Abstract
Purpose:
To assess the efficacy of novel Digital spectacles (DSpecs) to improve mobility of patients with peripheral visual field (VF) loss.
Design:
Prospective Case Series.
Methods:
Binocular VF defects were quantified with the DSpecs testing strategy. We implemented an algorithm that generated personalized visual augmentation profiles based on the measured VF. These profiles were achieved by relocating and resizing video signals to fit within the remaining VF in real time. We tested 20 patients with known binocular VF defects with static test images, followed by dynamic walking simulations to determine if they could identify objects and avoid obstacles in an environment mimicking a real-life situation. We assessed the effect of the DSpecs on the visual/hand coordination with object grasping tests. Patients performed these tests with and without the DSpecs correction profile.
Results:
The diagnostic binocular VF testing with the DSpecs was comparable to the integrated monocular standard automated perimetry based on point by point assessment with a mismatch error of 7.0%. Eighteen of 20 patients (90%) could identify peripheral objects in test images with the DSpecs that they could not previously. Visual/hand coordination was successful for 17 patients (85%) from the first trial. The object grasping performance improved to 100% by the third trial. Patient performance, judged by finding and identifying objects in the periphery in a simulated walking environment, was significantly better with the DSpecs (P= 0.02, Wilcoxon rank sum test).
Conclusions:
DSpecs may improve mobility by facilitating the ability of patients to better identify moving peripheral hazardous objects.
Keywords: Digital Glasses, Visual Field, Peripheral Visual Defects, Field Expansion, Simulation testing, mobility testing
INTRODUCTION
Peripheral visual field (VF) defects can be caused by several diseases, including glaucoma, cerebrovascular accidents, retinitis pigmentosa, and choroideremia. Over 75 million patients are projected to suffer irreversible VF loss secondary to glaucoma by 2020.1 Visual impairments complicate the course of 72% of all cerebrovascular accidents.2 Binocular VF losses are associated with reduced quality of life activities for many patients.3–5 Peripheral vision losses affect postural stability6,7, motion estimation8, and the ability to avoid hazardous peripheral obstacles. Reduced VF total area and narrower visual fields worsen mobility performance.9–13 Despite recent advances, no medical or surgical treatment can reverse existing damage associated with either glaucoma or stroke. Visual aids attempt to maximize patient functionality14,15, yet often fail to achieve this goal.16,17 Research to evaluate effectiveness of visual aids for patients with damaged peripheral visual field is limited.17,18
Current visual aids relocate or minify the captured scenes to fit within the assumed intact VF. To achieve these goals, investigators have used both optical or electronic/digital solutions. Optical approaches use prisms or magnifying components to expand the field of view. These strategies have not been widely accepted because they result in reduced resolution, overlapping image effects, and require the patient to scan the environment with head rather than eye movements.19–22
Electronic or digital based visual aids apply enhancement techniques to improve central vision by adjusting image contrast, brightness, color, and edge properties with head mounted displays (HMDs).17,21,23–25 Representative examples of this technology include Esight (Esight, Ontario, Canada), Jordy (Enhanced Vision Systems, CA, USA), Flipperport (Enhanced Vision Systems, CA, USA), and Oxsight (Oxsight, Oxford, UK) devices. Clinical trial results and studies performed with these aids are limited26,27, and have focused on testing central vision tasks, such as writing, reading, and identifying objects. Although these tests showed improvements in central vision related tasks, the ability to avoid collisions and restore mobility did not improve.27,28 Failure to achieve this goal is likely related to the fact that current aids do not quantify patient specific visual field defects nor apply a unique strategy to augment visual function.16,21,27 Some investigators have used HMDs to measure or screen monocular VFs21,29–31, but did not use this information to provide patient specific solutions. A uniformly applied criteria to define the benefit of a specific visual aid for improving mobility, independency, and safety would facilitate comparisons among devices.16 Consequently, a need exists for a new low vision aid to improve mobility.32
We developed and used new Digital Spectacles (DSpecs) that measured binocular VF defects and applied a digital image processing strategy unique to each patient to create a visual augmentation profile. Real time augmentation was performed by rescaling and shifting video images of patients’ scene to fit within the remaining intact VF. To demonstrate the improved ability of patients to avoid and identify moving objects located in the periphery, we tested the device in a dynamic walking simulation environment specifically designed to assess mobility improvements.
MATERIALS AND METHODS
We used a commercial virtual reality (VR) HMD to develop the DSpecs visual aid (HTC Vive, Xindian District, New Taipei City, Taiwan). The HMD was integrated with an eye tracking system (Tobii Technology, Danderyd, Sweden) that fed gaze data to the VF testing algorithm (Figure 1.a). The visual aid was also equipped with two high definition (HD) 2 megapixels miniature cameras (Camera sensor: OmniVision, Santa Clara, California, USA) (Figure 1.b). The two cameras provided a binocular view. The cameras provided a field of view of about 85 degrees in diameter, which matched the 80-degree VF testing range of the DSpecs. The VR HMD and the eye tracking system permit a 100-degree field of view (FOV) for each eye. The DSpecs is controlled by a VR enabled laptop (Intel Core i7, 2.8GHz Quad core, NVIDIA 1070, 32GB RAM) that runs the binocular VF testing program and video processing algorithm. The developed algorithms were implemented with MATLAB R2018b (MathWorks, Inc., Natick, MA, USA) and C# under Unity (Unity Technologies, San Francisco, California, USA).
