Abstract
Objective:
Although buprenorphine treatment reduces risk of overdose and death in opioid use disorder, most patients discontinue treatment within a few weeks or months. Adverse health outcomes following buprenorphine discontinuation were compared among patients who were successfully retained beyond 6 months of continuous treatment, a minimum treatment duration recently endorsed by the National Quality Forum.
Methods:
A retrospective longitudinal cohort analysis was performed using the MarketScan multi-state Medicaid claims database (2013–2017), covering 12 million beneficiaries annually. The sample included adults (18–64 years of age) who received buprenorphine continuously for ≥180 days by cohorts retained for 6–9 months, 9–12 months, 12–15 months, and 15–18 months. For outcome assessment in the postdiscontinuation period, patients had to be continuously enrolled in Medicaid for 6 months after buprenorphine discontinuation. Primary adverse outcomes included all-cause emergency department visits, all-cause in-patient hospitalizations, opioid prescriptions, and drug overdose (opioid or non-opioid).
Results:
Adverse events were common across all cohorts, and almost half of patients (42.1%—49.9%) were seen in the emergency department at least once. Compared with patients retained on buprenorphine for 6–9 months (N=4,126), those retained for 15–18 months (N=931) had significantly lower odds of emergency department visits (odds ratio=0.75, 95% CI = 0.65–0.86), in-patient hospitalizations (odds ratio=0.79, 95% CI=0.64–0.99), and filling opioid prescriptions (odds ratio=0.67, 95% CI=0.56–0.80) in the 6 months following discontinuation. Approximately 5% of patients across all cohorts experienced one or more medically treated overdoses.
Conclusions:
Risk of acute care service use and overdose were high following buprenorphine discontinuation irrespective of treatment duration. Superior outcomes became significant with treatment duration beyond 15 months, although rates of the primary adverse outcomes remained high.
As the opioid-related overdose death rate continues to make national headlines, increasing attention, such as in the recently announced National Institutes of Health’s HEAL Initiative (1), has focused on difficulties faced by an estimated 2.1 million patients with opioid use disorder in accessing evidence-based care (2, 3). Buprenorphine, approved for the treatment of opioid dependence by the U.S. Food and Drug Administration in 2002, is the most frequently prescribed medication for opioid use disorder in the United States, now dispensed to more than 700,000 individuals annually (4). However, the great majority of patients who initiate buprenorphine are not successfully retained in care (3, 4).
Between 50% and 80% of patients who initiate buprenorphine discontinue the medication within several weeks or months (5–7). Stigma (8), lack of physician training (9), and attitudinal factors can lead patients to attempt to taper off buprenorphine once their drug use diminishes and their lives begin to stabilize (10). Furthermore, insurance coverage limits and other policy constraints commonly restrict access to buprenorphine or limit use to 6 months or less in many jurisdictions (11, 12). As a result, several factors undermine long-term patient retention on buprenorphine.
Yet no empirical basis exists for defining the optimal length of treatment with pharmacotherapy for opioid use disorder. Expert consensus (13, 14) and practice guidelines (15) generally recommend use of buprenorphine, methadone, or extended-release naltrexone with no predefined length of treatment, as opioid use disorder is an enduring, relapsing condition (14). However, previous research has not evaluated differences in clinical outcomes by duration of treatment for patients retained in care beyond 6 months, given pragmatic limitations on prospective trials and the challenges of accessing sufficiently large data sources.
In part as a result of these constraints, there are few quality measures for the treatment of opioid use disorder or use of buprenorphine (16). To guide practice improvement, the RAND Corporation recently developed a quality measure, endorsed by the National Quality Forum (17), that assesses the proportion of patients with opioid use disorder who initiate medication for addiction treatment that continues for a minimum of 180 days. The 180-day threshold was based on expert consensus rather than empirical evidence. APA was recently awarded a Centers for Medicare and Medicaid Services grant to develop a set of measures to improve practice for the treatment of opioid use disorder, including successful long-term retention in treatment (18).
A limitation of previous observational studies of long-term buprenorphine treatment is their focus on general outcomes, such as overall service use and psychosocial functioning, without consideration of disorder-specific outcomes, such as opioid use or overdose (19, 20). Other work in the addiction field has shown that patients with substance use disorders often need a minimum of 1 year of abstinence before exceeding a 50% likelihood of sustaining abstinence for the following year (21). In a prospective study of outpatients with varying substance use disorders, the odds of successful long-term abstinence continued to accrue after 3–5 years of continuous abstinence, considerably longer than a 6-month time horizon (21). For patients specifically in treatment for opioid use disorder, risks stemming from treatment dropout may be magnified given substantial evidence from the United States (13–15) and other Western nations (7, 22) for the effectiveness of medications such as buprenorphine.
