Abstract
We investigated the contributions of obesity on multidimensional aspects of dyspnea on exertion (DOE) in patients referred for clinical cardiopulmonary exercise testing (CPET). Ratings of perceived breathlessness (RPB, Borg scale 0–10) were collected in obese (BMI ≥ 30; n = 47) and nonobese (BMI ≤ 25; n = 27) patients during two (one lower: ~30 W; and one higher: ~50 W) 4–6 min constant load cycling bouts. Multidimensional dyspnea profiles (MDP) were collected in the final 26 obese and 14 nonobese patients of the sample. RPB was greater (p < 0.05) in obese (3.3 ± 2.2 vs 2.4 ± 1.4) at lower work rates, but similar at higher work rates (4.9 ± 2.2 vs 4.4 ± 1.8). MDP sensory score including unpleasantness was 4.3 ± 2.2 in obese vs 2.5 ± 1.9 in nonobese (p < 0.001). The affective score was 1.9 ± 2.2 vs 0.7 ± 0.7, respectively (p < 0.01). Breathing sensations including ‘air hunger’, ‘effort’, and ‘breathing at lot’ were greater (p < 0.05) in obese, making these patients more frustrated/angry (p < 0.05). Obesity should be considered as a potential independent influencing factor that provokes DOE and unpleasantness when assessing breathlessness during CPET.
Keywords: Shortness of breath, Breathlessness, Obesity, Cardiorespiratory fitness, Exercise
1. Introduction
Obesity is an epidemic problem. In fact, two-thirds of adults in the United States are currently classified as overweight or obese (Yang et al., 2015). Obesity is associated with numerous health problems (Kenchaiah et al., 2002; Van Gaal et al., 2006; Azagury et al., 2011) and obesity alone exerts limitations to breathing capacity including decreased pulmonary function (Babb et al., 1989, 2002; DeLorey et al., 2005; El-Gamal et al., 2005), altered respiratory mechanics (Babb et al., 2002; DeLorey et al., 2005; Lotti et al., 2005; Lorenzo et al., 2013), increased work of breathing (Babb et al., 2008; Bernhardt et al., 2013), and increased metabolic demands of exercise (Babb et al., 1991; Wood et al., 2008). These effects could influence exercise tolerance and dyspnea on exertion (DOE) (Whipp et al., 1984; Sahebjami, 1998; Sin et al., 2002; Babb et al., 2008), which appears particularly common in otherwise healthy individuals with obesity. Indeed, we have shown that in 37–44 % of otherwise healthy obese men and women that DOE is a frequent complaint during constant-load cycling exercise (Bernhardt et al., 2013, 2014; Bernhardt et al., 2016).
Studies have also reported that DOE can be provoked by a number of illness-related abnormalities (Russel et al., 1998; de Voogd et al., 2011d; Dube et al., 2016), even in the absence of obesity. In this regard, healthcare providers are often confounded as to the origin of DOE in patients with obesity that have various underlying disease conditions; that is, it may be unclear if DOE is a consequence of disease, obesity, or a combination of both. Whether obese patients with or without underlying disease have an exaggerated rating of perceived breathlessness (RPB) as compared with nonobese patients with or without underlying disease is unknown. We propose that patients with obesity will have a greater level of breathlessness during exertion compared with nonobese patients no matter the underlying disease conditions.
Furthermore, DOE is a multidimensional and complex symptom (O’Donnell et al., 1997). Indeed, while DOE is often described in terms of intensity (i.e., RPB), it must be noted that DOE can also be described in terms of affective distress (i.e., unpleasantness and emotional responses). We recently reported that ratings of unpleasantness/negative emotions were higher in otherwise healthy obese individuals with DOE (Bernhardt et al., 2019; Marines-Price et al., 2019). Therefore, these findings support the notion that not only is it important to study the intensity of DOE, but also the negative emotions that can be ascribed to DOE. Currently, it is unknown how the affective dimension of DOE is perceived in obese patients that may have a number of illness-related abnormalities that could also influence DOE (Russel et al., 1998; de Voogd et al., 2011d; Dube et al., 2016).
Accordingly, we performed a retrospective analysis of obese and nonobese patients who were referred to our institution for clinical cardiopulmonary exercise testing (CPET) due to unexplained DOE (i.e., either DOE was present in the absence of underlying disease, or the symptoms of DOE were greater than would be expected for the patients given disease severity), and examined the contributions of obesity on the intensity and affective dimensions of DOE in these patients. We hypothesized that patients with obesity would have a greater rating of DOE and unpleasantness/negative emotions as compared with nonobese patients.
Some of the data included in this study have been previously published in abstract form (Bernhardt et al., 2016).
2. Methods
2.1. Patients
The patients were referred to our institution for clinical CPET primarily for unexplained DOE. Patients had undergone otherwise comprehensive diagnostic testing before referral for CPET at our laboratory. On arrival to our laboratory, patients were queried regarding current major symptoms during exertion and physical activity habits, which were subsequently recorded by a medical staff member. Retrospectively, data from 112 patients tested were separated into obese (BMI ≥ 30; n = 47; 24 F) and nonobese (BMI ≤ 25; n = 27; 20 F) groups. To better delineate the groups, patients were excluded from the analysis if their BMI classification fell within the National Institute of Health’s definition of overweight (25 < BMI < 29.9, n = 38).
2.2. Exercise testing
All patients cycled at two individualized constant-load work rates (i.e., one easier [submaximal 1] and one harder [submaximal 2]) for 4–6 min each. Constant load submaximal work rates were set based on the severity of patient’s symptoms (e.g., do you get short of breath during walking, running, climbing stairs?) and current physical activity habits (how often do you exercise?; what type of exercise do you do?), which was left up to the discretion of the supervising physician. Following a short rest period, patients then performed an individualized incremental (1 min per stage) maximal exercise test on the same cycle ergometer to exhaustion or until symptom limited (medical staff encouraged patients until they reached volitional exhaustion). Twelve-lead electrocardiogram (to measure heart rate [HR] and cardiac rhythm), pulmonary gas exchange, pulse oximetry, and end-tidal CO2 (PETCO2) were monitored at rest and during exercise. Cardiac output (CO) was measured by acetylene rebreathing technique at rest, during submaximal exercise, and at peak exercise. Stroke volume (SV) was calculated from dividing CO by the corresponding HR. To account for differences in body size, CO and SV were subsequently normalized to body surface area, yielding cardiac index (CI) stroke volume index (SVI), respectively. Inspiratory capacity (IC) was measured at rest, during each constant load exercise stage, and 30 s into each exercise incremental stage to determine operational lung volumes and placement of tidal flow-volume loops within the maximal flow-volume loop as previously described (Babb, 1997; Babb et al., 1997). Patients were instructed on how to perform the IC maneuver and when to inhale to total lung capacity (TLC) (Babb et al., 1993). Lactate concentration was collected from the index finger and measured at rest, at the end of each submaximal work rate, and 2 min after the incremental test. An end-of-study report was generated following the incremental exercise test (addressing functional capacity, cardiovascular responses, respiratory responses, metabolic responses, symptoms, and most likely reasons for limitations or symptoms). The attending physician objectively determined the primary and secondary causes (if applicable or multifactorial) of exercise limitation based on the data (described above) that were collected during the test.