Figure 1:
Digital Spectacles prototype: A) Eye tracking infrared light emitting diodes integrated with the display lens shown from the patient view. B) Two high definition cameras mounted on the virtual reality head mounted display.
PARTICIPANTS RECRUITMENT:
The University of Miami IRB approved the study before patient recruitment. Patients signed informed consent forms before commencing the study. The design was in accordance with the Declaration of Helsinki and all HIPAA regulations.
We examined a test group consisting of 20 patients recruited from glaucoma and neuro ophthalmology clinics at the Anne Bates Leach Eye Center, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine during their regular follow-up visits. All patients had previously performed either 24–2 or 30–2 testing strategy with the Humphrey Zeiss standard automated perimetry (SAP) (Carl Zeiss Meditec, California).
MEASURING THE BINOCULAR VISUAL FIELD:
The VF perimetry method with the DSpecs was based on the SAP static technique with a fast threshold strategy.34 Locations of stimuli were determined by 52 points of a circular grid that covered the central 40-degree radius of both eyes, with an interspace of 10 degrees between each stimulus location. Four contrast staircase stimuli sequences were presented ranging between 32 dB to 20 dB in steps of 4 dB. A value of 0 dB was assigned to the stimuli locations where the patient could not respond. This testing method utilized a bright background with an illumination of 100 lux, and the stimuli were dark dots. This method of testing was relatively accurate for monocular and binocular VF testing (Digital Glasses for Visual Rehabilitation of Glaucoma Patients suffering from Visual Fields Defects”, ARVO, BC, Canada, April 2019). The patient was asked to fixate on a central target, and the eye tracking system monitored fixation during the test. If the patient looked at the stimulus, the test was halted until fixation was restored based on feedback from the eye tracking system.
Based on a normal human FOV33–35, the common binocular area covered by both eyes is approximately the central 60-degrees radius. Our VF test covers the central 40-degrees radius area, a subset region of the total binocular area. Our binocular VF test presented the stimuli to both eyes at the same visual location and time, so that either the left or right eye would detect the stimulus, and the patient would respond accordingly. The stimulus would only be marked as “unseen”, if both eyes were functionally defective at the same visual location in the binocular VF.
VISUAL FIELD MEASUREMENTS ACCURACY:
To assess the accuracy of the DSpecs binocular VF testing method, an estimated binocular VF was first constructed based on the monocular SAP VFs of 20 patients. The estimated binocular VF was constructed by integrating the right and left eyes monocular VF. Monocular integration was performed by selecting the maximum sensitivity value at each position of the monocular VFs. This merging process was based on the maximum sensitivity merging model reported by Crabb and associates35 and the best location integrated binocular model reported by Nelson-Quigg and coworkers.36 The predicted binocular VF was used as a reference to evaluate the accuracy of the DSpecs binocular test. We used the most recent monocular SAP VF tests to construct integrated visual field (IVF) binocular plots. The central 24 or 30 degrees area, depending on previous testing, were compared between the IVF and DSpecs binocular VF. Measurement errors were based on pointwise mismatches between the two methods.
IMAGE REMAPPING:
Image remapping required shifting and resizing operations on the 85 degrees FOV input video signals to fit within the intact regions of the measured binocular VF to increase the functional FOV. The remapping method utilized VF test data to estimate a new scale and new center for the output video signals to be presented. Geometric image properties of the measured intact binocular VF were calculated, including the areas in pixels, bounding pixels, and the weighted center of mass. If multiple intact regions existed in the field, only the largest area was considered for remapping to maximize utilization of the remaining VF. The geometric calculations were fed to a mathematical function to calculate a new center and scale for the video signals to be specifically presented to each patient. Calculation of the patient unique remapping parameters and video processing (shifting and resizing) was performed in real time through the control program. Processed video signals were displayed at a speed of 24 frames per second.
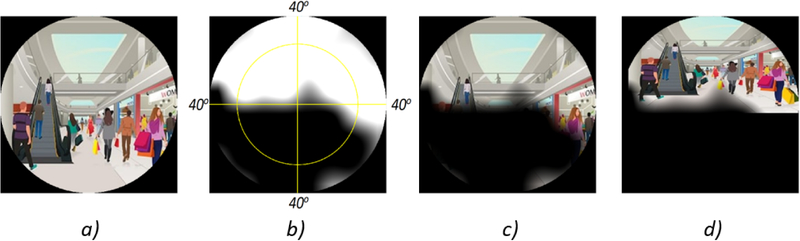
Figure 2 shows an example application of the video remapping process to fit input images within the intact region of a certain VF automatically. (Figure 2.A) shows a scene of a shopping mall as seen through normal vision. (Figure 2.B) is a VF with peripheral defects. (Figure 2.C) shows the scene without remapping where the escalator’s entrance could not be seen. (Figure 2.D) shows the remapped scene where the whole escalator can be noticed. VF was overlaid over the scenes in (Figures 2.C and D) for demonstration purposes only.