Medicaid covers approximately 20% of the U.S. population. It has become the largest single payer for addiction treatment, allowing for analysis of large populations of patients with opioid use disorder, with spending on prescription medications for treating opioid use disorder reaching nearly $1 billion per annum (23). We analyzed multi-state Medicaid claims to compare clinical outcomes among beneficiaries who were retained on buprenorphine for variable periods beyond a minimum of 6 months. Before data were analyzed, we hypothesized that among patients with at least 6 months of continuous treatment, those with longer as compared with shorter periods of buprenorphine treatment would demonstrate superior outcomes after buprenorphine discontinuation.
METHODS
Study Design and Participants
We analyzed data from 2013 to 2017 from the MarketScan database of Medicaid claims, which is a multi-state sample of insurance claims and enrollment information for approximately 12 million Medicaid enrollees each year. The de-identified data include enrollment information as well as comprehensive in-patient, outpatient, and emergency services and drug prescriptions billed to Medicaid. These data capture diagnostic codes, pharmacy claims, and billing codes across all providers for services paid by Medicaid in addition to patient demographic characteristics (age, sex, race) and insurance plan type. An advantage of using insurance claims data is that service codes reflect care across many different treatment settings rather than being restricted to a single provider or clinical site. As a result, patients who drop out of treatment can be observed in subsequent periods, provided they maintain enrollment.
Our sample included patients who were 18–64 years old at the time they initiated buprenorphine treatment (buprenorphine formulations approved for pain indications were excluded), who were continuously retained on buprenorphine for a minimum of 6 months (180 days), and who maintained Medicaid enrollment for at least 6 additional months following buprenorphine discontinuation, so that clinical outcomes could be assessed (Figure 1). Given these requirements, patients had to have initiated buprenorphine treatment before January 1, 2017, to be eligible for study inclusion.
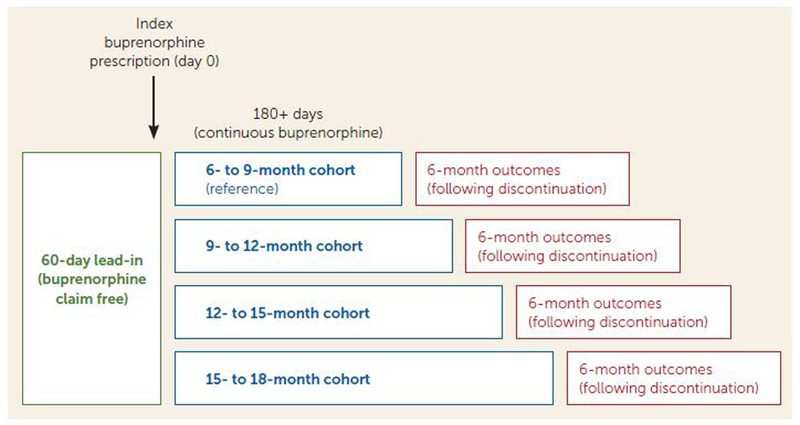
FIGURE 1. Method for designs of cohorts of Medicaid beneficiaries ages 18–64 retained on buprenorphine for ≥180 daysa.
aThe 6- to 9-month cohort was used as a reference group for the 9- to 12-month, 12- to 15-month, and 15- to 18-month cohorts. Buprenorphine refers to formulations approved for treatment of opioid use disorders and excludes those approved for pain indications. An index buprenorphine prescription was defined as a single buprenorphine prescription with no buprenorphine claim in the preceding 60 days, in order to capture new episodes. Discontinuation was determined after a 60-day lapse between refills and was defined as the last day of medication coverage. Four primary outcomes in the 6-month period following discontinuation were analyzed: all-cause emergency department visits, all-cause hospitalizations, opioid prescriptions, and medically treated overdoses.
We defined buprenorphine treatment initiation as an index buprenorphine claim following at least a 60-day observable baseline period without any buprenorphine claims. Similar to previous studies, buprenorphine discontinuation was defined as a gap >60 days beyond the number of days supplied for the last observed filled buprenorphine prescription. This liberal definition reflects clinical experience with long-term buprenorphine patients and prescriber habits (24, 25). Among individuals retained in buprenorphine treatment beyond 180 days, we partitioned the study sample into cohorts retained for 6–9 months (180–270 days), 9–12 months (271–364 days), 12–15 months (365–455 days), and 15–18 months (456–545 days), based on the number of days supplied for their buprenorphine prescriptions. Three-month cohorts were selected given common clinical practice and guidelines (e.g., quarterly treatment plans) and were prospectively chosen before we conducted analyses, as patients in long-term buprenorphine treatment may only be seen on a quarterly basis (26) and shorter intervals may categorize patients incorrectly. The cohorts were used to assess the strength of associations between buprenorphine treatment duration and outcomes following discontinuation.
Measures
Outcomes measured during the 6-month period following buprenorphine discontinuation (see Figure 1), included all-cause emergency department visits, all-cause in-patient hospital admissions, full-agonist opioid prescription fills, and opioid and nonopioid medically treated drug overdose (ICD-10 codes T36–T39, T40.5–T50; see Table S1 in the online supplement) (24). Prescription of a full-agonist opioid to a patient with opioid use disorder treated with buprenorphine is generally contraindicated and is a marker of relapse or poorly coordinated care (2).
