Abstract
Today, there are no medicines to treat enterovirus and rhinovirus infections. In the present study, a series of novel pleconaril derivatives with substitutions in the isoxazole and phenyl rings was synthesized and evaluated for their antiviral activity against a panel of pleconaril-sensitive and -resistant enteroviruses. Studies of the structure-activity relationship demonstrate the crucial role of the N,N-dimethylcarbamoyl group in the isoxazole ring for antiviral activity against pleconaril-resistant viruses. In addition, one or two substituents in the phenyl ring directly impact on the spectrum of antienteroviral activity. The 3-(3-methyl-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 10g was among the compounds exhibiting the strongest activity against pleconaril-resistant as well as pleconaril-susceptible enteroviruses with IC50 values from 0.02 to 5.25 μM in this series. Compound 10g demonstrated markedly less CYP3A4 induction than pleconaril, was non-mutagenic, and was bioavailable after intragastric administration in mice. These results highlight compound 10g as a promising potential candidate as a broad spectrum enterovirus and rhinovirus inhibitor for further preclinical investigations.
Keywords: Antivirals, drug design/discovery, capsid-binding inhibitor, structure-activity relationship analysis, coxsackie B3 virus, rhinovirus, resistance, CYP3A4 induction, pharmacokinetics
Graphical Abstract:

1. Introduction
Enteroviruses and rhinoviruses belong to the Enterovirus genus of the Picornaviridae family. There are about 100 serotypes of enteroviruses species A-D and more than 160 serotypes of rhinoviruses species A-C [1]. Enteroviruses cause a wide range of acute and chronic diseases, such as foot-hand-and-mouth disease, myocarditis, acute flaccid myelitis, cardiomyopathy, poliomyelitis, encephalitis, meningitis, bronchiolitis and pneumonia [2, 3]. Rhinoviruses are the major cause of the common cold, a mild illness of the upper respiratory tract, but can also induce more severe lower respiratory tract illnesses, for example, bronchitis, sinusitis and pneumonia and can exacerbate asthma, cystic fibrosis and chronic obstructive pulmonary disease [4–8]. Vaccine development for these viruses is complicated by the multiplicity of serotypes. Today, there are only vaccines to prevent poliomyelitis caused by polioviruses [9], two inactivated enterovirus A71 vaccines used in China for prevention of hand-foot-and-mouth human disease [10] and vaccine against foot-and-mouth disease virus [11]. Thus, development of effective antiviral small molecules acting on the viral life cycle represents a real clinical unmet need.
Enteroviruses, rhinoviruses A and B [12], as well as rhinoviruses C [13] have a hydrophobic pocket in capsid protein 1. The hydrophobic pocket of the majority of enteroviruses and rhinoviruses A and B is filled with a fatty acid (so called “pocket factor”), whereas pocket of rhinoviruses C is filled with multiple bulky residues [13]. The pocket factor is released during viral attachment to host cells. During the attachment, conformational changes in the viral capsid trigger the release of viral genomic RNA. Inhibitors competing with the pocket factor for binding to the hydrophobic pocket (capsid-binding inhibitors) prevent virus attachment and/or uncoating. Among the most extensively studied drug candidates with this capsid-binding mechanism of action against enteroviruses are pleconaril, vapendavir and pocapavir. The synthesis, biological studies and causes of clinical trial failures of these capsid-binding inhibitors were described comprehensively in a recently published review [14].
Resistance of entero- and rhinoviruses toward capsid-binding inhibitors is most commonly linked to substitutions of amino acid residues forming the hydrophobic pocket [15]. In addition, different amino acid substitutions of isoleucine in position 207 in the viral capsid protein 1 (I1207K/R/M/T), a position not directly involved in the formation of the hydrophobic pocket of the pleconaril-sensitive coxsackievirus B3 97927 were shown to induce resistance under experimental conditions [16, 17]. Generally, known naturally occurring and treatment-triggered resistance should be taken into account during the development of effective antienteroviral agents.
Up to now, there have been no regulatory agency approved antienteroviral medicines for clinical uses. Current developments in this field have not met safety and quality requirements. For example, pleconaril was not approved by the FDA for the treatment of common cold due to set of adverse effects (headache, diarrhea and nausea), cytochrome P-450 (CYP3A4) induction (increasing ethinyl estradiol metabolism) and existence of pleconaril-resistant viruses [18]. In February 2017, it was reported that another clinical candidate vapendavir, failed a phase IIb due to insufficient efficacy against RVs as compared with placebo [19]. In addition, a very high rate of resistance was observed (44% in the pocapavir treated group vs 10% in the placebo treated group) in a randomized blinded placebo-controlled study where poliovirus-infected patients were treated with pocapavir [20]. Therefore, development of potent selective broad-spectrum antienteroviral agents remains one of the priorities of medicinal chemists in this field.
We have previously synthesized several new pleconaril and [(biphenyloxy)propyl]isoxazole analogues where pleconaril resistance of some entero- and rhinoviruses was overcome by unsubstituted analogues or by monosubstitution in the central phenyl ring [21, 22]. In a recently published review, we tracked the evolution of the synthetic development of some significant capsid-binding inhibitors [14]. The consistent study of the structure-activity relationship helped us to select a scaffold as the basis for compounds overcoming resistance and lowering the risk of CYP3A4 induction.
In the present work, we show the influence of substituents in the 3-position of pleconaril and isoxazole ring on inhibition activity against a set of selected pleconaril-sensitive and -resistant entero- and rhinoviruses. We continued to examine the impact of substituents in the phenyl ring of pleconaril derivatives on antienteroviral activity for a better understanding of structure-activity relationships to overcome viral resistance to capsid-inhibitors. In addition, we show that the most active compound synthesized in the present study is a much weaker inducer of the CYP3A4 enzyme than pleconaril. In addition, it did not exert mutagenicity and showed high bioavailability after intragastric administration.
2. Results and discussion
2.1. Chemistry and chemical characterization of new pleconaril derivatives
The syntheses of target compounds are presented in Schemes 1–6.
Scheme 1.
Synthesis of 3-(4-(3-(3-carbalkoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazoles 6a–i. Reagents and conditions: (a) 5-chloro-1-pentyne, K2CO3, KI, N-methylpyrrolidone, 65 °C, 24h; (b) NH2OH·HCl, K2CO3, EtOHabs, reflux, 12h; (c) (CF3CO)2O, pyridine, 80–90 °C, 2–3h; (d) Et3N, dimethylformamide, 80–90 °C, 2–3h.
Scheme 6.
Synthesis of pyridinyl analogues. Reagents and conditions: (a) 4-pentyn-1-ol, K2CO3, N-methylpyrrolidone, 65 °C, 24h; (b) NH2OH·HCl, K2CO3, EtOHabs, reflux, 12h; (c) (CF3CO)2O, pyridine, 80–90 °C, 2–3h; (d) 5b, Et3N, dimethylformamide, 80–90 °C, 2–3h; (e) dimethylamine solution 17 wt. % in dioxane, 50–60 °C, 12h
O-Alkylation of hydroxybenzonitriles 1a,c–i with 5-chloro-1-pentyne in presence of potassium carbonate and potassium iodide in N-methylpyrrolidone at 65 °C provided the corresponding 4-(pent-4-yn-1-yloxy)benzonitriles 2a,c–i in good yields (Scheme 1). Treatment of 2a,c–i with hydroxylamine hydrochloride and potassium carbonate in absolute ethanol gave amidoximes 3a,c–i. Interestingly, two intermediates, 3d (Me) and 3e (MeO), were present in the form of two tautomeric structures, which is observed by characteristic peaks in their 1H NMR. It should also be noted that the presence or absence of tautometic structures in compounds 3a, c–i does not affect subsequent reactions. For cyclization into 1,2,4-oxadiazole compounds 3a,c–i were acylated with trifluoroacetic anhydride in pyridine with synthesis the appropriate (pent-4-yn-1-yloxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazoles 4a,c–i. Their reaction with 2-chloro-2-(hydroxyimino)acetic acid methyl ester 5a or 2-chloro-2-(hydroxyimino)acetic acid ethyl ester 5b in presence of triethylamine in dry dimethylformamide (DMF) led to 3-(4-(3-(3-carbalkoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazoles 6a–i.
3-(3,5-Dimethyl-4-(3-(3-carbisopropoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 7 was synthesized according to the two step procedure based on available chemicals shown in Scheme 2. Basic hydrolysis of previously prepared 3-(3,5-dimethyl-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 6b gave the corresponding acid and its subsequent Fischer esterification with i-propanol provided the target compound 7.
Scheme 2.
Synthesis of 3-(3,5-dimethyl-4-(3-(3-carbisopropoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 7. Reagents and conditions: (a) 1. LiOH, MeOH/H2O, 2. iPrOH, H2SO4cat, reflux.
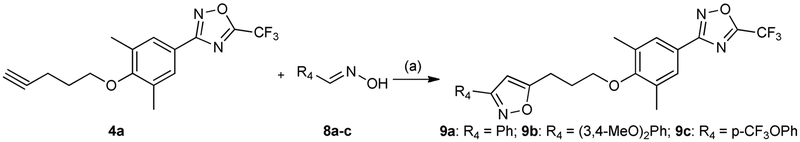
As shown in Scheme 3, unsubstituted or substituted (((phenylisoxazolyl)propoxy)phenyl)oxadiazoles 9a–c were prepared by cyclization of intermediate 4a with the corresponding benzaldehyde oximes 8a–c in the presence of N-chlorosuccinimide, triethylamine and pyridine as catalyst in dry dimethylformamide.
Scheme 3.
Synthesis of unsubstituted or substituted (((phenylisoxazolyl)propoxy)phenyl)oxadiazoles 9a–c. Reagents and conditions: (a) N-chlorosuccinimide, pyridinecat, Et3N, dimethylformamide, 80–90 °C.
Carbamoyl and substituted carbamoyl derivatives were prepared in two ways according to the Scheme 4. One way, (a), included treatment of 3-(3,5-dimethyl-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 6b with the corresponding amines in refluxing i-propanol to provide compounds 10a–b,m. Another way, (b), included substitution of the 3-carbethoxy group of (((isoxazolyl)propoxy)phenyl)oxadiazoles 6c–i with dimethylamine solution in water or dioxane to yield the respective dimethylcarbamoyl derivatives 10f–l. All of the reactions proceeded easily and quickly, and the final compounds 10a–m, which precipitate upon cooling of the reaction mixture as a well-formed solids were filtered and recrystallized from alcohols.
Scheme 4.
Synthesis of carbamoyl and substituted carbamoyl derivatives 10a–m. Reagents and conditions: (a) the corresponding amine, iPrOH, reflux, 18–24h; (b) dimethylamine solution 33 wt. % in H2O or 17 wt. % in dioxane, EtOH, 40–50 °C, 1h.
Reduction of nitro derivative 6f with stannum chloride dehydrate in ethanol gave the amino derivative 11 in excellent yield (Scheme 5). Firstly, this derivative was functionalized by acetic anhydride, 2,5-dimethoxyfuran and dimethylformamide-dimethylacetal (DMF-DMA) to provide the corresponding final compounds 12a–c. Also, derivate 11 was treated with dimethylamine solution in dioxane to gave 3-(3-amino-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 13, which was functionalized in the same manner as 12a,b to obtain target compounds 14a,b.
Scheme 5.
Synthesis of amino derivatives 11 and 13 and functionalized amino analogues 12a–c and 14a,b. Reagents and conditions: (a) SnCl2·2H2O, EtOH, rt; (b) Ac2O, 80 °C or 2,5-dimethoxyfuran, AcOH or DMF-DMA, EtOH, reflux; (c) dimethylamine solution 33 wt. % in H2O, EtOH, 40–50 °C, 1h; (d) Ac2O, 80 °C or 2,5-dimethoxyfuran, AcOH
It was discovered that the derivative 10i, containing a nitro group in the phenyl ring and a N,N-dimethylcarbamoyl group in the isoxazole, was active towards tested pleconaril-sensitive and - resistant viruses (see Table 2). Based on the similarity of pyridine to nitrobenzene, it seemed interesting to us to study how the exchange of the nitrogroup on insertion a nitrogen atom in the phenyl ring will affect the inhibition activity of compounds.
Table 2.