Figure 2:
An example showing the image remapping process: a) Example scene of a shopping mall as seen through normal vision. b) Visual Field (VF) with peripheral defects. c) The scene as shown without remapping: the escalator’s entrance cannot be seen. d) Image with remapping: the escalator can now be noticed. VF was overlaid over the image in c) and d) for demonstration purposes only.
Before commencing the walking simulation, we tested the effectiveness of image remapping to augment the functional FOV with static test images of incoming cars in different image quadrants. The test images were first presented without remapping, then with remapping activated. We asked patients to identify the exact nature of the object in the images.
WALKING SIMULATION TEST:
We designed and constructed a computer based walking simulator for testing of our visual aid. The walking simulator ensured the patient safety, where no fall could occur, before testing the use of DSpecs in a walking track.
Hand Coordination Test.
We determined if the DSpecs adversely affected hand coordination before performing the walking simulation test. Patient visual/hand coordination was tested with the DSpecs while activating the binocular video remapping strategy to ensure cognitive coordination between hand movements and visual perception with the remapped video signals. In this test, three objects with different sizes (Height × Width: 23×5, 13×4, and 7.5×3 cm, respectively), were randomly placed at different distances (51, 62, and 70 cm, respectively) on a table in front of the patient. Patients were required to grasp these three objects one at a time and the number of grasp trials was recorded for each object.
Walking simulation environment.
We constructed a walking computer simulation test environment to project a 80” × 60” image on the wall. The simulation environment was constructed geometrically with SketchUp software (Trimble, California, USA), and then converted to 3D and animated with SimLab VR software (Simulation Lab Software, Amman, Jordan). The image of the projector screen covered 80 degrees of the FOV, which matched the DSpecs VF testing range.
The participants performed the simulation while seated in front of the screen where a scene portrayed a long corridor with an equivalent length of 72 meters. Average simulated walking speed was 2.45 meters per second. The participants were able to navigate with a gaming joystick. All participants were instructed to look at the end of the corridor (center of the screen) as a fixation target to limit the confounding effect of eye and head movements scanning. We asked patients to identify the type and shape of eight peripheral objects located above the central horizontal line of sight in the walking corridor. These shapes were initially hidden, but appeared when the virtual walker was within approximately 2 meters of each object. The objects located on the walls included common shapes: a large clock and paintings with basic shapes (circles, triangles, and the letter ‘X’). The shapes were projected to test the two superior VF quadrants, in which each quadrant was stimulated four times during the test, yielding eight static shapes (Figure 3.A).
Figure 3:
A) 3D model of the simualted walking corridor. B) A parcipant performing the rendered walking simulation test with real-world dimensions.
We created six moving obstacles to block the passage of the patient from both sides (initially hidden obstacles: chairs, couches, and tables). The obstacles moved to block the virtual walker when within approximately 2 meters of each obstacle. Participants were asked to stop walking when they noticed the obstacle (Figure 3.B). The simulation software mimicked a collision situation and halted forward movements if the participant did not notice the moving obstacle. We used the moving obstacles to test functionality of the two inferior VF quadrants, where each quadrant was stimulated three times, therefore, the simulation environment had six moving obstacles.
We presented a demonstration version of the walking simulator that displayed sample obstacles and shape before performing the complete track, to demonstrate the test and describe the walking controls. The demonstration corridor was 28 meters long and patients took approximately 2 minutes to complete this learning phase, including explanations by the tester. The patients repeated the demonstration, two to four times, until they felt conformable with the simulation environment and understood how to respond. We believed that this step minimized the potential learning effect on the recorded visual identification responses.
Two walking testing strategies with different obstacles and shapes distributions were displayed. Immediately after completing the virtual walk with one strategy with the DSpecs, the patient would proceed through the other theme without the DSpecs. This was chosen to minimize memory or practice effects, so that the patient would not memorize landmarks from the first completed course. Patients were asked to walk through the corridor twice for each setup (with/without DSpecs). They performed two different themes with the DSpecs and two additional themes without the DSpecs. The order of the walking trials was randomly assigned. Each trial was completed in 1–2 minutes depending on how many times the patient stopped during the test and responded to different obstacles/shapes appearance. Both types of scores (obstacle avoidance/stopping scores and object identification scores) were recorded and calculated for all participants’ trials in both testing strategies by two observers to make sure that the correct scores are being recorded.
Descriptive and significance statistics were calculated using MATLAB R2018b (MathWorks, Inc., Natick, MA, USA). Means ± standard deviations (SD) were used to describe VF measurements errors. Wilcoxon rank-sum tests were used to test for significance between the patients walking simulation scores, with and without the DSpecs. We used Pearson’s linear correlation tests to assess the correlation of three VF defect severity measures and the walking simulation average scores. We included the Mean Deviation (MD), Pattern Standard Deviation (PSD), and the Visual Field Index (VFI) metrics in the correlation analysis, as being commonly reported VF defects characterization parameters.37,38 For each measure we used the best eye’s value in the analysis. P-values of less than 0.05 were considered to be statistically significant.