Covariates included patient sex, age, race/ethnicity, Medicaid plan type (fee for service or capitation), and comorbid psychiatric (ICD-10 codes F01–F09, F20–F99) and substance use disorders (ICD-10 codes F10, F12–F16, F18–F19) for which diagnoses were recorded on the date of buprenorphine initiation or during the 60-day baseline period before the index buprenorphine prescription (see Table S2 in the online supplement) (27).
Statistical Analysis
Tests of the equality of proportions were used to compare the baseline characteristics and associated outcomes of the 6- to 9-month, 9- to 12-month, 12- to 15-month, and 15- to 18-month cohorts. Separate logistic regressions were then used to estimate odds ratios for each outcome, comparing each cohort to the 6- to 9-month cohort (reference group). All regressions were adjusted for sociodemographic characteristics (age, sex, race/ethnicity), Medicaid plan type, and clinical characteristics (psychiatric diagnosis, alcohol use diagnosis, nonopioid substance use diagnosis, and initial buprenorphine dosage). Sensitivity analyses were restricted to individuals with a documented diagnosis of opioid use disorder during the 60-day baseline assessment or at any point during the study period, given that approximately 20%–30% of patients on buprenorphine for opioid use disorder may not have a concurrently documented opioid use disorder diagnosis in claims data (26). All analyses were conducted using Stata, version 15.
The project was reviewed and determined to be exempt from human subjects review by the Rutgers University Institutional Review Board.
RESULTS
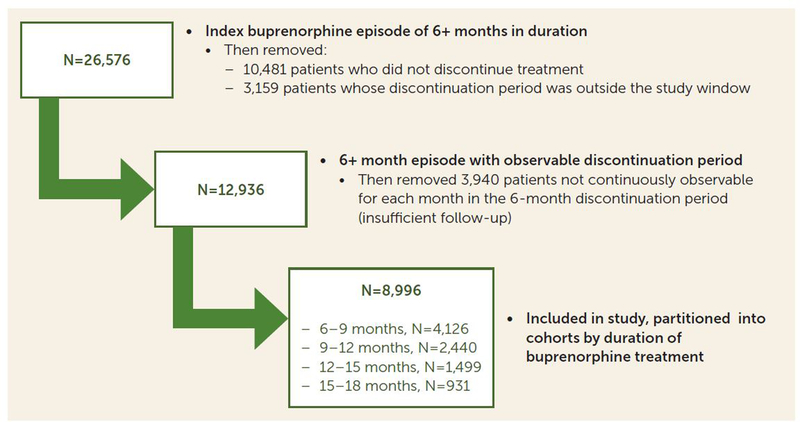
A total of 26,576 Medicaid beneficiaries were identified who had a new buprenorphine treatment episode that was continuous for >6 months. We excluded 10,481 individuals who did not discontinue treatment and 3,159 who discontinued with less than 6 months remaining in the study window (Figure 2), leaving 12,936 who discontinued treatment inside the study window with ≥6 covered months remaining for outcome analysis. Of this group, 3,940 were excluded because they lacked continuous enrollment throughout the follow-up period, yielding a final sample size of 8,996 individuals with observable data for analyses. A majority of the patients were women (61.0%), three-quarters were in the range of 25–44 years old (76.4%), and most were white (91.5%) (Table 1).
FIGURE 2. Flow chart of participant selection and treatment duration among Medicaid beneficiaries ages 18–64 with ≥180 days of buprenorphine treatment and a follow-up period of ≥6 months (2013–2017).
TABLE 1.