2.3. Intensity and quality of respiratory sensations during constand load cycling
During the last minute of each constant load exercise bout, patients rated their level of perceived breathlessness (RPB, Borg scale 0–10) and perceived exertion (RPE, Borg scale 6–20). All patients were given a standard written explanation of the rating procedure, which was followed up with a verbal confirmation of their understanding of the rating process. Following the maximal incremental exercise test, patients were asked to sit in a chair and rate their RPB and RPE at peak exercise. As part of our routine protocol, we implemented the multidimensional dyspnea profile (MDP) questionnaire to obtain more information regarding the quality of respiratory sensations including unpleasantness (sensory dimension of DOE), and perceived level of negative emotions (affective dimension of DOE) associated with DOE – depression, anxiety, frustration, anger, and fear. The final 52 (29F) patients out of the total 112 were asked to immediately complete the MDP questionnaire following exercise at rest with the focus period of when their perceived level of breathlessness was at its highest during peak exercise. For analysis, the 52 patients who completed the MDP questionnaire were subsequently divided into obese (BMI ≥ 30; n = 26; 12 F) and nonobese (BMI ≤ 25; n = 14; 11F) groups.
2.4. Data analysis
Differences between groups were determined by independent t-tests. Relationships among variables were examined by linear regression analysis. All data are presented as means ± SD and statistical significance was accepted at p≤0.05.
3. Results
3.1. Patients
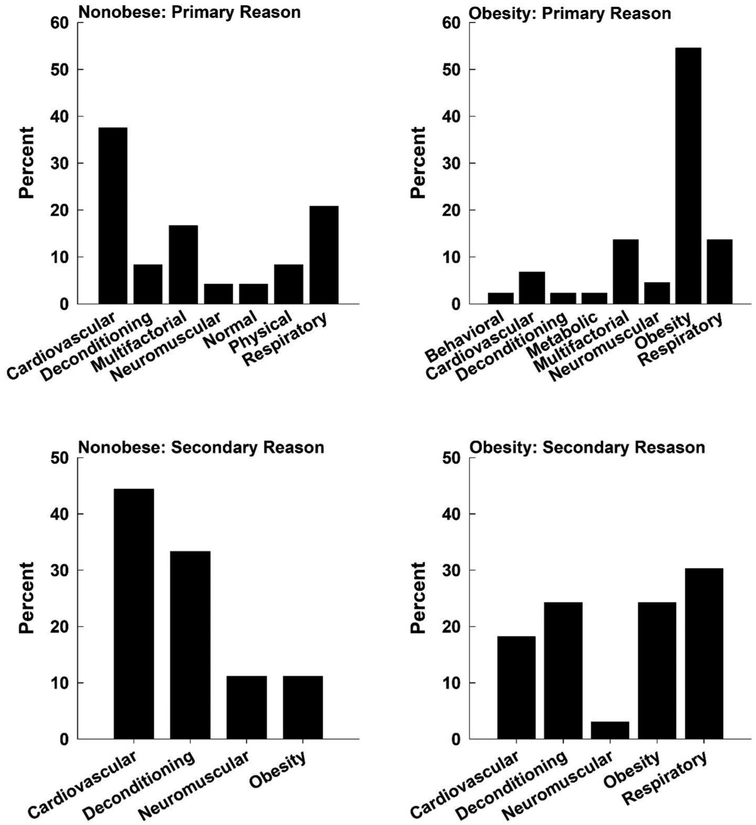
Patients were well-matched for age and height (Table 1, p > 0.05) although, as expected, weight and BMI were greater in obese compared with non-obese patients (Table 1, p < 0.0001). Fig. 1 shows a frequency plot of primary and secondary reasons for exercise intolerance and/or DOE for each patient group. While the majority of the nonobese patients had some primary cardiovascular or respiratory issue, obesity was the primary reason for limitation for over half of the patients with obesity. The secondary reasons for exercise intolerance and/or DOE were cardiovascular dysfunction (> 40 %) or deconditioning (> 30 %) in the nonobese patients, while respiratory dysfunction (~30 %) or deconditioning (> 20 %) were the main reasons in the patients with obesity. However, in the obese group, obesity was the secondary reason for limitation in 20 % of the obese patients. Thus, in over 70 % of the obese patients, obesity was felt to have played a primary or secondary role in their DOE and/or exercise intolerance.
Table 1.
Patient characteristics.
| Obese (n = 47, 24 F) | Nonobese (n = 27, 20 F) | P value | |
|---|---|---|---|
| Age (yr) | 57 ± 12 | 51 ± 20 | 0.19 |
| Height (cm) | 170 ± 11 | 167 ± 8 | 0.20 |
| Weight (kg) | 103 ± 16 | 62 ± 9 | < 0.0001 |
| BMI (kg/m2) | 36 ± 4 | 22 ± 2 | < 0.0001 |
Data are mean ± SD.
Fig. 1.
Frequency plots outlining the primary and secondary reasons for exercise intolerance or dyspnea on exertion. Cardiova=cardiovascular; Decondit=deconditioned; Multifac=multifactorial; Neuromus=neuromuscular; Respirat=respiratory; Behavior=Behavioral.
3.2. Cardiorespiratory responses at peak exercise
Peak work rate and peak oxygen uptake (peak V̇O2, L/min) were not different between the groups (Table 2, p > 0.05). When peak V̇O2 was expressed relative to actual body weight (in ml/min/kg) and as a percent of predicted based on actual weight, the patients with obesity appeared to have a lower exercise capacity (Table 2, p < 0.05). However, when peak V̇O2 was normalized to ideal body weight, there was no difference between the two patient groups (p > 0.05). In this case, cardiorespiratory fitness was not significantly different between the nonobese and obese patients. Moreover, minute ventilation (V̇O2) relative to CO2 production (V̇E/V̇CO2), respiratory exchange ratio (RER), CO, CI, HR, HR (%predicted), SV, and SVI, were different in obese compared with nonobese patients at peak exercise (Table 2, p > 0.05).