RESULTS
BINOCULAR VF MEASUREMENTS:
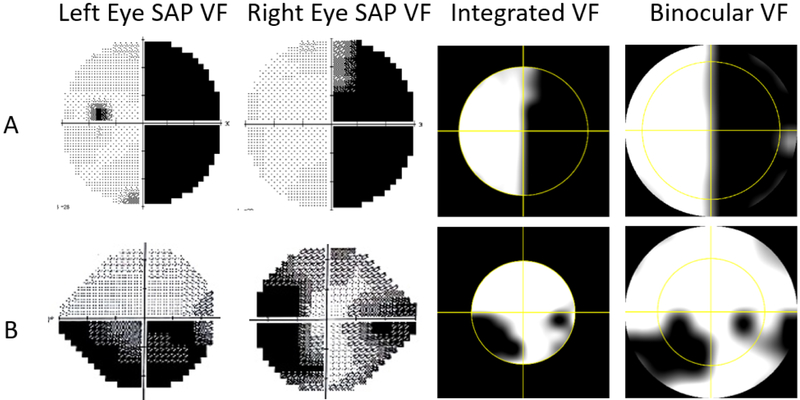
Patients age range was 13 to 89 years with a mean value of 63 ± 19 years old. The VF measurements were performed with SAP and DSpecs. The VF measurements of a patient with a residual left homonymous hemifield is shown for demonstration (Figure 4.A). The left blind spot is not detected, as the intact portion of the visual field in the right eye includes this area. A glaucomatous defect, where the superior hemifield of the left VF is intact, and nasal hemifield of the right eye exhibits moderate damage temporal to fixation is shown (Figure 4.B). Merging both fields revealed a common defect in the inferior-left quadrant, which was confirmed with the DSpecs VF measurements. Monocular SAP measurements supported the validity of using the DSpecs for defining binocular VFs.
Figure 4:
Binocular visual field (VF) measurements for two patients A) Hemianopia patient tested with 30–2 monocular Humphrey standard automated perimetry (SAP). B) Glaucoma patient tested with 24–2 Humphrey SAP. First column: left eyes monocular SAP. Second column: Right eyes Monocular SAP. Third column: binocular Integrated VF (IVF) constructed by merging the two monocular fields based on the maximum sensitivity model. Fourth column: Direct binocular VF measurement with the digital spectacles.
The IVF was used as a reference for calculating the DSpecs binocular measurement error. The mean error and standard deviation was found to be 3 ± 2 points (range: 0 to 6) for the number of mismatched error points, and 7 ± 4 % (range: 0 to 15 %) as a percentage of the total number of common test points between monocular SAPs and DSpecs VF methods.
IMAGE REMAPPING:
We initially tested the image remapping method with static images (Figure 5.B) to ensure potential benefit for each patient and the likelihood of improving the functional FOV. Eighteen out of 20 patients (90%) successfully identified the peripheral objects in the test images with the DSpecs remapping algorithm, while 2 patients (10%) correctly identified the objects without remapping. For example, in a patient with retinitis pigmentosa, he could not see the car before remapping (Figure 5.C) but could see the object after image remapping (Figure 5.D).
Figure 5:
A retinitis pigmentosa Patients’ image remapping results: A) Measured binocular visual field. B) Test image showing an incoming car as a safety hazard. C) Test image as seen by the patient: patient could see only a road. D) Remapped image: patient could identify the car. Visual field was overlaid over the test image in the last two images for demonstration purposes only.
WALKING COMPUTER SIMULATION:
Hand Coordination Test.
Seventeen out of 20 patients (85%) were able to grasp the 3 objects on the first trial. For the other three patients: two patients grasped one object in the first trial and 2 objects in the second trial, the third patient grasped two objects from the second trial and one object in the third trial. Those three patients repeated the test (two patients repeated one time and the third patient repeated twice) until they became adapted to the new perspective of the DSpecs and grasped the three objects successfully from the first time. The use of DSpecs did not negatively affect visual/hand coordination, although training was needed to achieve a good coordination for some patients.
Walking Simulation Results.