Baseline characteristics of Medicaid beneficiaries ages 18–64 who were retained on buprenorphine for ≥180 days, by treatment duration cohorta
| Treatment Duration Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total | 6–9 months | 9–12 months | 12–15 months | 15–18 months | ||||
| N | N | % | N | % | N | % | N | % | |
| 8,996 | 4,126 | 45.9 | 2,440 | 27.1 | 1,499 | 16.7 | 931 | 10.3 | |
| Sex | |||||||||
| Male | 3,512 | 1,641 | 39.8 | 934 | 38.3 | 583 | 38.9 | 354 | 38.0 |
| Female | 5,484 | 2,485 | 60.2 | 1,506 | 61.7 | 916 | 61.1 | 577 | 62.0 |
| Age group (years) | |||||||||
| 18–24 | 966 | 462 | 11.2 | 258 | 10.6 | 157 | 10.5 | 89 | 9.6 |
| 25–34 | 4,473 | 2,072 | 50.2 | 1,210 | 49.6 | 718 | 47.9 | 473 | 50.8 |
| 35–44 | 2,400 | 1,079 | 26.2 | 654 | 26.8 | 418 | 27.9 | 249 | 26.7 |
| 45–54 | 856 | 375 | 9.1 | 241 | 9.9 | 151 | 10.1 | 89 | 9.6 |
| 55–64 | 301 | 138 | 3.3 | 77 | 3.2 | 55 | 3.7 | 31 | 3.3 |
| Race/ethnicity | |||||||||
| White | 8,234 | 3,772 | 91.4 | 2,220 | 91.0 | 1,372 | 91.5 | 870* | 93.4 |
| Nonwhite | 762 | 354 | 8.6 | 220 | 9.0 | 127 | 8.5 | 61* | 6.6 |
| Medicaid plan type | |||||||||
| Fee for service | 2,681 | 1,237 | 30.0 | 762 | 31.2 | 409* | 27.3 | 273 | 29.3 |
| Capitation | 6,315 | 2,889 | 70.0 | 1,678 | 68.8 | 1,090* | 72.7 | 658 | 70.7 |
| Psychiatric diagnosis | 2,777 | 1,273 | 30.9 | 764 | 31.3 | 454 | 30.3 | 286 | 30.7 |
| Substance use diagnosis | |||||||||
| Alcohol use disorder | 430 | 205 | 5.0 | 107 | 4.4 | 77 | 5.1 | 41 | 4.4 |
| Nonopioid drug use disorder | 1,951 | 931 | 22.6 | 517 | 21.2 | 308 | 20.5 | 195 | 20.9 |
| Mean | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Initial buprenorphine dosage (mg/day) | 8.0 | 7.9 | 2.3 | 8.0 | 2.3 | 8.2** | 2.4 | 8.2** | 2.4 |
Data are from the multi-state MarketScan database of Medicaid claims, 2013–2017.
p<0.05.
p<0.01.
The 8,996 individuals included in the analysis were partitioned into cohorts based on duration of treatment, including 4,126 individuals retained for 6–9 months, 2,440 retained for 9–12 months, 1,499 retained for 12–15 months, and 931 retained for 15–18 months, reflecting decay in retention over time (Figure 2). Demographic characteristics were generally not associated with cohort membership; however, nonwhite patients were slightly less likely to be retained for 15–18 months compared with white patients (p=0.04). The mean initial buprenorphine dosage was slightly higher for patients in longer treatment episodes (overall mean dose across cohorts, 8.0 mg; range, 7.9–8.2 mg). Insurance plan type was not strongly related to buprenorphine duration group.
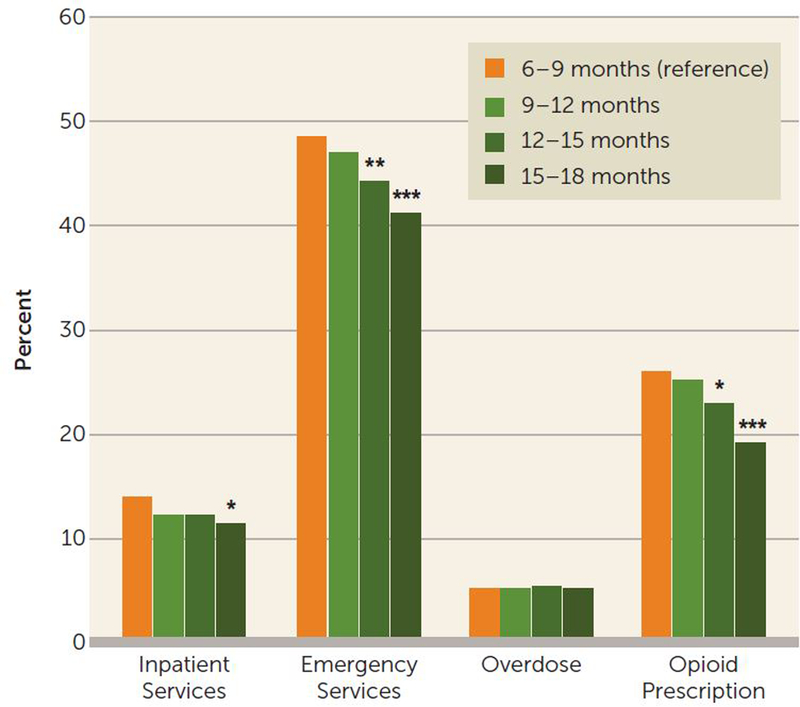
Across all cohorts, during the 6-month postdiscontinuation outcome period, rates of emergency department visits ranged from 41.2% to 48.6%, rates of all-cause in-patient hospitalization ranged from 11.3% to 13.9%, opioid prescriptions were received by 19.1%–25.9% of patients, and 5.1%–5.5% of patients experienced one or more medically treated overdoses.
Compared with the 6- to 9-month reference group, the 15–to 18-month cohort had significantly lower rates of adverse events—except for overdose, which was comparable across all cohorts—with proportionately fewer all-cause emergency department visits (41.2% compared with 48.6%, p<0.001), all-cause in-patient hospitalizations (11.3% compared with 13.9%, p<0.05), and opioid prescription claims (19.1% compared with 25.9%, p<0.001) (Figure 3). Similar patterns were observed for the 9- to 12-month and 12- to 15-month cohorts, but they did not reach the same level of statistical significance (Figure 3). Among all sampled individuals (N=8,996), 464 (5.2%) had at least one overdose in the 6 months following buprenorphine discontinuation. Among these cohorts retained for a minimum of 6 months, longer durations of buprenorphine treatment (i.e., up to 18 months) had no relationship with likelihood of overdose after treatment discontinuation.
