Abstract
Background:
Dietary fiber increases short-chain fatty acid (SCFA)-producing bacteria yet is often withheld in the intensive care unit (ICU). This study evaluated the safety and effect of fiber in ICU patients with sampling of the gut microbiome at ICU admission and after 72 hours.
Methods:
Consecutive adults 18 years or older were eligible if they were newly admitted to the ICU. Rectal swabs were performed at ICU admission and 72 hours later. The primary exposure was fiber intake over 72 hours, classified in tertiles and adjusted for caloric intake. The primary outcome was the relative abundance (RA) of SCFA-producers based on 16S rRNA sequencing and the tolerability of dietary fiber.
Results:
In 129 ICU patients, median fiber intake was 13.4 g (IQR 0–35.4g) over 72 hours. The high fiber intake group had less abdominal distension (11% high fiber vs 28% no fiber, p<0.01), and no increase in diarrhea (15% high fiber vs 13% no fiber, p=0.94) or other adverse events. After 72 hours, the median RA of SCFA producers was 0.40%, 0.50%, and 1.8% respectively for the no, low, and high fiber intake groups (p=0.05 for trend). After correcting for total caloric intake, the median RA of SCFA producers was 0.41%, 0.32% and 2.35% in the no, low and high corrected fiber categories (p<0.01). These associations remained significant after adjusting for clinical factors including antibiotics.
Conclusions:
During the 72 hours after ICU admission, fiber was well tolerated and higher fiber intake was associated with less decrease in SCFA-producing bacteria.
Keywords: dietary fiber, critically ill, gut microbiome, short chain fatty acid
INTRODUCTION
Pre- and probiotics are being explored in the ICU in the hope that they will modify the gut microbiome to prevent multidrug-resistant (MDR) bacterial colonization and reduce healthcare-associated infections (HAIs). In a meta-analysis of randomized trials, probiotics showed benefit in preventing ventilation associated pneumonia and Clostridiodes difficile infection in the ICU.1–3 However, probiotic trials have been heterogeneous in terms of both interventions and results, and some trials have suggested harm.2, 4–6
Dietary fiber is a prebiotic which increases the abundance of bacteria which produce butyrate and other short-chain fatty acids (SCFAs). These bacteria, or their metabolites, appear to have immunomodulatory benefits that are relevant for ICU patients.7–9 In bone marrow transplant recipients and select ICU cohorts, increased abundance of SCFA-producing bacteria was associated with decreased MDR organism colonization including decreased vancomycin-resistant Enterococcus.8, 10 In the ICU, diminished levels of SCFA producers have been associated with higher Enterococcus and risk for death.11 In healthy volunteers and ambulatory patients, fiber is well tolerated and increases SCFA producer levels.12
For these reasons, fiber may be an appropriate prebiotic to test in the ICU. However, concerns are often raised that fiber will increase bloating and contribute to diarrhea during critical illness.13, 14 Rare cases of bezoars and intestinal obstruction have been reported. Guidelines emphasize the selective use of fiber for diarrhea rather than for all ICU patients.15 In clinical practice, ICU patients often receive fiber-free enteral formulations. Overall, it is uncertain whether ICU patients who receive broad-spectrum antibiotics, mechanical ventilation, and other interventions will respond to dietary fiber in the same manner as healthy volunteers or ambulatory patients.
This study was conducted to assess the relationship between dietary fiber on SCFA-producing bacteria within the gastrointestinal microbiome of ICU patients, and also to assess its tolerability. Fiber intake and clinical factors were measured within a cohort of ICU patients whose gut microbiota was serially sampled.
METHODS
Population
This was a retrospective study nested within a longitudinal, prospective ICU-based cohort.8 In brief, adults 18 years old or more were eligible for the study if they were newly admitted to any one of 5 distinct medical or surgical ICUs and could be reached within 4 hours of ICU admission. Rectal swabs were performed at the time of ICU admission and 72 hours later (±4 hours), and clinical data was gathered. The study focused on the 72-hour window following ICU admission because of prior data indicating that interventions occurring early during critical illness are disproportionately important in patient outcome.16 Patients who were nil per os (NPO), who received enteral nutrition, or who took food by mouth were all included provided that their nutritional intake was clearly recorded and calculable. Patients were excluded if the 72-hour study assessment could not be completed due to death, discharge, or patient refusal. Informed consent was obtained from all subjects or from appropriate surrogates.17 This study was approved by the institutional review board of Columbia University Irving Medical Center.
