Abstract
Introduction
Patient-reported outcomes are routinely assessed in atrial fibrillation (AF) to evaluate efficacy of treatment and as clinical trial outcomes. The relation of depression to such measures has had limited study in AF.
Methods
In a cohort receiving treatment for AF, we assessed depression with the Patient Health Questionniare-9 (PHQ; 0–4, normal range; 5–9, mild depression; ≥10 moderate depression). We related depression to disease-specific quality of life with the AF Effect on QualiTy of life (AFEQT, range 0–100) and the Global Perceived Stress Scale (GPPS, range 0–24) in multivariable-adjusted models.
Results
In 260 individuals (age 71.7±10.1, 44.6% women) with AF, 51 (26.1%) had PHQ scores ≥5 and 17 (6.5%) ≥10. AFEQT scores decreased progressively with depression severity (normal range PHQ, 81.4±14.1; mild depression, 65.8±17.1; moderate depression, 50.6±19.3). Individuals without depression had lower GPPS scores (3.0±2.6) than those with mild (4.9±2.5) or moderate (8.9±4.0) depression. In multivariable-adjusted models mild depression was associated with a 12.1-point (95% confidence interval [CI], −17.2 to −6.9) decrease in AFEQT and 1.9-point (95% CI, 1.1 to 2.7) increase in GPSS, while moderate depression a 27.7-point (95% CI, −35.5 to −19.8) decrease in AFEQT and 5.5-point (95% CI, 4.2 to 6.8) increase in GPSS, relative to normal range PHQ. Regression analyses confirmed significant correlations between depression and AFEQT and GPPS scores in multivariable-adjusted models.
Conclusions
We determined that depression is associated with a step-wise, progressively adverse change in patient-centered outcomes in individuals with AF. Our findings suggest the importance of assessing depression in the evaluation of AF.
Keywords: health services, atrial fibrillation, risk factors, Atrial fibrillation, depression, quality of life, stress, patient-reported outcomes
Introduction
Atrial fibrillation (AF) is a challenging and chronic condition for patients that is associated with depression.1 The contribution of depression towards adverse clinical care and outcomes is recognized in multiple health conditions.2 Individuals with cardiac and non-cardiac conditions and depression have increased health care utilization and mortality relative to those without.3–5 There is further evidence that depression is associated with treatment adherence in cardiac and non-cardiac conditions.6,7 The contributions of depression towards adverse clinical outcomes and poor self-care in cardiac disease are well summarized.8
In AF, the prevalence of depression in AF is estimated as ranging from 20% to 40% and similarly associated with deleterious care metrics and outcomes. Depression increases with the severity of AF,9–12 and is correlated with health care utilization and mortality similar to other conditions.13,14 Depression likewise affects the clinical management of AF; individuals with AF and depression are less likely to receive anticoagulation as well as have decreased adherence to such agents.15–18
Depression may also have an important effect on patients’ subjective experience of AF. Individuals with depression have increased symptoms associated with AF, independent of the burden or severity of the arrhythmia,14,19 compared to those without depression. AF has a deleterious effect on quality of life, and may further impact psychological stress.20,21 Professional society guidelines have prioritized patient-reported outcomes as a central metric for the evaluation and management of AF.22 Hence, examining the contribution of depression to patient-reported outcomes may inform patient-centered care and guide interdisciplinary treatment strategies that address depression rather than a predominant focus on the arrhythmia.
In this study we examined depression in relation to central patient-reported outcomes that are relevant to the patient experience of AF. First, we examined the relation of depression to disease-specific quality of life, in contrast to other investigations that have employed general quality of life instruments. Second, we examined the contribution of depression to psychological stress, given the association of stress to symptom burden in AF.23 We hypothesized that individuals with AF and depression would have worse disease-specific quality of life and increased psychological stress compared to those without depression.