Cytotoxicity, anti-coxsackievirus B3 and anti-rhinovirus activity of synthesized phenyl-substituted (((carbethoxyisoxazolyl)propoxy)phenyl)oxadiazoles and (((N,N-dimethylcarbamoyl-isoxazolyl)propoxy)phenyl)oxadiazoles
| No | CC50, μM | IC50 towards coxsackievirus B3, μM |
IC50 towards Rhinovirus, μM |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 97927 | Nancy | A2 | B5 | B14 | |||||
| I1207 I1092 |
I1207K I1092 |
I1207M I1092 |
I1207T I1092 |
||||||
| 6c | >100 | 0.746 | n/a | 6.54 | 42.89 | n/a | n/a | n/a | 0.34 |
| 6d | 35.11 | 0.062 | n/a | 0.61 | 1.23 | 18.96 | n/a | n/a | 0.52 |
| 6e | >100 | 0.490 | n/a | 3.41 | 37.29 | n/a | 59.71 | 19.44 | 0.66 |
| 6f | >100 | 0.148 | n/a | 0.18 | 3.99 | n/a | 47.66 | n/a | 2.93 |
| 6g | 60.19 | 0.217 | n/a | 1.35 | 24.90 | n/a | n/a | n/a | 0.50 |
| 6h | 28.44 | 0.03 | n/a | 0.01 | 1.04 | n/a | 4.89 | n/a | n/a |
| 6i | >100 | 1.189 | n/a | 10.05 | 43.78 | n/a | 5.63 | n/a | 2.68 |
| 10f | 27.98 | 0.458 | 27.49 | 0.02 | 8.11 | 10.81 | 4.61 | 1.74 | 0.04 |
| 10g | 13.38 | 0.02 | 4.79 | 0.01 | 0.01 | 2.76 | 0.86 | 5.25 | 0.09 |
| 10h | 68.00 | 0.030 | 51.71 | 0.60 | 1.66 | 37.76 | 1.97 | 3.28 | 0.04 |
| 10i | 22.48 | 0.003 | 6.46 | 0.001 | 0.03 | 1.66 | 0.67 | 7.63 | 0.28 |
| 10j | 20.16 | 0.025 | n/a | 0.06 | 1.44 | 18.48 | 3.77 | 4.04 | 0.05 |
| 10k | 19.23 | 0.16 | 7.18 | 0.16 | 0.54 | 2.24 | 0.32 | n/a | 6.76 |
| 10l | 25.55 | 0.365 | n/a | 0.88 | 0.84 | 14.47 | 0.32 | n/a | 0.20 |
| 10m | >100 | 0.02 | n/a | 0.06 | 1.04 | 14.11 | 0.96 | n/a | n/a |
| 11 | >100 | 20.02 | n/a | n/a | n/a | n/a | n/a | n/a | 14.27 |
| 12a | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 12b | 57.26 | 10.22 | n/a | n/a | 14.29 | n/a | n/a | n/a | n/a |
| 12c | >100 | 19.95 | n/a | n/a | n/a | n/a | n/a | n/a | 29.26 |
| 13 | 90.82 | 4.47 | 51.58 | 2.08 | 14.31 | 27.83 | 18.17 | n/a | 0.33 |
| 14a | >100 | 30.14 | n/a | 16.07 | 73.16 | n/a | n/a | n/a | 18.14 |
| 14b | 21.24 | 0.82 | n/a | 1.06 | 3.55 | n/a | 3.21 | n/a | 7.34 |
For this purpose, the synthesis of pyridinyl analogues 19 and 20 started from chloronicotinonitrile 15, which was O-alkylated with 4-pentyn-1-ol in the presence of potassium carbonate in N-methylpyrrolidone to yield 6-(pent-4-yn-1-yloxy)nicotinonitrile 16 (Scheme 6). Consistent treatment of 16 with hydroxylamine hydrochloride and potassium carbonate in ethanol and trifluoroacetic anhydride in pyridine gave the corresponding (pyridinyl)oxadiazole 18. Cyclization of this intermediate with 2-chloro-2-(hydroxyimino)acetic acid ethyl ester 5b in dry dimethylformamide yielded 3-(4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)pyridin-3-yl)-5-trifluoromethyl-1,2,4-oxadiazole 19. Finally, the second target compound 20 was obtained by nucleophilic substitution with dimethylamine solution in dioxane.
2.2. Cytotoxicity and in vitro antiviral activity of synthesized compounds
Aiming to identify selective inhibitors against pleconaril-sensitive and pleconaril-resistant enteroviruses and rhinoviruses, we performed cytotoxic as well as antiviral assays in HeLa cells. The maximum tested concentration was 100 μM.
To exclude unspecific antiviral effects based on cytotoxicity, the effect of test compounds on HeLa cell viability was analyzed after 72 h of compound treatment as described previously [23]. Six wells with HeLa cells without compound served as cells control on each test plate. The test was stopped by adding a crystal violet/formalin/methanol solution [24]. After dye elution, cell viability of individual compound-treated wells was evaluated as the percentage of the mean value of optical density from the cell controls, which was set 100% cell viability. The 50% cytotoxic concentration (CC50) was defined as the compound concentration reducing the viability of cell controls by 50%.
The set of pleconaril-sensitive and -resistant enteroviruses applied in the antiviral assays, included coxsackieviruses B3 Nancy, coxsackieviruses B3 97927 wild type and 3 mutants thereof and 3 rhinovirus serotypes A2, B5, and B14. Whereas coxsackievirus B3 Nancy and rhinovirus B5 are naturally pleconaril-resistant and possess resistance-conferring amino acids within the hydrophobic pocket, coxsackievirus B3 97927, rhinoviruses A2 and B14 are pleconaril-sensitive [15, 25]. In contrast, the pleconaril-resistant mutants of coxsackievirus B3 97927 with resistance-conferring amino acids outside, but near to the hydrophobic pocket were selected in the presence of pleconaril under experimental conditions [17]. All test viruses replicated well in HeLa cells. Replication of coxsackieviruses B3 and rhinoviruses caused the complete destruction of infected, untreated HeLa cells (called a cytopathic effect). Thus, inhibition of cytopathic effect (CPE) by test compounds (corresponding to increase of cell viability) could be used as parameter for evaluation of antiviral activity. Compounds were added immediately before virus infection of HeLa cells. At 48 h or 72 h after infection with coxsackieviruses B3 or rhinoviruses, respectively, the test was stopped by adding a crystal violet/formalin/methanol solution [16, 23]. At this time a nearly complete cytopathic effect was observed microscopically in six virus-infected, untreated HeLa cells whereas a confluent HeLa cell monolayer was apparent in six uninfected, untreated cells controls. The percentage of antiviral activity of the tests compounds were calculated according to Pauwels et al. [26] using the following equation: antiviral activity = [(mean optical density of 6 cell controls - mean optical density of 6 virus controls)/(optical density of test - mean optical density of 6 virus controls)] × 100 %. The 50% inhibitory concentration (IC50) was determined as the compound concentration reducing the virus-induced CPE by 50% (50% viability of mock-treated, uninfected).
2.2.1. The effect of substituents in the 3-position of isoxazole ring on the antiviral activity
Firstly, we evaluated the influence of replacement of the methyl group in the isoxazole ring of pleconaril with various substituents, such as carbamoyl, substituted carbamoyl and carbalkoxy groups, phenyl and substituted phenyl rings, on the viral inhibition. Biological results are shown in Table 1.
Table 1.
Cytotoxicity, anti-coxsackievirus B3 and anti-rhinovirus activity of pleconaril and synthesized (dimethyl((isoxazolyl)propoxy)phenyl)oxadiazoles
| No | CC50, μM | IC50 towards coxsackievirus B3, μM |
IC50 towards rhinovirus, μM |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 97927 | Nancy | A2 | B5 | B14 | |||||
| I1207 I1092 |
I1207K I1092 |
I1207M I1092 |
I1207T I1092 |
||||||
| GH | n/t | 592.34 | 664.07 | 681.76 | 725.20 | 422.68 | n/t | n/t | n/t |
| P | 26.25 | 0.01 | n/a | 2.05 | 3.97 | n/a | 0.04 | n/a | 0.07 |
| 6a | 35.37 | 0.06 | 20.44 | 1.53 | 12.83 | 17.92 | 0.36 | n/a | 0.37 |
| 6b | 17.21 | 0.07 | 11.12 | 0.94 | 20.40 | n/a | n/t | n/t | n/t |
| 7 | 17.93 | 0.19 | n/a | 4.09 | n/a | n/t | 2.43 | n/t | 0.43 |
| 9a | >100 | 0.14 | n/a | 0.28 | 1.69 | n/t | 0.76 | n/a | 0.53 |
| 9b | 41.93 | n/a | n/a | n/a | n/a | n/t | 2.11 | n/t | 2.78 |
| 9c | >100 | n/a | n/a | n/a | n/a | n/t | n/a | n/t | n/t |
| 10a | >100 | 0.07 | n/a | 1.69 | n/a | n/t | 0.50 | n/t | 0.22 |
| 10b | >100 | 0.06 | n/a | 0.37 | n/a | n/t | 0.20 | n/t | 0.02 |
| 10c | >100 | 0.05 | n/a | 0.48 | n/a | n/t | 0.60 | n/t | 0.18 |
| 10d | >100 | 1.39 | n/a | 14.36 | n/a | n/t | 0.23 | n/t | 0.25 |
| 10e | 13.20 | 0.02 | 6.33 | 0.03 | 1.01 | n/t | 0.12 | n/t | 0.12 |
CC50 – 50% cytotoxic concentration, IC50 – 50% inhibitory concentration, GH – guanidine hydrochloride, P – pleconaril, n/t – not tested, n/a – not active
Replacement of the methyl group of isoxazole ring with a carbalkoxy (6a, b, 7) and carbamoyl groups (10a–e) revealed compounds, which have activity comparable to the reference compound – pleconaril. The increasing length and volume of the side alkyl chain (Me → Et → iPr) of ester derivatives moderately reduced activity: a carbmethoxy (6a) inhibited pleconaril-susceptible and pleconaril-resistant viruses in IC50 values from 0.06 to 20.44 μM, but at the same time no activity against pleconaril-resistant rhinovirus B5 was observed. It is interesting to note that compounds with carbamoyl and substituted carbamoyl groups (10a–e) were more active towards the coxsackievirus B3 97927 wild type and I1207M mutant than corresponding compounds with carbalkoxy groups (6a, b, and 7). In the case of coxsackievirus B3 I1207T inhibition confounding results of a structure-activity relationship were obtained: for 6a and 6b derivatives decreased activity was observed compared with pleconaril, and all of the carbamoyl derivatives, except for 10e, were totally inactive towards this mutant, whereas 10e was approximately 4-times more active than pleconaril (IC50 = 1.01 μM for 10e and 3.97 μM for pleconaril). Compound 10d with a bulky substituent (benzylcarbamoyl) was significantly less active against the studied viruses compared with other carbamoyl derivatives and pleconaril. The small alkyl groups, such as methyl (10b) and ethyl (10c) in the carbamoyl derivative (10a) retained activity towards tested viruses (except for I1207K and I1207T, not active). Interestingly, the dimethylcarbamoyl derivative (10e) exhibited the most potent activity (coxsackievirus B3 Nancy and rhinovirus B5 not tested) in this series.
It is worth noting that derivatives with another bulky groups, such as phenyl (9a), dimethoxyphenyl (9b) and 4-(trifluoromethoxy)phenyl (9c) were completely inactive against the majority of viruses.
2.2.2. The effect of substituents in the phenyl ring on the antiviral activity
Secondly, we examined the influence of substituents with various properties, e.g. electronic, steric or hydrophilic/hydrophobic, in the central phenyl ring or replacement of the phenyl with a pyridine. Carbethoxy and dimethylcarbamoyl groups were selected in the capacity of the constant substituents in the isoxazole ring. Biological results were showed in Tables 2 and 3.
Table 3.
Cytotoxicity and antiviral activity of synthesized pyridinyl analogues
| No | CC50, μM | IC50 towards coxsackievirus B3, μM |
IC50 towards Rhinovirus, μM |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 97927 | Nancy | A2 | B5 | B14 | |||||
| I1207 I1092 |
I1207K I1092 |
I1207M I1092 |
I1207T I1092 |
||||||
| 19 | >100 | 10.26 | n/a | n/a | n/a | n/a | n/a | n/a | 1.99 |
| 20 | 48.84 | 5.95 | 22.01 | 5.93 | 17.38 | 22.17 | 5.83 | 4.28 | 0.12 |
It was shown that derivatives 6c-i exhibited similar activity against enteroviruses and reduced activity against rhinoviruses like pleconaril. However, 6f and 6h were more active towards two mutants coxsackievirus B3 (I1207M and I1207T) than pleconaril (IC50 = 0.18 and 3.99 μM for 6f, 0.01 and 1.04 μM for 6h, 2.05 and 3.97 μM for pleconaril, respectively), whereas derivative 6d not only inhibited these viruses at a lower 50% concentration, but also was active against pleconaril-resistant coxsackievirus B3 Nancy (IC50 = 18.96 μM).
We observed that derivatives with the dimethylcarbamoyl group in the isoxazole ring 10f–m, 13, 14a,b were more potent than with carbethoxy 6c–i, 11, 12a–c and the reference compound regardless of the substitution nature in the phenyl ring again. Whereas 6c with carbethoxy group in the 3-position of isoxazole and without any substituents in phenyl ring was inactive against pleconaril-resistant viruses (coxsackievirus B3 I1207K and Nancy, rhinovirus B5), the IC50 of 10f with dimethylcarbamoyl group of isoxazole was 27.49, 10.81 and 1.74 μM for these viruses, respectively.
Introduction of second substituent in 5-position of phenyl ring (6i and 10l with 5-methoxy and 3-nitro groups in the phenyl ring) significantly reduced inhibition activity compared with monosubstituted derivatives 6f and 10i having 3-nitro group in the central ring.
It is interesting to note that the derivative 10m with the methylcarbamoyl group in isoxazole and the trifluoromethyl in phenyl ring had a CC50 value more than 100 μM whereas the CC50 of 10k with the dimethylcarbamoyl group in isoxazole and trifluoromethyl in the same position was 19.23 μM. Compound 10m exhibited no activity against coxsackievirus B3 I1207K mutant compared to 10k, which showed inhibition all of coxsackieviruses B3 with IC50 values of 0.16–7.18 μM. In addition, 10m did not totally inhibit rhinoviruses B5 and B14, while 10k was not active against rhinovirus B5 only.
The functionalization of the 3-amino group showed that derivatives 12a–c and 14a,b having bulky substituents in the phenyl ring were weakly active or completely inactive towards Coxsackie virus serotypes. Surprisingly, derivatives 12a–c with carbethoxy group in isoxazole moderately inhibited rhinoviruses, including pleconaril-resistant rhinovirus B5, while 14a,b having the N,N-dimethylcarbamoyl group in the same position were weakly active against rhinoviruses A2 and B14 and inactive against rhinovirus B5.
The 2-methyl analogue 10g demonstrated the best activity towards pleconaril-resistant as well as pleconaril-susceptible viruses with IC50 values of 0.02, 4.79, 0.01, 0.01, 2.76 (for coxsackieviruses B3 97927 wt, I1207K, I1207M, I1207T, Nancy, respectively), 0.86, 5.25 and 0.09 μM (for rhinoviruses A2, B5 and B14, respectively). The selectivity indexes (SI = CC50 / IC50) of the most promising compound in the series 10g are given in Table 4. In addition, the dose-response curves obtained in the eight biological assays with 10g are shown in Figure 2.