Table 2.
Cardiorespiratory responses during peak exercise.
| Nonobese | Obese | |
|---|---|---|
| Work Rate (W) | 101 ± 46 | 104 ± 60 |
| V̇O2 (L/min) | 1.37 ± 0.55 | 1.67 ± 0.79 |
| V̇O2 (ml/min/kg) | 22 ± 8 | 16 ± 6* |
| V̇O2 (%Predicted based on actual wt) | 76 ± 24 | 58 ± 21* |
| V̇O2 (%Predicted based on ideal body wt) | 82 ± 25 | 94 ± 30 |
| V̇E (L/min) | 73.08 ± 24.21 | 72.43 ± 31.32 |
| VT (L) | 1.70 ± 0.54 | 1.93 ± 0.83 |
| Fb (bpm) | 44.02 ± 11.32 | 39.31 ± 8.93 |
| V̇E/ V̇CO2 | 48.77 ± 9.89 | 41.89 ± 7.72* |
| PETCO2 (Torr) | 30 ± 5 | 36 ± 5 |
| RER | 1.15 ± 0.11 | 1.07 ± 0.11* |
| CO (L/min) | 10.32 ± 3.42 | 12.61 ± 3.98* |
| CI | 6.7 ± 1.7 | 6.3 ± 1.8 |
| HR (bpm) | 157 ± 32 | 137 ± 30* |
| HR (%Pred) | 90 ± 16 | 81 ± 15* |
| SV (ml) | 66 ± 23 | 93 ± 25Ω |
| SVI | 41 ± 10 | 47 ± 11 |
| a-vO2 Diff (%) | 13.69 ± 2.35 | 12.65 ± 3.24 |
| Lactate (mmol) | 7.12 ± 2.33 | 6.22 ± 3.03 |
| IC (L) | 2.77 1.86 | 2.57 0.82 |
| RPB (0–10) | 8.3 ± 1.8 | 8.5 ± 1.9 |
| RPE (6–20) | 18.1 ± 1.6 | 17.6 ± 1.9 |
Data are mean ± SD,
p < 0.05;
p<0.01;
p<0.001.
V̇O2 =oxygen uptake in L/min; V̇E =minute ventilation in L/min; VT=tidal volume in L; Fb=breathing frequency in beat per minute (bpm); V̇E/ V̇CO2=ventilator equivalent for carbon dioxide; PETCO2=end-tidal carbon dioxide in Torr; RER=respiratory exchange quotient; CO=cardiac output in L/min; CI=cardiac index; HR=heart rate in beats per minute (bpm); HR (%Pred)= heart rate as a percent of predicted maximal heart rate; SV=stroke volume in ml; SVI=stroke volume index; a-vO2 Diff=arterial-mixed venous oxygen difference in %; IC=inspiratory capacity in L; RPB=ratings of perceived breathlessness using the Borg 0–10 scale; and RPE=ratings of perceived exertion using the Borg 6–20 scale.
3.3. Cardiorespiratory responses to constant load exercise
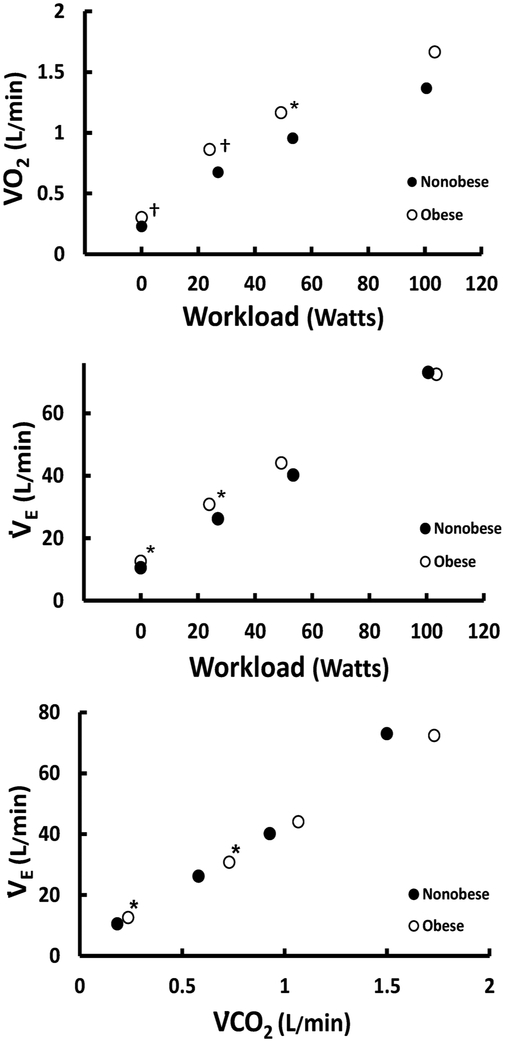
Submaximal work rates were not different between the two groups (Table 3, p > 0.05). In the obese patients, V̇O2 was significantly greater at rest (Table 3, p < 0.001) and during the two submaximal work rates (Table 3, p < 0.05). The relationship between V̇O2 and workload is shown in Fig. 2. V̇E was greater in the obese patients than in the nonobese patients at rest and during submaximal exercise bout one (Table 3, p < 0.05), however it was similar during the second submaximal exercise work rate and at peak exercise. The relationships between V̇E vs. workload and V̇E vs V̇CO2 are shown in Fig. 2. Breathing pattern – tidal volume (VT) and breathing frequency (fb) – was not different between the two groups at any level (Table 3, p > 0.05). V̇E/V̇CO2 tended to be lower in the obese patients but failed to reach significance until peak exercise. PETCO2 was overall similar in obese compared with nonobese, but was different during the second submaximal exercise bout (Table 3, p < 0.001). RER was significantly lower in the obese patients at the second submaximal exercise bout and peak exercise (Table 3, p < 0.05), probably reflecting the lower V̇E/V̇CO2 in the obese patients. Pulse oximetry was not different between the two groups at rest or at any level of exercise (data not shown). IC was greater in the obese patients as compared with the nonobese patients at rest only (Table 3, p < 0.01). Heart rate during the second submaximal work rate was lower in the obese group than the nonobese group (Table 3, p < 0.05). CO was increased in the obese patients at rest and both submaximal work rates (Table 3, p < 0.05). This was due to an increased SV, which was higher at rest and during each level of exercise (Table 3, p < 0.05). CI was not different between groups at rest or during exercise (Table 3, p > 0.05). While SVI was not different between groups at rest (Table 3, p > 0.05), SI was slightly larger in the obese compared with nonobese patients during both submaximal work rates (Table 3, p < 0.05). The arterial–mixed venous oxygen difference (a-vO2) difference was similar between the two groups, although it tended to be lower in the patients with obesity.