Methods
We enrolled individuals receiving care at ambulatory facilities affiliated with the University of Pittsburgh Medical Center in Pittsburgh, PA, a large, regional health care system. Individuals with prevalent, nonvalvular AF were identified by screening the electronic health record and direct contact during clinical visits, physician or provider referral, or self-referral. Eligibility criteria were age ≥18 years; history of non-valvular AF as documented by the electronic health record; CHA2DS2-VaSc24 (congestive heart failure, hypertension, age, diabetes, stroke, vascular disease [history of MI, PVD, or aortic atherosclerotic disease], and sex category) score ≥2; having been prescribed oral anticoagulation for AF; and English-speaking at a level appropriate to provide informed consent and participate in this research protocol. Exclusion criteria were history of ischemic or hemorrhagic stroke, AF secondary to non-cardiac issues, AF within 30 days of any cardiothoracic surgery, or those not able to pass a three-item screening instrument prior to informed consent. From 2016 through 2018, our study team identified 1093 eligible individuals from the electronic health record. Of these we were able to approach the 486 who presented at scheduled clinic visits and were amenable to discussing participation in this study. Of these, we enrolled 339 for participation in this study, of which 260 had complete measures for inclusion in this analysis. By design, our study is cross-sectional, such that we obtained all data presented here at a single encounter with participants.
Baseline demographics including age, sex, and race were obtained by self-report. Clinical history pertinent to the CHA2DS2-VaSc and body mass index were extracted from the electronic health record. AF treatment, consisting in history of electrical or pharmacologic cardioversion, pulmonary vein isolation, and anticoagulant and antiarrhythmic medications (amiodarone, dofetilide, flecainide, lidocaine, propafenone and sotalol) were identified from the electronic record. Social factors obtained from participant self-report included annual household income, categorized as <$19,000; $20,000-$49,999; $50,000-$99,999; >$100,000, and level of education (≤high school or vocational training; some part of college or an associate degree; bachelor’s degree; or any graduate or professional school degree or enrollment). Health literacy was measured with the Short-Test Of Functional Health Literacy in Adults (S-TOFHLA).25
Depression was measured with the Patient Health Questionnaire (PHQ), a 9-item, validated instrument for quantification of depression severity over the previous 2 weeks that ranges from 0 to 27 with higher scores indicating more severe depression and a score of 0–4 defined as normal range, 5–9 suggesting mild depression, and ≥10 major depression.26 Quality of life specific to AF was collected with the AF Effect on QualiTy of life instrument, a 20-item instrument scored from 0 to 100, where higher scores indicate greater quality of life over the prior 4 weeks.27 The AFEQT includes a global score and 4 domain scores (symptoms, daily activities, treatment concerns, and treatment satisfaction). Global perceived psychological stress (GPPS) was measured with a validated instrument that measures 12-month stress on a 4-point scale (0 to 3) across 8 domains (caring for others, employment, legal problems, medical problems, meeting basic needs, racism and discrimination, related to the neighborhood, and relationships) and ranging from 0–24.28,29
Statistical analysis
We summarized continuous variables according to their means and standard deviation and categorical variables by their distributions. We evaluated PHQ results, our independent variable, according to prespecified categories (0–4, 5–9, and ≥10) and as a continuous variable.26 We examined differences in the dependent variables (AFEQT and psychological stress score) by PHQ category using analysis of variance. We examined the associations of the PHQ and the AFEQT, AFEQT domains, and GPSS graphically using scatterplots and numerically with Pearson correlation coefficients. We employed multivariable-adjusted linear regression models to examine the relation of PHQ scores to AFEQT, AFEQT domains and psychological stress. Models adjusted for age, sex, race, body mass index, smoking history, heart failure, hypertension, diabetes, vascular disease, AF treatments, education and household income. All statistical analyses were conducted with SAS version 9.4 (Cary, North Carolina). Written informed consent was collected for each participant. The study was approved by the University of Pittsburgh institutional review board.
Results
Of 339 individuals enrolled in the study, 79 entered the study prior to the study’s adoption of the PHQ as its instrument for depression screening. As such, the 79 individuals recruited prior to the introduction of the PHQ lack an essential assessment and were consequently excluded from this analysis. In consequence, 260 participants were included in the present analysis (age 71.7±10.1 years, 116 [44.6%] women). The largest category of annual household income was in the $20-$49,999 range (n=74, 28.5%) and education for most participants was at the high school or vocational level (n=94, 36.2%). There were no significant differences in age, sex, race, level of education, or level of health literacy between participants by PHQ score category. Cohort characteristics for the cohort and are summarized by PHQ category in Table 1.
Table 1.