Table 4.
Cytotoxicity, anti-coxsackievirus B3, anti-rhinovirus activity and selectivity indexes of the most promising compound 10g
| No | CC50, μM | IC50 towards coxsackievirus B3 (mean, μM) and SI |
IC50 towards rhinovirus (mean, μM) and SI |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 97927 | Nancy | A2 | B5 | B14 | |||||||||||||
| I1207/I1092 | I1207K/I1092 | I1207M/I1092 | I1207T/I1092 | ||||||||||||||
| mean | SI | mean | SI | mean | SI | mean | SI | mean | SI | mean | SI | mean | SI | mean | SI | ||
| 10g | 13.38 | 0.02 | 669 | 4.79 | 2.8 | 0.01 | 1338 | 0.01 | 1338 | 2.76 | 4.9 | 0.86 | 15.6 | 5.25 | 2.6 | 0.09 | 149 |
Fig. 2.
Dose-dependent cytotoxicity (A), anti-coxsackievirus B3 (B), and anti-rhinovirus (C) activity of compound 10g in HeLa cells.
As for the pyridine analogues 19 and 20, it was clear that only the presence of one or another substituent in the isoxazole cycle played a critical role: thus, analogue 20 containing an N,N-dimethylcarbamoyl group showed moderate inhibition activity towards all of the studied viruses with IC50 values from 0.12 to 22.17 μM, while analog 19 with the carbethoxy group was active only for coxsackievirus B3 wt with IC50 = 10.26 μM and rhinovirus B14 with IC50 = 1.99 μM.
(A) In the cytotoxicity assay compound 10g was added at the indicated concentrations to two-day-old confluent HeLa cell monolayers for 3 days. Thereafter, the cells were fixed and stained with a crystal-violet/formalin/methanol solution. After eluting the dye, the optical density of compound treated and untreated wells with HeLa cells was measured. The mean optical density of six mock-treated HeLa cell controls was set 100% cell viability when calculating the viability of compound 10g-treated cells. In (B) and (C) compound 10g was added at the indicated concentrations to HeLa cells immediately before infecting the cells with coxsackieviruses B3 (CVB3) or rhinoviruses (RV). The applied multiplicity of infection of CVB3 and RV resulted in a complete cytopathic effect of the six untreated HeLa cells (negative controls) after an incubation time of 48 h (CVB3) or 72 h (RV). Thereafter, the cells were fixed, stained, and destained as in the cytotoxicity assay and the optical density was measured. The mean optical density of six mock-treated, virus-infected cell controls was used as blank. The remaining mean optical density of six mock-treated, uninfected cell controls was set 100% cell viability when calculating the viability of compound 10g-treated, infected cells (antiviral activity). The mean percentage values of cell viability and standard deviation calculated from at least 3 independently performed cytotoxicity and antiviral assays is shown.
2.3. Pharmacokinetics of compound 10g after intragastric administration
We determined the pharmacokinetic properties of the most promising compound in the series 10g after intragastric administration of 100 mg/kg as 0.5 mL of prepared suspension in 0.5% CMC with one drop Tween-80 in mice. The 10g plasma concentration was determined using HPLC-MS. The pharmacokinetic curve of 10g is presented in Figure 3. The plasma concentration-time data were analyzed by applying the model-independent method with the ESTRIP computer program. Pharmacokinetic parameters are shown in Table 5 (for details see Experimental section). These results show that 10g has very high bioavailability. The concentration of 10g in blood and good value of the half-life favors this compound as potential drug candidate.
Fig 3.
Mean pharmacokinetic curve after single dose intragastric administration of 10g (n = 5; 100 mg/kg)
Table 5.
Pharmacokinetic parameters of 10g
| AUC0-∞, ng/mL h | Cmax., ng/mL | Tmax., h | CL, h−1 | kel h−1 | T1/2, h | MRT, h | Vd, L |
|---|---|---|---|---|---|---|---|
| 3015.5 | 1710.5±489.2 | 0.5 | 0.66 | 0.4373 | 1.58 | 2.15 | 1.52 |
2.4. Compound 10g demonstrated less CYP3A4 induction compared to pleconaril
As CYP3A4 induction is a key issue for pleconaril failure in the clinical trials, compound 10g was also tested in cryopreserved hepatocytes using rifampicin as positive control and midazolam as the substrate for the CYP3A4 enzyme. The induction experiment procedures were described in [27]. At 10 μM compound 10g induction of CYP3A4 was 1.4x (7% of control) vs pleconaril 2.7x (30% of control) and positive control 10 μM rifampicin 6.6x. At 1 μM compound 10g resulted in 1.2x (3% of control) vs pleconaril 1.7x (13% of control). These results demonstrate compound 10g is less likely to represent a CYP3A4 induction risk compared with pleconaril, which is a major differentiating factor that will impact clinical use.
2.5. Exclusion of genotoxicity of compound 10g
In the SOS-chromotest, none of the tested compounds appeared to be genotoxic in E. coli PQ37 at 10 mg/mL. Moreover, no inhibition of E. coli growth was detected (Table 6). The positive control, 4NQO, inhibited as expected the growth of E. coli PQ37, the zone of inhibition has 25 mm of diameter and the presence of a blue halo was detected indicative that X-Gal was metabolized (DNA damage). The negative control DMSO in this test, does not inhibit the growth of E. coli PQ37.
Table 6.
Mutagenicity of the compounds, DMSO and 4NQO. DMSO was used as negative control. 4NQO was used as positive control. All agents were tested at 10 mg/mL and DMSO 99%.
| Compound | Concentration | Mutagenicity | Zone of inhibition, mm |
|---|---|---|---|
| 10b | 10 mg/mL | NO | 0 |
| 10e | 10 mg/mL | NO | 0 |
| 10i | 10 mg/mL | NO | 0 |
| 10g | 10 mg/mL | NO | 0 |
| 10k | 10 mg/mL | NO | 0 |
| 4NQO | 10 mg/mL | YES | 25 |
| DMSO | 99% | NO | 0 |
With regard to the potential mutagenicity of these compounds the results showed that none of them are mutagenic and they are not inhibiting the growth of Escherichia Coli bacteria.
3. Conclusion
Entero- and rhinoviruses cause a wide range of socially significant acute and chronic diseases. Up to now, there are three well-known capsid-binding agents against enteroviruses – pleconaril, vapendavir and pocapavir – but none of these inhibitors have been approved as antivirals because of CYP3A4 induction (pleconaril), lack of statistically significant antiviral effect (vapendavir) or high rate of viral resistance (pocapavir) observed in clinical trials. It is also worth noting that vaccine development for enteroviruses is complicated by the multiplicity of serotypes (about 260 serotypes). Thus, there is a real need to develop the potent broad-spectrum direct-acting antienteroviral small molecules.
For this purpose, a series of novel (((isoxazolyl)propoxy)phenyl)oxadiazoles and pyridinyl analogues was synthesized and evaluated for their antiviral activity against a panel of pleconaril-resistant and pleconaril-susceptible viruses – coxsackievirus B3 Nancy, coxsackievirus B3 97927 wild type and mutants thereof, rhinoviruses A2, B5 B14. The results showed that derivatives with N,N-dimethylcarbamoyl group in isoxazole ring were more active than the corresponding compounds with ethoxycarbonyl group in the same place. Significant impact of substituents in the central phenyl ring for antiviral activity was also found. Pleconaril resistance of coxsackievirus B3 97927 I1207K variant, coxsackievirus B3 Nancy and rhinovirus B5 was overcome by the unsubstituted analogue 10f, by monosubstitution with 3-methyl 10g, 3-nitro 10i and 3-F 10g or replacement of the phenyl with a pyridine 20. The 3-methyl derivative 10g inhibited all of the investigated viruses in micro- or nanomolar concentrations. Moreover, the lack of induction of CYP3A4 and the acceptable bioavailability after oral administration warrant further detailed preclinical investigations with 10g as well as assessment of other closely related viruses. We will next perform in vivo efficacy assessments in mice infected with representative viruses as well as performing further tests to confirm the mechanism of action is identical to pleconaril. The development of a drug for enteroviruses and rhinoviruses is an unmet medical need and we can learn from the failures of prior candidates in order to offer a higher probability of success in future clinical studies.
4. Experimental section
4.1. Chemistry
All reagents and solvents were purchased from commercial suppliers and used without further purification. 1H spectra were measured on a Varian Unity +400 operated at 200–300 MHz. Chemical shifts were measured in DMSO-d6 or CDCl3, using tetramethylsilane as an internal standard. Mass spectra were obtained on a Finnigan SSQ-700 with direct injection. A Waters Micromass ZQ detector was used in ESI MS for identification of various products. Melting points were determined on Electrothermal 9001 and are uncorrected. Merck silica gel 60 F254 plates were used for analytical TLC; column chromatography was performed on Merck silica gel 60 (70–230 mesh).
Hydroxybenzonitriles 1a, c–e, g, h were synthesized from corresponding phenols by N-bromosuccinimide (NBS) bromination and subsequent change bromine atom to cyano group by copper (I) cyanide in dry dimethylformamide [28, 29]. 4-Hydroxy-3-methoxy-5-nitrobenzonitrile 1i was synthesized by nitration of 4-hydroxy-3-methoxybenzonitrile 1e [30]. 4-Hydroxy-3-nitrobenzonitrile 1f was obtained by nitration of 4-hydroxybenzonitrile with nitric acid/acetic acid [31].
2-Сhloro-2-(hydroxyimino)acetic acid methyl ester 5a and 2-chloro-2-(hydroxyimino)acetic acid ethyl ester 5b were synthesized from corresponding glycine ester hydrochloride by nitrosation with sodium nitrite and hydrochloric acid [32]. Substituted and unsubstituted benzaldehyde oximes 8a–c were obtained by oximation of corresponding benzaldehydes [33]. 6-Chloronicotinonitrile 17 was synthesized by dehydration of 6-chloronicotinamide with thionyl chloride [34, 35].
4.1.1. General procedure for the synthesis of compounds 2a,c–i
A mixture of corresponding hydroxybenzonitrile 1a,c–i (1 mol), finely divided K2CO3 (5 mol), KI (0.01 mol), 5-chloro-1-pentyne (1.5 mol) and N-methylpyrrolidone-2 was heated at 65 °C for 24 h. The cooled reaction mixture was treated by cold water and stirred for 3–4 h. The solid was collected and recrystallized from the corresponding solvent.
4.1.1.1. 3,5-Dimethyl-4-(pent-4-yn-1-yloxy)benzonitrile 2a
White solid, yield 74 %, mp 60–63 °C (EtOH). Mass (EI), m/z (Irelat.(%)): 213.2750 [M]+ (35). C14H15NO. 1H NMR (DMSO-d6): δ 1.91 (2H, dt, J = 6.5, 7.7, CH2CH2CH2O), 2.23 (6H, s, (CH3)2Ph), 2.35 (2H, t, CH2CH2CH2O), 2.85 (1H, s, CHCCH2), 4.15 (2H, t, J = 6.5, CH2CH2CH2O), 7.54–7.69 (2H, m, H3, H5) ppm.
4.1.1.2. 4-(Pent-4-yn-1-yloxy)benzonitrile 2c
White solid, yield 70 %, mp 86–89 °C (EtOH). Mass (EI), m/z (Irelat.(%)): 185.2218 [M]+ (44). C12H11NO. 1H NMR (DMSO-d6): δ 1.89 (2H, dt, J = 6.5, 7.7, CH2CH2CH2O), 2.30 (2H, t, CH2CH2CH2O), 2.81 (1H, s, CHCCH2), 4.05 (2H, t, J = 6.5, CH2CH2CH2O), 7.03 (2H, d, J = 8.8, H2, H6), 7.50 (2H, d, J = 8.8, H3, H5) ppm.
4.1.1.3. 3-Methyl-4-(pent-4-yn-1-yloxy)benzonitrile 2d
White solid, yield 92 %, mp 84–86 °C (EtOH). Mass (EI), m/z (Irelat.(%)): 199.2484 [M]+ (69). C13H13NO. 1H NMR (DMSO-d6): δ 1.91 (2H, dt, J = 6.5, 7.5, CH2CH2CH2), 2.16 (3H, s, СН3Ph), 2.34 (2H, t, CH2CH2CH2O), 2.81 (1H, s, CHCCH2), 4.09 (2H, t, J = 6.5, CH2CH2CH2O), 7.09 (1H, d, J = 8.3, H6), 7.55–7.65 (2H, m, H3, H5) ppm.
4.1.1.4. 3-Methoxy-4-(pent-4-yn-1-yloxy)benzonitrile 2e
White solid, yield 93 %, mp 103–106 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 215.2478 [M]+ (61). C13H13NO2. 1H NMR (DMSO-d6): δ 1.92 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.36 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 3.81 (3H, s, CH3O), 4.21 (2H, t, J = 6.5, CH2CH2CH2O), 6.91 (1H, d, J = 8.1, H6), 7.47 (1H, d, J = 2.0, H3), 7.63 (1H, dd, J = 2.0, 8.1, H5) ppm.
4.1.1.5. 3-Nitro-4-(pent-4-yn-1-yloxy)benzonitrile 2f
White solid, yield 91 %, mp 118–121 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 230.2194 [M]+ (55). C12H10N2O3. 1H NMR (DMSO-d6): δ 1.91 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.32 (2H, t, J = 7.5, CH2CH2CH2O), 2.79 (1H, s, CHCCH2), 4.32 (2H, t, J = 6.5, CH2CH2CH2O), 7.21 (1H, d, J = 8.3, H6), 8.15 (1H, dd, J = 1.5, 8.3, H5), 8.45 (1H, d, J = 1.5, H3) ppm.