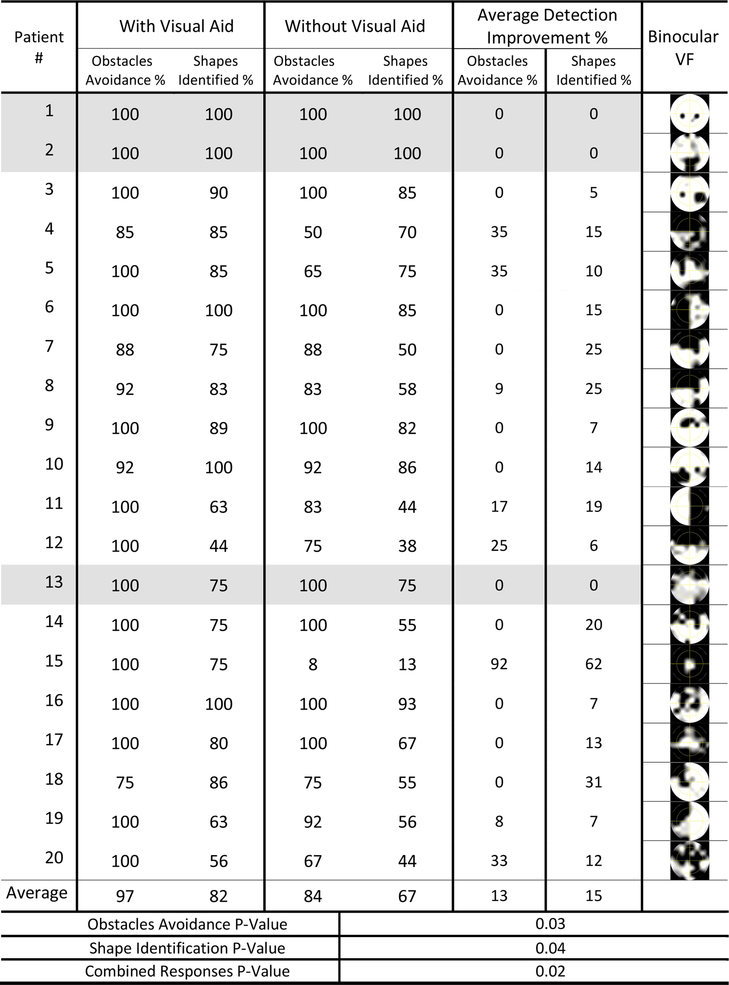
We asked patients to perform the walking simulation experiment, and respond to the simulation events. Patients responded by stopping if they saw an obstacle or to verbally identify a sign or shape. Responses as percentage of successful obstacles avoidances and signs identifications were calculated (Figure 6). Improvements in the patient’s ability to perform both tasks were calculated as the difference between the scores in the with and without DSpecs conditions. These calculations reveal average improvements of 13% in the obstacle avoidance task, and 15% for the shapes identification task. The use of DSpecs improved detection in 85% of subjects (17 out of 20 patients), but did not benefit three patients (patients 1,2 and 13 in Figure 6). To illustrate the difference between the walking simulation scores, with and without the visual aid, the effects were calculated for three response categories: obstacles avoidance, peripheral shapes identification, and combined responses calculated by averaging the first two response categories. Assuming no data normality, the Wilcoxon rank sum test was significant (P-value: 0.03, 0.04, and 0.02) for the three response categories, respectively.
Figure 6:
Walking simulation test scores for 20 patients with and without the digital spectacles.
Correlation analysis was performed between walking simulation mean scores, calculated by averaging the scores in columns 6 and 7 of (Figure 6), and three standard global parameters characterizing SAP VF tests (MD, VFI, and PSD). Pearson’s linear correlation analysis demonstrated a significant correlation (r −0.55; P-value 0.01) between the MD metric and DSpecs walking improvement scores. The analysis also showed a marginal significance of the VFI metric (r −0.45; P-value 0.05). On the other hand, no significant correlation was found between the PSD parameter and DSpecs improvements (r −0.18; P-value 0.46). Patient’s age was not correlated with the walking scores (r −0.02; P-value 0.95).
DISCUSSION
We designed and tested the DSpecs, to measure binocular VF defects and accordingly generate a unique patient visual augmentation profile. We hypothesized that these personalized augmentation strategies may expand peripheral awareness and improve mobility.9,10 The ability of our patients to identify and avoid moving objects in a walking simulation environment was improved with the DSpecs. They could successfully coordinate their hand movements with the new visual aid.
Peli and coworkers39–42 used an HMD to augment restricted visual fields by displaying expanded contours of objects, but did not use real images. Their approach increased the FOV for patients with tunnel vision due to retinitis pigmentosa to less than 40 degrees. This technique did not improve the ability of the patients to avoid collisions nor improve mobility in a walking simulation experiment.28 More recently, they reported expansion of vision with optical prisms that improved collision detection rates tested with virtual reality walking simulations.43,44 Binocular visual field expansion was limited to 11 to 39 degree through small apertures and binocular visual confusion due to the prismatic field expansion method was not tolerated by some patients.43 Other available visual aids21,26,27,45,46 apply image magnification, brightness and contrast enhancements without considering the pattern of the VF defect. These aids were reported to provide limited benefits for many functional activities that rely on the peripheral VF.16,17,21 Wittich and coworkers investigated a modern visual aid where mobility tests did not show significant improvement.27 Experimental validation of mobility improvements with HMDs has not been reported.
In review of current visual aids, we could not identify a similar approach to measure a VF defect pattern and apply a unique visual device to improve mobility. This feature has been reported as a possible future innovation.46 Our method of binocular VF testing with DSpecs is advantageous over the standard Esterman method, that has been reported to underestimate the field defect extent, especially in the superior hemifield, due to fewer number of testing points in the superior hemifield.47,48 With Estermann binocular visual field testing, we believe that the results would have affected the accuracy of the image remapping process. Our device does not exhibit image overlap nor optical confusion artifacts, as the entire test image is remapped.