Characteristics of study cohort, total and according to Patient Health Questionnaire-9 (PHQ) category.
| All Participants | PHQ 0–4 | PHQ 5–9 | PHQ≥10 | |
|---|---|---|---|---|
| Characteristic | n = 260 | n = 192 | n = 51 | n = 17 |
| Age | 71.7 ± 10.1 | 71.9 ± 10.3 | 72.7 ± 9.3 | 67.4 ± 10.4 |
| Female Sex | 116 (44.6%) | 83 (43.2%) | 25 (49.0%) | 8 (47.1%) |
| White Race | 247 (95.0%) | 183 (95.3%) | 49 (96.1%) | 15 (88.2%) |
| Body mass index (m/kg2) | 31.4 ± 7.3 | 30.3 ± 6.4 | 35.3 ± 8.9 | 32.4 ± 8.2 |
| Smoking History | ||||
| Never | 126 (48.5%) | 101 (52.6%) | 19 (37.3%) | 6 (35.3%) |
| Former | 120 (46.2%) | 86 (44.8%) | 28 (54.9%) | 6 (35.3%) |
| Current | 14 (5.4%) | 5 (2.6%) | 4 (7.8%) | 5 (29.4%) |
| Heart Failure | 42 (16.2%) | 25 (13.0%) | 14 (27.5%) | 3 (17.6%) |
| Preserved | 22 (8.5%) | 12 (6.3%) | 8 (15.7%) | 2 (11.8%) |
| Reduced | 10 (3.8%) | 6 (3.1%) | 4 (7.8%) | 0 (0.0%) |
| Not Specified | 10 (3.8%) | 7 (3.6%) | 2 (3.9%) | 1 (5.9%) |
| Hypertension | 184 (70.8%) | 130 (67.7%) | 42 (82.4%) | 12 (70.6%) |
| Diabetes | 59 (22.7%) | 39 (20.3%) | 16 (31.4%) | 4 (23.5%) |
| Vascular Disease | 32 (12.3%) | 24 (12.5%) | 7 (13.7%) | 1 (5.9%) |
| History of Cardioversion | 68 (26.2%) | 46 (24.0%) | 18 (35.3%) | 4 (23.5%) |
| History of ablation for AF | 67 (25.8%) | 56 (29.2%) | 9 (17.6%) | 2 (11.8%) |
| Prescribed antiarrhythmic medication | 63 (24.2%) | 45 (23.4%0 | 16 (31.4%) | 2 (11.8%) |
| Education | ||||
| High school/Vocational | 94 (36.2%) | 61 (31.8%) | 26 (51.0%) | 7 (41.2%) |
| Some College | 48 (18.5%) | 38 (19.8%) | 8 (15.7%) | 2 (11.8%) |
| Bachelor’s | 60 (23.1%) | 45 (23.4%) | 11 (21.6%) | 4 (23.5%) |
| Graduate | 58 (22.3%) | 48 (25.0%) | 6 (11.8%) | 4 (23.5%) |
| Income | ||||
| <$19,999 | 33 (12.7%) | 15 (7.8%) | 11 (21.6%) | 7 (41.2%) |
| $20,000–49,999 | 74 (28.5%) | 53 (27.6%) | 15 (29.4%) | 6 (35.3%) |
| $50,000–99,999 | 71 (27.3%) | 55 (28.6%) | 14 (27.5%) | 2 (11.8%) |
| >$100,000 | 48 (18.5%) | 45 (23.4%) | 2 (3.9%) | 1 (5.9%) |
| Refused/No Answer | 34 (13.1%) | 24 (12.5%) | 9 (17.6%) | 1 (5.9%) |
| S-TOFHLA | 29.4 ± 5.1 | 29.8 ± 4.9 | 28.6 ± 5.9 | 28.1 ± 5.7 |
Continuous variables presented as mean±standard deviation and categorical by number (percentage). PHQ indicates Patient Health Questionnaire; TIA, transient ischemic attack; S-TOFHLA, Short-Test Of Functional Health Literacy in Adults.
