematological toxicity was reported in 16/22 (73%) patients. High Ki-67 (>15%) and prior PRRT therapy because of progression constituted negative predictive factors for OS whereas only high Ki-67 (>15%) constituted negative predictive factor for PFS.
In a recently published prospective study by Kolasinska-Cwikla et al., 13 inoperable or metastatic SDHx-related PPGL patients (SDHB, n=5; SDHD, n=8) underwent a 2.5 mean cycles of 90Y-DOTATATE (mean 3.4 GBq/cycle). All 13 patients treated were progressive at start. According to RECIST 1.0, after 1-year of PRRT therapy, nine (82%) of 11 patients had stable disease, whereas 2/11 (18%) patients had progressive disease and none of the patients demonstrated partial response. Grade 3 renal toxicity occurred in 2 patients who also demonstrated grade 3 anemia. Median PFS and OS were 35 and 68 months, respectively; however, they were 12 and 25 months, respectively for SDHB patients and did not reach for SDHD patients. The OS was 25 months and PFS was 10 and 12 months for patients with liver and bone metastases, respectively. In long-term follow up (duration unspecified), all SDHB patients and 3 SDHD patients progressed with 4 (80%) deaths in SDHB and 2 (25%) deaths in SDHD patients.
In a retrospective study by Yadav et al., 25 patients received a median of 3 (range: 2–8) cycles of PRRT with 177Lu-DOTATATE and concomitant capecitabine (1250 mg/m2 for 15 consecutive days commencing on the morning of 177Lu-DOTATATE therapy) (Yadav, et al. 2019). According to RECIST 1.1, 7 (28%) patients had partial response, 14 (56%) patients had stable disease, and the remaining 4 (16%) patients had progressive disease. The biochemical response was evaluated with a reduction in CgA observed in 23 (92%) of patients with >50% reduction in 7 (28%) of patients whereas increase in CgA was seen in 2 (8%) patients. The symptomatic response was evaluated by change in anti-hypertensive drugs. Fourteen of 25 patients were on anti-hypertensive drugs and its reduction was observed in 6 (43%) patients whereas no change occurred in 8 (58%) patients. The median PFS was 32 months and the median OS was not reached (7 deaths), however the median OS in progressive group was 16 months (95% CI, 14–24 months). All the patients reported improved general well-being without occurrence of severe hematotoxicity or nephrotoxicity in any of the patients. They concluded that concomitant therapy did not demonstrate superiority in comparison to reported outcomes of published PRRT monotherapy. Furthermore, a case report describing metastatic mediastinal PGL improved by combination of 177Lu-DOTATATE and capecitabine has also been reported (Ashwathanarayana, et al. 2017).
In conclusion, overall results suggest favorable objective disease control, reflected mainly by partial response or stable disease (Figure 5). The rate of objective response on conventional imaging seems to be lower than that for gastroenteropancreatic NETs. One could speculate about possible lower radiosensitivity of PPGL, but this question cannot be answered currently and would require specific prospective evaluation. Patients should undergo both 123I-MIBG and 68Ga-DOTA-SSA imaging and based on the results, the first-line therapy should be chosen (Vyakaranam et al. 2019). In case of mosaic expression of the two tracers, treatment with both can be considered, and if any lesion/s lack uptake of both the tracers, then it can be targeted by additional beam radiation (Vyakaranam et al. 2019). Furthermore, PRRT with combined and/or maintenance cold SSA therapy should be considered as it demonstrated superior results compared to PRRT monotherapy in NETs (Yordanova et al. 2018). However, currently there is no data supporting this in the setting of PPGL. These data have provided a valuable impetus for studying the wider application of the 68Ga/177Lu-DOTATATE strategy in the management of NETs and various SSTR expressing tumors.
Given the favorable efficacy, further prospective PRRT trials, including use of radiosensitizing chemotherapy, are warranted. There is currently an ongoing phase II study at the NIH () that evaluates 177Lu-DOTATATE for metastatic/inoperable SDHx and apparently sporadic PPGLs. Interestingly, inclusion requires progressive disease by RECIST 1.1 with or without symptoms within the last 12 months. The primary objective relies on 6-month PFS. Further, future prospective trials comparing PRRT and HSA 131I-MIBG therapy determining their efficacy and radiation safety profile can be studied.
Potential indications of radiotherapeutic in the management of PPGL
Head and neck PGL
Most of head and neck paragangliomas (HNPGLs) exhibit an indolent behavior and almost always do not secrete catecholamines and hence need not always show symptoms of catecholamines excess. The application of targeted internal radiotherapy should therefore be discussed in limited situations in non-metastatic patients. In large HNPGL, surgical management is often challenging due to complex anatomy and proximity to critical structures. This is particularly true for jugular PGLs arising from the adventitia of the dome of the jugular bulb (glomus jugulare PGLs) that may extend into the pars nervosa of the jugular foramen, jugular vein, sigmoid sinus, carotid artery, middle ear, mastoid space, and posterior cerebellar fossa. In few patients, subtotal surgical resection can be considered for limiting cranial nerve morbidity. Surgery of vagal nerve PGL is also questionable since it always requires the sacrifice of the vagus nerve. In the recent years, various forms of radiotherapy have been reported in these tumors, with favorable outcomes. Vagal nerve PGL is usually related to SDHx mutation (mainly SDHD) in approximately 40% of patients. The impact of detecting multiple HNPGLs, even millimetric in size are of a major clinical importance. It is important to recall that bilateral neurologic injuries affect respiration, deglutition and phonation, and are associated with chronic complications and vital risks (Suarez, et al. 2013). In these patients with tumor multifocality, any post-operative complications may compromise subsequent interventions. The initial staging of an HNPGL is currently based on the use of anatomical and functional imaging approaches to determine the tumor extension into the bone and surrounding soft tissue, to determine tumor multiplicity not only in the head and neck area but also elsewhere in the body, and finally, to exclude any metastases. Although the locoregional extension of HNPGLs is well determined and delineated by anatomic imaging (temporal bone CT, MR angiography), PET imaging using specific tracers performs better for detecting multiple lesions and can increase diagnosis confidence for tumor extensions classified as doubtful on anatomic imaging. Furthermore, previous treatments can lead to artifacts that can seriously degrade the quality of anatomic imaging.
An individual management approach is absolutely necessary in HNPGL patients, especially those with multifocality with vagus nerve involvement. In all patients, a wait-and-scan policy could be the primary option for defining the growth pattern. Patients undergoing such an approach should be informed that many tumors continue to grow, and may eventually require treatment. In head and neck due to the presence of important structures and nerves, at times even a minor growth may lead to symptoms and complications. External beam radiotherapy (EBRT) should be considered as an option in patients with inoperable HNPGL (i.e. unresectability of the primary tumor or tumor relapse due to invasion into surrounding tissue) having progressive and/or symptomatic disease. It has been reported that higher doses are associated with improved local control (Breen, et al. 2018; Vogel, et al. 2014). The advantage of PRRT over radiotherapy is potentially to treat multiple tumors during the same session without any risk of nerve palsy. In patient with a single inoperable HNPGL, the indication of systemic PRRT vs locoregional treatment with EBRT needs to be discussed on an individual basis, depending on potential benefits and estimated risks of both strategies. EBRT can be provided first and PRRT could be given in cases who progress on EBRT. PRRT possibly could act synergistically after debulking surgery of large PGL since it may have more limited effect on very large tumors. In cases of large PGL, PRRT with 90Y only or alternating it with 177Lu to prevent higher renal toxicity can be considered.
Retroperitoneal PPGL
The majority of these patients are clinically symptomatic due to catecholamines excess which may get controlled by surgery, regardless of the surgical approach (open or endoscopic). Although minimally invasive surgery can also be performed in most retroperitoneal PGLs, surgery can be more challenging due to possible adhesions to major retroperitoneal vessels leading to surgical conversions. Therefore, surgical strategy should be tailored to each situation with the knowledge of the genetic status since upto 70% of patients with extraadrenal retroperitoneal PGL may carry germline mutations in SDHx or VHL genes (Amar et al. 2005, Amar et al. 2007, Brouwers et al. 2006, Gimenez-Roqueplo et al. 2003, Neumann et al. 2004). Large PPGLs and those associated to SDHB mutation are associated with a higher risk for metastatic spread and imaging is required to fully localize the extent of disease at whole-body scale. Surgery should always be considered as first-line therapy. In patients with non- or incompletely resectable large PGL, a radiotherapeutic management could be considered (also as complementary therapy after debulking surgery), especially for patients with SDHB mutation which are prone to develop metastases and symptomatic patients (e.g. pain, uncontrolled hypertension). The choice of the therapeutic radiopharmaceutical can be tailored to diagnostic scans using their uptake pattern on companion diagnostics. The use of PRRT using 177Lu-DOTA-SSA will be better suited to SDHx-related PPGL than 131I-MIBG.