FIGURE 3. Unadjusted 6-month outcomes following discontinuation among Medicaid beneficiaries ages 18–64 retained on buprenorphine for ≥180 days, by treatment duration cohort (2013–2017)a.
a All comparisons are with the reference group (the 6- to9-month cohort).
*p<0.05.**p<0.01. ***p<0.001.
Logistic regression controlling for baseline characteristics revealed that compared with the reference group (with 6–9 months of buprenorphine treatment), patients retained for 15–18 months had significantly lower adjusted odds of emergency department visits (odds ratio=0.75, 95% CI=0.65–0.86, p<0.001), in-patient hospitalizations (odds ratio=0.79, 95% CI=0.64–0.99, p<0.05), and opioid prescription fills (odds ratio=0.67, 95% CI=0.56–0.80, p<0.001) in the 6 months following treatment discontinuation (Table 2). Patients retained for 12–15 months had significantly lower adjusted odds of emergency department visits (odds ratio=0.84, 95% CI=0.75–0.95, p<0.01) and opioid prescription fills (odds ratio=0.84, 95% CI=0.73–0.97, p<0.05). Similar trends for all outcomes were observed for those retained 9–12 months, but these did not reach significance (Table 2). Odds of overdose were comparable across all cohorts.
TABLE 2.
Outcomes associated with Medicaid beneficiaries ages 18–64 retained on buprenorphine for ≥180 days
| In-patient Hospitalization | Emergency Department Visit | Overdose | Opioid Prescription | |||||
|---|---|---|---|---|---|---|---|---|
| Measure | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI |
| Treatment episode duration | ||||||||
| 6–9 months (N=4,126) | Ref. | Ref. | Ref. | Ref. | ||||
| 9–12 months (N=2,440) | 0.86 | 0.74, 1.01 | 0.94 | 0.85, 1.04 | 1.04 | 0.82, 1.30 | 0.95 | 0.85, 1.07 |
| 12–15 months (N=1,499) | 0.86 | 0.72, 1.03 | 0.84** | 0.75, 0.95 | 1.10 | 0.84, 1.43 | 0.84* | 0.73, 0.97 |
| 15–18 months (N=931) | 0.79* | 0.64, 0.99 | 0.75*** | 0.65, 0.86 | 1.04 | 0.75, 1.44 | 0.67*** | 0.56, 0.80 |
| Sex | ||||||||
| Male | Ref. | Ref. | Ref. | Ref. | ||||
| Female | 1.10 | 0.96, 1.26 | 1.16*** | 1.06, 1.27 | 0.65*** | 0.54, 0.79 | 1.39*** | 1.25, 1.54 |
| Age group (years) | ||||||||
| 18–24 | Ref. | Ref. | Ref. | Ref. | ||||
| 25–34 | 0.94 | 0.76, 1.16 | 0.98 | 0.85, 1.12 | 0.83 | 0.61, 1.15 | 1.20* | 1.01, 1.43 |
| 35–44 | 0.94 | 0.74, 1.18 | 1.03 | 0.88, 1.20 | 0.95 | 0.68, 1.33 | 1.52*** | 1.26, 1.83 |
| 45–54 | 1.39* | 1.07, 1.82 | 1.04 | 0.86, 1.25 | 0.76 | 0.50, 1.16 | 2.14*** | 1.72, 2.67 |
| 55–64 | 1.79*** | 1.27, 2.57 | 1.37* | 1.05, 1.78 | 0.95 | 0.55, 1.64 | 2.76*** | 2.07, 3.69 |
| Race/ethnicity | ||||||||
| White | Ref. | Ref. | Ref. | Ref. | ||||
| Nonwhite | 1.22 | 0.99, 1.50 | 1.33*** | 1.14, 1.54 | 1.06 | 0.76, 1.48 | 1.09 | 0.92, 1.30 |
| Medicaid Plan | ||||||||
| Fee for service | Ref. | Ref. | Ref. | Ref. | ||||
| Capitation | 1.19* | 1.03, 1.37 | 1.09 | 0.99, 1.20 | 1.21 | 0.97, 1.50 | 1.00 | 0.90, 1.12 |
| Psychiatric diagnosis | 1.56*** | 1.37, 1.78 | 1.49*** | 1.36, 1.64 | 1.61*** | 1.32, 1.96 | 1.58*** | 1.42, 1.75 |
| Substance use diagnosis | ||||||||
| Alcohol use disorder | 1.61*** | 1.26, 2.05 | 1.34** | 1.09, 1.63 | 2.02*** | 1.47, 2.79 | 0.98 | 0.78, 1.23 |
| Nonopioid drug use disorder | 1.70*** | 1.48, 1.95 | 1.26*** | 1.13, 1.39 | 1.51*** | 1.22, 1.86 | 1.07 | 0.95, 1.21 |
| Initial buprenorphine dosageb | 0.99 | 0.96, 1.02 | 0.98 | 0.97, 1.00 | 1.00 | 0.97, 1.04 | 0.99 | 0.97, 1.01 |
Data are from the multi-state MarketScan database of Medicaid claims, 2013–2017.