Table 3.
Cardiorespiratory responses during submaximal and maximal exercise.
| Variables | Rest | Submaximal 1 | Submaximal 2 | |||
|---|---|---|---|---|---|---|
| Nonobese | Obese | Nonobese | Obese | Nonobese | Obese | |
| Work Rate (W) | – | – | 27 ± 13 | 24 ± 14 | 53 ± 22 | 49 ± 28 |
| V̇O2 (L/min) | 0.23 ± 0.06 | 0.30 ± 0.07Ω | 0.68 ± 0.18 | 0.86 ± 0.26Ω | 0.96 ± 0.30 | 1.17 ± 0.42* |
| %V̇O2max | 18 ± 6 | 21 ± 7 | 53 ± 11 | 56 ± 13 | 74 ± 11 | 72 ± 11 |
| V̇E (L/min) | 10.56 ± 4.33 | 12.57 ± 3.51* | 26.22 ± 6.42 | 30.81 ± 7.48* | 40.19 ± 10.50 | 44.07 ± 12.50 |
| VT (L) | 0.96 ± 0.33 | 0.83 ± 0.25 | 1.24 ± 0.27 | 1.39 ± 0.58 | 1.48 ± 0.41 | 1.65 ± 0.74 |
| Fb (bpm) | 14.47 ± 4.03 | 15.37 ± 5.13 | 22.93 ± 7.07 | 24.77 ± 9.14 | 29.11 ± 9.40 | 28.95 ± 7.74 |
| VE/VCO2 | 58.07 ± 11.37 | 53.53 ± 11.09 | 45.30 ± 10.37 | 42.26 ± 8.81 | 43.37 ± 8.79 | 41.33 ± 8.71 |
| PETCO2 (Torr) | 36 ± 6 | 37 ± 6 | 38 ± 6 | 40 ± 5 | 37 ± 6 | 39±5Ω |
| RER | 0.80 ± 0.09 | 0.79 ± 0.12 | 0.89 ± 0.11 | 0.85 ± 0.13 | 1.0 ± 0.09 | 0.95 ± 0.09* |
| CO (L/min) | 3.57 ± 1.02 | 4.28 ± 1.08* | 6.84 ± 1.72 | 8.81 ± 2.61Ω | 8.19 ± 2.30 | 10.05 ± 2.92* |
| CI | 2.2 ± 0.5 | 2.1 0.5 | 4.0 ± 0.8 | 4.3 ± 1.2 | 4.8 ± 1.1 | 4.7 ± 1.2 |
| HR (bpm) | 83 ± 20 | 80 ± 16 | 103 ± 22 | 98 ± 19 | 126 ± 28 | 112 ± 24* |
| HR (%Pred) | 48 ± 9 | 47 ± 9 | 60 ± 11 | 57 ± 9 | 73 ± 13 | 66 ± 12* |
| SV (ml) | 45 ± 15 | 56 ± 16* | 69 ± 22 | 91 ±26Ω | 67 ± 23 | 92 ± 25Ω |
| SVI | 25 ± 6 | 28 ± 8 | 38 ± 10 | 46 ± 11* | 36 ± 9 | 45 ± 11* |
| a-vO2 Diff (%) | 6.66 ± 1.70 | 7.46 ± 2.01 | 10.32 ± 2.65 | 10.00 ± 2.70 | 12.08 ± 2.40 | 11.78 ± 3.14 |
| Lactate (mmol) | 1.57 ± 0.69 | 1.33 ± 0.61 | 2.04 ± 0.79 | 2.35 ± 0.73 | 3.73 ± 1.28 | 2.92 ± 1.36* |
| IC (L) | 2.27 ± 0.67 | 2.79 ± 0.81† | 2.38 ± 0.75 | 2.79 ± 0.96 | 2.39 ± 0.80 | 2.74 ± 0.94 |
| RPB (0–10) | 1.1 ± 1.3 | 1.5 ± 2.0 | 2.4 ± 1.4 | 3.2 ± 2.2* | 4.4 ± 1.8 | 4.9 ± 2.2 |
| RPE (6–20) | – | – | 10.2 ± 2.4 | 11.7 ± 3.0* | 13.4 ± 2.5 | 13.9 ± 2.5 |
Data are mean ± SD,
p < 0.05;
p<0.01;
p<0.001.
V̇O2=oxygen uptake in L/min; V̇E=minute ventilation in L/min; VT=tidal volume in L; Fb=breathing frequency in beat per minute (bpm); V̇E/ V̇CO2=ventilatory equivalent for carbon dioxide; PETCO2=end-tidal carbon dioxide in Torr; RER=respiratory exchange quotient; CO=cardiac output in L/min; CI=cardiac index; HR=heart rate in beats per minute (bpm); HR (%Pred)= heart rate as a percent of predicted maximal heart rate; SV=stroke volume in ml; SI=stroke volume index; a-vO2 Diff=arterial-mixed venous oxygen difference in %; IC=inspiratory capacity in L; RPB=ratings of perceived breathlessness using the Borg 0–10 scale; and RPE=ratings of perceived exertion using the Borg 6–20 scale.
Fig. 2.
The relationship between V̇O2 and workload (top), V̇E and workload(middle), and V̇E and V̇CO2 (bottom). V̇O2=oxygen uptake; V̇E=minute ventilation; V̇CO2=carbon dioxide. *p < 0.05; †p < 0.01.