Fiber Intake
The primary exposure was total fiber intake from the time of ICU admission when the first sample was taken until 72 hours later when the second sample was taken. Fiber intake was measured by manually extracting data from electronic nursing flow sheets. For patients receiving enteral feeds, the type of feed, its nutritional values, the hourly infusion rate and the duration of infusion were collected. Interruptions of feeding were recorded to the hour by the nursing staff. The amount of soluble fiber in each type of feed was obtained from the manufacturer’s published nutritional information sheet. Fiber intake over the 72-hour period was then calculated by multiplying the total volume of feeds by the amount of soluble fiber per unit. For patients receiving oral diets, the type of diet and the number of meals were extracted from the electronic orders. Nutritional values for each meal type were obtained from the Department of Food and Nutrition, including the fiber content of that meal. In the participating ICUs, nurses visually inspect the meal tray after each mealtime and record the percentage of each meal consumed in quartiles. The percentage of meal consumption as recorded by the patient’s nurse was extracted from the nursing flow sheets. Short-term withholding of meals (e.g., before procedures) was also accounted for. The amount of fiber intake over the 72-hour period was then calculated as number of meals received multiplied by the percentage of meal consumption from the nursing flowsheet and by the amount of fiber contained in each meal. The calculated total fiber intake was analyzed in tertiles or as a continuous measure.
Other Nutritional Intake
Total caloric intake was calculated in a similar fashion as fiber intake. A patient-specific calorie target was calculated by a registered clinical dietician using a hospital-wide nutritional assessment protocol taking into consideration age, body mass index, ventilation status, and comorbidities adapted from the American Society of Parenteral and Enteral Nutrition (ASPEN) guidelines.15 Additional details are described in the Supplemental Methods. The actual caloric intake over the 72-hour period was then divided by total calorie target to obtain the percentage of target calories received. The amount of fiber consumed depends on total caloric consumption so, to explore the possibility that relative consumption of fiber was a determinant of fecal microbial composition, a calorie-corrected “fiber index” was calculated. The fiber index was defined as fiber intake over the 72-hour period divided by the percentage of calorie target received.
Short Chain Fatty Acid-Producing Bacteria
The primary outcome was the relative abundance of SCFA-producing bacteria after 72 hours in the ICU based on 16S rRNA gene sequencing results from the 72-hour rectal swab. This was defined as the sum total of the relative abundance of the following taxa within Clostridial Clusters IV/XIVa, with all taxa specified at the lowest possible hierarchical level: Faecalibacterium prausnitzii, Eubacterium rectale, Ruminococcus, Blautia, Coprococcus, and Roseburia.7–9 Additional sequencing details are described in the Supplemental Methods. Sequencing data is available in the short-read archive section of the National Center for Biotechnology Information (accession number SRP149563).
Gastrointestinal Microbiome
An untargeted hierarchical linear discriminant analysis (LDA) effect size algorithm (LEfSe)18 was used with sequencing results to identify altered taxa based on fiber intake. Additionally, Enterococcus relative abundance was pre-specified as a secondary outcome of interest within the gastrointestinal microbiome because our previous study identified it as an independent predictor of death and infection in the ICU study.11 Standard microbiome alpha diversity measures (Shannon and Chao index) were assessed, also based on 16S rRNA gene sequencing.
Co-Variables
Demographic information, laboratory data, and data related to ICU interventions was extracted from the electronic medical record. Baseline comorbidities were recorded including immunosuppression and sepsis as the admitting diagnosis. ICU interventions were recorded including proton pump inhibitors, mechanical ventilation, vasopressor support and hemodialysis (either intermittent or continuous). Antibiotic use (any dose or duration) was recorded and divided into narrow- and broad-spectrum based on previous studies reporting the extent of antibiotic disruption to the gut microbiota.19–22 For clinical data, the following data was extracted: presence of edema on physical exam, abdominal distention, nausea/vomiting (either by symptoms recorded in the physician’s notes or the administration of antiemetic agents), diarrhea, bowel obstruction confirmed by radiography, history of gastrointestinal surgery, and severe malnutrition as diagnosed by a clinical nutritionist. Clinical and laboratory data were used to estimate acute severity of illness as the Acute Physiologic Assessment and Chronic Health Evaluation (APACHE IV) score.23
Statistical Analysis
Comparisons of clinical data were performed using Fisher’s exact test or a chi-squared tests for categorical data. T tests or rank-sum tests were used for continuous variables. For multiple groups, analysis of variance (ANOVA) or Kruskal-Wallis tests were used. To assess the independent effect of fiber after adjusting for additional exposures, a non-parametric least squares regression model was constructed for the outcome of relative abundance of SCFA producers.24, 25 Exposure variables were included in this model if they had an independent relationship with SCFA producer level (p<0.10). Alpha was 0.05 for all analyses unless otherwise specified.