4.1.1.6. 3-Fluoro-4-(pent-4-yn-1-yloxy)benzonitrile 2g
White solid, yield 98 %, mp 68–73 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 203.2123 [M]+ (55). C12H10FNO.1H NMR (DMSO-d6): δ 1.90 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.35 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.18 (2H, t, J = 6.5, CH2CH2CH2O), 7.09 (1H, d, J = 8.3, H6), 7.75 (1H, dd, J = 1.5, 8.3, H5), 7.77 (1H, d, J = 1.5, H3) ppm.
4.1.1.7. 3-Trifluoromethyl-4-(pent-4-yn-1-yloxy)benzonitrile 2h
White solid, yield 100 %, mp 78–80 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 253.2198 [M]+ (56). C13H10F3NO. 1H NMR (DMSO-d6): δ 1.91 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.34 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.21 (2H, t, J = 6.5, CH2CH2CH2O), 7.10 (1H, d, J = 8.3, H6), 7.63 (1H, dd, J = 1.5, 8.3, H5), 7.72 (1H, d, J = 1.5, H3) ppm.
4.1.1.8. 3-Methoxy-5-nitro-4-(pent-4-yn-1-yloxy)benzonitrile 2i
White solid, yield 56 %, mp 105–107 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 260.2454 [M]+ (46). C13H12N2O4. 1H NMR (DMSO-d6): δ 1.93 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.36 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 3.77 (3H, s, CH3O), 4.21 (2H, t, J = 6.5, CH2CH2CH2O), 7.11 (1H, d, J = 8.3, H6), 7.64 (1H, dd, J = 1.5, 8.3, H5), 7.71 (1H, d, J = 1.5, H3) ppm.
4.1.2. General procedure for the synthesis of compounds 3a,c–i and 17
Compounds were obtained according to the previously described method [22].
4.1.2.1. N′-hydroxy-3,5-dimethyl-4-(pent-4-yn-1-yloxy)benzimidamide 3a
White solid, yield 81 %, mp 103–105 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 246.3049 [M]+ (36). C14H18N2O2. 1H NMR (DMSO-d6): δ 1.96 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O,), 2.29 (6H, s, (CH3)2Ph), 2.46 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.21 (2H, t, J = 6.5, CH2CH2CH2O), 4.96 (1H, s, NOH), 5.08 (2H, brs, NH2), 7.33 (2H, m, H3, H5) ppm.
4.1.2.2. N′-hydroxy-4-(pent-4-yn-1-yloxy)benzimidamide 3c
White solid, yield 79 %, mp 93–94 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 218.2518 [M]+ (45). C12H14N2O2. 1H NMR (DMSO-d6): δ 1.94 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.46 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.16 (2H, t, J = 6.5, CH2CH2CH2O), 4.95 (1H, s, NOH), 5.06 (2H, brs, NH2), 7.30 (2H, d, J = 8.8, H2, H6), 7.57 (2H, d, J = 8.8, H3, H5) ppm.
4.1.2.3. N′-hydroxy-3-methyl-4-(pent-4-yn-1-yloxy)benzimidamide 3d
White solid, yield 32 %, mp 110 °C (decomp.) (hexane/EtOAc). Mass (EI), m/z (Irelat.(%)): 232.2783 [M]+ (65). C13H16N2O2. 1H NMR (CDCl3): δ 2.05 (4H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.20 (3H, s, СН3Ph), 2.46 (2H, t, J = 7.5, CH2CH2CH2O), 2.86 (2H, s, CHCCH2), 4.18 (2H, t, J = 6.5, CH2CH2CH2O), 4.94 (1H, brs, NOH), 5.06 (1Н, brs, NHOH), 5.83 (1H, brs, NH), 6.81 (2H, m, NH2), 7.25 (1H, s, NHOH), 7.39 (2H, d, J = 8.3, H6), 7.49 (2H, d, J = 8.3, H5), 7.67 (2H, s, H3) ppm.
4.1.2.4. N′-hydroxy-3-methoxy-4-(pent-4-yn-1-yloxy)benzimidamide 3e
White solid, yield 67 %, mp 82 °C (decomp.) (EtOH). Mass (EI), m/z (Irelat. (%)): 248.2777 [M]+ (66). C13H16N2O3. 1H NMR (CDCl3): δ 1.96 (4H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.46 (4H, t, J = 7.5, CH2CH2CH2O), 2.77 (2H, s, CHCCH2), 3.80 (3H, s, CH3O), 4.20 (4H, t, J = 6.5, CH2CH2CH2O), 4.98 (1H, brs, NOH), 5.08 (1Н, brs, NHOH), 5.85 (1H, brs, NH), 6.87 (2H, m, NH2), 7.29 (1H, s, NHOH), 7.87 (2H, d, J = 8.3, H6), 7.29 (2H, dd, J = 1.5, 8.3, H5), 7.40 (2H, d, J = 1.5, H3) ppm.
4.1.2.5. N′-hydroxy-3-nitro-4-(pent-4-yn-1-yloxy)benzimidamide 3f
White solid, yield 71 %, mp 100–102 °C (hexane/EtOAc). Mass (EI), m/z (Irelat.(%)): 263.2493 [M]+ (61). C12H13N3O4. 1H NMR (DMSO-d6): δ 1.98 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.46 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.30 (2H, t, J = 6.5, CH2CH2CH2O), 4.98 (1H, s, NOH), 5.09 (2H, brs, NH2), 7.20 (1H, d, J = 8.3, H6), 7.84 (1H, dd, J = 1.5, 8.3, H5), 8.61 (1H, d, J = 1.5, H3) ppm.
4.1.2.6. N′-hydroxy-3-fluoro-4-(pent-4-yn-1-yloxy)benzimidamide 3g
White solid, yield 86 %, mp 93–97 °C (EtOH/H2O). Mass (EI), m/z (Irelat. (%)): 236.2422 [M]+ (42). C12H13FN2O2. 1H NMR (DMSO-d6): δ 1.97 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.46 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.19 (2H, t, J = 6.5, CH2CH2CH2O), 4.99 (1H, s, NOH), 5.05 (2H, brs, NH2), 6.92 (1H, d, J = 8.3, H6), 7.45 (1H, dd, J = 1.5, 8.3, H5), 7.52 (1H, d, J = 1.5, H3) ppm.
4.1.2.7. N′-hydroxy-3-trifluoromethyl-4-(pent-4-yn-1-yloxy)benzimidamide 3h
White solid, yield 44 %, mp 118–121 °C (hexane/EtOAc). Mass (EI), m/z (Irelat.(%)): 286.2497 [M]+ (41). C13H13F3N2O2. 1H NMR (DMSO-d6): δ 1.95 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.46 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.21 (2H, t, J = 6.5, CH2CH2CH2O), 4.93 (1H, s, NOH), 5.07 (2H, brs, NH2),7.10 (1H, d, J = 8.3, H6), 7.52 (1H, dd, J = 1.5, 8.3, H5), 7.61 (1H, d, J = 1.5, H3) ppm.
4.1.2.8. N′-hydroxy-3-methoxy-5-nitro-4-(pent-4-yn-1-yloxy)benzimidamide 3i
White solid, yield 67 %, mp 128–130 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 293.2753 [M]+ (37). C13H15N3O5. 1H NMR (DMSO-d6): δ 1.98 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.47 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 3.78 (3H, s, CH3O), 4.29 (2H, t, J = 6.5, CH2CH2CH2O), 4.95 (1H, s, NOH), 5.08 (2H, brs, NH2), 7.03 (1H, d, J = 8.1, H2), 7.41 (1H, d, J = 2.0, H6) ppm.
4.1.2.9. N’-Hydroxy-6-(pent-4-yn-1-yloxy)nicotinimidamide 17
White solid, yield 38 %, mp 138–140 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 219.2398 [M]+ (48). C11H13N3O2. 1H NMR (DMSO-d6): δ 1.84 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.30 (2H, t, J = 7.6, CH2CH2CH2O), 2.78 (1H, s, CHCCH2), 4.29 (2H, t, J = 6.5, CH2CH2CH2O), 4.98 (1H, s, NOH), 5.06 (2H, brs, NH2), 6.76 (1H, d, J = 7.8, H3), 7.95 (1H, dd, J = 1.5, 7.8, H4), 8.45 (1H, d, J = 1.5, H6) ppm.
4.1.3. General procedure for the synthesis of compounds 4a,c–i and 18
Compounds were obtained according to the previously described method [22]. For compounds 4g and 18, the residue was purified by column chromatography with hexane: EtOAc = 2:1 as eluent. The product fractions were concentrated in vacuo. Compounds 4g and 18 were obtained as oils.
4.1.3.1. 3-(3,5-Dimethyl-4-(pent-4-yn-1-yloxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 4a
White solid, yield 61 %, mp 42–44 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 324.2977 [M]+ (49). C16H15F3N2O2. 1H NMR (DMSO-d6): δ 1.98 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.19 (6H, s, (CH3)2Ph), 2.46 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.20 (2H, t, J = 6.5, CH2CH2CH2O), 7.33 (2H, m, H3, H5) ppm.
4.1.3.2. 3-(4-(Pent-4-yn-1-yloxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 4c
White solid, yield 94 %, mp 43–45 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 296.2445 [M]+ (46). C14H11F3N2O2. 1H NMR (DMSO-d6): δ 1.97 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.47 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.18 (2H, t, J = 6.5, CH2CH2CH2O), 7.27 (2H, d, J = 8.8, H2, H6), 7.86 (2H, d, J = 8.8, H3, H5) ppm.
4.1.3.3. 3-(3-Methyl-4-(pent-4-yn-1-yloxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 4d
Colorless oil, yield 96 %. Mass (EI), m/z (Irelat. (%)): 310.2711 [M]+ (54). C15H13F3N2O2. 1H NMR (DMSO-d6): δ 1.98 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.17 (3H, s, СН3Ph), 2.46 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.19 (2H, t, J = 6.5, CH2CH2CH2O), 7.08 (1H, d, J = 8.3, H6), 7.62–7.69 (2H, m, H3, H5) ppm.
4.1.3.4. 3-(3-Methoxy-4-(pent-4-yn-1-yloxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 4e
White solid, yield 78 %, mp 67–71 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 326.2705 [M]+ (64). C15H13F3N2O3. 1H NMR (DMSO-d6): δ 1.98 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.44 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 3.84 (3H, s, CH3O), 4.19 (2H, t, J = 6.5, CH2CH2CH2O), 7.03 (1H, d, J = 8.3, H6), 7.41 (1H, dd, J = 1.5, 8.3, H5), 7.52 (1H, d, J = 1.5, H3) ppm.
4.1.3.5. 3-(3-Nitro-4-(pent-4-yn-1-yloxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 4f
White solid, yield 83 %, mp 82–84 °C (hexane/EtOAc). Mass (EI), m/z (Irelat. (%)): 341.2421 [M]+ (69). C14H10F3N3O4. 1H NMR (DMSO-d6): δ 1.99 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.35 (2H, t, J = 7.5, CH2CH2CH2O), 2.81 (1H, s, CHCCH2), 4.26 (2H, t, J = 6.5, CH2CH2CH2O), 7.51 (1H, d, J = 8.3, H6), 8.28 (1H, dd, J = 1.5, 8.3, H5), 8.50 (1H, d, J = 1.5, H3) ppm.
4.1.3.6. 3-(3-Fluoro-4-(pent-4-yn-1-yloxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 4g
Colorless oil, yield 91 %. Mass (EI), m/z (Irelat. (%)): 314.2350 [M]+ (72). C14H10F4N2O2. 1H NMR (DMSO-d6): δ 1.97 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.47 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.22 (2H, t, J = 6.5, CH2CH2CH2O), 7.17 (1H, d, J = 8.3, H6), 7.61 (1H, dd, J = 1.5, 8.3, H5), 7.74 (1H, d, J = 1.5, H3) ppm.
4.1.3.7. 3-(3-Trifluoromethyl-4-(pent-4-yn-1-yloxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 4h
White solid, yield 79 %, mp 37–40 °C (EtOH/H2O). Mass (EI), m/z (Irelat. (%)): 364.2425 [M]+ (42). C15H10F6N2O2. 1H NMR (DMSO-d6): δ 1.98 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.44 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 4.23 (2H, t, J = 6.5, CH2CH2CH2O), 7.28 (1H, d, J = 8.3, H6), 7.70 (1H, dd, J = 1.5, 8.3, H5), 7.89 (1H, d, J = 1.5, H3) ppm.
4.1.3.8. 3-(3-Methoxy-5-nitro-4-(pent-4-yn-1-yloxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 4i
White solid, yield 83 %, mp 70–75 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 371.2681 [M]+ (47). C15H12F3N3O5. 1H NMR (DMSO-d6): δ 1.98 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.44 (2H, t, J = 7.5, CH2CH2CH2O), 2.77 (1H, s, CHCCH2), 3.84 (3H, s, CH3O), 4.19 (2H, t, J = 6.5, CH2CH2CH2O), 7.03 (1H, d, J = 8.1, H3), 7.41 (1H, d, J = 2.0, H5) ppm.
4.1.3.9. 3-(6-(Pent-4-yn-1-yloxy)pyridin-3-yl)-5-trifluoromethyl-1,2,4-oxadiazole 18
Colorless oil, yield 66%. Mass (EI), m/z (Irelat. (%)): 297.2326 [M]+ (51). C13H10F3N3O2. 1H NMR (DMSO-d6): δ 1.93 (2H, dt, J = 6.5, 7.6, CH2CH2CH2), 2.33 (2H, t, J = 7.6, CH2CH2CH2O), 2.80 (1H, s, CHCCH2), 4.42 (2H, t, J = 6.5, CH2CH2CH2O), 7.03 (1H, d, J = 7.8, H3), 8.28 (1H, dd, J = 1.5, 7.8, H4), 8.82 (1H, d, J = 1.5, H6) ppm.