Table 2 presents the distribution of AF-specific quality of life, total and by AFEQT domain, and GPSS scores and their distributions by PHQ category. We noted a progressive reduction in mean AFEQT scores, both total and domain, with progressive increase in PHQ category of depression. Individuals with a normal range PHQ had a total AFEQT 81.4±14.1, in contrast to a score of 65.8±17.1 for those with PHQ scores 5–9, and 50.5±19.3 for those with scores ≥10. The largest contrast in AFEQT was seen in the domain of daily activities, where those with normal range PHQ scored 76.5±21.8 compared to 36.9±20.0 in those with PHQ ≥10. Participants with PHQ scores ≥10 consistently scored lower across AFEQT domains than those with PHQ scores in the 5–9 range. Likewise, those with PHQ scores ≥10 had a nearly three-fold increased GPSS (8.9±4.0) relative those with normal range PHQ scores (3.0±2.6).
Table 2.
Distribution of AF-specific quality of life and psychological stress score, according to presence of absent or mild depression as measured by the Patient Health Questionnaire.
| Patient Health Questionnaire | ||||
|---|---|---|---|---|
| 0–4 | 5–9 | ≥10 | P-Value | |
| AFEQT | ||||
| Total | 81.4 ± 14.1 | 65.8 ± 17.1 | 50.6 ± 19.3 | <0.001 |
| Symptoms | 87.8 ± 14.7 | 81.5 ± 19.1 | 66.9 ± 28.9 | <0.001 |
| Daily Activities | 76.5 ± 21.8 | 53.0 ± 23.2 | 36.9 ± 20.0 | <0.001 |
| Treatment Concerns | 83.5 ± 16.3 | 71.3 ± 21.1 | 52.5 ± 25.1 | <0.001 |
| Treatment Satisfaction | 82.4 ± 21.4 | 71.1 ± 21.7 | 67.2 ± 23.3 | <0.001 |
| Global Perceived Stress Score | 3.0 ± 2.6 | 4.9 ± 2.5 | 8.9 ± 4.0 | <0.001 |
AFEQT indicates Atrial Fibrillation Effect on QualiTy of life measure.
Table 3 summarizes the magnitude of difference by PHQ category in AFEQT and GPSS measurements in multivariable-adjusted linear regression models. The beta coefficients present the difference in AFEQT score, total and by domain, and GPSS comparing each of the PHQ categories of mild depression (5–9) and major depression (≥10) those with normal range (0–4) PHQ scores. These data summarily demonstrate the magnitude of difference when comparing AFEQT and GPSS by PHQ category. For all measures, those with PHQ≥10 had a significant decrease in AFEQT score and increase in GPSS. Of note, the estimate of the significant increase in GPSS between those with major depression (β = 5.5, 95% CI, 4.2 to 6.9) relative to normal range PHQ scores did not diminish with progressive multivariable adjustment.
Table 3.
Association of Patient Health Questionnaire-9 (categorized as 0–4, 5–9, and ≥10) with AF-specific quality of life (AFEQT) and Global Psychological Stress Score.
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| AFEQT | β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value |
| Total | |||||||||
| PHQ 5–9 (vs. 0–4) | −15.4 | (−20.0, −10.7) | <0.001 | −13.0 | (−18.1, −7.8) | <0.001 | −12.1 | (−17.2, −6.9) | <0.001 |
| PHQ ≥10 (vs. 0–4) | −29.6 | (−37.0, −22.1) | <0.001 | −28.1 | (−35.6, −20.5) | <0.001 | −27.7 | (−35.5, −19.8) | <0.001 |
| Symptom | |||||||||