RESULTS
Population
A total of 178 patients were enrolled in the original study cohort including 142 who completed both study assessments and were considered for this study (36 patients died or refused the second sample collection). An additional thirteen patients were excluded because there was insufficiently detailed dietary information recorded in the nursing flowsheets, leaving 129 patients who were analyzed.
Fiber Intake
Median cumulative fiber intake during the 72 hours after ICU admission was 13.4 g (IQR 0–35.4g). Excluding patients who received no fiber, the median cumulative fiber intake was 27.3 g over 72 hours (IQR 11.6–39.3g). Patients who received more fiber were less acutely ill (lower APACHE IV scores) and less likely to receive broad-spectrum antibiotics (Table 1). They also had a higher total caloric intake and attained a higher percentage of goal calories (Table 2). There were no differences in gastrointestinal events based on fiber intake category including no difference in bowel obstruction, nausea or vomiting, enteric infections, edema, or diarrhea (Table 3). Patients with higher fiber intake were less likely to have abdominal distension (11% high fiber vs 4% low fiber vs 28% no fiber, chi-squared p<0.01).
Table 1.
Baseline and ICU characteristics, stratified by fiber intake.
| CHARACTERISTICS (N, %) | NO FIBER (N=36) | LOW FIBER (N=46) | HIGH FIBER (N=47) | P-VALUE |
|---|---|---|---|---|
| Baseline | ||||
| Female sex | 17 (47) | 21 (48) | 19 (40) | 0.51 |
| Age | 0.09 | |||
| <60 | 14 (39) | 12 (26) | 21 (45) | |
| 60–70 | 10 (28) | 24 (52) | 13 (28) | |
| >70 | 12 (33) | 10 (22) | 13 (28) | |
| ICU type | 0.23 | |||
| Medical | 21 (58) | 20 (43) | 28 (60) | |
| Surgical | 15 (42) | 26 (57) | 19 (40) | |
| Immunosuppression | 6 (30) | 4 (42) | 8 (39) | 0.44 |
| Kidney disease | 6 (13) | 7 (15) | 9 (19) | 0.72 |
| Diabetes | 16 (34) | 16 (34) | 14 (30) | 0.38 |
| Pulmonary disease | 3 (6) | 2 (4) | 4 (8) | 0.49 |
| Intensive care unit | ||||
| Sepsis | 16 (34) | 12 (26) | 7 (15) | <0.01 |
| APACHE IV score | <0.01 | |||
| Lowest tertile (< 45) | 7 (19) | 14 (30) | 25 (53) | |
| Medium tertile (45–70) | 9 (25) | 16 (35) | 12 (26) | |
| Highest tertile (>70) | 20 (56) | 16 (35) | 10 (21) | |
| Antibiotics | 0.02 | |||
| None | 2 (6) | 10 (22) | 17 (36) | |
| Narrow-spectrum | 12 (33) | 10 (22) | 10 (21) | |
| Broad-spectrum | 22 (61) | 26 (56) | 20 (42) | |
| Proton pump inhibitors | 16 (44) | 22 (48) | 25 (53) | 0.72 |
| Mechanical ventilation | 14 (39) | 4 (9) | 3 (6) | <0.01 |
| Hemodialysis | 6 (17) | 3 (6) | 1 (2) | <0.01 |
| Vasopressors | 14 (30) | 8 (17) | 5 (11) | <0.01 |
Table 2.
Nutrition during the 72 hours after ICU admission, stratified by fiber intake.
| NUTRITIONAL CHARACTERISTICS OVER 72 HOURS | NO FIBER (N=36) | LOW FIBER (N=46) | HIGH FIBER (N=47) | P-VALUE |
|---|---|---|---|---|
| Total fiber (median g, IQR) | 0.0 | 11.2 (3.8–18.2) | 39.3 (34.7–50.2) | <0.01 |
| (mean g, standard deviation) | 0.0 (0.0) | 11.0 (7.5) | 43.4 (13.2) | |
| Nutritional intake (n, %) | <0.01 | |||
| NPO | 25 (69) | 0 | 0 | |
| Enteral feeds | 11 (31) | 3 (9) | 1 (2) | |
| By mouth | 0 | 43 (91) | 46 (98) | |
| Total calories (median kcal, IQR) | 0 (0–128) | 1082 (674–1574) | 3215 (2698–3769) | <0.01 |
| Target calories (median kcal, IQR) | 0.0% (0.0–2.4%) | 16.9% (9.9–27.2%) | 61.3% (46.2–71.7%) | <0.01 |
| Fiber index (MEDIAN G/%, IQR) | 0.0 | 49.7 (28.9–76.0) | 74.0 (63.6–82.3) | <0.01 |
| Total protein (median g, IQR) | 0.0 (0.0–6.6) | 46.5 (6.3–68.7) | 153.5 (133.7–177.4) | <0.01 |
| Total fat (median g, IQR) | 0.0 (0.0–3.6) | 33.2 (4.7–54.6) | 105.7 (94.0–129.4) | <0.01 |
| Diagnosis of malnutrition (n, %) | 1 (2) | 2 (4) | 0 | 0.37 |
Table 3.