3.4. Dyspnea on exertion
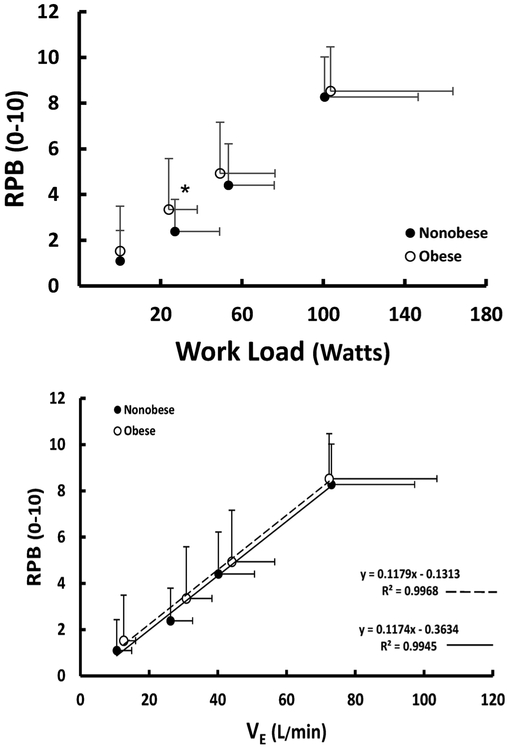
RPB did not differ between groups at rest; however, RPB was ~33 percent greater at a similar absolute work rate during the first submaximal exercise bout (Fig. 3, p < 0.05). During the second submaximal exercise bout and at peak exercise, there was no difference in RPB between groups. Furthermore, the relationship between RPB and V̇E was similar between groups (Fig. 3, p > 0.05). The relationships between RPB and: BMI, V̇O2 (%peak exercise capacity), and peak exercise V̇O2 (%predicted based on predicted weight) were modest for both groups (r2 ≤ 0.36). In contrast, the relationship between RPB and RPE was strong in both the obese (r = 0.75 and 0.66, respectively, p < 0.001) and nonobese patients (r = 0.46 and 0.67, respectively, p < 0.001) at both submaximal exercise levels.
Fig. 3.
Top: RPB (0–10 Borg Scale) at rest, submaximal work rates, and peak exercise for nonobese and obese patients; Bottom: Relationship between RPB (0–10 Borg scale) and V̇E (, L/min) for nonobese and obese patients. RPB=rating of perceived breathlessness; V̇E=minute ventilation. *p < 0.05.
3.5. Multidimensional dyspnea profile
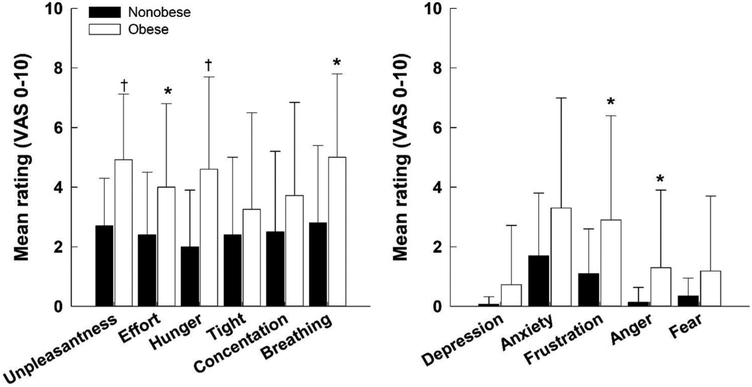
In the subset of patients that completed the prescribed breathlessness questionnaire to assess the quality of respiratory sensations and perceived level of unpleasantness (sensory dimension) and negative emotions associated with DOE (affective dimension), effort, air hunger, and breathing sensations were rated higher in the obese compared to nonobese (Fig. 4, left panel, p < 0.05). Ratings of depression, anxiety, and fear were not different between groups; however, ratings of frustration and anger were higher in the obese compared with nonobese patients (Fig. 4, right panel, p < 0.05). Overall, the mean sensory score (including unpleasantness) was 4.3 ± 2.2 in obese compared to 2.5 ± 1.9 in nonobese patients (p < 0.01) and the mean affective score was 1.9 ± 2.2 in obese compared to 0.7 ± 0.7 in nonobese patients (p < 0.01). At submaximal work rate 1, there were modest and positive correlations between RPB and unpleasantness (r = 0.67, p < 0.0001), effort (r = 0.60, p < 0.0001), air hunger (r = 0.67, p < 0.0001), tight (r = 0.35, p = 0.02), and breathing (r = 34, p = 0.03). At submaximal work rate 2, there also modest and positive correlations between RPB and unpleasantness (r = 0.66, p < 0.0001), effort (r = 0.63, p < 0.0001), air hunger (r = 0.60, p < 0.0001), tight (r = 0.63, p < 0.0001), and breathing (r = 0.60, p < 0.0001). In contrast, RPB (at submaximal work rate 1 and 2) did not correlate with any negative emotions. RPB was also correlated with V̇O2 (%peak V̇O2) at submaximal work rates 1 (r = 0.62, p < 0.0001) and 2 (r = 0.55, p < 0.0001).
Fig. 4.
Sensory dimensions of dyspnea including unpleasantness (left) and affective dimensions of dyspnea (right) recorded from a visual analog scale (VAS 0–10) for obese and nonobese patients. *p < 0.05; †p < 0.01.
4. Discussion
While obese patients had greater breathlessness during the lowest level of exercise, they had a similar level of breathlessness during the higher submaximal exercise work rate and at peak exercise compared with nonobese patients with significant cardiorespiratory limitations to exercise. Thus, obesity may independently provoke similar intensities of DOE compared with those nonobese patients with significant levels of cardiorespiratory limitation. Moreover, DOE was associated with higher levels of unpleasantness and negative emotions in the obese patients. Therefore, these findings not only highlight the need to test patients at lower constant load submaximal work rates where the temporal dynamics of respiratory sensations can be accurately determined, but also suggest that CPET evaluations and interpretations must consider the strong possibility that obesity is an independent confounding mechanism of DOE and/or the sole reason for breathlessness during exercise.
4.1. Patients
The patients included in the retrospective analysis were a diverse group of individuals with various diseases/limitations. Indeed, patients were referred for CPET due to unexplained DOE and had been through extensive diagnostic testing prior to referral to our laboratory. However, in many of the patients, DOE was greater than what is usually associated with their cardiorespiratory condition. Thus, this is a very different patient population than is usually studied regarding breathlessness during exercise, where patients with specific types of lung and heart disease are studied (e.g., COPD or heart failure patients) (Travers et al., 2008; Jensen et al., 2009; Ora et al., 2009). Lastly, the relative distribution of women was higher in the nonobese patients; however, there does not appear to be a difference in the prevalence or magnitude of breathlessness during exercise between otherwise healthy obese men and women (Bernhardt et al., 2016). Therefore, it is unlikely that the proportion of women in the nonobese group affected the results in a meaningful manner.
4.2. Cardiorespiratory fitness
When peak V̇O2 was expressed as a percent of predicted peak V̇O2 (based on predicted ideal body weight), peak exercise capacity in the patients with obesity was higher than that of the nonobese patients. This method of determining cardiorespiratory fitness has been recommended for many years for individuals with obesity (Buskirk et al., 1957; Wasserman et al., 1987; Jones, 1988). Recently, the EACPR/AHA Scientific Statement for CPET has also recommended reporting peak V̇O2 as percent predicted in patients with obesity (Guazzi et al., 2016). In contrast, when peak V̇O2 was indexed to body weight (i.e., ml/min/kg), the patients with obesity, as compared with the nonobese patients, appeared to have a reduced exercise capacity (i.e., decreased physical fitness). Thus, while the patients with obesity in the present study had reduced physical fitness (i.e., ability to perform physical activities), they were not deconditioned or exercise limited as compared with the nonobese patients. This finding could have been overlooked without the correct normalization of peak V̇O2, as recommended (Guazzi et al., 2016). Notably, accurately assessing maximal exercise capacity is one of the most important reasons for completing CPET.