Metastatic PPGL
The management of patients with metastatic PPGL can be challenging and may rely on surgery, interventional radiology, chemotherapy, external beam radiation, or other therapeutic options (Nolting, et al. 2018). Primary tumor resection can be recommended based on careful evaluation of tumor burden as well as the extent of metastatic disease. Exercising this option helps to reduce cardiovascular and other such risks from high catecholamine levels and alleviate/prevent symptoms from the tumor’s invasion of surrounding structures. Treatment of metastases with a curative intent to treat may also be considered in individual patients with oligometastatic disease which remains a rare situation. In rapidly progressive metastatic PPGL, patients can be treated with various chemotherapeutic agents. Although there exist various options as well as biases in the literature, currently CVD is still considered as the first line therapeutic modality, especially for rapidly progressive disease (Niemeijer, et al. 2014). In SDHB patients, temozolomide may be considered for tumor stabilization as a maintenance regime subsequent to CVD chemotherapy (Hadoux, et al. 2014). Sunitinib is currently under evaluation (). Palliative surgery followed by external radiotherapy can be indicated in metastatic PPGL with a high risk for neurologic compression (e.g. spinal cord compression). In metastatic PPGL with slow pace of growth and limited tumor volume, 131I-MIBG or PRRT using 177Lu-DOTA-SSA can be indicated in patients with positive scans for the respective radiopharamaceuticals. Additionally, combined 177Lu-DOTA-SSA and cold SSA can be considered in SSTR positive patients and its efficacy can be determined in future trials. However, currently there is no data supporting this in the setting of PPGL. Moreover, patients with tumor phenotype showing both or discordant molecular targets on various lesions may theoretically benefit from a combined or sequential radionuclide therapeutic approach using PRRT and 131I-MIBG, but further trials are required to assess efficacy and potential toxicity of this regimen.
Healthcare environment
Management of PPGL is complex and it is therefore strongly encouraged to refer these patients to experienced centers with high-volume surgical procedures. Core members of the team should include various specialities (oncologists, radiation oncologists, endocrinologists, otorhinolaryngologist/endocrine surgeons, radiologists/nuclear physicians, pediatricians, clinical geneticists, and pathologists) who work in the same center and have meetings on the regular basis. Imaging scans can be performed elsewhere if available and then can be interpreted by this core team.
Research avenues
Dosimetry-based regimens
Dosing of targeted radionuclides is often based on empirical observations and is not subject to the precise, patient-specific treatment planning that is standard for external beam radiotherapy. Based on published studies, it is possible that better outcomes could be achieved with increased cumulative dose delivery to tumors. The main challenge for systemic radiation therapy remains to optimize the dose delivery to a tumor, while minimizing normal tissue irradiation. In therapeutic nuclear medicine, the term “dose” is a misnomer that is often used for describing the administered activity [expressed in units of Becquerel (Bq)] of a radiopharmaceutical. The “absorbed dose” [expressed in units of gray (Gy)] is dependent on many factors including the administered activity but most importantly on tumor characteristics (target expression, metabolic activity, volume, and perfusion). Calculation of the absorbed dose is more complex than for external radiotherapy since the cells and organs are irradiated not only for seconds or minutes, but continuously, over a long period of time with everchanging dose rate. Therefore, it relies on repeated blood/urinary collections and imaging procedures following therapy. Several modern dosimetry models have been developed in order to optimize PRRT for individual patients (Del Prete, et al. 2018; Huizing, et al. 2018; Taieb, et al. 2015). In order to circumvent some difficulties for estimating the absorbed dose by the tumor, two main options have been proposed for achieving radiation dose intensification (Cremonesi, et al. 2018). One is the standard 7.4 GBq per cycle with variation in number of cycles until the individual limit of kidney/bone marrow biologic effective dose is reached (Sandstrom, et al. 2013; Sundlov, et al. 2017) or the other is a 4 fixed-cycles with variable activity per cycle to reach the dose limits (Del Prete, et al. 2017). The time interval between therapeutic cycles could also be tailored to individual situations.
Preliminary results of a trial in NETs (P-PRRT, ) have shown that the use of patient-specific PRRT dosimetry might increase absorbed dose delivered to a tumor (median 1.26-fold; range: 0.47–2.7-fold) compared to fixed-dose regimen, without increased toxicity (oral communication OP-181, Del Prete et al, EANM 2018 congress). A prospective evaluation of PRRT in PPGL patients with integration of a dosimetry-based approach (at voxel level analysis) would be an important step towards treating PPGL patients. Roadmaps to improvements not only include the use of synergistic effects of radiosensitizers but also knowledge regarding the relationships between tumor genotype and cellular sensitivity to ionizing radiations.
Biomarkers of response to PRRT
Therapeutic response to PRRT is often delayed and post-therapy scans or regular 68Ga-DOTA-SSA PET/CT scans often show disease stabilization with some clinical and biological improvement. Furthermore, bone metastases are often detectable only on molecular imaging and not measurable according to CT criteria and therefore not evaluable on RECIST. Assessment of response to PRRT would probably require a specific composite scoring with the integration of tumor uptake as a continuous variable. Beyond classical uptake parameters, it is of prime importance to find new biomarkers of response. One of the great advantages of PET relative to other imaging modalities is its inherent quantitative nature. Traditionally, PET image analysis in the field of oncologic applications has been restricted to the use of semi-quantitative indices, such as SUV or SUV-derived indices. Currently, imaging is ideally placed via radiomics approaches (Lambin et al. 2012, Kumar et al. 2012, O’Connor et al. 2015, Garcia-Carbonero et al. 2015). Over the last few years, a lot of interest has been concentrated on the development of PET image segmentation algorithms allowing the robust and reproducible determination of 3D tumor volumes from PET reconstructed images. As such, the use of PET image derived 3D tumor volumes has in turn increased the potential of further exploring the information available in PET tumor images. Further analysis consists of assessing the PET tumor shape and/or heterogeneity in intratumoral activity. Intratumoral heterogeneity has been found to demonstrate superior prognostic performance in predicting both PFS and OS in 141 patients of NETs (Werner et al. 2016) and OS in 31 patients of pancreatic NETs (Werner et al. 2019). These new parameters that may reflect, depending upon the radiotracer used, various biological processes that could be used for assessement of response to PRRT (Werner et al. 2016, Werner et al. 2019). We can anticipate that combination of radiomics, tumor grade, and molecular biomarkers (e.g., transcriptomics, microRNA, DNA, metabolomics) will offer an optimal multiparametric approach for more individualized management to patients (Bodei, et al. 2018). It is expected that machine learning technology will help to improve parameter selection and combination compared to more traditional statistical analysis, albeit the need for extensive validation and evaluation across centers and patient populations. Together with dosimetry, these biomarkers of treatment response will enable more individualized treatment plans (Werner et al. 2018).
Alpha emitters
Until now, experience with therapeutic applications of nuclear medicine were based on beta-emitting radionuclides. Beta emitters are characterized by a path length in the range of millimeters and a low linear energy transfer (LET) (Navalkissoor et al. 2019). The biological effectiveness of the deposited radiation is inversely correlated with the LET. From a radiobiological standpoint, there are significant advantages of using alpha emitters due to their shorter path length of 0–100 microns and much higher LET (Navalkissoor et al. 2019). As a result, alpha emitters deposit much more energy along their path, resulting in greater cytotoxicity than seen with beta radiation, which manifests itself through mechanisms such as apoptosis, autophagy, necrosis, and mitotic catastrophe (Baidoo et al. 2013, Wadas et al. 2014). Until recently, alpha-emitters were only used in experimental settings. PPGL as many NETs present or develop bone metastases, which may lead to adverse skeletal-related events (SRE) including pain and neurological compression (Ayala-Ramirez et al. 2013). SREs can lead to significant morbidity, impact functional status, and alter patient’s quality of life. The high incidence of osteoblastic metastatic bone disease should allow for the use of bone-seeking radiopharmaceuticals in carefully selected patients who have bone scan-positive lesions. Radium-223 dichloride (223Ra) is a bone-seeking calcium mimetic alpha emitter that accumulates in areas of increased bone turnover and has been approved for the treatment of metastatic prostate cancer to the bone. Following the results of the ALSYMPCA (ALpharadin in SYMptomatic Prostate CAncer) Phase III 223Ra multinational trial (Parker, et al. 2013), the FDA granted full approval in May 2013 with subsequent approval by the European Union. In contrast to other bone-seeking radiopharmaceuticals, 223Ra therapy demonstrates significant survival improvements compared to the placebo. New clinical trials are underway to examine 223Ra efficacy in breast cancer, lung cancer and osteosarcoma. Further studies are necessary to specifically assess the role of 223Ra in palliation of extensive metastatic bone disease in endocrine malignancies such as PPGL, which present with bone lesions in more than 50% of metastatic patients (Zelinka, et al. 2008). A case report has shown an improvement in pain control, mobility, and overall quality of life in a 26-year-old woman with extensive bone disease in a SDHB-related metastatic PGL treated with 223Ra (Makis, et al. 2016).