Gastrointestinal events during the 72 hours after ICU admission, stratified by fiber intake.
| GASTROINTES TINAL EVENTS (N, %) | NO FIBER (N=36) | LOW FIBER (N=46) | HIGH FIBER (N=47) | P-VALUE, HIGH VS. NO FIBER | P-VALUE, HIGH VS. MEDIUM FIBER |
|---|---|---|---|---|---|
| Abdominal distension | 13 (36) | 2 (4) | 5 (11) | <0.01 | <0.01 |
| High gastric residuals | 0 | 0 | 0 | --- | --- |
| Bowel obstruction | 0 | 0 | 0 | --- | --- |
| Nausea or vomiting | 8 (22) | 21 (46) | 21 (45) | 0.04 | 0.23 |
| Enteric infections | 2 (6) | 4 (9) | 0 | 0.18 | 0.69 |
| Edema | 10 (28) | 10 (22) | 12 (26) | >0.99 | 0.61 |
| Diarrhea | 6 (17) | 8 (17) | 7 (15) | >0.99 | >0.99 |
Fiber Index
Total fiber intake tends to be correlated with total caloric intake, so a fiber index was calculated to assess the impact of fiber on the gut microbiome after correcting for total caloric intake. Like high fiber patients, high fiber index patients were less acutely ill and less likely to receive broad-spectrum antibiotics compared to low fiber index patients, although these differences were less marked compared to total fiber intake (Supplemental Table 1).
Fiber Intake and SCFA Producing Bacteria
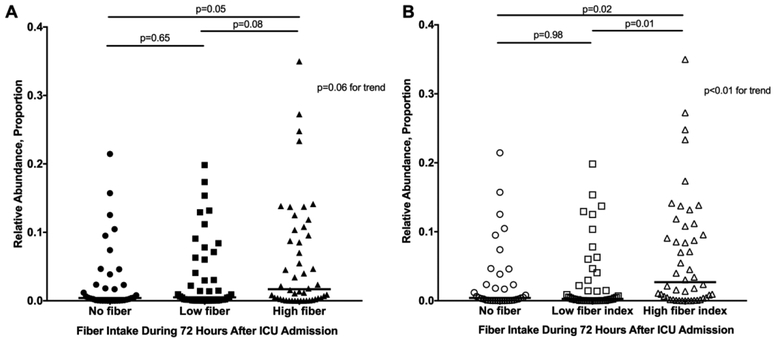
The median relative abundance of SCFA producers was 2.8% (IQR 0.42–7.9%) at the time of ICU admission and 0.80% (IQR 0.062–6.3%) 72 hours later (median change −0.33%, Wilcoxon p=0.02). Relative abundance of SCFA producers was similar at ICU admission across fiber intake categories (Supplemental Figure 1). After 72 hours, higher levels of SCFA producers were seen in patients with higher fiber intake (median 1.8% high fiber vs 0.40% no fiber, Figure 1A). Results were similar based on calorie-corrected fiber index rather than fiber intake (Figure 1B).
Figure 1.
Relative abundance of short chain fatty acid-producing bacteria 72 hours after ICU admission. This is shown stratified by (A) fiber intake and (B) fiber index during the 72 hours after ICU admission.