Not all patients benefitted with the use of the DSpecs, including three patients with relatively mild binocular defects. The first patient had monocular VF defects with the normal blind spots shown to be defective in the binocular VF, and the other two patients had moderately dense defects in the inferior and superior hemifields, respectively. It is worth mentioning that the DSpecs augmentation profile was not beneficial for the first two patients in Figure 6 in both the static images and walking simulation tests. This suggests that testing with static images was indicative of the walking simulation performance.
Although we instructed all patients to focus at the end of the corridor, center of the projector screen, while virtually walking and expect appearance of the objects, many continued to scan the environment with eye, head, and body movements to compensate for their VF losses. The efficient use of scanning likely explains the failure of the DSpecs to improve visual function in these patients. This behavior became an involuntary habit and controlling or stopping it was hard for them.49–52
All other patients scored higher with the DSpecs, and some patients had very substantial improvements in both tasks. For example, two patients had much better avoidance and identification score with the DSpecs, and it was significantly different than without it: patient 15 (retinitis pigmentosa) and patient 20 (glaucoma). Therefore, two factors may have affected the performance of the DSpecs augmentation process. First, how profound the VF defect was regarding depth and pattern. More severe and homogeneous peripheral defects were found to be more beneficial, as can be clearly noticed in the objects detection improvement percentages shown in (Figure 6). Correlation analysis suggests that the MD and VFI metrics might be predictive factors for potential functional improvements with the DSpecs. Second, patients scanning behavior was responsible for not noticing improvements, as they compensate for their visual defects.
Our study has several limitations. The current prototype was based on a bulky and heavy VR HMD that isolates patients from their surroundings and is not socially acceptable. The application of augmented reality (AR) technology will likely address these VR technology limitations and provide a see-through, light-weight display. The image remapping algorithm was based on geometrical calculations to basically perform automatic image operations and create the required augmentation profile. The current process is a two degrees of freedom algorithm that involves both resizing and shifting of the images. The preliminary results have shown that this remapping method will likely not be sufficient to account for all the blind areas. An optimization method incorporating more degrees of freedom and considering different image transformations, such as fish eye transformation, may be required to maximize the usage of the remaining VF.
Hand coordination tests were performed only with the DSpecs. Although, this confirmed a level of motor/visual coordination and accordingly we proceeded with the walking simulation experiment, it did not determine if the DSpecs affected or improved the baseline coordination before the DSpecs were used. Future studies will incorporate coordination assessments that include with and without DSpecs testing setups.
Future studies to improve peripheral awareness include increasing the DSpecs FOV. The current DSpecs prototype captures a VF of 80 degrees, and displays approximately 85 degrees FOV with images generated by the forward facing digital cameras. These field ranges cover a large portion of the mid-periphery VF, which is responsible for the highest collision risks53, and they were adequate to demonstrate the feasibility of this proof of concept research to avoid obstacles. However, they are considered to be relatively limited ranges for daily life activities. A more useful FOV would be at least 110 degrees, which matches the FOV of prescription glasses. The walking computer simulation was a preliminarily effective and safe test to judge effectiveness of vision augmentation with the DSpecs, but did not account for head and body coordination with the new visual aid. A standardized and validated real mobility track with physical objects that mimics the real world environment and permits measurements for all eye, head, and body kinematic movements while walking will be the subject of future studies.
We believe that our digital glasses technology may aid patients with peripheral VF defects improve their peripheral visual awareness. A virtual walking environment has been verified and found to be practical for assessing the effect of peripheral vision loss. The demonstrated results showed that the DSpecs detected different binocular VF defects. Functional tests demonstrated a successful hand coordination with the visual aid, and significant improvements to the ability to avoid obstacles and identify moving objects in the simulated walking test. These findings suggest the potential benefit of the DSpecs to improve recognition of peripherally moving objects, augment mobility, facilitate independence, and quality of life.
FUNDING/SUPPORT:
This research has been partially supported by the National Institute of Health (NIH) under Grant # K23 KEY026118A. Research and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
FINANCIAL DISCLOSURES:
United States Non-Provisional Pending Patent (Application No. 16/144,995) (MA) and United States Non-Provisional Filed Patents (Application No. 16/367,633, 16/367,687 and 16/367,751) (MA, AS).