Alpha-particle emitting radiopharmaceuticals have also be evaluated in small series for intra-arterial (IA) PRRT in patients with liver metastases. IA PRRT resulted in a 4-fold higher intrahepatic tumor accumulation compared to intravenous infusion due to high first-pass extraction, decreasing to 1.3 times greater at 72 hours (Kratochwil, et al. 2011). In a pilot study of patients (in 6 patients) who previously failed treatment with a beta emitter PRRT, IA therapy with 213Bi-DOTATOC for liver metastases produced durable responses with acceptable toxicity in NETs (Kratochwil, et al. 2014). By contrast, systemic administration of alpha-particle emitting SSA would possibly be an interesting approach in PPGL but this would require to perform phase I studies in experienced medical centers.
Recently, strong anti-tumor effects of α-emitting meta-211At-astato-benzylguanidine has been reported in a PC12-cell line mouse xenograft PHEO-bearing mouse model (Ohshima, et al. 2018).
Combined strategy
In the recent years, immunotherapy has gradually gained an important role in oncology. Checkpoint inhibitors (PD-1/PD-L1) have opened up exciting new treatment pathways in metastatic cancers (Galluzzi, et al. 2018). Predictors of response are multiple (e.g. high mutation burden/neoantigen burden, high PD-L1 expression, and presence of pro-immunogenic driver mutations). A recent study has shown that the expression of PD-L1 and extent of immune infiltration of small bowel NETs is heterogeneous (Cives, et al. 2019). The effect of PPGL driver mutations on these parameters and response to immunotherapy would be of particular interest. Immunity can be dissected with large-scale technologies (e.g. mutational landscape, transcriptome, major histocompatibility complex-associated peptidome of tumor cells, T-cell receptor repertoire, presence of tumor-infiltrating lymphocytes and its detailed characteristics, evaluation of cytokines, and microenvironment assessment). For tumors with low tumor mutation burden such as NETs, one approach for increasing treatment efficacy would be to increase mutational/neoantigen load (Chauhan et al. 2018, Lawrence et al. 2013). A preclinical study has shown that PRRT leads to increased infiltration of antigen-presenting cells and natural killer cells into tumors, suggesting a mechanistic role for the inflammatory response followed by increased innate immunity in the action of PRRT (Wu, et al. 2013). The immunotherapy with external beam radiation, including targeted internal therapy could synergistically play a significant role, which needs to be further evaluated. An emerging challenge is how to combine radiation therapy with immunotherapy effectively (Citrin 2017; Serre, et al. 2016). In metastatic PPGL, this approach would have to be powered by various studies showing that indeed PPGL have specific immune targets since they are generally considered as immunogenically cold.
New ligands or targets for PRRT
More recently, there has been an evidence that radiolabeled SSTR2 antagonists generate higher tumor doses and more DNA double-strand breaks than agonists, resulting in better treatment efficacy despite poor internalisation. An open-label study to evaluate safety, tolerability, biodistribution, dosimetry and preliminary efficacy of 177Lu-OPS201 for the therapy of somatostatin receptor-positive NETs is currenty recruiting patients (). In this clinical trial, malignant unresectable PHEO or PGL are also eligible.
An evans blue (EB) modification of DOTA-octreotate, resulted in a radionuclide 177Lu-DOTA-EB-TATE which markedly increased binding to circulating serum albumin, a slower clearance through the urinary tract and thus, an increased half-life in blood was observed thereby increasing tumor retention. In a pilot study by Zhang et al in advanced NET patients, 177Lu-DOTA-EB-TATE demonstrated a 7.9-fold increase in delivered tumor doses when comparable total body effective doses of 177Lu-DOTATATE (0.174 millisievert/megabecquerel (mSv/MBq), 3 patients) and 177Lu-DOTA-EB-TATE (0.205 mSv/MBq, 5 patients) (Zhang, et al. 2018). However, significant increase in dose delivery to the kidneys (3.2-fold) and bone marrow (18.2-fold) were noted in patients receiving 177Lu-DOTA-EB-TATE than those receiving 177Lu-DOTATATE (Zhang et al.2018, JNM). Subsequently, 4 NET patients were treated with single low-dose of 177Lu-DOTA-EB-TATE (0.66 gigabecquerel (GBq), 4 patients) and were compared to a control group undergoing 177Lu-DOTATATE (3.98 GBq, 3 patients) (Wang, et al. 2018). The response was evaluated by assessing difference in SUVmax of 68Ga-DOTATATE before and 3-month after therapy and no difference was found between 177Lu-DOTA-EB-TATE and 177Lu-DOTATATE groups. Further, the 177Lu-DOTA-EB-TATE group approximately received 1/6 of the total radiation exposure compared to 177Lu-DOTATATE group. Also, single low-dose 177Lu-DOTA-EB-TATE was found to be safe and effective in treatment of NETs. These encouraging results merit further studies with increasing dose and frequency of 177Lu-DOTA-EB-TATE in NETs, including PPGL.
With the expanding use of prostate-specific membrane antigen (PSMA) based PET imaging, several reports have shown uptake in nonprostatic benign and malignant entities including PGL of urinary bladder in a patient with metastatic prostate cancer (Parihar, et al. 2018). Since PSMA targeting represents a theranostic approach, it could be interesting to evaluate PSMA expression in a series of PPGLs with various genotypes.
In conclusion, nuclear medicine has gained an increasing role in the personalized approach to PPGL patients. This has been recently augmented by a number of excellent PET radiopharmaceuticals, which target different functional and molecular pathways that often reflect the diverse genetic landscape of PPGL. The visualization and quantification of tumor targets by imaging at the whole-body level (e.g., somatostatin receptors, norepinephrine transporter, and bone turnover), mirroring “in vivo immunohistochemistry”, enabling selection of candidates for potential targeted radionuclide therapy (Lutathera®, Azedra®, and Xofigo®). The very recently obtained FDA approvals in this area has generated interest by various interest groups from researchers to industries and will give a new impetus to the development of therapeutic radiopharmaceuticals. Despite increased availability of diagnostic and therapeutic radiophamaceuticals, it is important to remember that management of PPGL is complex and thepatients should be referred to experienced centers for their management. The future of therapeutic nuclear medicine will also depend upon the identification of new targets, new schedules, and potential synergistic combinations.
Learning points.
On successful completion of this activity, participants should be able to:
Know the molecular taxonomy of pheochromocytoma and/or paraganglioma (PPGL) and the relationship between various genotypes and imaging phenotypes
Describe imaging features of SDHx-related PPGL
Understand pathophysiologic basis of theranostics using iodine-123/131 (123/131I)-metaiodobenzylguanidine (123/131I-MIBG) and peptide receptor radionuclide therapy (177Lu or 90Y-DOTA somatostatin analogs) and know the results of clinical studies
Understand the main areas of improvement of radiotherapeutics in the management of PPGL
Key messages.
Positron emission tomography imaging using gallium-68 (68Ga)-labeled somatostatin receptor analogs (SSAs) is the most sensitive approach for PPGL localization and can select patients for peptide receptor radionuclide therapy (PRRT).
Approximately 234 (n=166, 71% patients with progressive disease) PPGL patients have been treated on compassionate grounds with PRRT and promising results have been reported mostly in the setting of retrospective small case reports or case series.
Following a multicentric phase II study, high-specific-activity iodine-131 (131I)-MIBG (Azedra®) has received FDA approval for metastatic or locally aggressive PPGL.
Potential indications of radiotherapeutics in PPGL include inoperable PPGL with progressive and/or symptomatic metastatic PPGL.
The future perspectives include the introduction of dosimetry and biomarkers for therapeutic responses for more individualized treatment plans, α-emitting isotopes and combination of targeted radionuclide therapy with other forms of treatment, including immunotherapy.