Gastrointestinal Microbiome
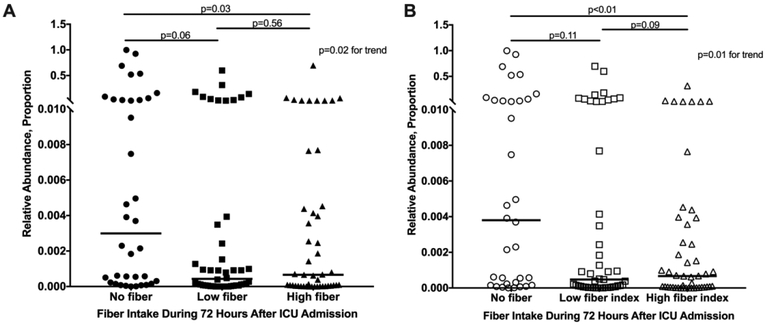
An untargeted algorithm (LEfSe) was used to test for differences in the relative abundance across all bacterial taxa based on fiber intake. The SCFA producers Blautia (4.0-fold difference) and F. prausnitzii (3.9-fold difference) were among 7 taxa that were enriched in the high fiber group compared to the no fiber group. Enterococcus (4.8-fold difference) was the only taxon that was enriched in the no fiber group compared to the high fiber group. Comparing the high fiber group to the low fiber group, Blautia was among 3 taxa that were enriched in the high fiber group (3.7-fold difference). Median relative abundance of Enterococcus was 0.0076% at ICU admission (IQR 0.0016–0.13%) and 0.070% (IQR 0.0072–0.77%) 72 hours later (median change 0.010%, Wilcoxon p<0.01). Higher fiber intake associated with lower abundance of Enterococcus after 72 hours (median 0.066% high fiber vs 0.30% no fiber, Figure 2A). Results were similar based on fiber index rather than fiber intake (Figure 2B).
Figure 2.
Relative abundance of Enterococcus 72 hours after ICU admission. This is shown stratified by (A) fiber intake and (B) fiber index during the 72 hours after ICU admission.
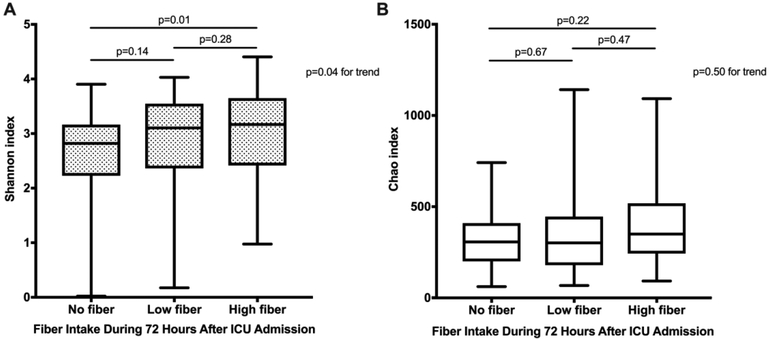
The diversity and richness of the gut microbiota was analyzed using the Shannon and Chao indices. Over the 72-hour period, there was a significant within-individual decline in Shannon diversity (median −0.20, 95% CI −0.34 to −0.063, p<0.01) and Chao richness (median −26, 95% CI −48 to −3.7, p=0.02). Higher fiber intake was associated with higher fecal microbial diversity at 72 hours yet not richness (p=0.04 and 0.50 respectively, Figures 3A and 3B). A similar result was seen based on fiber index (data not shown).
Figure 3.
(A) Fecal bacterial diversity (Shannon index) and (B) fecal bacterial richness (Chao) 72 hours after ICU admission, stratified by fiber intake during the 72 hours after ICU admission.
Multivariable Model
In a multivariable model, higher fiber intake remained associated with higher relative abundance of SCFA producers after adjusting for antibiotics, admission APACHE IV score, and exposure to mechanical ventilation or use of vasopressors (Table 4). There was a 0.30% median increase in SCFA producers after 72 hours per additional 10g of fiber intake (p<0.01).
Table 4.
Multivariable model for relative abundance (RA) of short chain fatty acid (SCFA) producers after 72 hours in the ICU.
| Exposure Variables | Median Increase in RA of SCFA Producers | Estimate IQR | p-value |
|---|---|---|---|
| Fiber, per 10 g increase | +0.30% | +0.10 to +0.46% | <0.01 |
| Antibiotics | |||
| None | Reference | --- | --- |
| Narrow-spectrum only | +1.0% | −2.4 to +4.4% | 0.56 |
| Broad-spectrum | −0.62% | −3.6 to +2.4% | 0.69 |
| APACHE IV score at admission, per 10-point increase | +0.27% | −0.15 to +0.69% | 0.21 |
| Mechanical ventilation | +0.40% | −3.1 to +3.9% | 0.82 |
| Vasopressors | −1.7% | −4.7 to +1.3% | 0.26 |
This kernel-based least squares regression model estimates the median independent effect of each exposure variable on the outcome of relative abundance of SCFA producers after 72 hours in the ICU. This model was selected because the relative abundance of SCFA producers is non-linear (left skewed) data. See text for additional details. IQR: interquartile range (25th to 75th percentile).