4.1.4. General procedure for the synthesis of compounds 6a–i and 19
To a solution of 5a or 5b (3 mmol) in DMF was added a solution of corresponding pentynyloxyphenyl-oxadiazoles 4a,c–i in DMF for 20–30 min and stirred for 40–50 min at room temperature. To the reaction solution was added Et3N (3 mmol) in DMF for 2h at 80–90 °C. The mixture was stirred for 1–2h at 80–90 °C and for 12h at room temperature. The reaction mixture was diluted with water and extracted with ethyl acetate (3-times). The combined organic phases were washed with water, dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was treated by water and stored in the refrigerator for 2–4 h. Crystals were collected and recrystallized from the corresponding solvent.
4.1.4.1. 3-(3,5-Dimethyl-4-(3-(3-carbmethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 6a
White solid, yield 46 %, mp 76–78 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 425.3585 [M]+ (45). C19H18F3N3O5. 1H NMR (DMSO-d6): δ 2.18 (2H, dt, J = 6.5, 7.7, CH2CH2CH2O), 2.30 (6H, s, (CH3)2Ph), 3.11 (2H, t, J = 7.7, CH2CH2CH2O), 3.84 (2H, t, J = 6.5, CH2CH2CH2O), 3.89 (3H, s, CH3O), 6.80 (1H, s, isoxazole), 7.75 (2H, s, H3, H5) ppm.
4.1.4.2. 3-(3,5-Dimethyl-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 6b
White solid, yield 45 %, mp 74–76 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 439.3851 [M]+ (59). C20H20F3N3O5. 1H NMR (DMSO-d6): δ 1.31 (3H, t, J = 7.1, CH3CH2O), 2.18 (2H, dt, J = 6.5, 7.7, CH2CH2CH2O), 2.30 (6H, s, (CH3)2Ph), 3.11 (2H, t, J = 7.7, CH2CH2CH2O), 3.86 (2H, t, J = 6.5, CH2CH2CH2O), 4.35 (2H, q, J = 7.1, CH3CH2O), 6.81 (1H, s, isoxazole), 7.75 (2H, s, H3, H5) ppm.
4.1.4.3. 3-(4-(3-(3-Carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 6c
White solid, yield 58 %, mp 71–75 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 411.3319 [M]+ (53). C18H16F3N3O5. 1H NMR (DMSO-d6): δ 1.31 (3H, t, J = 7.1, CH3CH2O), 2.16 (2H, dt, J = 6.5, 7.5 CH2CH2CH2O), 3.00 (2H, t, J = 7.5, CH2CH2CH2O), 4.14 (2H, t, J = 6.5, CH2CH2CH2O), 4.35 (2H, q, J = 7.1, CH3CH2O), 6.76 (1H, s, isoxazole), 7.15 (2H, d, J = 8.8, H2, H6), 8.00 (2H, d, J = 8.8, H3, H5) ppm.
4.1.4.4. 3-(3-Methyl-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 6d
White solid, yield 91 %, mp 66–68 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 425.3585 [M]+ (47). C19H18F3N3O5. 1H NMR (DMSO-d6): δ 1.27 (3H, t, J = 7.1, CH3CH2O), 2.15–2.25 (5H, m, CH2CH2CH2O, CH3Ph), 3.02 (2H, t, J = 7.5, CH2CH2CH2O), 4.18 (2H, t, J = 6.5, CH2CH2CH2O), 4.35 (2H, q, J = 7.1, CH3CH2O), 6.78 (1H, s, isoxazole), 7.18 (1H, d, J = 8.3, H6), 7.80–7.90 (2H, m, H3, H5) ppm.
4.1.4.5. 3-(3-Methoxy-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 6e
White solid, yield 64 %, mp 75–77 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 441.3579 [M]+ (45). C19H18F3N3O6. 1H NMR (DMSO-d6): δ 1.31 (3H, t, J = 7.1, CH3CH2O), 2.18 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 3.02 (2H, t, J = 7.5, CH2CH2CH2O), 3.86 (3H, s, CH3O), 4.13 (2H, t, J = 6.5, CH2CH2CH2O), 4.35 (2H, q, J = 7.1, CH3CH2O), 6.78 (1H, s, isoxazole), 7.18 (1H, d, J = 8.1, H3), 7.51 (1H, d, J = 2.0, H6), 7.65 (1H, dd, J = 2.0, 8.1, H4) ppm.
4.1.4.6. 3-(3-Nitro-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 6f
White solid, yield 60 %, mp 98–100 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 456.0893 [M]+ (41). C18H15F3N4O7. 1H NMR (DMSO-d6): δ 1.31 (3H, t, J = 7.1, CH3CH2O), 2.25 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 3.02 (2H, t, J = 7.5, CH2CH2CH2O), 4.35 (2H, q, J = 7.1, CH3CH2O), 4.42 (2H, t, J = 6.5, CH2CH2CH2O), 6.75 (1H, s, isoxazole), 7.52 (1H, d, J = 8.3, H6), 8.26 (1H, dd, J = 1.5, 8.3, H5), 8.50 (1H, d, J = 1.5, H3) ppm.
4.1.4.7. 3-(3-Fluoro-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 6g
White solid, yield 57 %, mp 68–75 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 429.3224 [M]+ (49). C18H15F4N3O5. 1H NMR (DMSO-d6): δ 1.27 (3H, t, J = 7.1, CH3CH2O), 2.20 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 3.01 (2H, t, J = 7.5, CH2CH2CH2O), 4.24 (2H, t, J = 6.5, CH2CH2CH2O), 4.36 (2H, q, J = 7.1, CH3CH2O), 6.78 (1H, s, isoxazole), 7.45 (1H, t, J = 8.6, H3), 7.79–7.91 (2H, m, H4, H6) ppm.
4.1.4.8. 3-(3-Trifluoromethyl-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 6h
White solid, yield 99 %, mp 76–78 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 479.3299 [M]+ (46). C19H15F6N3O5. 1H NMR (DMSO-d6): δ 1.31 (3H, t, J = 7.1, CH3CH2O), 2.17 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 3.01 (2H, t, J = 7.6, CH2CH2CH2O), 4.25–4.40 (4H, m, CH2CH2CH2O, CH3CH2O), 6.75 (1H, s, isoxazole), 7.51 (1H, d, J = 8.0, H6), 8.20 (1H, s, H3), 8.32 (1H, d, J = 8.0, H5) ppm.
4.1.4.9. 3-(3-Methoxy-5-nitro-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 6i
White solid, yield 100 %, mp 48–50 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 486.3555 [M]+ (53). C19H17F3N4O8. 1H NMR (DMSO-d6): δ 1.32 (3H, t, J = 7.1, CH3CH2O), 2.13 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 3.01 (2H, t, J = 7.5, CH2CH2CH2O), 4.01 (3H, s, CH3O), 4.24 (2H, t, J = 6.5, CH2CH2CH2O), 4.36 (2H, q, J = 7.1, CH3CH2O), 6.74 (1H, s, isoxazole), 7.86 (1H, d, J = 2.0, H3), 8.08 (1H, d, J = 2.0, H5) ppm.
4.1.4.10. 3-(4-(3-(3-Carbethoxy-isoxazol-5-yl)propoxy)pyridin-3-yl)-5-trifluoromethyl-1,2,4-oxadiazole 19
White solid, yield 10 %, mp 45–48 °C (hexane). Mass (EI), m/z (Irelat. (%)): 412.3200 [M]+ (62). C17H15F3N4O5. 1H NMR (DMSO-d6): δ 1.28 (3H, t, J = 7.1, CH3CH2O), 2.18 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 3.00 (2H, t, J = 7.6, CH2CH2CH2O), 4.28 (2H, q, J = 7.1, CH3CH2O), 4.45 (2H, t, J = 6.5, CH2CH2CH2O), 6.75 (1H, s, isoxazole), 7.01 (1H, d, J = 7.8, H3), 8.29 (1H, dd, J = 1.5, 7.8, H4), 8.80 (d, 1H, J = 1.5, H6) ppm.
4.1.5. Synthesis of 3-(3,5-dimethyl-4-(3-(3-carbisopropoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 7
A solution of 6b (0.1 mmol) and LiOH (0.16 mmol) in ethanol-water mixture (1:1) was stirred for 3 h at room temperature. Reaction mixture was concentrated in vacuo. The remaining aqueous solution was acidified by the addition of 6 M hydrochloric acid. The precipitated white solid was collected and washed with water. The acid obtained was used without other purification.
A solution of acid obtained previously, one-two drops of H2SO4 and isopropanol was refluxed for 4 h. After completion of reaction (monitored by TLC) the reaction mixture was concentrated in vacuo. The precipitate was collected, washed with water and recrystallized from ethanol. White solid, yield 78 %, mp 69–74 °C (EtOH). Mass (EI), m/z (Irelat. (%)): 453.4117 [M]+ (54). C21H22F3N3O5. 1H NMR (DMSO-d6): δ 1.42 (6H, d, J = 7.0, (CH3)2CH), 2.18 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.30 (6H, s, (CH3)2Ph), 3.11 (2H, t, J = 7.7, CH2CH2CH2O), 3.84 (2H, t, J = 6.5, CH2CH2CH2O), 4.87 (1H, sept, J = 7.0, (CH3)2CH), 6.80 (1H, s, isoxazole), 7.75 (2H, s, H3, H5) ppm.
4.1.6. General procedure for the synthesis of compounds 9a–c
To a solution of NCS (2 mmol) and 1–2 drops of pyridine in DMF was added a solution of the corresponding benzaldehyde oximes 8a–c (1 mmol) in DMF for 20 min and stirred for 1h at room temperature, then to a solution was added a solution 4a in DMF for 20 min. To resulted mixture was added Et3N (2 mmol) in DMF for 1h at 80–90 °C and stirred for 3–4h at 80–90 °C. The reaction mixture was diluted with water and extracted with ethyl acetate (3-times). The combined organic phases were washed with water, dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was recrystallized from the corresponding solvent.
4.1.6.1. 3-(3,5-Dimethyl-4-(3-(3-phenylisoxazol-5-yl)propoxy)phenyl)-5-(trifluoromethyl)-1,2,4- oxadiazole 9a
White solid, yield 24 %, mp 74–76 °C (hexane). Mass (EI), m/z (Irelat.(%)): 443.4184 [M]+ (54). C23H20F3N3O3. 1H NMR (DMSO-d6): δ 2.20 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.30 (6H, s, (CH3)2Ph), 3.10 (2H, t, J = 7.5, CH2CH2CH2O), 3.92 (2H, t, J = 6.5, CH2CH2CH2O), 6.93 (1H, s, isoxazole), 7.50 (2H, m, m-Ph, p-Ph), 7.75 (2H, s, H3, H5), 7.90 (2H, m, o-Ph) ppm.
4.1.6.2. 3-(4-(3-(3-(3,4-Dimethoxyphenyl)isoxazol-5-yl)propoxy)-3,5-dimethylphenyl)-5- (trifluoromethyl)-1,2,4-oxadiazole 9b
White solid, yield 38 %, mp 112–114 °C (EtOH). Mass (EI), m/z (Irelat.(%)): 503.4704 [M]+ (67). C25H24F3N3O5. 1H NMR (DMSO-d6): δ 2.23 (2H, dt, J = 6.5, 7.5, CH2CH2CH2O), 2.30 (6H, s, (CH3)2Ph), 3.14 (2H, t, J = 7.5, CH2CH2CH2O), 3.80 (3H, s, CH3O), 3.85 (3H, s, CH3O), 3.92 (2H, t, J = 6.5, CH2CH2CH2O), 6.83 (1H, s, isoxazole), 7.08 (1H, d, J = 8.0, H2’), 7.41 (1H, d, J = 1.5, H6’), 7.44 (1H, dd, J = 1.5, 8.0, H5’), 7.75 (2H, s, H3, H5) ppm.
4.1.6.3. 3-(3,5-Dimethyl-4-(3-(3-(4-(trifluoromethoxy)phenyl)isoxazol-5-yl)propoxy)phenyl)-5- (trifluoromethyl)-1,2,4-oxadiazole 9c
White solid, yield 39 %, mp 65–67 °C (EtOH/H2O). Mass (EI), m/z (Irelat.(%)): 527.4158 [M]+ (49). C24H19F6N3O4. 1H NMR (DMSO-d6): δ 2.22 (2H, dt, J = 6.5, 7.7, CH2CH2CH2O), 2.30 (6H, s, (CH3)2Ph), 3.10 (2H, t, J = 7.7, CH2CH2CH2O), 3.94 (2H, t, J = 6.5, CH2CH2CH2O), 6.93 (1H, s, isoxazole), 7.50 (2H, d, J = 8.4, H2’, H6’), 7.75 (2H, s, H3, H5), 8.00 (2H, d, J = 8.4, H3’, H5’) ppm.
4.1.7. General procedure for the synthesis of compounds 10a–e,m
A mixture of 6b (1 mmol), the corresponding amine (2 mmol) and iPrOH was refluxed for 18–36h. After completion of reaction (monitored by TLC) the reaction mixture was cooled. The precipitate was collected by filtration and recrystallized from the corresponding solvent.