Funding
This research was supported, in part, by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Amar L, Bertherat J, Baudin E, Ajzenberg C, Bressac-de Paillerets B, Chabre O, Chamontin B, Delemer B, Giraud S, Murat A, et al. 2005. Genetic Testing in Pheochromocytoma or Functional Paraganglioma. Journal of Clinical Oncology 23 8812–8818. [DOI] [PubMed] [Google Scholar]
- Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, Bertagna X, Schlumberger M, Jeunemaitre X, Gimenez-Roqueplo AP, et al. 2007. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. Journal of Clinical Endocrinology and Metabolism 92 3822–3828. [DOI] [PubMed] [Google Scholar]
- Archier A, Varoquaux A, Garrigue P, Montava M, Guerin C, Gabriel S, Beschmout E, Morange I, Fakhry N, Castinetti F, et al. 2016. Prospective comparison of (68)Ga-DOTATATE and (18)F-FDOPA PET/CT in patients with various pheochromocytomas and paragangliomas with emphasis on sporadic cases. European journal of nuclear medicine and molecular imaging 43 1248–1257. [DOI] [PubMed] [Google Scholar]
- Ashwathanarayana AG, Biswal CK, Sood A, Parihar AS, Kapoor R & Mittal BR 2017. Imaging-Guided Use of Combined (177)Lu-DOTATATE and Capecitabine Therapy in Metastatic Mediastinal Paraganglioma. J Nucl Med Technol 45 314–316. [DOI] [PubMed] [Google Scholar]
- Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, Busaidy N, Cote GJ, Perrier N, Phan A et al. 2011. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. Journal of Clinical Endocrinology and Metabolism. 96 717–725. [DOI] [PubMed] [Google Scholar]
- Baidoo KE, Yong K, Brechbiel MW. 2013. Molecular pathways: targeted α-particle radiation therapy. Clin Cancer Res. 19 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Ranade R & Thapa P 2016. Metastatic Neuroendocrine Tumor with Extensive Bone Marrow Involvement at Diagnosis: Evaluation of Response and Hematological Toxicity Profile of PRRT with (177)Lu-DOTATATE. World journal of nuclear medicine 15 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum RP, Kulkarni HR, Singh A, Kaemmerer D, Mueller D, Prasad V, Hommann M, Robiller FC, Niepsch K, Franz H, et al. 2018. Results and adverse events of personalized peptide receptor radionuclide therapy with (90)Yttrium and (177)Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 9 16932–16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, Croxson M, Dahia PL, Elston M, Grimm O, et al. 2006. Clinical Presentation and Penetrance of Pheochromocytoma/Paraganglioma Syndromes. Journal of Clinical Endocrinology and Metabolism 91 827–836. [DOI] [PubMed] [Google Scholar]
- Benn DE, Robinson BG & Clifton-Bligh RJ 2015. 15 years of paraganglioma: clinical manifestations of paraganglioma syndromes types 1–5. Endocrine-Related Cancer 22 T91–T103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma H, Konijnenberg MW, Kam BL, Teunissen JJ, Kooij PP, de Herder WW, Franssen GJ, van Eijck CH, Krenning EP & Kwekkeboom DJ 2016a. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging 43 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma H, Konijnenberg MW, van der Zwan WA, Kam BL, Teunissen JJ, Kooij PP, Mauff KA, Krenning EP & Kwekkeboom DJ 2016b. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging 43 1802–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma H, van Lom K, Raaijmakers M, Konijnenberg M, Kam B, Teunissen JJM, de Herder WW, Krenning EP & Kwekkeboom DJ 2018. Persistent Hematologic Dysfunction after Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE: Incidence, Course, and Predicting Factors in Patients with Gastroenteropancreatic Neuroendocrine Tumors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 59 452–458. [DOI] [PubMed] [Google Scholar]
- Black JRM, Atkinson SR, Singh A, Evans J & Sharma R 2018. The inflammation-based index can predict response and improve patient selection in NETs treated with PRRT: a pilot study. J Clin Endocrinol Metab 104 285–292 [DOI] [PubMed] [Google Scholar]
- Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, Baio SM, Sansovini M & Paganelli G 2008. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging 35 1847–1856. [DOI] [PubMed] [Google Scholar]
- Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, Lepensky C, Kwekkeboom DJ, Baum RP, Krenning EP, et al. 2015. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging 42 5–19. [DOI] [PubMed] [Google Scholar]
- Bodei L, Cremonesi M, Kidd M, Grana CM, Severi S, Modlin IM & Paganelli G 2014a. Peptide receptor radionuclide therapy for advanced neuroendocrine tumors. Thorac Surg Clin 24 333–349. [DOI] [PubMed] [Google Scholar]
- Bodei L, Cremonesi M & Paganelli G 2014b. Yttrium-based therapy for neuroendocrine tumors. PET Clin 9 71–82. [DOI] [PubMed] [Google Scholar]
- Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, Lepensky C, Kwekkeboom DJ, Baum RP, Krenning EP, et al. 2015a. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. European journal of nuclear medicine and molecular imaging 42 5–19. [DOI] [PubMed] [Google Scholar]
- Bodei L, Kidd M, Prasad V & Modlin IM 2015b. Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors. Front Horm Res 44 198–215. [DOI] [PubMed] [Google Scholar]
- Bodei L, Modlin IM, Luster M, Forrer F, Cremonesi M, Hicks RJ, Ezziddin S, Kidd M & Chiti A 2016. Myeloid neoplasms after chemotherapy and PRRT: myth and reality. Endocr Relat Cancer 23 C1–7. [DOI] [PubMed] [Google Scholar]
- Bodei L, Kidd MS, Singh A, van der Zwan WA, Severi S, Drozdov IA, Cwikla J, Baum RP, Kwekkeboom DJ, Paganelli G, et al. 2018. PRRT genomic signature in blood for prediction of (177)Lu-octreotate efficacy. European journal of nuclear medicine and molecular imaging 45 1155–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, van Eijck CHJ, Franssen GJH, Krenning EP & Kwekkeboom DJ 2017. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res 23 4617–4624. [DOI] [PubMed] [Google Scholar]
- Breen W, Bancos I, Young WF Jr., Bible KC, Laack NN, Foote RL & Hallemeier CL 2018. External beam radiation therapy for advanced/unresectable malignant paraganglioma and pheochromocytoma. Adv Radiat Oncol 3 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers FM, Eisenhofer G, Tao JJ, Kant JA, Adams KT, Linehan WM, Pacak K. 2006. High frequency of SDHB germline mutations in patients with malignant catecholamine-producingparagangliomas: implications for genetic testing. Journal of Clinical Endocrinology and Metabolism 91 4505–4509. [DOI] [PubMed] [Google Scholar]
- Carrasquillo JA, Pandit-Taskar N, Chen CC. I-131 Metaiodobenzylguanidine Therapy of Pheochromocytoma and Paraganglioma. Semin Nucl Med. 46 203–14 [DOI] [PubMed] [Google Scholar]
- Casey RT, Warren AY, Martin JE, Challis BG, Rattenberry E, Whitworth J, Andrews KA, Roberts T, Clark GR, West H, et al. 2017. Clinical and Molecular Features of Renal and Pheochromocytoma/Paraganglioma Tumor Association Syndrome (RAPTAS): Case Series and Literature Review. J Clin Endocrinol Metab 102 4013–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Vega LJ, Buffet A, De Cubas AA, Cascon A, Menara M, Khalifa E, Amar L, Azriel S, Bourdeau I, Chabre O, et al. 2014. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Human molecular genetics 23 2440–2446. [DOI] [PubMed] [Google Scholar]
- Chang CA, Pattison DA, Tothill RW, Kong G, Akhurst TJ, Hicks RJ & Hofman MS 2016. (68)Ga-DOTATATE and (18)F-FDG PET/CT in Paraganglioma and Pheochromocytoma: utility, patterns and heterogeneity. Cancer Imaging 16 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Horn M, Magee G, Hodges K, Evers M, Arnold S, Anthony L. 2018. Immune checkpoint inhibitors in neuroendocrine tumors: A single institution experience with review of literature. Oncotarget. 9 8801–8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherk MH, Kong G, Hicks RJ, Hofman MS. 2018. Changes in biodistribution on 68Ga-DOTA- Octreotate PET/CT after long acting somatostatin analogue therapy in neuroendocrine tumour patients may result in pseudoprogression. Cancer Imaging 18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cistaro A, Quartuccio N, Caobelli F, Piccardo A, Paratore R, Coppolino P, Sperandeo A, Arnone G, Ficola U. 2015. 124I-MIBG: a new promising positron-emitting radiopharmaceutical for the evaluation of neuroblastoma. Nuclear Medicine Review 18 102–106. [DOI] [PubMed] [Google Scholar]
- Citrin DE 2017. Recent Developments in Radiotherapy. The New England journal of medicine 377 1065–1075. [DOI] [PubMed] [Google Scholar]