| PHQ 5–9 (vs. 0–4) | −6.2 | (−11.2, −1.03) | 0.019 | −6.2 | (−11.0, −0.5) | 0.034 | −5.2 | (−10.9, 0.5) | 0.073 |
| PHQ ≥10 (vs. 0–4) | −19.3 | (−27.5, −10.9) | <0.001 | −19.3 | (−27.8, −10.7) | <0.001 | −18.8 | (−27.6, −10.1) | <0.001 |
| Daily Activities | |||||||||
| PHQ 5–9 (vs. 0–4) | −23.1 | (−29.9, −16.2) | <0.001 | −17.9 | (−25.2, −10.5) | <0.001 | −16.7 | (−24.1, −9.3) | <0.001 |
| PHQ ≥10 (vs. 0–4) | −39.2 | (−50.2, −28.1) | <0.001 | −35.7 | (−46.6, −24.6) | <0.001 | −35.3 | (−46.6, −23.9) | <0.001 |
| Treatment concerns | |||||||||
| PHQ 5–9 (vs. 0–4) | −12.1 | (−17.4, −6.7) | <0.001 | −11.4 | (−17.1, −5.5) | <0.001 | −10.7 | (−16.6, −4.8) | <0.001 |
| PHQ ≥10 (vs. 0–4) | −28.2 | (−36.7, −19.6) | <0.001 | −27.9 | (−36.5, −19.2) | <0.001 | −27.5 | (−36.5, −18.6) | <0.001 |
| Treatment satisfaction | |||||||||
| PHQ 5–9 (vs. 0–4) | −11.2 | (−17.9, −4.4) | 0.001 | −9.4 | (−16.7, −1.9) | 0.014 | −8.3 | (−15.9, −0.8) | 0.031 |
| PHQ ≥10 (vs. 0–4) | −15.7 | (−26.6, −4.8) | 0.005 | −15.3 | (−26.4, −4.14) | 0.008 | −14.1 | (−25.7, −2.6) | 0.017 |
| GPSS | |||||||||
| PHQ 5–9 (vs. 0–4) | 1.9 | (1.1, 2.7) | <0.001 | 1.8 | (0.9, 2.7) | <0.001 | 1.9 | (1.0, 2.7) | <0.001 |
| PHQ ≥10 (vs. 0–4) | 5.5 | (4.2, 6.8) | <0.001 | 5.6 | (4.3, 6.9) | <0.001 | 5.6 | (4.2, 6.9) | <0.001 |
AFEQT indicates Atrial Fibrillation Effect on QualiTy of life measure; GPSS, Global Psychological Stress Score; CI, confidence interval. Estimates of difference in outcome measures (AFEQT/GPSS) by PHQ categorized in multivariable linear regression models. Each beta coefficient represents the estimated difference in each respective outcome for patients with PHQ≥10 versus PHQ<10. For example, a patient with PHQ≥10 has an estimated AFEQT total score 26.4 points (95% CI, −34.4, −18.4) lower than a patient with PHQ<10 after adjustment for age, sex, and race (Model 1).
Model 1 adjusted for age, sex, race.
Model 2 adjusted for age, sex, race, body mass index, smoking history, heart failure, hypertension, diabetes, and vascular disease.
Model 3 adjusted for Model 2 covariates and AF treatment (antiarrhythmics and pulmonary vein isolation), education, and estimated household income.
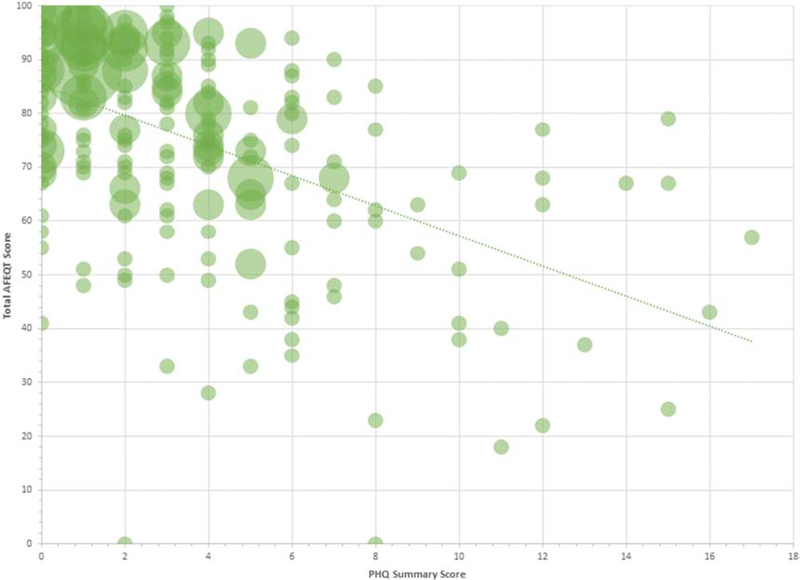
Supplementary Table 1 summarizes the effect of just a single-point increase in PHQ in relation to AFEQT and GPSS measures. For every 1-point increase in PHQ, we observed consistent decreases in AFEQT total and domain scores, all meeting our threshold for statistical significance. A 1-point increase in PHQ was likewise associated with a 0.5 increase in GPSS. These associations did not attenuate and remained statistically significant with progressive multivariable adjustment. In Figure 1 we present the distributions of PHQ and total AFEQT measures, showing that patients with worse depressive symptoms tended to have lower AF-related quality of life (Pearson correlation=−0.52, p<0.01). The Supplementary Figure provides a similar graphical presentation of the GPSS results, demonstrating a significant correlation between depression as determined by PHQ and psychological stress (Pearson correlation=0.53, p<0.01).