DISCUSSION
In 129 critically ill patients sampled at the time of ICU admission and 72 hours later, higher fiber intake was associated with higher levels of SCFA-producing bacteria. Fiber intake was not associated with adverse gastrointestinal events including diarrhea. The association between fiber intake and SCFA producers was seen even though 77% of the study cohort received antibiotics and even after accounting for acute severity of illness. The association also remained even after correcting as much as possible for the fact that patients with a lower caloric intake also inevitably had a lower fiber intake.
Considerable efforts are being expended to develop pre- or probiotics suitable for critically ill patients. SCFA producers are among the leading targets for such therapies, and therefore the results of this study provide encouraging data that dietary fiber—a simple, inexpensive treatment—may promote the growth of beneficial gut micro-organisms in the ICU. However, it must be recognized that this study was observational and intervention studies with randomization to fiber should be performed before concluding that a causal relationship exists between fiber intake and SCFA producers.
The timing and the density of caloric delivery in the ICU has been studied extensively, usually with disappointing results. Multiple randomized controlled trials (RCTs) have shown negligible clinical differences between early versus late enteral nutrition, and between calorie-dense versus calorie-sparse feeding strategies.26–31 Comparatively few studies have examined the specific effects of dietary fiber. In 15 patients receiving chronic enteral nutrition, fiber had little impact on SCFA producers yet increased the SCFAs including acetate and butyrate.32 Interestingly, fiber was associated with decreased levels of Enterococcus, similar to this study. Another RCT involving 22 critically ill patients found no increase in levels of SCFA producers after a low (7g) daily dose of fiber, although the study failed to meet its enrollment target.33
While ICU studies are conflicting, ambulatory studies have consistently shown that fiber supplementation increases SCFA producer levels.34 In two placebo-controlled studies, fiber was associated with a doubling of F. prausnitzii and other SCFA-producers and concurrent decreases in candidate biomarkers for infection such as serum C-reactive protein (CRP) and lipopolysaccharide (LPS).35, 36 In another placebo-controlled study, the fiber arm had higher post-treatment total SCFA levels and butyrate levels, lower circulating LPS concentrations, and decreased gene expressions of pro-inflammatory cytokines in whole blood.37 In this study, the associations between higher fiber intake and SCFA producer level were large in magnitude, with 4-fold higher F. prausnitzii and Blautia in the highest compared to the lowest fiber intake group. Future investigations should test whether these changes were also associated with biomarkers like CRP or LPS.
Animal studies and data from ambulatory patients suggest that increased SCFA levels may have clinical benefits that are relevant for ICU patients.8 In animals, SCFA producers confer colonization resistance against common enteric pathogens including vancomycin-resistant Enterococcus (VRE).38–43 This study also observed an inverse relationship between SCFA producers and Enterococcus. In addition to higher SCFA producers, dietary fiber intake was associated with improved fecal microbial diversity. Diversity within the microbiome may be a surrogate for colonization resistance against VRE or other pathogens. In ICU patients, fecal microbial diversity tends to be low on admission and to decline with prolonged hospitalization.44, 45 In bone marrow transplant recipients, low diversity and low SCFA producer levels prior to transplant have been associated with VRE bacteremia, respiratory infections, and increased risk for death.7, 10, 46, 47
Historically, fiber has often been withheld in the ICU for fear of diarrhea or bowel obstruction. This study showed no difference in gastrointestinal-related side effects in patients with high fiber intake, although there were relatively few gastrointestinal events overall. To date, there are no reports of serious adverse effects due to soluble fiber in the ICU.48 In a dose-ranging study, ICU patients received open-label wheat dextran for 5 weeks at doses up to 36 g/day with good tolerance.49 Patients had higher SCFA-producers and fecal SCFAs seen comparing post- versus pre-fiber. Like these studies, this study provides reassurance given the concerns occasionally raised regarding prebiotics in patients with altered small bowel or colonic motility.6, 50
There are several strengths to our study. It is one of the first to explore the effect of a specific dietary component in critically ill patients. A rigorous protocol was implemented for sample collection and fiber intake measurement. A broad range of clinical data was collected to ensure comprehensive analysis and to control for important differences between patient groups, including use of antibiotics and acute severity of illness. It also has limitations. Patients in the study were not randomized to different amounts of fiber, and conclusions regarding causality should therefore be cautious. The dietary fibers used in this study varied by chain length, fermentability and solubility. We hope that well-designed RCTs follow up on our findings. Another important limitation of the study design is that fiber intake cannot be completely separated from caloric intake although we made substantial efforts to do so. In addition, it was not possible to collect whole stools immediately at the time of ICU admission and therefore we were unable to directly measure the level of SCFAs.