- Cives M, Strosberg J, Al Diffalha S & Coppola D 2019. Analysis of the immune landscape of small bowel neuroendocrine tumors. Endocr Relat Cancer 26 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremonesi M, Ferrari ME, Bodei L, Chiesa C, Sarnelli A, Garibaldi C, Pacilio M, Strigari L, Summers PE, Orecchia R, et al. 2018. Correlation of dose with toxicity and tumour response to (90)Y- and (177)Lu-PRRT provides the basis for optimization through individualized treatment planning. European journal of nuclear medicine and molecular imaging 45 2426–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crona J, Taieb D & Pacak K 2017. New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification. Endocr Rev 38 489–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crona J, Lamarca A, Ghosal S, Welin S, Skogseid B, Pacak K. Genotype-phenotype correlations in pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2019;pii: ERC-19–0024.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahia PL 2014. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nature reviews. Cancer 14 108–119. [DOI] [PubMed] [Google Scholar]
- Darr R, Nambuba J, Del Rivero J, Janssen I, Merino M, Todorovic M, Balint B, Jochmanova I, Prchal JT, Lechan RM, et al. 2016. Novel insights into the polycythemia-paraganglioma-somatostatinoma syndrome. Endocr Relat Cancer 23 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete M, Arsenault F, Saighi N, Zhao W, Buteau FA, Celler A & Beauregard JM 2018. Accuracy and reproducibility of simplified QSPECT dosimetry for personalized (177)Lu-octreotate PRRT. EJNMMI Phys 5 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete M, Buteau FA & Beauregard JM 2017. Personalized (177)Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: a simulation study. European journal of nuclear medicine and molecular imaging 44 1490–1500. [DOI] [PubMed] [Google Scholar]
- Delpassand ES, Samarghandi A, Zamanian S, Wolin EM, Hamiditabar M, Espenan GD, Erion JL, O’Dorisio TM, Kvols LK, Simon J, et al. 2014. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas 43 518–525. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Lenders JW, Linehan WM, Walther MM, Goldstein DS, Keiser HR. 1999. Plasma Normetanephrine and Metanephrine for Detecting Pheochromocytoma in von Hippel–Lindau Disease and Multiple Endocrine Neoplasia Type 2. The New England journal of medicine 340 1872–1879. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Pacak K, Huynh TT, Qin N, Bratslavsky G, Linehan WM, Mannelli M, Friberg P, Grebe SK, Timmers HJ, et al. 2011. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer 18 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevao R, Duarte H, Lopes F, Fernandes J & Monteiro E 2015. Peptide receptor radionuclide therapy in head and neck paragangliomas - Report of 14 cases. Rev Laryngol Otol Rhinol (Bord) 136 155–158. [PubMed] [Google Scholar]
- Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grunwald F, Guhlke S, Biersack HJ & Sabet A 2014. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 55 183–190. [DOI] [PubMed] [Google Scholar]
- Favier J, Amar L & Gimenez-Roqueplo AP 2015. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nature Reviews. Endocrinology 11 101–111. [DOI] [PubMed] [Google Scholar]
- Fishbein L, Leshchiner I, Walter V, Danilova L, Robertson AG, Johnson AR, Lichtenberg TM, Murray BA, Ghayee HK, Else T, et al. 2017. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 31 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonte JS, Robles JF, Chen CC, Reynolds J, Whatley M, Ling A, Mercado-Asis LB, Adams KT, Martucci V, Fojo T et al. 2012. False-negative 123I-MIBG SPECT is most commonly found in SDHB-related pheochromocytoma or paraganglioma with high frequency to develop metastatic disease. Endocrine-Related Cancer 19 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrer F, Riedweg I, Maecke HR & Mueller-Brand J 2008. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q J Nucl Med Mol Imaging 52 334–340. [PubMed] [Google Scholar]
- Gabriel M, Nilica B, Kaiser B & Virgolini IJ 2018. Twelve-year Follow-up after Peptide Receptor Radionuclide Therapy (PRRT). Journal of nuclear medicine : official publication, Society of Nuclear Medicine. doi: 10.2967/jnumed.118.215376 [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Chan TA, Kroemer G, Wolchok JD & Lopez-Soto A 2018. The hallmarks of successful anticancer immunotherapy. Sci Transl Med 10. doi: 10.1126/scitranslmed.aat7807 [DOI] [PubMed] [Google Scholar]
- Garcia-Carbonero R, Garcia-Figueiras R, Carmona-Bayonas A, Sevilla I, Teule A, Quindos M, Grande E, Capdevila J, Aller J, Arbizu J, et al. 2015. Imaging approaches to assess the therapeutic response of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): current perspectives and future trends of an exciting field in development. Cancer and Metastasis Reviews 34 823–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gild ML, Naik N, Hoang J, Hsiao E, McGrath RT, Sywak M, Sidhu S, Delbridge LW, Robinson BG, Schembri G, et al. 2018. Role of DOTATATE-PET/CT in preoperative assessment of phaeochromocytoma and paragangliomas. Clinical endocrinology. doi: 10.1111/cen.13737. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, Khau Van Kien P, Corvol P, Plouin PF, Jeunemaitre X 2003. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant pheochromocytomas. Cancer Research 635615–5621. [PubMed] [Google Scholar]
- Gonias S, Goldsby R, Matthay KK, Hawkins R, Price D, Huberty J, Damon L, Linker C, Sznewajs A, Shiboski S, et al. 2009. Phase II Study of High-Dose [131I] Metaiodobenzylguanidine Therapy for Patients With Metastatic Pheochromocytoma and Paraganglioma. J Clin Oncol 27 4162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Singla S, Karunanithi S, Damle N & Bal C 2014. Peptide receptor radionuclide therapy with (177)Lu DOTATATE in a case of recurrent carotid body paraganglioma with spinal metastases. Clinical nuclear medicine 39 440–441. [DOI] [PubMed] [Google Scholar]
- Hadoux J, Favier J, Scoazec JY, Leboulleux S, Al Ghuzlan A, Caramella C, Deandreis D, Borget I, Loriot C, Chougnet C, et al. 2014. SDHB mutations are associated with response to temozolomide in patients with metastatic pheochromocytoma or paraganglioma. Int J Cancer 135 2711–2720. [DOI] [PubMed] [Google Scholar]
- Hamidi O, Young WF Jr, Iniguez-Ariza NM, Kittah NE, Gruber L, Bancos C, Tamhane S & Bancos I & Bancos I 2017. Malignant pheochromocytoma and paraganglioma 2017 malignant pheochromocytoma and paraganglioma: 272 patients over 55 years. Journal of Clinical Endocrinology and Metabolism 102 3296–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Suh CH, Woo S, Kim YJ & Lee JJ 2019. Performance of (68)Ga-DOTA-Conjugated Somatostatin Receptor Targeting Peptide PET in Detection of Pheochromocytoma and Paraganglioma: A Systematic Review and Meta-Analysis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 60 369–376. [DOI] [PubMed] [Google Scholar]
- Hartung-Knemeyer V, Rosenbaum-Krumme S, Buchbender C, Pöppel T, Brandau W, Jentzen W, Antoch G, Forsting M, Bockisch A, Kühl H, et al. 2012. Malignant Pheochromocytoma Imaging with [124I]mIBG PET/MR. Journal of Clinical Endocrinology and Metabolism 97 3833–3834. [DOI] [PubMed] [Google Scholar]
- Hartung-Knemeyer V, Rosenbaum-Krumme S, Buchbender C, Pöppel T, Brandau W, Jentzen W, Antoch G, Forsting M, Bockisch A, Kühl H, et al. 2012. Malignant Pheochromocytoma Imaging with [124I] mIBG PET/MR. Journal of Clinical Endocrinology and Metabolism 97 3833–3834. [DOI] [PubMed] [Google Scholar]
- Crona J, Lamarca A, Ghosal S, Welin S, Skogseid B, Pacak K. Genotype-phenotype correlations in pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2019;pii: ERC-19–0024.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendifar AE, Delpassand ES, Kittleson MM & Tuli R 2018. Cardiac Toxicity in a Patient Receiving Peptide Receptor Radionuclide Therapy. Pancreas 47 e55–e56. [DOI] [PubMed] [Google Scholar]
- Hofman MS, Lau WF & Hicks RJ 2015. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics : a review publication of the Radiological Society of North America, Inc 35 500–516. [DOI] [PubMed] [Google Scholar]
- Hescot S, Curras-Freixes M, Deutschbein T, van Berkel A, Vezzosi D, Amar L, de la Fouchardière C, Valdes N, Riccardi F, Do Cao C, et al. 2019. Prognosis of Malignant Pheochromocytoma and Paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors Retrospective Study. J Clin Endocrinol Metab. 104 2367–2374. [DOI] [PubMed] [Google Scholar]
- Hofman MS & Hicks RJ 2015. Moving Beyond “Lumpology”: PET/CT Imaging of Pheochromocytoma and Paraganglioma. Clin Cancer Res 21 3815–3817. [DOI] [PubMed] [Google Scholar]