4.3. Dyspnea on exertion and respiratory sensations
DOE is a multidimensional and complex symptom (Parshall et al., 2012; Banzett et al., 2015; Bernhardt et al., 2017), and distinguishing the origin of breathing discomfort is even more difficult when the patient is obese. Even in otherwise healthy obese adults, one in three individuals with obesity present with an increased rating of dyspnea during low level constant load cycling exercise (Babb et al., 2008; Bernhardt et al., 2013, Bernhardt et al., 2014). Based on our findings, patients with obesity have similar, or even higher levels of DOE, as patients who are nonobese with significant limitations due primarily or secondarily to cardiovascular or respiratory causes. Thus, in order to correctly evaluate DOE in a patient with obesity, it may be necessary to first exclude heart and lung limitations before obesity can be diagnosed as the primary cause of DOE. Furthermore, while the intensity of breathlessness (i.e., RPB) seemed to be similar between groups during the higher submaximal exercise work rate and at peak exercise, ratings of the quality of breathlessness and negative emotions associated with DOE were greater in the obese patients. Specifically, DOE in the obese patients induced greater feelings of ‘unpleasantness’, ‘effort’, ‘air hunger’, and ‘breathing’ which overall appeared to make these patients more angry and frustrated with their DOE when compared with nonobese patients. As such, these findings highlight that the intensity and quality of breathlessness are independently perceived (Bernhardt et al., 2013), and that measurements of the affective dimensions of DOE are essential to obtain in obese patients since the unpleasantness/negative emotions associated with DOE are indeed not reflected simply by measuring RPB (Marines-Price, 2019). Furthermore, whether similar findings would be observed in younger individuals and/or children with obesity is currently unclear and further studies are warranted to investigate this notion directly.
Based on the data in the present study, we may only speculate on the potential mechanisms responsible for differences in the emotional response to DOE. Over the past decade, it has been proposed that respiratory sensations are regulated by neural gating systems that control afferent respiratory information flow to the cerebral cortex (Banzett et al., 2000; Gerlach et al., 2013). A gating system known as “affective processing” regulates how afferent respiratory information is associated with anger or frustration (Davenport et al., 2009). This is an integral component for determining the emotional response to DOE, which is primarily based on individuals’ previous experiences/expectations (Davenport et al. 2009). Therefore, in the absence of any physiological and/or mechanical differences related to breathing in obese adults, it stands to reason that differences in respiratory neural gating and/or past experiences/expectations may explain the higher emotional response to DOE observed in obese compared with nonobese patients. However, these mechanisms deserve further study, particularly since it is the affective unpleasantness/negative emotions that may motivate patients to decrease levels of physical activity (Lansing et al., 2009). This behavior can be detrimental to patients’ health-related quality of life given that physical activity is an essential component in the management of treatment of obesity (Donnelly et al., 2009).
With respect to obtaining an accurate representation of the affective dimension of DOE specifically, it may be prudent to include a constant-load exercise stage, similar to what has previously been described by Sutton and Jones (1974), prior to the incremental CPET. Although this may be a costly expense given the current obesity epidemic where two out of every three patients are obese or overweight, it is indeed a critical step in assessing the major contributors in DOE in patients with obesity. Indeed, the constant-load exercise stage can be easily added to a maximal incremental exercise protocol (i.e.,CPET) as a warm-up stage (Bernhardt and Babb, 2016). The constant-load exercise stage is required prior to entering the incremental stages of CPET given that the temporal dynamics of respiratory sensations are slower to establish than those for physiological responses (Banzett, 1996; Moosavi et al., 2004). Not only will an adequate amount of time at a given lower level exercise work rate ensure that the respiratory sensation(s) reach a temporal steady-state (Bernhardt et al., 2016), but exercise at a constant load work rate is also more reflective of the patient’s symptom provoking activities of daily living. This representation of respiratory sensations cannot be achieved if the duration of the exercise stage is too short, the exercise intensity is too high, or if the intensity of the exercise stage is changed too often. This may be why the obese patients in the present study had an increased rating of breathlessness and exertion during the lower work rate but not during the higher work rates in the present study. At the higher work rate, the patients with obesity were less likely to be at a temporal steady state, especially since they were exercising at a higher oxygen demand. The same lack of temporal latency is true during an incremental exercise test, where the exercise intensity and ventilatory demands change quickly. Nevertheless, obesity can provoke as much dyspnea as substantial cardiorespiratory disease at higher work rates.
The lack of meaningful correlation between RPB and body fatness (BMI) suggests that general measures of obesity will not always indicate which patients will have a greater degree of DOE. The association between RPB and body composition, and RPB and cardiorespiratory measures including respiratory mechanics are also low in otherwise healthy obese adults (Bernhardt et al., 2016). However, the high correlation observed between RPB and RPE in the obese group suggests that a patient’s perceived exertion may be closely related to the breathing discomfort a patient feels (Bernhardt et al., 2016). Surprisingly, there was not a meaningful correlation between DOE and the intensity of constant load exercise in the present study. This could have more to do with the affective dimension of DOE than with the absolute or relative exercise level (i.e., exercise intensity). Whilst this relationship deserves further investigation in future studies, it is unlikely that any one cardiorespiratory factor is the cause of DOE, but rather it is due to a interplay of complex factors to which the individual is sensitive (Bernhardt et al., 2016).
4.4. Cardiorespiratory responses
Some of the DOE in the patients with obesity can be attributed to an increase in V̇O2 per given work rate and the subsequent increase in ventilatory demand (Fig. 2). Overall however, the ventilatory ratio for the obese patients was lower than one might expect (Table 2 and 3), perhaps contributing to a higher than expected PETCO2. Nevertheless, differences in DOE at the lowest work rate appeared to be partially ameliorated when related to the ventilatory demand; this has been suggested before in other studies of obese patient populations (Ofir et al., 2007; Faisal et al., 2016).