4.1.7.1. 3-(3,5-Dimethyl-4-(3-(3-carbamoyl-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4- oxadiazole 10a
White solid, yield 64%, mp 147–152 °С (EtOH). Mass (EI), m/z (Irelat.(%)): 410.3473 [M]+ (67). C18H17F3N4O4. 1H NMR (DMSO-d6): δ 2.23 (2H, dt, J = 6.5, 7.7, CH2CH2CH2), 2.30 (6H, s, (CH3)2Ph), 3.12 (2H, t, J = 7.7, CH2CH2CH2O), 3.80 (2H, t, J = 6.5, CH2CH2CH2O), 6.62 (1H, s, isoxazole), 7.75 (3H, m, H3, H5, NH), 8.07 (1H, brs, NH) ppm.
4.1.7.2. 3-(3,5-Dimethyl-4-(3-(3-methylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl- 1,2,4-oxadiazole 10b
White solid, yield 67 %, mp 121–123 °С (EtOH). Mass (EI), m/z (Irelat.(%)): 424.3739 [M]+ (78). C19H19F3N4O4. 1H NMR (DMSO-d6): δ 2.13 (2H, dt, J = 6.5, 7.7, CH2CH2CH2O), 2.30 (6H, s, (CH3)2Ph), 2.75 (3H, d, J = 4.0, NCH3), 3.12 (2H, t, J = 7.7, CH2CH2CH2O), 3.82 (2H, t, J = 6.5, CH2CH2CH2O), 6.70 (1H, s, isoxazole), 7.75 (2H, s, H3, H5), 8.60 (1H, br.d, J = 4.0, NH) ppm.
4.1.7.3. 3-(3,5-Dimethyl-4-(3-(3-ethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl- 1,2,4-oxadiazole 10c
White solid, yield 73%, mp 110–112 °С (EtOH). Mass (EI), m/z (Irelat.(%)): 438.4007 [M]+ (59). C20H21F3N4O4. 1H NMR (DMSO-d6): δ 1.22 (3H, t, J = 7.1, CH3CH2), 2.13 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.30 (6H, s, (CH3)2Ph), 3.10 (2H, q, J = 7.1, CH3CH2N), 3.14 (2H, t, J = 7.6, CH2CH2CH2O), 3.92 (2H, t, J = 6.5, CH2CH2CH2O), 6.83 (1H, s, isoxazole), 7.70 (2H, s, H3, H5) ppm.
4.1.7.4. 3-(3,5-Dimethyl-4-(3-(3-benzylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl- 1,2,4-oxadiazole 10d
White solid, yield 52 %, mp 102–104 °С (EtOH). Mass (EI), m/z (Irelat.(%)): 500.4699 [M]+ (42). C25H23F3N4O4. 1H NMR (DMSO-d6): δ 2.13 (2H, dt, J = 6.5, 7.7, CH2CH2CH2O), 2.30 (6H, s, (CH3)2Ph), 3.14 (2H, t, J = 7.7, CH2CH2CH2O), 3.92 (2H, t, J = 6.5, CH2CH2CH2O), 4.53 (2H, s, NCH2), 6.85 (1H, s, isoxazole), 7.26–7.31 (5H, m, Ph), 7.70 (2H, s, H3, H5) ppm
4.1.7.5. 3-(3,5-Dimethyl-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5- trifluoromethyl-1,2,4-oxadiazole 10e
White solid, yield 33 %, mp 77–85 °С (EtOH). Mass (EI), m/z (Irelat.(%)): 438.4005 [M]+ (81). C20H21F3N4O4. 1H NMR (DMSO-d6): δ 2.20 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.30 (3H, s, CH3Ph), 2.50 (3H, s, CH3Ph), 2.88–3.14 (8H, m, N(CH3)2, CH2CH2CH2O), 3.87 (2H, t, J = 6.5, CH2CH2CH2O), 6.54 (1H, s, isoxazole), 7.75 (2H, s, H3, H5) ppm.
4.1.7.6. 3-(3-Trifluoromethyl-4-(3-(3-methylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5- trifluoromethyl-1,2,4-oxadiazole 10m
White solid, yield 22 %, mp 102–104 °С (iPrOH). Mass (EI), m/z (Irelat.(%)): 464.3187 [M]+ (73). C18H14F6N4O4. 1H NMR (DMSO-d6): δ 2.17 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.77 (3H, d, J = 2.4, NCH3), 2.98 (2H, t, J = 7.6, CH2CH2CH2O), 4.30 (2H, t, J = 6.5, CH2CH2CH2O), 6.60 (1H, s, isoxazole), 7.51 (1H, d, J = 8.0, H6), 8.24 (1H, s, H3), 8.32 (d, 1H, J = 8.0, H5), 8.61 (1H, brs, NH) ppm.
4.1.8. General procedure for the synthesis of compounds 10f–l, 13, 20
A mixture of 6c–i or 11 or 19 (1 mmol) and dimethylamine solution 33 wt. % in H2O or 17 wt. % in dioxane (5 mmol) was heated at 50–60 °C for 1–12 h. The cooled reaction mixture was concentrated in vacuo. The residue was treated by water and stored in the refrigerator for 12 h. Crystals were collected and recrystallized from the corresponding solvent.
4.1.8.1. 3-(4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4- oxadiazole 10f
White solid, yield 60 %, mp 95–98 °С (EtOH). Mass (EI), m/z (Irelat.(%)): 410.3473 [M]+ (39). C18H17F3N4O4. 1H NMR (DMSO-d6): δ 2.15 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.95 (3H, s, NCH3), 3.00 (2H, t, J = 7.6, CH2CH2CH2O), 3.05 (3H, s, NCH3), 4.20 (2H, t, J = 6.5, CH2CH2CH2O), 6.50 (1H, s, isoxazole), 7.20 (2H, d, J = 7.50, H2, H6), 8.00 (2H, d, J = 7.5, H3, H5) ppm.
4.1.8.2. 3-(3-Methyl-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5- trifluoromethyl-1,2,4-oxadiazole 10g
White solid, yield 70 %, mp 88–89 °С (EtOH/H2O). Mass (EI), m/z (Irelat.(%)): 424.3739 [M]+ (37). C19H19F3N4O4. 1H NMR (DMSO-d6): δ 2.22 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.24 (3H, s, CH3Ph), 3.00 (3H, s, NCH3), 3.05 (2H, t, J = 7.6, CH2CH2CH2O), 3.10 (3H, s, NCH3), 4.20 (2H, t, J = 6.5, CH2CH2CH2O), 6.50 (1H, s, isoxazole), 7.20 (1H, d, J = 8.8, H6), 7.80 (1H, d, J = 1.5, H5), 7.81 (1H, dd, J = 1.5, 8.8, H3) ppm.
4.1.8.3. 3-(3-Methoxy-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5- trifluoromethyl-1,2,4-oxadiazole 10h
White solid, yield 53 %, mp 92–94 °С (EtOH/H2O). Mass (EI), m/z (Irelat.(%)): 440.3733 [M]+ (94). C19H19F3N4O5. 1H NMR (DMSO-d6): δ 2.18 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.98 (3H, s, NCH3), 3.00 (2H, t, J = 7.6, CH2CH2CH2O), 3.11 (3H, s, NCH3), 3.85 (3H, s, OMe), 4.20 (2H, t, J = 6.5, CH2CH2CH2O), 6.50 (1H, s, isoxazole), 7.23 (1H, d, J = 8.8, H6), 7.51 (1H, d, J = 1.5, H3), 7.65 (1H, dd, J = 1.5, 8.8, H5) ppm.
4.1.8.4. 3-(3-Nitro-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5- trifluoromethyl-1,2,4-oxadiazole 10i
White solid, yield 37%, mp 62–64 °С (EtOH). Mass (EI), m/z (Irelat.(%)): 455.3449 [M]+ (52). C18H16F3N5O6. 1H NMR (DMSO-d6): δ 2.20 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.98 (3H, s, NCH3), 3.00 (2H, t, J = 7.6, CH2CH2CH2O), 3.05 (3H, s, NCH3), 4.40 (2H, t, J = 6.5, CH2CH2CH2O), 6.51 (1H, s, isoxazole), 7.55 (1H, d, J = 8.8, H6), 8.32 (1H, dd, J = 1.5, 8.8, H5), 8.50 (1H, d, J = 1.5, H3) ppm.
4.1.8.5. 3-(3-Fluoro-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5- trifluoromethyl-1,2,4-oxadiazole 10j
White solid, yield 42 %, mp 83–85 °С (hexane). Mass (EI), m/z (Irelat.(%)): 428.3378 [M]+ (29). C18H16F4N4O4. 1H NMR (DMSO-d6): δ 2.21 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.99 (3H, s, NCH3), 3.10 (3H, s, NCH3), 3.05 (2H, t, J = 7.6, CH2CH2CH2O), 4.25 (2H, t, J = 6.5, CH2CH2CH2O), 6.52 (1H, s, isoxazole), 7.40 (1H, t, J = 8.3, H3), 7.80–7.90 (2H, m, H5, H6) ppm.
4.1.8.6. 3-(3-Trifluoromethyl-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5- trifluoromethyl-1,2,4-oxadiazole 10k
White solid, yield 71 %, mp 160–162 °С (iPrOH). Mass (EI), m/z (Irelat.(%)): 478.3453 [M]+ (68). C19H16F6N4O4. 1H NMR (DMSO-d6): δ 2.17 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.93 (3H, s, NCH3), 2.98 (2H, t, J = 7.6, CH2CH2CH2O), 3.05 (3H, s, NCH3), 4.29 (2H, t, J = 6.5, CH2CH2CH2O), 6.52 (1H, s, isoxazole), 7.54 (1H, d, J = 8.0, H6), 8.20 (1H, s, H3), 8.34 (1H, d, J = 8.0, H5) ppm.
4.1.8.7. 3-(3-Methoxy-5-nitro-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5- trifluoromethyl-1,2,4-oxadiazole 10l
White solid, yield 92 %, mp 76–78 °С (EtOH/H2O). Mass (EI), m/z (Irelat.(%)): 485.3709 [M]+ (49). C19H18F3N5O7. 1H NMR (DMSO-d6): δ 2.13 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.97 (3H, s, NCH3), 3.00 (2H, t, J = 7.6, CH2CH2CH2O), 3.03 (3H, s, NCH3), 4.00 (3H, s, OMe), 4.25 (2H, t, J = 6.5, CH2CH2CH2O), 6.50 (1H, s, isoxazole), 7.80 (1H, d, J = 2.0, H6), 8.07 (1H, d, J = 2.0, H4) ppm.
4.1.8.8. 3-(3-Amino-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5- trifluoromethyl-1,2,4-oxadiazole 13
Light-brown solid, yield 54 %, mp 84–85 °С (EtOH). Mass (EI), m/z (Irelat.(%)): 425.3618 [M]+ (59). C18H18F3N5O4. 1H NMR (DMSO-d6): δ 2.18 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.99 (3H, s, NCH3), 3.00 (2H, t, J = 7.6, CH2CH2CH2O), 3.05 (3H, s, NCH3), 4.15 (2H, t, J = 6.5, CH2CH2CH2O), 5.15 (2H, brs, NH2), 6.50 (1H, s, isoxazole), 6.95 (1H, d, J = 8.0, H6), 7.25 (1H, d, J = 8.0, H5), 7.40 (1H, s, H3) ppm.
4.1.8.9. 3-(4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)pyridin-3-yl)-5-trifluoromethyl- 1,2,4-oxadiazole 20
White solid, yield 25 %, mp 74–75 °С (hexane). Mass (EI), m/z (Irelat.(%)): 411.3352 [M]+ (49). C17H16F3N5O4. 1H NMR (DMSO-d6): δ 2.18 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.95 (2H, t, J = 7.6, CH2CH2CH2O), 3.00 (3H, s, NCH3), 3.05 (3H, s, NCH3), 4.45 (2H, t, J = 6.5, CH2CH2CH2O), 6.51 (1H, s, isoxazole), 7.02 (1H, d, J = 7.8, H3), 8.27 (1H, dd, J = 1.5, 7.8, H4), 8.79 (1H, d, J = 1.5, H6) ppm.
4.1.9. Synthesis of 3-(3-amino-4-(3-(3-carbetoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl- 1,2,4-oxadiazole 11
A mixture compound 6f (1 mmol) and SnCl2·2H2O (5 mmol) in ethanol was stirred for 12 h at room temperature. The reaction mixture was diluted with ammonia water solution and extracted with ethyl acetate (3-times). The combined organic phases were washed with water, dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was treated by water and stored in the refrigerator for 2 h. Crystals were collected and recrystallized from ethanol. White solid, yield 93%, mp 56–58 °С (EtOH). Mass (EI), m/z (Irelat.(%)): 426.3466 [M]+ (61). C18H17F3N4O5. 1H NMR (DMSO-d6): δ 1.31 (3H, t, J = 7.1, CH3CH2O), 2.17 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 3.05 (2H, t, J = 7.6, CH2CH2CH2O), 4.10 (2H, t, J = 6.5, CH2CH2CH2O), 4.35 (2H, q, J = 7.1, CH3CH2O), 5.10 (2H, brs, NH2), 6.74 (1H, s, isoxazole), 6.98 (1H, d, J = 8.0, H6), 7.25 (1H, d, J = 8.0, H5), 7.35 (1H, s, H3) ppm.
4.1.10. General procedure for the synthesis of compounds 12a and 14a
A solution of 11 or 13 (1 mmol) and acetic anhydride (0.04 mol) was heated at 80 °C for 2–2.5 h. The cooled reaction mixture was treated by cold water and stirred for 1–2 h. The precipitate was collected by filtration and recrystallized from the corresponding solvent.
4.1.10.1. 3-(3-Acetylamino-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl- 1,2,4-oxadiazole 12a
White solid, yield 90 %, mp 132–134 °С (EtOH). Mass (EI), m/z (Irelat.(%)): 468.3833 [M]+ (61). C20H19F3N4O6. 1H NMR (DMSO-d6): δ 1.32 (3H, t, J = 7.1, CH3CH2O), 2.10 (3H, s, CH3CO), 2.25 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 3.04 (2H, t, J = 7.6, CH2CH2CH2O), 4.25 (2H, t, J = 6.5, CH2CH2CH2O), 4.35 (2H, q, J = 7.1, CH3CH2O), 6.60 (1H, s, isoxazole), 7.25 (1H, d, J = 8.0, H6), 7.85 (1H, d, J = 8.0, H5), 8.80 (1H, s, H3), 9.20 (1H, s, NH) ppm.