- Huizing DMV, de Wit-van der Veen BJ, Verheij M & Stokkel MPM 2018. Dosimetry methods and clinical applications in peptide receptor radionuclide therapy for neuroendocrine tumours: a literature review. EJNMMI Res 8 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri M, Whitworth J, Rattenberry E, Vialard L, Kilby G, Kumar AV, Izatt L, Lalloo F, Brennan P, Cook J, et al. 2013. Evaluation of SDHB, SDHD and VHL gene susceptibility testing in the assessment of individuals with non-syndromic phaeochromocytoma, paraganglioma and head and neck paraganglioma. Clinical endocrinology 78 898–906. [DOI] [PubMed] [Google Scholar]
- Janssen I, Blanchet EM, Adams K, Chen CC, Millo CM, Herscovitch P, Taieb D, Kebebew E, Lehnert H, Fojo AT, et al. 2015. Superiority of [68Ga]-DOTATATE PET/CT to Other Functional Imaging Modalities in the Localization of SDHB-Associated Metastatic Pheochromocytoma and Paraganglioma. Clin Cancer Res 21 3888–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Chen CC, Millo CM, Ling A, Taieb D, Lin FI, Adams KT, Wolf KI, Herscovitch P, Fojo AT, et al. 2016a. PET/CT comparing (68)Ga-DOTATATE and other radiopharmaceuticals and in comparison with CT/MRI for the localization of sporadic metastatic pheochromocytoma and paraganglioma. European journal of nuclear medicine and molecular imaging 43 1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Chen CC, Taieb D, Patronas NJ, Millo CM, Adams KT, Nambuba J, Herscovitch P, Sadowski SM, Fojo AT, et al. 2016b. 68Ga-DOTATATE PET/CT in the Localization of Head and Neck Paragangliomas Compared with Other Functional Imaging Modalities and CT/MRI. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 57 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Chen CC, Zhuang Z, Millo CM, Wolf KI, Ling A, Lin FI, Adams KT, Herscovitch P, Feelders RA, et al. 2017. Functional Imaging Signature of Patients Presenting with Polycythemia/Paraganglioma Syndromes. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 58 1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A, Ling A, Millo C, Chen C, Gupta G, Viana B, Gonzales M, Adams K, Herscovitch P, Lin F, et al. 2018a. Superiority of 68Ga-DOTATATE PET/CT to other functional and anatomic imaging modalities in the detection of SDHD-related pheochromocytoma and paraganglioma - A comparative prospective study. Journal of Nuclear Medicine 59 46–46. [Google Scholar]
- Jha A, Ling A, Millo C, Gupta G, Viana B, Lin FI, Herscovitch P, Adams KT, Taieb D, Metwalli AR, et al. 2018b. Superiority of (68)Ga-DOTATATE over (18)F-FDG and anatomic imaging in the detection of succinate dehydrogenase mutation (SDHx)-related pheochromocytoma and paraganglioma in the pediatric population. European journal of nuclear medicine and molecular imaging 45 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A, de Luna K, Balili CA, Millo C, Paraiso CA, Ling A, Gonzales MK, Viana B, Alrezk R, Adams KT, et al. 2019a. Clinical, Diagnostic, and Treatment Characteristics of SDHA-Related Metastatic Pheochromocytoma and Paraganglioma. Front Oncol 9 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A, Ling A, Millo C, Chen C, Gonzales M, Patel M, Tena I, Mamilla D, Knue M, Geraldine O’Sullivan C, et al. 2019b. Diagnostic performance of PET/CT utilizing 68Ga-DOTATATE, 18F-FDG, 18F-DOPA, and 18F-FDA, and anatomic imaging in the detection of sporadic primary pheochromocytoma - A comparative prospective study Journal of Nuclear Medicine 60 439.30936253 [Google Scholar]
- Jochmanova I & Pacak K 2018. Genomic Landscape of Pheochromocytoma and Paraganglioma. Trends Cancer 4 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y, Zhang S, Wang W, Liu J, Yang J & Wang Z 2018. (68)Ga-somatostatin receptor analogs and (18)F-FDG PET/CT in the localization of metastatic pheochromocytomas and paragangliomas with germline mutations: a meta-analysis. Acta Radiol. 59 1466–1474. [DOI] [PubMed] [Google Scholar]
- Kelkar SS, Reineke TM. 2011. Theranostics: combining imaging and therapy. Bioconjugate Chem. 22 1879–1903 [DOI] [PubMed] [Google Scholar]
- Kesavan M, Claringbold PG, Turner JH. 2014. Hematological toxicity of combined 177Lu-octreotate radiopeptide chemotherapy of gastroenteropancreatic neuroendocrine tumors in long-term follow-up. Neuroendocrinology. 99 108–17. [DOI] [PubMed] [Google Scholar]
- Kesavan M & Turner JH 2016. Myelotoxicity of Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors: A Decade of Experience. Cancer Biother Radiopharm 31 189–198. [DOI] [PubMed] [Google Scholar]
- King KS, Prodanov T, Kantorovich V, Fojo T, Hewitt JK, Zacharin M, Wesley R, Lodish M, Raygada M, Gimenez-Roqueplo AP, et al. 2011. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. Journal of Clinical Oncology 294 137–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasinska-Cwikla A, Peczkowska M, Cwikla JB, Michalowska I, Palucki JM, Bodei L, Lewczuk-Myslicka A, Januszewicz A. 2019. A Clinical Efficacy of PRRT in Patients with Advanced, Nonresectable, Paraganglioma-Pheochromocytoma, Related to SDHx Gene Mutation. J Clin. Med 8, 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Grozinsky-Glasberg S, Hofman MS, Callahan J, Meirovitz A, Maimon O, Pattison DA, Gross DJ & Hicks RJ 2017. Efficacy of Peptide Receptor Radionuclide Therapy for Functional Metastatic Paraganglioma and Pheochromocytoma. J Clin Endocrinol Metab 102 3278–3287. [DOI] [PubMed] [Google Scholar]
- Kong G, Schenberg T, Yates CJ, Trainer A, Sachithanandan N, Iravani A, Ravi Kumar A, Hofman MS, Akhurst T, Michael M, et al. 2019. The role of 68Ga-DOTA-Octreotate (GaTate) PET/CT in follow-up of SDH-associated pheochromocytoma and paraganglioma (PPGL). J Clin Endocrinol Metab. doi: 10.1210/jc.2019-00018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R, Murphy K, Haberkorn U & Morgenstern A 2014. (2)(1)(3)Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. European journal of nuclear medicine and molecular imaging 41 2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochwil C, Lopez-Benitez R, Mier W, Haufe S, Isermann B, Kauczor HU, Choyke PL, Haberkorn U & Giesel FL 2011. Hepatic arterial infusion enhances DOTATOC radiopeptide therapy in patients with neuroendocrine liver metastases. Endocrine-Related Cancer 18 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroiss A, Putzer D, Frech A, Decristoforo C, Uprimny C, Gasser RW, Shulkin BL, Url C, Widmann G, Prommegger R, et al. 2013. A retrospective comparison between 68Ga-DOTA-TOC PET/CT and 18F-DOPA PET/CT in patients with extra-adrenal paraganglioma. European journal of nuclear medicine and molecular imaging 40 1800–1808. [DOI] [PubMed] [Google Scholar]
- Kroiss A, Shulkin BL, Uprimny C, Frech A, Gasser RW, Url C, Gautsch K, Madleitner R, Nilica B, Sprinzl GM, et al. 2015. (68)Ga-DOTATOC PET/CT provides accurate tumour extent in patients with extraadrenal paraganglioma compared to (123)I-MIBG SPECT/CT. European journal of nuclear medicine and molecular imaging 42 33–41. [DOI] [PubMed] [Google Scholar]
- Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, Forster K, Aerts HJ, Dekker A, Fenstermacher D, et al. 2012. Radiomics: the process and the challenges. Magn Reson Imaging. 30 1234–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, et al. 2012. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carte SL, Stewart C, Mermel CH, Roberts SA, et al. 2013. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 499 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makis W, McCann K, McEwan AJ & Sawyer MB 2016. Palliation of Extensive Metastatic Bone Disease With 223Ra-Dichloride alpha-Particle Therapy in a Patient With Malignant Hereditary Paraganglioma-Pheochromocytoma Syndrome With SDHB Mutation. Clinical nuclear medicine 41 144–147. [DOI] [PubMed] [Google Scholar]
- Mei L, Khurana A, Al-Juhaishi T et al. 2019. Prognostic factors of malignant pheochromocytoma and paraganglioma: a combined SEER and TCGA databases review. Horm Metab Res 51 451–457 [DOI] [PubMed] [Google Scholar]
- Naji M, Zhao C, Welsh SJ, Meades R, Win Z, Ferrarese A, Tan T, Rubello D & Al-Nahhas A 2011. 68Ga-DOTA-TATE PET vs. 123I-MIBG in identifying malignant neural crest tumours. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging 13 769–775. [DOI] [PubMed] [Google Scholar]
- Nambuba J, Därr R, Janssen I, Bullova P, Adams KT, Millo C, Bourdeau I, Kassai A, Yang C, Kebebew E, et al. 2015. Functional Imaging Experience in a Germline Fumarate Hydratase Mutation–Positive Patient With Pheochromocytoma and Paraganglioma. AACE Clinical Case Reports 2 e176–e181. [Google Scholar]