REFERENCES
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and metaanalysis. Ophthalmology. 2014;121(11):2081–2090. [DOI] [PubMed] [Google Scholar]
- 2.Rowe FJ, Hepworth LR, Hanna K, Howard C. Point prevalence and incidence of visual impairment following stroke. Invest Ophth Vis Sci. 2017;58(8). [Google Scholar]
- 3.Parrish RK 2nd, Gedde SJ, Scott IU, et al. Visual function and quality of life among patients with glaucoma. Arch Ophthalmol-Chic. 1997;115(11):1447–1455. [DOI] [PubMed] [Google Scholar]
- 4.Parrish RK 2nd,. Visual impairment, visual functioning, and quality of life assessments in patients with glaucoma. Trans Am Ophthalmol Soc. 1996;94:919–1028. [PMC free article] [PubMed] [Google Scholar]
- 5.Viswanathan AC, McNaught AI, Poinoosawmy D, et al. Severity and stability of glaucoma - Patient perception compared with objective measurement. Arch Ophthalmol-Chic. 1999;117(4):450–454. [DOI] [PubMed] [Google Scholar]
- 6.Kotecha A, Chopra R, Fahy RTA, Rubin GS. Dual Tasking and Balance in Those With Central and Peripheral Vision Loss. Invest Ophth Vis Sci. 2013;54(8):5408–5415. [DOI] [PubMed] [Google Scholar]
- 7.Elliott DB, Patla AE, Flanagan JG, et al. The Waterloo Vision and Mobility Study - Postural Control Strategies in Subjects with Arm. Ophthal Physl Opt. 1995;15(6):553–559. [PubMed] [Google Scholar]
- 8.Stoffregen TA, Schmuckler MA, Gibson EJ. Use of Central and Peripheral Optical-Flow in Stance and Locomotion in Young Walkers. Perception. 1987;16(1):113–119. [DOI] [PubMed] [Google Scholar]
- 9.Loviekitchin J, Mainstone J, Robinson J, Brown B. What Areas of the Visual-Field Are Important for Mobility in Low Vision Patients. Clin Vision Sci. 1990;5(3):249–263. [Google Scholar]
- 10.Chung DC, McCague S, Yu ZF, et al. Novel mobility test to assess functional vision in patients with inherited retinal dystrophies. Clin Exp Ophthalmol. 2018;46(3):247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peli E, Apfelbaum H, Berson EL, Goldstein RB. The risk of pedestrian collisions with peripheral visual field loss. J Vision. 2016;16(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salive ME, Guralnik J, Glynn RJ, Christen W, Wallace RB, Ostfeld AM. Association of visual impairment with mobility and physical function. J Am Geriatr Soc. 1994;42(3):287–292. [DOI] [PubMed] [Google Scholar]
- 13.Turano KA, Rubin GS, Quigley HA. Mobility performance in glaucoma. Invest Ophthalmol Vis Sci. 1999;40(12):2803–2809. [PubMed] [Google Scholar]
- 14.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans K, Law SK, Walt J, Buchholz P, Hansen J. The quality of life impact of peripheral versus central vision loss with a focus on glaucoma versus age-related macular degeneration. Clin Ophthalmol. 2009;3:433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patodia Y, Golesic E, Mao A, Hutnik CM. Clinical effectiveness of currently available low-vision devices in glaucoma patients with moderate-to-severe vision loss. Clin Ophthalmol. 2017;11:683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrlich JR, Ojeda LV, Wicker D, et al. Head-Mounted Display Technology for Low-Vision Rehabilitation and Vision Enhancement. Am J Ophthalmol. 2017;176:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrlich JR, Spaeth GL, Carlozzi NE, Lee PP. Patient-Centered Outcome Measures to Assess Functioning in Randomized Controlled Trials of Low-Vision Rehabilitation: A Review. Patient. 2017;10(1):39–49. [DOI] [PubMed] [Google Scholar]
- 19.Fasce FBP, Luca G, Brancato R. Effects of minification on visual performance in advanced glaucoma. Vision Rehabilitation: Assessment, Intervention and Outcomes. 2000:177–179. [Google Scholar]
- 20.Peli E. Vision multiplexing: an optical engineering concept for low-vision aids - art. no. 66670C. Current Developments in Lens Design and Optical Engineering Viii. 2007;6667:C6670–C6670. [Google Scholar]
- 21.Markowitz SN. State-of-the-art: low vision rehabilitation. Can J Ophthalmol. 2016;51(2):59–66. [DOI] [PubMed] [Google Scholar]
- 22.Apfelbaum H, Peli E. Tunnel Vision Prismatic Field Expansion: Challenges and Requirements. Transl Vis Sci Technol. 2015;4(6):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis CW, Mathers DR, Hilkes RG, Munger RJYB, Colbeck RP, Inventors; Esight Corp, Assignee. Apparatus and method for augmenting sight. US patent 8,494,298 B2, 2013. [Google Scholar]
- 24.Pr Antaki, Dunn R, Lemburg R, Inventors; Evergaze Inc, Assignee. Apparatus and method for improving, augmenting or enhancing vision. EP3108444A1, 2015. [Google Scholar]
- 25.Hilkes R, Jones F, Rankin K, Inventors. Apparatus and method for enhancing human visual performance in a head worn video system. EP2674805A2, 2013. [Google Scholar]
- 26.Culham LE, Chabra A, Rubin GS. Clinical performance of electronic, head-mounted, low-vision devices. Ophthalmic Physiol Opt. 2004;24(4):281–290. [DOI] [PubMed] [Google Scholar]
