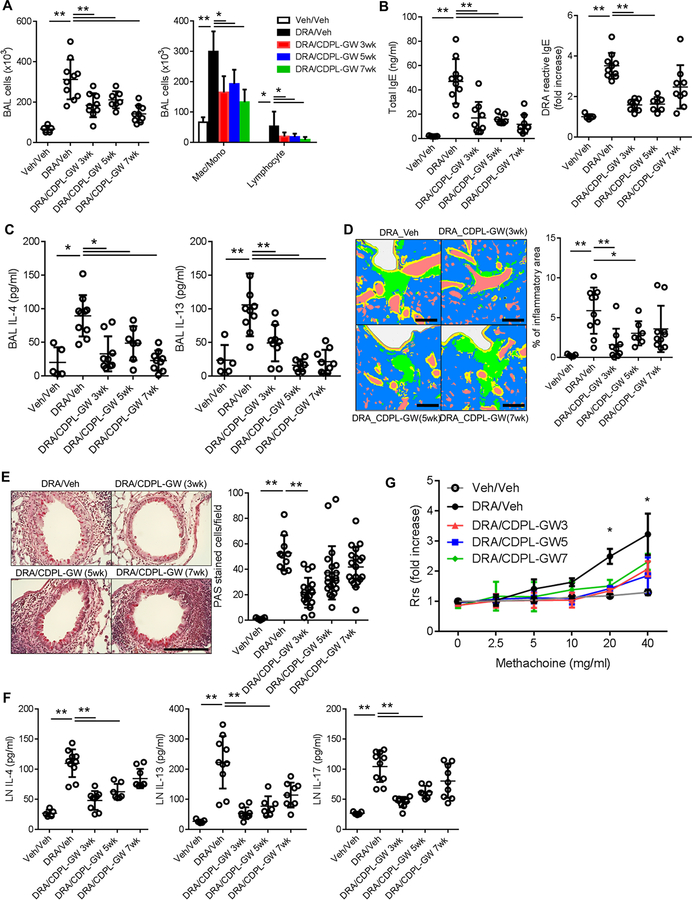
Figure 6. Intranasal delivery of CDPL-GW nanoprobe abolishes chronic allergenic lung inflammation in the DRA model.
Mice were subjected to the chronic DRA model, and then treated with CDPL-GW (1ng/mouse via the i.n. route, twice a week) in three different designs as depicted in Figure E8. CDPL-GW 3wk indicates that the treatment with CDPL-GW was started at week 3 of the chronic DRA model and maintained until week 8 (total of 6 weeks). CDPL-GW 5wk and 7wk were done in a similar fashion. All of the measurements were done at week 11. (n=5–10 mice per group) (A) Treatment with CDPL-GW markedly reduced the DRA-induced BAL cellular recruitment which was mainly comprised of macrophages and lymphocytes. (B-E) CDPL-GW treatment starting at 3 and 5 weeks of the chronic DRA model (CDPL-GW 3wk and 5wk) effectively blocked the increases in total and DRA-reactive serum IgE, BAL IL-4 and IL-5, and attenuated lung inflammation and goblet cell metaplasia. However, the CDPL-GW 7wk group showed similar trends, but differences failed to reach statistical significance in terms of DRA-reactive serum IgE, lung inflammation and goblet cell metaplasia. Scale bars represent 200μm. (F) Single cell suspensions of regional LNs were re-stimulated with DRA for 72 hours. DRA re-stimulation increased the secretion of IL-4, IL-13 and IL-17, but CDPL-GW treatment blocked these increases. However, cytokine levels from cells obtained from the CDPL-GW 7wk group did not reach statistical significance compared to those of the sham-treated DRA group, although they showed a declining trend. (G) Airway resistance was measured using increasing doses of methacholine in the sham and CDPL-GW treated groups after DRA challenge. All of the three CDPL-GW treated groups had significantly lower airway resistance compared to the sham treated chronic DRA group.