Initial buprenorphine dosage was a continuous variable, and odds ratios are associated with each 1 mg increment in initial buprenorphine dose.
p<0.05.
p<0.01.
p<0.001.
The results of sensitivity analyses restricted to the subsample with a documented opioid use disorder diagnosis (total N=8,077) during either the 60-day baseline period or the treatment episode were similar to the main findings, with lower rates of emergency department visits (odds ratio=0.74, 95% CI=0.64–0.86, p<0.001), all-cause in-patient hospital admissions (odds ratio=0.79, 95% CI=0.63–0.99, p<0.05), and opioid prescription fills (odds ratio=0.68, 95% CI=0.56–0.82, p<0.001) in the 15- to 18-month cohort compared with the 6- to 9-month reference group (see Table S3 in the online supplement). In the sensitivity analysis, the odds of overdose remained equivalent across all cohorts, with an overall observed rate of 5.6% in the 6 months following treatment discontinuation.
DISCUSSION
Among patients retained long term on buprenorphine, irrespective of treatment duration, the 6-month window following buprenorphine discontinuation was a high-risk period for adverse events, especially among patients with comorbid mental illness. Notably, rates of opioid prescription claims and emergency department visits were high for all cohorts, averaging more than 25% and 45%, respectively. Of particular concern is that medically treated overdose rates after buprenorphine discontinuation were around 5% for all cohorts, suggesting that overdose events in the subacute period following buprenorphine discontinuation remain common irrespective of treatment duration.
Compared with patients retained on buprenorphine for 6–9 months, those retained for 15–18 months had superior clinical outcomes in the 6-month period following buprenorphine discontinuation, with lower odds of opioid prescription fills, emergency department visits, and hospital admissions. However, this association did not extend to risk of medically treated overdose.
To our knowledge, this is one of the first studies to empirically demonstrate superior outcomes in the period following buprenorphine discontinuation for patients who are maintained in buprenorphine treatment beyond 6 months. The findings suggest that greater lengths of treatment are warranted than are usually achieved in clinical practice or reflected in existing quality measures (16). Patients with opioid use disorder may require an extended period of medication adherence and stabilization to consolidate improved function across multiple domains in life, including comprehensive treatment of co-occurring mental disorders. While cohort comparisons may be confounded by unmeasured patient-level or environmental variables related to the timing of treatment discontinuation, the results are consistent with a growing literature underscoring the protective effects of long-term pharmacotherapy for opioid use disorder as opposed to short-term use or brief detoxification (14). Regardless, eventual treatment discontinuation was associated with high rates of potentially fatal adverse events in the ensuing months.
Among persons with opioid use disorder, periods when patients receive pharmacotherapy (i.e., buprenorphine or methadone) have been found to be associated with lower risk of overdose mortality compared with periods when they are not in treatment (28, 29). Our study refines this finding, demonstrating that the period following buprenorphine discontinuation is associated with rates of overdose approximately two to three times higher than those observed in a general sample of patients with opioid use disorder (29). Although our results do not demonstrate a significant reduction in medically treated overdoses following discontinuation among patients retained in treatment for longer periods, this may be related to insufficient power and warrants follow-up studies with larger samples.
The rate of overdose in our sample was within the approximate annual rate of 5%–7.5% for subsequent overdoses reported in previous studies (2) for patients who have experienced a past-year overdose. Emergency department visits and use of opioids have also been related to increased risk of subsequent overdose (2). Unlike previous studies, ours is the first designed specifically to evaluate outcomes among patients retained for a minimum of 6 months and to examine risks during the 6-month period following buprenorphine discontinuation rather than the period during buprenorphine treatment.
Little is known about patterns of buprenorphine adherence over the long-term. Gordon and colleagues (30) reported that among Pennsylvania Medicaid patients, only 21% persistently refilled buprenorphine for 12 months. That cohort, compared with one that persisted for only 3–5 months, had an 18% lower risk of hospitalization and a 14% lower risk of emergency department use. These findings are consistent with a recent review of 55 studies on retention that found that most patients in community practice who initiate buprenorphine treatment discontinue before 3–6 months (6, 7). Consistent with previous studies of Medicaid patients (31), we found that nonwhite patients were somewhat less likely to receive buprenorphine for extended durations, underscoring the need to identify factors that contribute to these disparities and develop interventions to support extending treatment in minority patient populations.