4.1.10.2. 3-(3-Acetylamino-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5- trifluoromethyl-1,2,4-oxadiazole 14a
White solid, yield 34 %, mp 135–137 °С (hexane/EtOAc). Mass (EI), m/z (Irelat.(%)): 467.3985 [M]+ (60). C20H20F3N5O5. 1H NMR (DMSO-d6): δ 2.20 (3H, s, CH3CO), 2.26 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.98 (3H, s, NCH3), 3.00 (2H, t, J = 7.6, CH2CH2CH2O), 3.10 (3H, s, NCH3), 4.23 (2H, t, J = 6.5, CH2CH2CH2O), 6.52 (1H, s, isoxazole), 7.26 (1H, d, J = 8.0, H6), 7.75 (1H, d, J = 8.0, H5), 8.75 (1H, s, H3), 9.20 (1H, brs, NH) ppm.
4.1.11. General procedure for the synthesis of compounds 12b and 14b
To a solution of 11 or 13 (1 mmol) in acetic acid was added a solution of 2,5-dimethoxyfuran (1.1 mmol) in acetic acid drop-by-drop at room temperature and then the mixture was stirred at 50 °C for 12–16 h. The cooled reaction mixture was treated by cold water, stirred for 1 h and stored in the refrigerator for 12 h. The precipitate was collected by filtration and recrystallized from the corresponding solvent.
For compound 14b, the cooled reaction mixture was treated by cold water and extracted with ethyl acetate (3-times). The combined organic phases were washed with water, dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography with chloroform as eluent. The product fractions were concentrated in vacuo. Compound 14b was obtained as oil.
4.1.11.1. 3-(3-(1H-Pyrrol-1-yl)-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5- trifluoromethyl-1,2,4-oxadiazole 12b
Light-brown solid, yield 17 %, mp 95–97 °С (EtOH/H2O). Mass (EI), m/z (Irelat.(%)): 476.4053 [M]+ (47). C22H19F3N4O5. 1H NMR (DMSO-d6): δ 1.27 (3H, t, J = 7.1, CH3CH2O), 2.17 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.95 (2H, t, J = 7.6, CH2CH2CH2O), 4.20 (2H, t, J = 6.5, CH2CH2CH2O), 4.35 (2H, q, J = 7.1, CH3CH2O), 6.25 (2H, m, H3’,H4’), 6.55 (1H, s, isoxazole), 7.20 (2H, m, H2’, H5’), 7.50 (1H, d, J = 8.0, H6), 8.00 (1H, d, J = 8.0, H5), 7.90 (1H, s, H3) ppm.
4.1.11.2. 3-(3-(1H-Pyrrol-1-yl)-4-(3-(3- N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 14b
Colorless oil, yield 40 %. Mass (EI), m/z (Irelat.(%)): 475.4205 [M]+ (52). C22H20F3N5O4. 1H NMR (DMSO-d6): δ 2.17 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.93 (2H, t, J = 7.6, CH2CH2CH2O), 3.00 (3H, s, NCH3), 3.11 (3H, s, NCH3), 4.24 (2H, t, J = 6.5, CH2CH2CH2O), 6.25 (2H, m, H3’, H4’), 6.50 (1H, s, isoxazole), 7.20 (2H, m, H2’, H5’), 7.49 (1H, d, J = 8.0, H6), 7.94 (1H, s, H3), 8.01 (1H, d, J = 8.0, H5) ppm.
4.1.12. Synthesis of 3-(3-(((dimethylamino)methylene)amino)-4-(3-(3-carbethoxy-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 12c
A solution of 11 (1 mmol) and DMF-DMA (1.4 mmol) in ethanol was refluxed for 3.5–4 h. The cooled reaction mixture was treated by cold water and stirred for 12 h. The solid was collected and recrystallized from hexane. White solid, yield 13 %, mp 79–82 °С. Mass (EI), m/z (Irelat.(%)): 481.4251 [M]+ (45). C21H22F3N5O5. 1H NMR (DMSO-d6): δ 1.32 (3H, t, J = 7.1, CH3CH2O), 2.12 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.98 (3H, s, NCH3), 3.00 (3H, s, NCH3), 3.05 (2H, t, J = 7.6, CH2CH2CH2O), 4.10 (2H, t, J = 6.5, CH2CH2CH2O), 4.35 (2H, q, J = 7.1, CH3CH2O), 6.74 (1H, s, isoxazole), 7.18 (1H, d, J = 8.0, H6), 7.56 (1H, d, J = 8.0, H5), 7.45 (1H, s, H3), 7.75 (1H, s, NCH) ppm.
4.1.13. Synthesis of 6-(pent-4-yn-1-yloxy)nicotinonitrile 16
A mixture 6-chloronicotinonitrile 15 (1 mol), 4-pentyn-1-ol (1.2 mol), finely divided K2CO3 (2 mol) in DMF was heated at 100 °C for 8–9 h. The cooled mixture was diluted with water and extracted with ethyl acetate (3-times). The combined organic phases were washed with water, dried over anhydrous Na2SO4 and concentrated in vacuo to an oil, which was dissolved in CHCl3, filtered though a short column of Silicagel 60, and concentrated in vacuo. The residue was treated by water and stored in the refrigerator for 2 h. Crystals were filtered off and recrystallized from ethanol. Yellow solid, yield 47 %, mp 55–57 °C. Mass (EI), m/z (Irelat.(%)): 186.2099 [M]+ (45). C11H10N2O. 1H NMR (DMSO-d6): 1.99 (2H, dt, J = 6.5, 7.6, CH2CH2CH2O), 2.26 (2H, t, J = 7.6, CH2CH2CH2O), 2.75 (1H, s, CHCCH2), 4.26 (2H, t, J = 6.5, CH2CH2CH2O), 7.00 (1H, d, J = 7.8, H3), 8.01 (1H, dd, J = 1.5, 7.8, H4), 8.52 (1H, d, J = 1.5, H6) ppm.
4.2. In vitro antiviral testing
4.2.1. Compounds
Stock solutions (10,000 μM) of test and reference compounds (pleconaril and guanidine hydrochloride) were prepared in DMSO and stored at 4 °C until use in cytotoxicity and antiviral studies.
4.2.2. Cells and viruses
HeLa Ohio (human cervix carcinoma; FlowLabs) were used in cytotoxic and anti-viral studies as well. HeLa cells were grown in Eagle’s minimal essential medium (MEM/E; Cambrex, Verviers, Belgium) supplemented with 10% fetal calf serum (FCS: HeLa Ohio, PAA, Pasching, Austria). The medium in cytotoxicity and antiviral studies (test medium) contained only 2% of FCS.
Virus stocks of the coxsackievirus B3 prototype strain Nancy (CVB3 Nancy; Chumakov Institute of Poliomyelitis and Viral Encephalitides, Moscow, Russia), the clinical coxsackievirus B3 isolate 97927 (Robert Koch Institute, Berlin, Germany), the three pleconaril-resistant mutants of coxsackievirus B3 97927 [17], rhinoviruses A2, B5 (kindly provided by Dr. J. Seipelt, Greenhills Biotechnology, Ltd., Vienna, Austria), rhinovirus B14 (kindly provided by Dr. H.-P. Grunert, Universitatsklinikum Benjamin Franklin, Berlin, Germany) were prepared by incubating confluent HeLa cell monolayers (FlowLabs) grown in cell culture flasks with a certain amount of virus at 37 °C (coxsackieviruses B3 and rhinovirus B14) or 33°C (rhinoviruses A2 and B5) and 5% CO2. When a nearly complete cytopathic effect became microscopically visible, infected cells were frozen at − 80 °C and thawed three times, the cell debris was removed by centrifugation, and aliquots of the virus-containing supernatant were stored as virus stock at −80 °C until use. Titers of virus stocks were determined by endpoint dilution in HeLa cells according to Reed and Muench [36].
4.2.3. Determination of cytotoxicity
To determine the 50% cytotoxic concentration (CC50), 2-day-old confluent HeLa cell monolayer grown in the internal 60 wells of 96-well plates were incubated with serial half-log dilutions of compounds (100 μM, 31.6 μM, 10 μM, 3.16 μM etc.) in test medium for 72 h (37 °C, 5% CO2) in test medium or test medium without compound (six mock-treated cell controls). After an incubation time of 72 h, the cells were fixed and stained with a crystal violet formalin solution as described previously [21]. The crystal violet-stained monolayers were treated for 20 min with 100 μL of lysis buffer (0.8979 g of sodium citrate and 1.25 mL of 1N HCl in 98.05 mL 47.5 % ethanol) to elute the crystal violet. After dye elution, the optical density of individual wells was quantified spectrophotometrically at 550/630 nm with a microplate reader (Dynex, Guernsey, GB). Cell viability of individual compound-treated wells was evaluated as the percentage of the mean value of optical density resulting from the six mock-treated cell controls which was set 100% cell viability. The 50% cytotoxic concentration (CC50) was defined as the compound concentration reducing the mean viability of cell controls by 50%. Mean CC50 values were calculated from at least 3 tests.
4.2.4. Cytopathic effect (CPE)-inhibition assay
The CPE-inhibition assays have been performed as described previously with some modifications [21]. Briefly, the tests were carried out in 1-day-old (all rhinoviruses) or 2-day-old (all coxsackieviruses B3) HeLa cell monolayers growing in the inner 60 wells of 96-well flat-bottomed microtiter plates. After removal of culture medium, 50 μL of compound solution (serial half-log dilutions of compounds: 100 μM, 31.6 μM, 10 μM, 3.16 μM etc. in test medium) and 50 μL of a virus suspension with a certain multiplicity of infection were added to the cells. The multiplicity of infection was 0.015–0.028 for coxsackievirus B3 97927, 0.015–0.02 for coxsackievirus B3 97927 I1207K/I1092, 0.002–0.0025 for coxsackievirus B3 97927 I1207M/I1092, 0.002–0.0025 for coxsackievirus B3 97927 I1207M/I1092, 0.01–0.015 for coxsackievirus B3 97927 I1207T/I1092, 0.002 for coxsackievirus B3 Nancy, 0.03 for rhinovirus A2, 0.01–0.04 for rhinovirus B5, and 0.005 for rhinovirus B14. Six wells of non-infected and six wells of infected cells without the test compound served as cell and virus control, respectively, on each plate. Guanidine hydrochloride was included as positive control compounds for all CVB3. With the exception of rhinovirus B5 (Pleconaril resistant), pleconaril was used as reference compound for rhinoviruses. Serial dilutions of test compounds were included in each assay run for assay validation. Using the crystal violet uptake assay described for cytotoxic investigations, the inhibition of the virus-induced CPE was scored 48 h (all coxsackieviruses B3) or 72 h (all rhinoviruses) post infection when untreated infected control cells showed maximum cytopathic effect and the positive control compound-treated wells a 50% or 100% protection. The percentage of antiviral activity of the tests compounds were calculated according to Pauwels et al. [26]. The mean IC50 values of the antiviral active compounds were calculated from at least 3 separate experiments.
4.3. Pharmacokinetic study in mice
All animal procedures were approved by the Institutional Animal Care and Use Committee of Central Research Institute of Tuberculosis (Moscow, Russia) and conducted in accordance with the state industry standards GOST 33215–2014 and GOST 33216–2014 (in harmonization to the European Directive 2010/63/ЕС).
4.3.1. Experimental animals
Twenty two white Balb/C male mice with average body weight of 20 g were selected for the study. All the test animals had free access to water but were deprived of food for 1 hour before administration of the compound. This regimen was continued for another hour after the administration.
4.3.2. Compound administration and sample collection and preparation
Compound in a dose of 100 mg/kg of body weight was administered as 0.5 ml of prepared suspension in 0.5% CMC with one drop Tween-80 by intragastric intubation. The animals were euthanized by decapitation for blood and brain sampling. Blood and brain samples (3 animals per time point) were taken at 0.5, 1, 2, 3, 5 and 7 h after administration for pharmacokinetic evaluation. Blood was collected in heparinized tubes and centrifuged at 3500 RPM for 15 minutes. Plasma was separated from formed elements and immediately frozen at −20 °C in freezer. This storage continued before transferring of plasma for analysis. Serum from 6 untreated animals was used as control and for equipment calibration with compound. Mice brain was immediately frozen at −120 °C. A mixture of 50 μL of plasma and 150 μL of MeCN by intensive shaking (Vortex) for 30 sec. Centrifugation 14000g for 4 min. After centrifugation the 150 μL of organic layer was injected into the HPLC column.
4.3.3. HPLC-MS Conditions
Chromatographic separation was achieved on an Agilent Eclipse XDB-C18 column (4.6 mm × 100 mm, 3.5 μm) using an Agilent 1290 Infinity HPLC system equipped with the binary pump, auto-sampler, column oven and degasser. The mobile phase containing 0.1% formic acid in acetonitrile (A) and 0.2% formic acid in water (B) was at a flow rate of 0.4 mL/min. The gradient program was as follows: 0.0–3.0 min, 60% B (separation); 3.0–4.0 min, 60 → 97% B (washing and regeneration); 4.0–6.0 min, 97% B; 6.0–6.1 min, 97 → 60% B; 6.1–9.0 min, 60% B. The separation temperature was set at 40 °C and the sample injection volume was 2 μL.