The higher CO and SV observed in the obese patients has not been highly cited. However, it is believed to be ascribed to an increased preloading of the heart secondary to the obesity-related increases in central blood volume, as well as increases in muscle mass that can enhance venous return via a greater muscle pump effect. Since the obese patients in the present study exhibited a higher SV and SI, the exercising HR was lower although the response was within normal limits. On the other hand, the trend for a lower a-vO2 difference could be the reason for increased CO. Indeed, it has been suggested that skeletal muscle/metabolic/circulation limitations contribute to an increase in CO (Schaeffer et al., 2014). Further research is required to investigate these notions directly since this study was not designed to address these questions.
4.5. Limitations
Because the patients were a mixed clinical population, there could have been confounding factors. Overall though, the patients with obesity seemed to be limited by their obesity more so than respiratory and cardiovascular disease, which was likely the primary reason causing DOE in the nonobese patients. Therefore, it may be argued that even in the presence of potential disease-related comorbidities, obesity contributes largely to DOE and provokes greater levels of unpleasantness and negative emotions during exercise. Moreover, that we included a mixed patient population referred for CPET in the present study does not limit the extrapolation of our findings related to DOE and obesity to specific patient populations. Finally, our findings cannot be extrapolated to younger obese individuals with similar underlying disease conditions.
5. Conclusions
In summary, the findings of the present study suggest that obesity can provoke greater DOE and is most discernable at lower constant work rates consistent with the temporal latency of respiratory sensations. While obesity can also provoke just as much DOE as significant levels of cardiorespiratory disease at higher work rates, DOE was associated with greater levels of unpleasantness and negative emotions in the obese compared with nonobese patients. Thus, in patients with obesity, healthcare providers must consider the possibility of obesity as an important confounding factor, or as the primary reason for breathlessness during exertion. Further, CPET may be helpful to separate the effects of obesity from that of significant breathing/exercise limitations, especially with the use appropriate testing methodology (i.e., the need to test patients at lower constant load submaximal work rates where the temporal dynamics of respiratory sensations can be accurately determined). In doing so, this will subsequently provide aid to healthcare providers in determining the specific origin of DOE, particularly in patients with obesity.
Acknowledgments
The authors wish to acknowledge the assistance of Simba Walker-Williams with data analysis and Anastasia Pyz with graphics. The authors also wish to thank all of the clinical staff and physicians who participate in the clinical CPET laboratory.
Funding
King Charitable Foundation Trust; Cain Foundation; Texas Health Presbyterian Hospital Dallas, and National Institutes of Health [Grants HL096782, HL136643].
Footnotes
Guarantor
Dr. Babb is the guarantor of the content of the manuscript, including data and analysis.
Declaration of Competing Interest
Dr. Balmain has no conflicts of interest to disclose. Mr. Weinstein has no conflicts of interest to disclose. Dr. Bernhardt has no conflicts of interest to disclose. Dr. Marines-Prince has no conflicts of interest to disclose. Dr. Tomlinson has no conflicts of interest to disclose. Dr. Babb has no conflicts of interest to disclose.
References
- Azagury DE, Lautz DB, 2011. Obesity overview: epidemiology, health and financial impact, and guidelines for qualification for surgical therapy. Gastrointest. Endosc. Clin 21 (2), 189–201. [DOI] [PubMed] [Google Scholar]
- Babb TG, 1997. Ventilation and respiratory mechanics during exercise in younger subjects breathing CO2 or HeO2. Respir. Physiol 109 (1), 15–28. [DOI] [PubMed] [Google Scholar]
- Babb TG, Buskirk ER, Hodgson JL, 1989. Exercise end-expiratory lung volumes in lean and moderately obese women. Int. J. Obes 13, 11–19. [PubMed] [Google Scholar]
- Babb TG, DeLorey DS, Wyrick BL, Gardner PP, 2002. Mild obesity does not limit change in end-expiratory lung volume during cycling in young women. J. Appl. Physiol 92, 2483–2490. [DOI] [PubMed] [Google Scholar]
- Babb TG, Korzick D, Meador M, Hodgson JL, Buskirk ER, 1991. Ventilatory response of moderately obese women to submaximal exercise. Int. J. Obes 15, 59–65. [PubMed] [Google Scholar]
- Babb TG, Long KA, Rodarte JR, 1997. The relationship between maximal expiratory flow and increases of maximal exercise capacity with exercise training. Am. J. Respir. Crit. Care Med. 156 (1), 116–121. [DOI] [PubMed] [Google Scholar]
- Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B, 2008. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am. J. Respir. Crit. Care Med. 178, 116–123. [DOI] [PubMed] [Google Scholar]
- Babb TG, Rodarte JR, 1993. Estimation of ventilatory capacity during submaximal exercise. J. Appl. Physiol 74, 2016–2022. [DOI] [PubMed] [Google Scholar]
- Banzett RB, 1996. Dynamic response characteristics of CO2-induced air hunger. Respir. Physiol 105 (1–2), 47–55. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Dempsey JA, O’Donnell DE, Wamboldt MZ, 2000. Symptom perception and respiratory sensation in asthma. Am. J. Respir. Crit. Care Med. 162, 1178–1182. [DOI] [PubMed] [Google Scholar]
- Banzett RB, O’Donnell CR, Guilfoyle TE, Parshall MB, Schwartzstein RM, Meek PM, Gracely RH, Lansing RW, 2015. Multidimensional Dyspnea Profile: an instrument for clinical and laboratory research. Eur. Respir. J 45 (6), 1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Babb TG, 2014. Respiratory symptom perception differs in obese women with strong or mild breathlessness during constant-load exercise. Chest 145 (2), 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Babb TG, 2016. Exertional dyspnoea in obesity. Eur. Respir. Rev 25 (142), 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Bhammar DM, Marines-Price R, Babb TG, 2019. Weight loss reduces dyspnea on exertion and unpleasantness of dyspnea in obese men. Respir. Physiol. Neurobiol 261, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Marines-Price R, Weinstein K, Walker-Williams S, Tomlinson A, Babb TG, 2017. Dyspnea on exertion in nonobese and obese patients. Med. Sci. Sports Exerc. 49 (5S). [Google Scholar]