We chose to focus on patients who were retained on buprenorphine for a minimum of 6 months, an understudied population. Although we controlled for several patient characteristics that are associated with the cohort membership and adverse outcomes, residual confounding remains a possible problem. It was not possible to ascertain indicators of addiction severity or response to treatment from claims data. However, covariates associated with addiction severity were included in all analyses (e.g., comorbid substance use disorder, mental illness), and the sample likely represents relatively stable patients, since they are among a minority of Medicaid patients who reach at least 6 months of treatment (6, 30, 31). Similarly, the study patients also likely differ from the large number of individuals who discontinue buprenorphine in the first few weeks or months of treatment. Prior treatment history, level of concern about relapse, and beliefs about the effectiveness of maintenance treatment have been associated with treatment discontinuation in addition to relapse events (10). The outcomes of patients receiving longer-term maintenance treatment can inform us on system design elements needed to appropriately treat opioid use disorder under a longitudinal care model to improve long-term retention (3, 14). An additional limitation concerns lack of data regarding vital status. We were unable to distinguish fatal from nonfatal overdose events. However, given that overdoses occurred at similar rates across cohorts, it is unlikely that fatal overdoses were differentially distributed across the study cohorts. Beneficiaries who experienced fatal overdoses in the community during their care episode or in the 6 months following discontinuation would have lost Medicaid eligibility and therefore been ineligible for analysis in this study. Our study was limited to Medicaid beneficiaries, excluding those who also received Medicare, and our results also may not generalize to other publicly or commercially insured, or uninsured, populations. Finally, MarketScan Medicaid claims contain data from multiple states anonymously reporting data, thereby complicating external generalizability to other locations.
CONCLUSIONS
For a great majority of patients with opioid use disorder, risk of relapse following treatment discontinuation is high, yet there is no consensus on the treatment duration necessary to achieve long-term recovery. In this study, among individuals retained in treatment for at least 6 months, high rates of adverse events occurred after treatment discontinuation, especially among patients with comorbid mental illness. Longer as compared with shorter retention in buprenorphine treatment (i.e., a minimum of 15–18 months) was associated with superior outcomes. Although the study design cannot establish a causal relationship between longer retention and clinical outcomes, the results suggest that postdiscontinuation benefits may not begin to accrue until well after the 6-month mark. This would imply that longer lengths of treatment are warranted than are currently reflected in clinical practice (3,5,6,30) or referenced in existing quality measures (16).
Given high rates of early treatment discontinuation among patients who initiate buprenorphine treatment, often exceeding 50% within 3–6 months (3, 6), greater efforts at the clinical and systems levels are needed to improve patient retention. Priority should be given to redesigning systems of care to emphasize chronic disease management models under collaborative care teams with emergency response capabilities for reaching patients who discontinue medication or disengage from care. Public and private insurance benefit design, utilization management, and clinical policies could be leveraged to enhance long-term retention in buprenorphine treatment. Finally, structural interventions, such as placement of care coordinators, development and routine monitoring of quality measures, and capitated or enhanced provider reimbursement for extended buprenorphine treatment, should also be considered to promote improved outcomes for this patient population.
Supplementary Material
Acknowledgments
Supported by NIDA grants K23 DA044342, T32 DA031099, and 1R01 DA047347–01, by Agency for Healthcare Research and Quality grants R18 HS03258, U19 HS021112, and R18HS02346, and by NIH’s National Center for Advancing Translational Sciences under award number UL1TR003017.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Dr. Samples has received consulting fees from the American Society for Addiction Medicine. The other authors report no financial relationships with commercial interests.
Contributor Information
Arthur Robin Williams, Department of Psychiatry, New York State Psychiatric Institute, Columbia University Medical Center, New York
Hillary Samples, Department of Epidemiology, Columbia University Mailman School of Public Health, New York
Stephen Crystal, Institute for Health, Health Care Policy, and Aging Research, Rutgers University, New Brunswick, N.J.