Mass detection was operated on an Agilent 6460 triple quadrupole mass spectrometer coupled with electrospray ionization (ESI) source. The mass spectrometer was set in positive ion mode. The optimum MS parameters were maintained as follows: nebulizer, 35 psi; drying gas (N2) flow rate, 12 L/min; capillary temperature, 350 °C.
4.3.4. Calibration
The calibration curve was based on the model serum (45 μL of serum + 5 μL of standard solution). Five levels of concentrations were used: 50 ng/mL, 250 ng/mL, 500 ng/mL, 1000 ng/mL, 5000 ng/mL.
4.3.5. Calculation of pharmacokinetic parameters
Pharmacokinetic parameters were calculated with the ESTRIP computer program using the model-independent method. The area under the plasma concentration-time curve from time zero to infinity (AUC0-∞) was calculated by the trapezium method with extrapolation to time infinity. The maximum concentration Cmax and the time when it occurred (Tmax) were obtained by visual inspection of the pharmacokinetic curve. The total clearance CL was calculated as dose/AUC0-∞. The volume of distribution Vd was calculated as CL/kel, where kel is the elimination constant. The half-life T1/2 was calculated using equation ln(2)/kel = 0.693/kel. The mean drug retention time in systemic bloodstream MRT was calculated by AUMC/AUC, where AUMC is the area under the first moment curve.
4.4. CYP3A4 Induction
Compound 10g and pleconaril were tested in cryopreserved hepatocytes (BioIVT, Westbury, NY) (at 1, 10, 100 μM) using 10 μM rifampicin as positive control and midazolam as the substrate for the enzyme (Eurofins Panlabs, Inc. St. Charles MO).
4.5. SOS chromotest
The SOS chromotest experiment was reported in [37]. In brief, Escherichia coli PQ37 strain was grown overnight at 37 °C, with shaking, in Luria Broth Base supplemented with 50 μg/mL Ampicillin. Bacteria were grown to mid-logarithmic phase and adjusted to an optical density of 0.4. The second day, Luria Broth Base supplemented with 1.5% Agar was autoclaved for 15 minutes at 121 °C and then put into a water bath at 55 °C for 1 hour. 80 mL of warm agar was transferred into a plastic bottle (Milian, PETG 2019–0125) supplemented with 50 mg/mL ampicillin and 0.005% X- Gal (dissolved into DMF). 4 mL of E. coli PQ37 was added to the mixture and 70 mL of it was transferred into a square petri dish (Grenier, 120 × 120 mm). The Petri dish containing the mixture was dried 15 minutes at RT and once the mixture became solid, 6 mm absorbent disks were stuck on the plate. 5 μL of each compound were added on the disk with a pipetman (Gilson P20) and the petri dish was incubated overnight at 37 °C. The third day, zones of inhibition were recorded with a ruler and a picture of the plate was taken with a scanner (Canon 8800F). The zone of inhibition was calculated by eye using a ruler and presence/absence of blue halo was reporter.
Supplementary Material
Fig 1.
Structures of well-known antipicornaviral drug candidates, pleconaril and vapendavir (A) and our structure-activity relationship (SAR) study of pleconaril-like compounds described in this article (B)
Highlights.
A series of novel pleconaril derivatives were synthesized and evaluated.
Analogues with monosubstitution in the phenyl ring are more active than with disubstitution.
Dimethylamido group in isoxazole ring is critical for inhibition of pleconaril-resistant viruses.
Compound 10g is the most potent antienteroviral compound in this series.
This derivative also show good bioavailability in mice.
Acknowledgments
Sean Ekins kindly acknowledges NIH funding: R21TR001718 from NIH/NCATS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
Sean Ekins is CEO of Collaborations Pharmaceuticals, Inc. Vadim Makarov, Michaela Schmidtke and Sean Ekins are co-inventors on a US provisional patent relating to this work.
Declaration of interests
The authors declare that they have no known competing financialinterestsor personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Miller EK, Mackay IM. From sneeze to wheeze: what we know about rhinovirus Cs, J. Clin. Virol 57 (2013) 291–299. 10.1016/j.jcv.2013.04.015. [DOI] [PubMed] [Google Scholar]
- [2].Melnick JL, Poliovirus and other enteroviruses, in: Evans AS, Kaslow RA (Eds.), Viral infections of humans: epidemiology and control, Plenum Publishing, New York, 1997, pp. 583–663. [Google Scholar]
- [3].Pallansch M, Roos R, Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses In: Knipe D, Howley P (Eds.), Fields Virology. Lippincott Williams Wilkins, Philadelphia, 2007, pp. 839–894. [Google Scholar]
- [4].Gern JE. The ABCs of rhinoviruses, wheezing, and asthma, J. Virol 84 (2010) 7418–7426. 10.1128/JVI.02290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hayden FG. Rhinovirus and the lower respiratory tract, Rev. Med. Virol 14 (2004) 17–31. 10.1002/rmv.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Busse WW, Lemanske RF Jr., Gern JE, The role of viral respiratory infections in asthma and asthma exacerbations, Lancet. 376 (2010) 826–834. 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jackson DJ, Johnston SL, The role of viruses in acute exacerbations of asthma, J. Allergy Clin. Immunol 125 (2010) 1178–1187. 10.1016/j.jaci.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thibaut HJ, Lacroix C, De Palma AM, Franco D, Decramer M, Neyts J, Toward antiviral therapy/prophylaxis for rhinovirus-induced exacerbations of chronic obstructive pulmonary disease: challenges, opportunities, and strategies, Rev. Med. Virol 26 (2016) 21–33. 10.1002/rmv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].World Health Organization, Poliomyelitis. https://www.who.int/immunization/diseases/poliomyelitis/en/, 2017. (accessed 11 June 2019).
- [10].Yi EJ, Shin YJ, Kim JH, Kim TG, Chang SY, Enterovirus 71 infection and vaccines, Clin. Exp. Vaccine Res. 6 (2017) 4–14. 10.7774/cevr.2017.6.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].World Organisation for Animal Health, Foot & Mouth disease (FMD). https://www.oie.int/en/animal-health-in-the-world/animal-diseases/foot-and-mouth-disease/, 2018. (accessed 18 December 2019).
- [12].Rossmann MG, He Y, Kuh RJ, Picornavirus-receptor interactions, Trends Microbiol. 10 (2002) 324–331. 10.1016/S0966-842X(02)02383-1. [DOI] [PubMed] [Google Scholar]
- [13].Liu Y, Hill MG, Klose T, Chen Z, Watters K, Bochkov YA, Jiang W, Palmenberg AC, Rossmann MG, Atomic structure of a rhinovirus C, a virus species linked to severe childhood asthma, Proc. Natl. Acad. Sci. USA 113 (2016) 8997–9002. 10.1073/pnas.1606595113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Egorova A, Ekins S, Schmidtke M, Makarov V, Back to the future: Advances in development of broad-spectrum capsid-binding inhibitors of enteroviruses, Eur. J. Med. Chem 178 (2019) 606–622. 10.1016/j.ejmech.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ledford RM, Patel NR, Demenczuk TM, Watanyar A, Herbertz T, Collett MS, Pevear DC, VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds, J. Virol 78 (2004) 3663–3674. 10.1128/jvi.78.7.3663-3674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Makarov VA, Braun H, Richter M, Riabova OB, Kirchmair J, Kazakova ES, Seidel N, Wutzler P, Schmidtke M, Pyrazolopyrimidines: Potent Inhibitors Targeting the Capsid of Rhino- and Enteroviruses, ChemMedChem. 10 (2015) 1629–1634. 10.1002/cmdc.201500304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Braun H, Kirchmair J, Williamson MJ, Makarov VA, Riabova OB, Glen RC, Sauerbrei A, Schmidtke M, Molecular mechanism of a specific capsid binder resistance caused by mutations outside the binding pocket, Antiviral Res. 123 (2015) 138–145. 10.1016/j.antiviral.2015.09.009. [DOI] [PubMed] [Google Scholar]
- [18].Hayden FG, Herrington DT, Coats TL, Kim K, Cooper EC, Villano SA, Liu S, Hudson S, Pevear DC, Collett M, McKinlay M, Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials, Clin. Infect Dis. 36 (2003) 1523–1532. 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Genetic Engineering & Biotechnology News, Aviragen Antiviral Drug Vapendavir Fails Phase IIb Study in Asthma Patients. https://www.genengnews.com/topics/drug-discovery/aviragen-antiviral-drug-vapendavir-fails-phase-iib-study-in-asthma-patients/, 2017. (accessed 22 October 2019).
- [20].Collett MS, Hincks JR, Benschop K, Duizer E, van der Avoort H, Rhoden E, Liu H, Oberste MS, McKinlay MA, Hartford M, Antiviral activity of pocapavir in a randomized, blinded, placebo-controlled human oral poliovirus vaccine challenge model, J. Infect. Dis, 215 (2017) 335–343. 10.1093/infdis/jiw542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Makarov VA, Riabova OB, Granik VG, Wutzler P, Schmidtke M, Novel [(biphenyloxy)propyl]isoxazole derivatives for inhibition of human rhinovirus 2 and coxsackievirus B3 replication, J. Antimicrob. Chemother 55 (2005) 483–488. 10.1093/jac/dki055. [DOI] [PubMed] [Google Scholar]
- [22].Schmidtke M, Wutzler P, Zieger R, Riabova OB, Makarov VA, New pleconaril and [(biphenyloxy)propyl]isoxazole derivatives with substitutions in the central ring exhibit antiviral activity against pleconaril-resistant coxsackievirus B3, Antiviral Res. 81 (2009) 56–63. 10.1016/j.antiviral.2008.09.002. [DOI] [PubMed] [Google Scholar]
- [23].Schmidtke M, Schnittler U, Jahn B, Dahse H, Stelzner A, A rapid assay for evaluation of antiviral activity against coxsackie virus B3, influenza virus A, and herpes simplex virus type 1, J. Virol. Methods 95 (2001) 133–43. 10.1016/s0166-0934(01)00305-6. [DOI] [PubMed] [Google Scholar]
- [24].Nain M, Hinder F, Gong JH, Schmidt A, Bender A, Sprenger H, Gemsa D, Tumor necrosis factor-alpha production of influenza A virus-infected macro-phages and potentiating effect of lipopolysaccharides, J. Immunol 145 (1990), 1921–1928. [PubMed] [Google Scholar]
- [25].Schmidtke M, Hammerschmidt E, Schüler S, Zell R, Birch-Hirschfeld E, Makarov VA, Riabova OB, Wutzler P, Susceptibility of coxsackievirus B3 laboratory strains and clinical isolates to the capsid function inhibitor pleconaril: antiviral studies with virus chimeras demonstrate the crucial role of amino acid 1092 in treatment, J. Antimicrob. Chemother 56 (2005) 648–656. 10.1093/jac/dki263. [DOI] [PubMed] [Google Scholar]
- [26].Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E, Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds, J. Virol. Methods 20 (1988), 309–321. 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- [27].Lu C, Li AP, Species comparison in P450 induction: effects of dexamethasone, omeprazole, and rifampin on P450 isoforms 1A and 3A in primary cultured hepatocytes from man, Sprague-Dawley rat, minipig, and beagle dog, Chem. Biol. Interact 134 (2001) 271–281. 10.1016/s0009-2797(01)00162-4. [DOI] [PubMed] [Google Scholar]
- [28].Zysman-Colman E, Arias K, Siegel JS, Synthesis of arylbromides from arenes and N-bromosuccinimide (NBS) in acetonitrile – A convenient method for aromatic bromination, Can. J. Chem 87 (2009) 440–447. 10.1139/V08-176. [DOI] [Google Scholar]
- [29].Friedman L, Shechter H, Dimethylformamide as a Useful Solvent in Preparing Nitriles from Aryl Halides and Cuprous Cyanide; Improved Isolation Techniques, J. Org. Chem 26 (1961) 2522–2524. 10.1021/jo01351a092. [DOI] [Google Scholar]
- [30].Kikuchi C, Tabata Y, Yamakawa T, Matsuhira T, Watanabe N, Kubota N, Kaneda K, inventors; Meiji Seika Pharma Co Ltd, assignee. PDE4 inhibitor. US patent 2016/0159783 A1 June 9 2016.
- [31].Steinkopf W, Jurgens B, Uber die Konstitution der aci-Nitrokorper, J. Prakt. Chem 84 (1911) 686–713. [Google Scholar]
- [32].Kozikowski AP, Adamcz M, Methods for the stereoselective cis-cyanohydroxylation and - carboxyhydroxylation of olefins, J. Org. Chem 48 (1983) 366–372. 10.1021/jo00151a017. [DOI] [Google Scholar]
- [33].Said SB, Skaržewski J, Młchowski J, Oxidative Conversion of Aldoximes into Carboxylic Acid Esters, Synth. Commun 22 (1992) 1851–1862. 10.1080/00397919208021316. [DOI] [Google Scholar]
- [34].Forrest HS, Walker J, Chemotherapeutic agents of the sulphone type. Part V. 2: 5-Disubstituted derivatives of pyridine, J. Chem. Soc (1948) 1935–1945. 10.1039/JR9480001939. [DOI] [PubMed] [Google Scholar]
- [35].Ramachandran U, Mital A, Bharatam PV, Khanna S, Rao PR, Srinivasan K, Kumar R, Chawla HP, Kaul CL, Raichur S, Chakrabarti R, Studies on some glitazones having pyridine as the linker unit, Bioorg. Med. Chem 12 (2004) 655–662. 10.1016/j.bmc.2003.11.027. [DOI] [PubMed] [Google Scholar]
- [36].Reed LJ, Muench H, A simple method of estimating fifty percent endpoints, Am. J. Epidemiol 27 (1938) 493–497 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- [37].Quillardet P, Hofnung M, The SOS chromotest: a review, Mutat Res. 7 (1993) 235–279. 10.1016/0165-1110(93)90019-j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.