- Bernhardt V, Stickford JL, Bhammar DM, Babb TG, 2016. Aerobic exercise training without weight loss reduces dyspnea on exertion in obese women. Respir. Physiol. Neurobiol 221, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Wood HE, Moran RB, Babb TG, 2013. Dyspnea on exertion in obese men. Respir. Physiol. Neurobiol 185 (2), 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskirk ER, Taylor HL, 1957. Maximal oxygen intake and its relation to body composition, with special reference to chronic physical activity and obesity. J. Appl. Physiol 11 (1), 72–78. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Vovk A, 2009. Cortical and subcortical central neural pathways in respiratory sensations. Respir. Physiol. Neurobiol 167 (1), 72–86. [DOI] [PubMed] [Google Scholar]
- de Voogd JN, Sanderman R, Postema K, van Sonderen E, Wempe JB, 2011d. Relationship between anxiety and dyspnea on exertion in patients with chronic obstructive pulmonary disease. Anxiety Stress Coping 24 (4), 439–449. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Wyrick BL, Babb TG, 2005. Mild-to-moderate obesity: implications for respiratory mechanics at rest and during exercise in young men. Int. J. Obes 29, 1039–1047. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, American M College of Sports, 2009. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 41 (2), 459–471. [DOI] [PubMed] [Google Scholar]
- Dube BP, Agostoni P, Laveneziana P, 2016. Exertional dyspnoea in chronic heart failure: the role of the lung and respiratory mechanical factors. Eur. Respir. Rev 25 (141), 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gamal H, Khayat A, Shikora S, Unterborn JN, 2005. Relationship of dyspnea to respiratory drive and pulmonary function tests in obese patients before and after weight loss. Chest 128 (6), 3870–3874. [DOI] [PubMed] [Google Scholar]
- Faisal A, Alghamdi BJ, Ciavaglia CE, Elbehairy AF, Webb KA, Ora J, Neder JA, O’Donnell DE, 2016. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am. J. Respir. Crit. Care Med. 193 (3), 299–309. [DOI] [PubMed] [Google Scholar]
- Gerlach Y, Williams MT, Coates AM, 2013. Weighing up the evidence – a systematic review of measures used for the sensation of breathlessness in obesity. Int. J. Obes. (Lond) 37 (3), 341–349. [DOI] [PubMed] [Google Scholar]
- Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ, 2016. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. [DOI] [PubMed] [Google Scholar]
- Jensen D, Ofir D, O’Donnell DE, 2009. Effects of pregnancy, obesity and aging on the intensity of perceived breathlessness during exercise in healthy humans. Respir. Physiol. Neurobiol 167, 87–100. [DOI] [PubMed] [Google Scholar]
- Jones NL, 1988. Clinical Exercise Testing. W.B. Saunders, Philadelphia. [Google Scholar]
- Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vassan RS, 2002. Obesity and the risk of heart failure. N. Engl. J. Med 347 (5), 305–313. [DOI] [PubMed] [Google Scholar]
- Lansing RW, Gracely RH, Banzett RB, 2009. The multiple dimensions of dyspnea: review and hypotheses. Respir. Physiol. Neurobiol 167 (1), 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo S, Babb TG, 2013. Ventilatory responses at peak exercise in endurance-trained obese adults. Chest 144 (4), 1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti P, Gigliotti F, Tesi F, Stendardi L, Grazzini M, Duranti R, Scano G, 2005. Respiratory muscles and dyspnea in obese nonsmoking subjects. Lung 183 (5), 311–323. [DOI] [PubMed] [Google Scholar]
- Marines-Price R, Bernhardt V, Bhammar DM, Babb TG, 2019. Dyspnea on exertion provokes unpleasantness and negative emotions in women with obesity. Respir. Physiol. Neurobiol 260, 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosavi SH, Banzett RB, Butler JP, 2004. Time course of air hunger mirrors the biphasic ventilatory response to hypoxia. J. Appl. Physiol 97 (6), 2098–2103 1985. [DOI] [PubMed] [Google Scholar]
- O’Donnell DE, Bertly JC, Chau LKL, Webb KA, 1997. Qualitative aspects of exertional breathlessness in chronic airflow limitation. Am. J. Respir. Crit. Care Med. 1555, 109–115. [DOI] [PubMed] [Google Scholar]
- Ofir D, Laveneziana P, Webb KA, O’Donnell DE, 2007. Ventilatory and perceptual responses to cycle exercise in obese women. J. Appl. Physiol 102, 2217–2226. [DOI] [PubMed] [Google Scholar]
- Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O’Donnell DE, 2009. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am. J. Respir. Crit. Care Med. 180, 964–971. [DOI] [PubMed] [Google Scholar]
- Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, Mahler DA, Meek PM, O’Donnell DE, On behalf of the American Thoracic Society Committee on dyspnea, 2012. An official American thoracic society statement: update on the mechanisms, assessment, and management of dyspnea. Am. J. Respir. Crit. Care Med. 185 (4), 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel SD, McNeer FR, Higginbotham MB, 1998. Exertional dyspnea in heart failure: a symptom unrelated to pulmonary function at rest or during exercise. Am. Heart J. 135, 398–405. [DOI] [PubMed] [Google Scholar]
- Sahebjami H, 1998. Dyspnea in obese healthy men. Chest 114, 1373–1377. [DOI] [PubMed] [Google Scholar]
- Schaeffer MR, Mendonca CT, Levangie MC, Andersen RE, Taivassalo T, Jensen D, 2014. Physiological mechanisms of sex differences in exertional dyspnoea: role of neural respiratory motor drive. Exp. Physiol 99 (2), 427–441. [DOI] [PubMed] [Google Scholar]
- Sin DD, Jones RL, Man SF, 2002. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch. Intern. Med 162 (13), 1477–1481. [DOI] [PubMed] [Google Scholar]
- Sutton JR, Jones NL, 1974. Exercise testing in health and disease. Can. Fam. Physician 20 (4), 66–69. [PMC free article] [PubMed] [Google Scholar]
- Travers J, Dudgeon DJ, Amjadi K, McBride I, Dillon K, Laveneziana P, Ofir D, Webb KA, O’Donnell DE, 2008. Mechanisms of exertional dyspnea in patients with cancer. J. Appl. Physiol 104, 57–66. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE, 2006. Mechanisms linking obesity with cardiovascular disease. Nature 444 (7121), 875–880. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Hansen JE, Sue DY, Whipp BJ, 1987. Principles of Exercise Testing and Interpretation. Lea and Febiger, Philadelphia. [Google Scholar]
- Whipp BJ, Davis JA, 1984. The ventilatory stress of exercise in obesity. Am. Rev. Respir. Dis 129, S90–S92. [DOI] [PubMed] [Google Scholar]
- Wood HE, Semon TL, Comeau LA, Schwartz B, MacDougall RM, Klocko MN, Babb TG, 2008. The ventilatory response to exercise does not differ between obese women with and without dyspnea on exertion. Adv. Exp. Med. Biol 605, 514–518. [DOI] [PubMed] [Google Scholar]
- Yang L, Colditz GA, 2015. Prevalence of overweight and obesity in the United States, 2007–2012. JAMA Intern. Med 175 (8), 1412–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]