- Nastos K, Cheung VTF, Toumpanakis C, Navalkissoor S, Quigley AM, Caplin M & Khoo B 2017. Peptide Receptor Radionuclide Treatment and (131)I-MIBG in the management of patients with metastatic/progressive phaeochromocytomas and paragangliomas. Journal of surgical oncology 115 425–434. [DOI] [PubMed] [Google Scholar]
- Navalkissoor S, Grossman A 2019. Targeted Alpha Particle Therapy for Neuroendocrine Tumours: The Next Generation of Peptide Receptor Radionuclide Therapy. Neuroendocrinology. 108 256–264. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M, Buchta M, Franke G, Klisch J, Bley TA et al. 2004. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. Journal of the American Medical Association 292 943–951. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Erlic Z, Boedeker CC, Rybicki LA, Robledo M, Hermsen M, Schiavi F, Falcioni M, Kwok P, Bauters C, et al. 2009. Clinical predictors for germline mutations in head and neck paraganglioma patients: cost reduction strategy in genetic diagnostic process as fall-out. Cancer research 69 3650–3656. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Young WF Jr, Krauss T, Bayley JP, Schiavi F, Opocher G, Boedeker CC, Tirosh A, Castinetti F, Ruf J, et al. 2018. 65 YEARS OF THE DOUBLE HELIX: Genetics informs precision practice in the diagnosis and management of pheochromocytoma. Endocrine-Related Cancer 25 T201–T219. [DOI] [PubMed] [Google Scholar]
- Niemeijer ND, Alblas G, van Hulsteijn LT, Dekkers OM & Corssmit EP 2014. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf) 81 642–651. [DOI] [PubMed] [Google Scholar]
- Nolting S, Grossman A & Pacak K 2018. Metastatic Phaeochromocytoma: Spinning Towards More Promising Treatment Options. Exp Clin Endocrinol Diabetes. 127 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JPB, Rose CJ, Waterton JC, Carano RAD, Parker GJM, Jackson A. 2015. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res. 21 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y, Sudo H, Watanabe S, Nagatsu K, Tsuji AB, Sakashita T, Ito YM, Yoshinaga K, Higashi T & Ishioka NS 2018. Antitumor effects of radionuclide treatment using alpha-emitting meta-(211)At-astato-benzylguanidine in a PC12 pheochromocytoma model. European journal of nuclear medicine and molecular imaging 45 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksuz MO, Winter L, Pfannenberg C, Reischl G, Mussig K, Bares R & Dittmann H 2014. Peptide receptor radionuclide therapy of neuroendocrine tumors with (90)Y-DOTATOC: is treatment response predictable by pre-therapeutic uptake of (68)Ga-DOTATOC? Diagn Interv Imaging 95 289–300. [DOI] [PubMed] [Google Scholar]
- Pandit-Taskar N, Zanzonico P, Staton KD, Carrasquillo JA, Reidy- Lagunes D, Lyashchenko S, Burnazi E, Zhang H, Lewis JS, Blasberg R, et al. 2018. Biodistribution and dosimetry of (18) F-meta-fluorobenzylguanidine: a first-in-human PET/CT imaging study of patients with neuroendocrine malignancies. Journal of Nuclear Medicine 59 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathomas TG, Gaal J, Corssmit EP, Oudijk L, Korpershoek E, Heimdal K, Bayley JP, Morreau H, van Dooren M, Papaspyrou K, et al. 2014. Non-pheochromocytoma (PCC)/paraganglioma (PGL) tumors in patients with succinate dehydrogenase-related PCC-PGL syndromes: a clinicopathological and molecular analysis. European journal of endocrinology / European Federation of Endocrine Societies 170 1–12. [DOI] [PubMed] [Google Scholar]
- Parihar AS, Vadi SK, Mittal BR, Kumar R, Bal A & Singh SK 2018. 68Ga-PSMA-HBED-CC-Avid Synchronous Urinary Bladder Paraganglioma in a Patient With Metastatic Prostate Carcinoma. Clinical nuclear medicine 43 e329–e330. [DOI] [PubMed] [Google Scholar]
- Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, Seke M, et al. 2013. Alpha emitter radium-223 and survival in metastatic prostate cancer. The New England journal of medicine 369 213–223. [DOI] [PubMed] [Google Scholar]
- Pasini B, McWhinney SR, Bei T, Matyakhina L, Stergiopoulos S, Muchow M, Boikos SA, Ferrando B, Pacak K, Assie G, et al. 2008. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. European journal of human genetics : EJHG 16 79–88. [DOI] [PubMed] [Google Scholar]
- Pinato DJ, Black JR, Ramaswami R, Tan TM, Adjogatse D & Sharma R 2016. Peptide receptor radionuclide therapy for metastatic paragangliomas. Med Oncol 33 47. [DOI] [PubMed] [Google Scholar]
- Pryma DA, Chin BB, Noto RB, Dillon JS, Perkins S, Solnes L, Kostakoglu L, Serafini AN, Pampaloni MH, Jensen J, et al. 2018. Efficacy and Safety of High-Specific-Activity I-131 MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. doi: 10.2967/jnumed.118.217463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouin PF, Amar L, Dekkers OM, Fassnacht M, Gimenez-Roqueplo AP, Lenders JW, Lussey-Lepoutre C, Steichen O; Guideline Working Group. 2016. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur J Endocrinol. 174 G1–G10. [DOI] [PubMed] [Google Scholar]
- Puranik AD, Kulkarni HR, Singh A & Baum RP 2015. Peptide receptor radionuclide therapy with (90)Y/ (177)Lu-labelled peptides for inoperable head and neck paragangliomas (glomus tumours). European journal of nuclear medicine and molecular imaging 42 1223–1230. [DOI] [PubMed] [Google Scholar]
- Riff BP, Yang YX, Soulen MC, Pryma DA, Bennett B, Wild D, Nicolas G, Teitelbaum UR & Metz DC 2015. Peptide Receptor Radionuclide Therapy-Induced Hepatotoxicity in Patients With Metastatic Neuroendocrine Tumors. Clin Nucl Med 40 845–850. [DOI] [PubMed] [Google Scholar]
- Sabet A, Ezziddin K, Pape UF, Ahmadzadehfar H, Mayer K, Poppel T, Guhlke S, Biersack HJ & Ezziddin S 2013a. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med 54 1857–1861. [DOI] [PubMed] [Google Scholar]
- Sabet A, Khalaf F, Haslerud T, Al-Zreiqat A, Sabet A, Simon B, Poppel TD, Biersack HJ & Ezziddin S 2013b. Bone metastases in GEP-NET: response and long-term outcome after PRRT from a follow-up analysis. American journal of nuclear medicine and molecular imaging 3 437–445. [PMC free article] [PubMed] [Google Scholar]
- Sabet A, Ezziddin K, Pape UF, Reichman K, Haslerud T, Ahmadzadehfar H, Biersack HJ, Nagarajah J & Ezziddin S 2014. Accurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with (177)Lu-octreotate. Eur J Nucl Med Mol Imaging 41 505–510. [DOI] [PubMed] [Google Scholar]
- Sandstrom M, Garske-Roman U, Granberg D, Johansson S, Widstrom C, Eriksson B, Sundin A, Lundqvist H & Lubberink M 2013. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 54 33–41. [DOI] [PubMed] [Google Scholar]
- Schovanek J, Martucci V, Wesley R, Fojo T, Del Rivero J, Huynh T, Adams K, Kebebew E, Frysak Z, Stratakis CA et al. 2014. The size of the primary tumor and age at initial diagnosis are independent predictors of the metastatic behavior and survival of patients with SDHB-related pheochromocytoma an d paraganglioma: a retrospective cohort study. BMC Cancer. 14:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidlin SM, Marinelli LD & Oshry E 1946. Radioactive iodine therapy; effect on functioning metastases of adenocarcinoma of the thyroid. J Am Med Assoc 132 838–847. [DOI] [PubMed] [Google Scholar]
- Serre R, Benzekry S, Padovani L, Meille C, Andre N, Ciccolini J, Barlesi F, Muracciole X & Barbolosi D 2016. Mathematical Modeling of Cancer Immunotherapy and Its Synergy with Radiotherapy. Cancer research 76 4931–4940. [DOI] [PubMed] [Google Scholar]
- Sharma P, Dhull VS, Arora S, Gupta P, Kumar R, Durgapal P, Malhotra A, Chumber S, Ammini AC, Kumar R, et al. 2014. Diagnostic accuracy of (68)Ga-DOTANOC PET/CT imaging in pheochromocytoma. European journal of nuclear medicine and molecular imaging 41 494–504. [DOI] [PubMed] [Google Scholar]
- Sharma P, Mukherjee A, Karunanithi S, Naswa N, Kumar R, Ammini AC & Bal C 2015. Accuracy of 68Ga DOTANOC PET/CT Imaging in Patients With Multiple Endocrine Neoplasia Syndromes. Clinical nuclear medicine 40 e351–356. [DOI] [PubMed] [Google Scholar]
- Sharma P, Thakar A, Suman Kc S, Dhull VS, Singh H, Naswa N, Reddy RM, Karunanithi S, Kumar R, Malhotra A, et al. 2013. 68Ga-DOTANOC PET/CT for Baseline Evaluation of Patients with Head and Neck Paraganglioma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 54 841–847. [DOI] [PubMed] [Google Scholar]
- Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, et al. 2017. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. The New England journal of medicine 376 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, Baum RP, Kunz P, Hobday T, Hendifar A, et al. 2018. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With (177)Lu-Dotatate in the Phase III NETTER-1 Trial. J Clin Oncol 36 2578–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C, Rodrigo JP, Bodeker CC, Llorente JL, Silver CE, Jansen JC, Takes RP, Strojan P, Pellitteri PK, Rinaldo A, et al. 2013. Jugular and vagal paragangliomas: Systematic study of management with surgery and radiotherapy. Head & neck 35 1195–1204. [DOI] [PubMed] [Google Scholar]
- Sundlov A, Sjogreen-Gleisner K, Svensson J, Ljungberg M, Olsson T, Bernhardt P & Tennvall J 2017. Individualised (177)Lu-DOTATATE treatment of neuroendocrine tumours based on kidney dosimetry. European journal of nuclear medicine and molecular imaging 44 1480–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb D, Garrigue P, Bardies M, Abdullah AE & Pacak K 2015. Application and Dosimetric Requirements for Gallium-68-labeled Somatostatin Analogues in Targeted Radionuclide Therapy for Gastroenteropancreatic Neuroendocrine Tumors. PET Clin 10 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb D, Hicks RJ & Pacak K 2016. Nuclear Medicine in Cancer Theranostics: Beyond the Target. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 57 1659–1660. [DOI] [PubMed] [Google Scholar]
- Taieb D, Jha A, Guerin C, Pang Y, Adams KT, Chen CC, Romanet P, Roche P, Essamet W, Ling A, et al. 2018. 18F-FDOPA PET/CT Imaging of MAX-Related Pheochromocytoma. J Clin Endocrinol Metab 103 1574–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb D, Hicks RJ, Hindie E, Guillet BA, Avrram A, Ghedini P, Timmers HJ, Scott AT, Elojjeimy S, Rubello D, et al. 2019. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. European journal of nuclear medicine and molecular imaging doi: 10.1007/s00259-019-04398-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TH, Hussein Z, Saad FF & Shuaib IL 2015. Diagnostic performance of (68) Ga-DOTATATE PET/CT, (18)F-FDG PET/CT and (131) I-MIBG scintigraphy in mapping metastatic pheochromocytoma and paraganglioma. Nuclear Medicine and Molecular Imaging 49 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, King KS, Rao JU, Wesley RA, Adams KT, et al. 2012. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. Journal of the National Cancer Institute 104 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh A, Papadakis GZ, Millo C, Hammoud D, Sadowski SM, Herscovitch P, Pacak K, Marx SJ, Yang L, Nockel P, et al. 2018. Prognostic Utility of Total 68Ga-DOTATATE-Avid Tumor Volume in Patients With Neuroendocrine Tumors. Gastroenterology 154 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkova H, Prodanov T, Maly M, Martucci V, Adams K, Widimsky J Jr., Chen CC, Ling A, Kebebew E, Stratakis CA, et al. 2016. Characteristics and Outcomes of Metastatic Sdhb and Sporadic Pheochromocytoma/Paraganglioma: An National Institutes of Health Study. Endocr Pract 22 302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Essen M, Krenning EP, Kooij PP, Bakker WH, Feelders RA, de Herder WW, Wolbers JG & Kwekkeboom DJ 2006. Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 47 1599–1606. [PubMed] [Google Scholar]
- van Hulsteijn LT, Niemeijer ND, Dekkers OM & Corssmit EP 2014. (131)I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: systematic review and meta-analysis. Clinical endocrinology 80 487–501. [DOI] [PubMed] [Google Scholar]
- Viglianti BL, Wale DJ, Wong KK, Johnson TD, Ky C, Frey KA, Gross MD. 2018. Effects of Tumor Burden on Reference Tissue Standardized Uptake for PET Imaging: Modification of PERCIST Criteria. Radiology. 287 993–1002. [DOI] [PubMed] [Google Scholar]
- Vogel J, Atanacio AS, Prodanov T, Turkbey BI, Adams K, Martucci V, Camphausen K, Fojo AT, Pacak K & Kaushal A 2014. External beam radiation therapy in treatment of malignant pheochromocytoma and paraganglioma. Front Oncol 4 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyakaranam AR, Crona J, Norlen O, Granberg D, Garske-Roman U, Sandström M, Fröss-Baron K, Thiis-Evensen E, Hellman P, Sundin A. 2019. Favorable Outcome in Patients with Pheochromocytoma and Paraganglioma Treated with 177Lu-DOTATATE. Cancers (Basel). 11 doi: 10.3390/cancers11070909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadas TJ, Pandya DN, Solingapuram Sai KK, Mintz A. 2014. Molecular targeted alpha-particle therapy for oncologic applications. AJR Am J Roentgenol. 203 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cheng Y, Zhang J, Zang J, Li H, Liu Q, Wang J, Jacobson O, Li F, Zhu Z, et al. 2018. Response to Single Low-dose (177)Lu-DOTA-EB-TATE Treatment in Patients with Advanced Neuroendocrine Neoplasm: A Prospective Pilot Study. Theranostics 8 3308–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welander J, Söderkvist P & Gimm O 2011. Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas. Endocrine-Related Cancer 18 R253–R276. [DOI] [PubMed] [Google Scholar]
- Werner RA, Lapa C, Ilhan H, Higuchi T, Buck AK, Lehner S, Bartenstein P, Bengel F, Schatka I, Muegge DO, et al. 2017. Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity. Oncotarget 8 7039–7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner RA, Ilhan H, Lehner S, Papp L, Zsoter N, Schatka I, Muegge DO, Javadi MS, Higuchi T, Buck AK, et al. 2019. Pre-therapy Somatostatin Receptor-Based Heterogeneity Predicts Overall Survival in Pancreatic Neuroendocrine Tumor Patients Undergoing Peptide Receptor Radionuclide Therapy. Mol. Imaging Biol 2018. [DOI] [PubMed] [Google Scholar]
- Wolf KI, Jha A, van Berkel A, Wild D, Janssen I, Millo CM, Janssen MJR, Gonzales MK, Timmers HJKM & Pacak K 2019. Eruption of Metastatic Paraganglioma After Successful Therapy with 177Lu/90Y-DOTATOC and 177Lu-DOTATATE. Nuclear Medicine and Molecular Imaging. doi: 10.1007/s13139-019-00579-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Pfeifer AK, Myschetzky R, Garbyal RS, Rasmussen P, Knigge U, Bzorek M, Kristensen MH & Kjaer A 2013. Induction of Anti-Tumor Immune Responses by Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE in a Murine Model of a Human Neuroendocrine Tumor. Diagnostics (Basel) 3 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav MP, Ballal S & Bal C 2019. Concomitant (177)Lu-DOTATATE and capecitabine therapy in malignant paragangliomas. EJNMMI Res 9 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova A, Mayer K, Brossart P, Gonzalez-Carmona MA, Strassburg CP, Essler M & Ahmadzadehfar H 2017. Safety of multiple repeated cycles of (177)Lu-octreotate in patients with recurrent neuroendocrine tumour. Eur J Nucl Med Mol Imaging 44 1207–1214. [DOI] [PubMed] [Google Scholar]
- Yordanova A, Wicharz MM, Mayer K, Brossart P, Gonzalez-Carmona MA, Strassburg CP, Fimmers R, Essler M & Ahmadzadehfar H 2018. The Role of Adding Somatostatin Analogues to Peptide Receptor Radionuclide Therapy as a Combination and Maintenance Therapy. Clin Cancer Res 24 4672–4679. [DOI] [PubMed] [Google Scholar]
- Zandee WT, Feelders RA, Smit Duijzentkunst D, Hofland J, Metselaar RM, Oldenburg RA, van Linge A, Kam BLR, Teunissen J, Korpershoek E, et al. 2019. Treatment of inoperable or metastatic paragangliomas and pheochromocytomas with peptide receptor radionuclide therapy using 177Lu-DOTATATE. Eur J Endocrinol. doi: 10.1530/EJE-18-0901. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zelinka T, Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Reynolds JC, Ling A, Eisenhofer G, Lazurova I, Adams KT, et al. 2008. Role of positron emission tomography and bone scintigraphy in the evaluation of bone involvement in metastatic pheochromocytoma and paraganglioma: specific implications for succinate dehydrogenase enzyme subunit B gene mutations. Endocr Relat Cancer 15 311–323. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang H, Jacobson O, Cheng Y, Niu G, Li F, Bai C, Zhu Z & Chen X 2018. Safety, Pharmacokinetics, and Dosimetry of a Long-Acting Radiolabeled Somatostatin Analog (177)Lu-DOTA-EB-TATE in Patients with Advanced Metastatic Neuroendocrine Tumors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 59 1699–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT & Pacak K 2012. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. The New England journal of medicine 367 922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovato S, Kumanova A, Dematte S, Sansovini M, Bodei L, Di Sarra D, Casagranda E, Severi S, Ambrosetti A, Schiavi F, et al. 2012. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL). Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 44 411–414. [DOI] [PubMed] [Google Scholar]