Mark Olfson, Department of Psychiatry, New York State Psychiatric Institute, Columbia University Medical Center, New York
REFERENCES
- 1.Collins FS, Koroshetz WJ, Volkow ND: Helping to end addiction over the long term: the research plan for the NIH HEAL initiative. JAMA 2018; 320:129–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larochelle MR, Bernson D, Land T, et al. : Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med 2018; 169:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams AR, Nunes EV, Bisaga A, et al. : Development of a cascade of care for responding to the opioid epidemic. Am J Drug Alcohol Abuse 2019; 45:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones CM, Campopiano M, Baldwin G, et al. : National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health 2015; 105:e55–e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meinhofer A, Williams AR, Johnson P, et al. : Prescribing decisions at buprenorphine treatment initiation: do they matter for treatment discontinuation and adverse opioid-related events? J Subst Abuse Treat 2019; 105:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timko C, Schultz NR, Cucciare MA, et al. : Retention in medication-assisted treatment for opiate dependence: a systematic review. J Addict Dis 2016; 35:22–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eastwood B, Strang J, Marsden J: Effectiveness of treatment for opioid use disorder: a national, five-year, prospective, observational study in England. Drug Alcohol Depend 2017; 176:139–147 [DOI] [PubMed] [Google Scholar]
- 8.Barry CL, McGinty EE, Pescosolido B, et al. : Stigma, discrimination, treatment effectiveness, and policy: public views about drug addiction and mental illness. Psychiatr Serv 2014; 65: 1269–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin FR: Bisaga A, Sullivan MA, et al. : A review of a national training initiative to increase provider use of MAT to address the opioid epidemic. Am J Addict 2016; 25:603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bentzley BS, Barth KS, Back SE, et al. : Discontinuation of buprenorphine maintenance therapy: perspectives and outcomes. J Subst Abuse Treat 2015; 52:48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark RE, Samnaliev M, Baxter JD, et al. : The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Aff (Millwood) 2011; 30: 1425–1433 [DOI] [PubMed] [Google Scholar]
- 12.McKenna RM: Treatment use, sources of payment, and financial barriers to treatment among individuals with opioid use disorder following the national implementation of the ACA. Drug Alcohol Depend 2017; 179:87–92 [DOI] [PubMed] [Google Scholar]
- 13.Volkow ND, Frieden TR, Hyde PS, et al. : Medication-assisted therapies: tackling the opioid-overdose epidemic. N Engl J Med 2014; 370:2063–2066 [DOI] [PubMed] [Google Scholar]
- 14.National Academies of Sciences, Engineering, and Medicine: Medications for Opioid Use Disorders Save Lives. Washington, DC, National Academies Press, 2019 [PubMed] [Google Scholar]
- 15.American Society of Addiction Medicine (ASAM): The ASAM Performance Measures for the Addiction Specialist Physician. Chevy Chase, Md, American Society for Addiction Medicine, 2014 [Google Scholar]
- 16.Williams AR, Nunes EV, Bisaga A, et al. : Developing an opioid use disorder treatment cascade: a review of quality measures. J Subst Abuse Treat 2018; 91:57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Quality Forum (NQF): Behavioral Health 2016–2017: Technical Report. Washington, DC, Department of Health and Human Services, 2017(https://www.qualityforum.org/Publications/2017/08/BehavioraLHealth_2016-2017_Final_Report.aspx) [Google Scholar]
- 18.American Psychiatric Association: APA wins award from CMS to develop quality measures. Psychiatr News, October 12, 2018. ( 10.1176/appi.pn.2018.10b19) [DOI] [Google Scholar]
- 19.Lo-Ciganic WH, Gellad WF, Gordon AJ, et al. : Association between trajectories ofbuprenorphine treatment and emergency department and in-patient utilization. Addiction 2016; 111:892–902 [DOI] [PubMed] [Google Scholar]
- 20.Parran TV, Adelman CA, Merkin B, et al. : Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend 2010; 106:56–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis ML, Foss MA, Scott CK: An eight-year perspective on the relationship between the duration of abstinence and other aspects of recovery. Eval Rev 2007; 31:585–612 [DOI] [PubMed] [Google Scholar]
- 22.Carrieri MP, Amass L, Lucas GM, et al. : Buprenorphine use: the international experience. Clin Infect Dis 2006; 43(suppl 4):S197–S215 [DOI] [PubMed] [Google Scholar]
- 23.Clemans-Cope L, Epstein M, Kenney GM: Rapid Growth in Medicaid Spending on Medications to Treat Opioid Use Disorder and Overdose. Washington, DC, Urban Institute, 2017. (https://www.urban.org/sites/default/files/publication/91521/2001386-rapid-growth-in-medicaid-spending-on-medications-to-treat-opioid-use-disorder-and-overdose_2.pdf) [Google Scholar]
- 24.Saloner B, Daubresse M, Caleb Alexander G: Patterns of buprenorphine-naloxone treatment for opioid use disorder in a multi-state population. Med Care 2017; 55:669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadland SE, Bagley SM, Rodean J, et al. : Receipt of timely addiction treatment and association of early medication treatment with retention in care among youths with opioid use disorder. JAMA Pediatr 2018; 172:1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Substance Abuse and Mental Health Services Administration: Federal Guidelines for Opioid Treatment Programs. HHS Publication No (SMA) PEP15-FEDGUIDEOTP. Rockville, Md, Substance Abuse and Mental Health Services Administration, 2015 [Google Scholar]
- 27.World Health Organization: ICD-10: International Classification of Diseases and Related Health Problems, 10th Revision. Geneva, World Health Organization, 2015 [Google Scholar]
- 28.Degenhardt L, Bucello C, Mathers B, et al. : Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction 2011; 106:32–51 [DOI] [PubMed] [Google Scholar]
- 29.Sordo L, Barrio G, Bravo MJ, et al. : Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017; 357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon AJ, Lo-Ciganic WH, Cochran G, et al. : Patterns and quality of buprenorphine opioid agonist treatment in a large Medicaid program. J Addict Med 2015; 9:470–477 [DOI] [PubMed] [Google Scholar]
- 31.Samples H, Williams AR, Olfson M, et al. : Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abuse Treat 2018; 95:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.