In sum, in this ICU-based cohort study, fiber intake during the initial 72 hours in the ICU was not associated with gastrointestinal intolerance and was correlated with higher levels of SCFA-producing bacteria, even after adjusting for antibiotics and acute severity of illness. SCFA-producing bacteria have been associated with health benefits in animals and humans, including improved colonization resistance against enteric pathogens. Fiber may be a simple candidate therapy for critically ill patients, and future controlled studies should test whether fiber can modulate the gut microbiome to confer benefit in ICU patients.
Supplementary Material
Clinical Relevancy Statement.
Probiotics have been tested in the intensive care unit (ICU) setting in the hope of enriching the gut microbiome in beneficial micro-organisms. Dietary fiber is a potent prebiotic which has beneficial effects on the gut microbiome of healthy volunteers, but the safety and effect of dietary fiber on the gut microbiome in the ICU is uncertain. In this study, fiber was safe in the ICU and was associated with relative preservation in putatively beneficial short-chain fatty acid producing bacteria.
Support:
Dr. Freedberg was supported in part by a Research Scholar Award from the American Gastroenterological Association, by the National Institutes of Health (K23 DK111847) and by the Feldstein Medical Foundation. Dr. Abrams was supported by the National Institutes of Health (U54 CA163006).
Footnotes
Conflict: The authors of the study disclosed no conflict of interest.
References
- 1.Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. December 19 2017;12:CD006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. October 15 2010;182(8):1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen NT, Maw A, Tmanova LL, et al. Timely Use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review With Meta-Regression Analysis. Gastroenterology. June 2017;152(8):1889–1900 e1889. [DOI] [PubMed] [Google Scholar]
- 4.Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. June 2006;83(6):1256–1264; quiz 1446–1257. [DOI] [PubMed] [Google Scholar]
- 5.Hempel S, Newberry S, Ruelaz A, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess (Full Rep). April 2011(200):1–645. [PMC free article] [PubMed] [Google Scholar]
- 6.Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. February 23 2008;371(9613):651–659. [DOI] [PubMed] [Google Scholar]
- 7.Haak BW, Littmann ER, Chaubard JL, et al. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood. June 28 2018;131(26):2978–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livanos AE, Snider EJ, Whittier S, et al. Rapid gastrointestinal loss of Clostridial Clusters IV and XIVa in the ICU associates with an expansion of gut pathogens. PLoS One. 2018;13(8):e0200322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van den Abbeele P, Belzer C, Goossens M, et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. May 2013;7(5):949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. August 14 2014;124(7):1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedberg DE, Zhou MJ, Cohen ME, et al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. August 01 2018;44(8):1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salonen A, Lahti L, Salojarvi J, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. November 2014;8(11):2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elia M, Engfer MB, Green CJ, Silk DB. Systematic review and meta-analysis: the clinical and physiological effects of fibre-containing enteral formulae. Aliment Pharmacol Ther. January 15 2008;27(2):120–145. [DOI] [PubMed] [Google Scholar]
- 14.Turza KC KJ, Sawyer RG. Enteral feeding and vasoactive agents: suggested guidelines for clinicians. Pract Gastroenterol. 2009(78):11–22. [Google Scholar]
- 15.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. February 2016;40(2):159–211. [DOI] [PubMed] [Google Scholar]
- 16.Levy MM, Macias WL, Vincent JL, et al. Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med. October 2005;33(10):2194–2201. [DOI] [PubMed] [Google Scholar]
- 17.Terry MA, Freedberg DE, Morris MC. An Alternative Consent Process for Minimal Risk Research in the ICU. Crit Care Med. September 2017;45(9):1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. June 24 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. March 15 2011;108 Suppl 1:4554–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rea MC, Dobson A, O’Sullivan O, et al. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc Natl Acad Sci U S A. March 15 2011;108 Suppl 1:4639–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis. September 2001;1(2):101–114. [DOI] [PubMed] [Google Scholar]
- 22.Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. December 2010;120(12):4332–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. May 2006;34(5):1297–1310. [DOI] [PubMed] [Google Scholar]
- 24.Hainmueller J, Hazlett C. Kernel Regularized Least Squares: Reducing Misspecification Bias with a Flexible and Interpretable Machine Learning Approach. Political Analysis. Spr 2014;22(2):143–168. [Google Scholar]