- 27.Wittich W, Lorenzini MC, Markowitz SN, et al. The Effect of a Head-mounted Low Vision Device on Visual Function. Optom Vis Sci. 2018;95(9):774–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo G, Woods RL, Peli E. Collision Judgment When Using an Augmented-Vision Head-Mounted Display Device. Invest Ophth Vis Sci. 2009;50(9):4509–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsapakis S, Papaconstantinou D, Diagourtas A, et al. Visual field examination method using virtual reality glasses compared with the Humphrey perimeter. Clin Ophthalmol. 2017;11:1431–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wroblewski D, Francis BA, Sadun A, Vakili G, Chopra V. Testing of visual field with virtual reality goggles in manual and visual grasp modes. Biomed Res Int. 2014;2014:206082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollander DA, Volpe NJ, Moster ML, et al. Use of a portable head mounted perimetry system to assess bedside visual fields. Br J Ophthalmol. 2000;84(10):1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrlich JR, Moroi SE. Comment on “Clinical effectiveness of currently available low-vision devices in glaucoma patients with moderate-to-severe vision loss”. Clin Ophthalmol. 2017;11:1119–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrington DO. The visual fields; a textbook and atlas of clinical perimetry. 6 ed. St. Louis,: Mosby; 1990. [Google Scholar]
- 34.Anderson DR. Automated static perimetry. St. Louis: Mosby Year Book; 1992. [Google Scholar]
- 35.Crabb DP, Viswanathan AC, McNaught AI, Poinoosawmy D, Fitzke FW, Hitchings RA. Simulating binocular visual field status in glaucoma. Br J Ophthalmol. 1998;82(11):1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson-Quigg JM, Cello K, Johnson CA. Predicting binocular visual field sensitivity from monocular visual field results. Invest Ophthalmol Vis Sci. 2000;41(8):2212–2221. [PubMed] [Google Scholar]
- 37.Ng M, Sample PA, Pascual JP, et al. Comparison of visual field severity classification systems for glaucoma. J Glaucoma. 2012;21(8):551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145(2):343–353. [DOI] [PubMed] [Google Scholar]
- 39.Peli E. Vision multiplexing: an engineering approach to vision rehabilitation device development. Optom Vis Sci. 2001;78(5):304–315. [DOI] [PubMed] [Google Scholar]
- 40.Luo G, Peli E. Use of an augmented-vision device for visual search by patients with tunnel vision. Invest Ophthalmol Vis Sci. 2006;47(9):4152–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang AD, Peli E. An augmented-reality edge enhancement application for Google Glass. Optom Vis Sci. 2014;91(8):1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peli E, Luo G, Bowers A, Rensing N. Applications of Augmented Vision Head-Mounted Systems in Vision Rehabilitation. J Soc Inf Disp. 2007;15(12):1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu C, Jung JH, Tuccar-Burak M, Spano L, Goldstein R, Peli E. Measuring Pedestrian Collision Detection With Peripheral Field Loss and the Impact of Peripheral Prisms. Transl Vis Sci Technol. 2018;7(5):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houston KE, Bowers AR, Peli E, Woods RL. Peripheral Prisms Improve Obstacle Detection during Simulated Walking for Patients with Left Hemispatial Neglect and Hemianopia. Optom Vis Sci. 2018;95(9):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hilkes Robert; Rankin Kevin, Inventors; ESight Corp., Assignee. Apparatus and Method for Enhancing Human Visual Performance in a Head Worn Video System. US Patent 10,225,526 B2, 2019. [Google Scholar]
- 46.Deemer AD, Bradley CK, Ross NC, et al. Low Vision Enhancement with Head-mounted Video Display Systems: Are We There Yet? Optom Vis Sci. 2018;95(9):694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crabb DP, Fitzke FW, Hitchings RA, Viswanathan AC. A practical approach to measuring the visual field component of fitness to drive. Br J Ophthalmol. 2004;88(9):1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jampel HD, Friedman DS, Quigley H, Miller R. Correlation of the binocular visual field with patient assessment of vision. Invest Ophthalmol Vis Sci. 2002;43(4):1059–1067. [PubMed] [Google Scholar]
- 49.Houston KE, Woods RL, Goldstein RB, Peli E, Luo G, Bowers AR. Asymmetry in the Collision Judgments of People With Homonymous Field Defects and Left Hemispatial Neglect. Invest Ophth Vis Sci. 2015;56(6):4135–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dijkerman HC, McIntosh RD, Milner AD, Rossetti Y, Tilikete C, Roberts RC. Ocular scanning and perceptual size distortion in hemispatial neglect: effects of prism adaptation and sequential stimulus presentation. Exp Brain Res. 2003;153(2):220–230. [DOI] [PubMed] [Google Scholar]
- 51.de Haan GA, Melis-Dankers BJM, Brouwer WH, Tucha O, Heutink J. The Effects of Compensatory Scanning Training on Mobility in Patients with Homonymous Visual Field Defects: A Randomized Controlled Trial. Plos One. 2015;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vega RP, van Leeuwen PM, Velez ER, Lemij HG, de Winter JCF. Obstacle Avoidance, Visual Detection Performance, and Eye-Scanning Behavior of Glaucoma Patients in a Driving Simulator: A Preliminary Study. Plos One. 2013;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peli E, Apfelbaum H, Berson EL, Goldstein RB. Residual Peripheral Fields in RP and the Risk for Pedestrian Collisions. Invest Ophth Vis Sci. 2015;56(7). [Google Scholar]