- 25.Ferwerda J, Hainmueller J, Hazlett CJ. Kernel-Based Regularized Least Squares in R (KRLS) and Stata (krls). J Stat Softw. 2017-July-13 2017;79(3):26 [Google Scholar]
- 26.Alberda C, Gramlich L, Jones N, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. October 2009;35(10):1728–1737. [DOI] [PubMed] [Google Scholar]
- 27.Arabi YM, Aldawood AS, Haddad SH, et al. Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. N Engl J Med. June 18 2015;372(25):2398–2408. [DOI] [PubMed] [Google Scholar]
- 28.Braunschweig CA, Sheean PM, Peterson SJ, et al. Intensive nutrition in acute lung injury: a clinical trial (INTACT). JPEN J Parenter Enteral Nutr. January 2015;39(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N, Rice TW, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. February 22 2012;307(8):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Target Investigators ftACTG, Chapman M, Peake SL, et al. Energy-Dense versus Routine Enteral Nutrition in the Critically Ill. N Engl J Med. November 8 2018;379(19):1823–1834. [DOI] [PubMed] [Google Scholar]
- 31.Wei X, Day AG, Ouellette-Kuntz H, Heyland DK. The Association Between Nutritional Adequacy and Long-Term Outcomes in Critically Ill Patients Requiring Prolonged Mechanical Ventilation: A Multicenter Cohort Study. Crit Care Med. August 2015;43(8):1569–1579. [DOI] [PubMed] [Google Scholar]
- 32.Schneider SM, Girard-Pipau F, Anty R, et al. Effects of total enteral nutrition supplemented with a multi-fibre mix on faecal short-chain fatty acids and microbiota. Clin Nutr. February 2006;25(1):82–90. [DOI] [PubMed] [Google Scholar]
- 33.Majid HA, Cole J, Emery PW, Whelan K. Additional oligofructose/inulin does not increase faecal bifidobacteria in critically ill patients receiving enteral nutrition: a randomised controlled trial. Clin Nutr. December 2014;33(6):966–972. [DOI] [PubMed] [Google Scholar]
- 34.Tap J, Furet JP, Bensaada M, et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ Microbiol. December 2015;17(12):4954–4964. [DOI] [PubMed] [Google Scholar]
- 35.Dewulf EM, Cani PD, Claus SP, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. August 2013;62(8):1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hooda S, Boler BM, Serao MC, et al. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J Nutr. July 2012;142(7):1259–1265. [DOI] [PubMed] [Google Scholar]
- 37.Lecerf JM, Depeint F, Clerc E, et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr. November 28 2012;108(10):1847–1858. [DOI] [PubMed] [Google Scholar]
- 38.Garner CD, Antonopoulos DA, Wagner B, et al. Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica serovar typhimurium murine model of infection. Infect Immun. July 2009;77(7):2691–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobson A, Lam L, Rajendram M, et al. A Gut Commensal-Produced Metabolite Mediates Colonization Resistance to Salmonella Infection. Cell Host Microbe. August 8 2018;24(2):296–307 e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brugiroux S, Beutler M, Pfann C, et al. Genome-guided design of a defined mouse microbiota that confers colonization resistance against Salmonella enterica serovar Typhimurium. Nat Microbiol. November 21 2016;2:16215. [DOI] [PubMed] [Google Scholar]
- 41.Lawhon SD, Maurer R, Suyemoto M, Altier C. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol. December 2002;46(5):1451–1464. [DOI] [PubMed] [Google Scholar]
- 42.Caballero S, Kim S, Carter RA, et al. Cooperating Commensals Restore Colonization Resistance to Vancomycin-Resistant Enterococcus faecium. Cell Host Microbe. May 10 2017;21(5):592–602 e594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morowitz MJ, Di Caro V, Pang D, et al. Dietary Supplementation With Nonfermentable Fiber Alters the Gut Microbiota and Confers Protection in Murine Models of Sepsis. Crit Care Med. May 2017;45(5):e516–e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaborin A, Smith D, Garfield K, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. MBio. September 23 2014;5(5):e01361–01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamarche D, Johnstone J, Zytaruk N, et al. Microbial dysbiosis and mortality during mechanical ventilation: a prospective observational study. Respir Res. December 7 2018;19(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris B, Morjaria SM, Littmann ER, et al. Gut Microbiota Predict Pulmonary Infiltrates after Allogeneic Hematopoietic Cell Transplantation. Am J Respir Crit Care Med. August 15 2016;194(4):450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. October 2012;55(7):905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. J Criti Care. August 19 2016;20(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Keefe SJ, Ou J, Delany JP, et al. Effect of fiber supplementation on the microbiota in critically ill patients. World J Gastrointest Pathophysiol. December 15 2011;2(6):138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McIvor AC, Meguid MM, Curtas S, Warren J, Kaplan DS. Intestinal obstruction from cecal bezoar; a complication of fiber-containing tube feedings. Nutrition. Jan-Feb 1990;6(1):115–117. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.