Figure 1.
Plot showing correlation between depression, as measured by the Patient Health Questionnaire (PHQ), and quality of life specific to atrial fibrillation (AF), determined with the total Atrial Fibrillation Effect on QualiTy of life (AFEQT) score. The regression line indicates the significant negative correlation between depression and AF-specific quality of life (Pearson correlation −.52, P<0.01).
Discussion
In this moderate-sized cohort of individuals with prevalent AF, we identified significant and consistent associations between depression and both AF-specific quality of life and psychological stress. We identified step-wise associations between depression and these patient-reported outcomes. As such, both the AFEQT and GPSS scores were modified significantly across categories of normal range, mild and major depression. Those with major depression showed the poorest AFEQT scores in both total and the individual domains, and likewise significantly increased GPSS scores. Our results were not modified by multivariable adjustment for clinical or socioeconomic variables or by the treatment approach and regimen for AF. The persistent associations seen here between depression and AF-specific quality of life and psychological stress suggest that depression has critical relevance to how patients experience AF.
We noted critical difference in those with and without depression in our cohort. Individuals with less education and lower income were far more likely to experience depression than those with bachelor’s or graduate education and higher income. Further, we identified differences in depression by AF treatment such that individuals who had undergone cardioversion or were prescribed antiarrhythmic medication had less likelihood of minor or major depression as identify by the PHQ. Conducting our study in a larger cohort would facilitate our performing a mediation analysis to identify how demographics, comorbid conditions, and social determinants may influence depression in AF.
Clinical significance of these findings
Large amounts of health care resources are dedicated to procedures to treat symptomatic AF. Such treatment is highly specialized and includes catheter ablation and advanced pharmacologic therapies with the objective of managing and controlling patient-reported symptoms. A recent clinical trial evaluating the 12-month effect of catheter ablation versus pharmacologic management on disease-specific quality of life in AF reported a 5.3-point (95% CI, 3.7 to 6.9) difference in individuals receiving ablation.30 In comparison our fully adjusted multivariable model identified a 12-point difference in AF-specific quality of life scores in those with mild depression and 28-point difference in those with major depression, relative to normal range PHQ. Changes in AFEQT scores of about 5 points, in either a positive or negative direction, have been considered clinically meaningful.31 Our findings imply that treatment of depression – a highly prevalent comorbid condition in AF – may improve disease-specific quality of life and the psychological stress that may accompany this chronic arrhythmia. Furthermore, evaluation of depression is relevant to the model of integrated AF management articulated by current professional society guidelines.32
Recognizing and treating depression has been documented as crucial to improving outcomes and quality of life in cardiac diseases such as coronary artery disease and congestive heart failure.33–37 In contrast, depression has not been well addressed by professional society guidelines for treatment of AF. An American Heart Association advisory statement on coronary heart disease has advocated routine screening for depression in individuals with coronary disease, given the significant contributions of depression towards adverse coronary disease prognosis.38 Depression is recognized as one of the most common comorbid conditions in Medicare beneficiaries with AF. However, the guidelines for management of AF do not mention the relevance of screening and treating comorbid depression.22
AF and depression are both highly prevalent and the successful results of treatment of coronary disease and depression argues for enhanced strategies to address AF and comorbid depression. Potential avenues span screening individuals with symptomatic AF for depression and appropriate mental health referral; application of cognitive behavioral therapy as part of routine care as demonstrated in a limited-sized cohort39; and a collaborative care model similar to what has been successfully implemented and evaluated in heart failure and coronary disease.40–42
Our study further identified that individuals with mild and major depression experienced greater levels of psychological stress. AF is a challenging condition for patients with variable symptoms, complex treatments, and the necessity of long-term adherence for a chronic disease. Prior studies evaluating the association of psychological stress to incident AF have had variable results.43,44 However, psychological stress has been related to symptoms associated with AF, regardless of the arrhythmia burden or ventricular rate during episodes of AF,23 and similarly with increased risk of cardiovascular events and prognosis.45–47 Our findings additionally support the importance of considering psychological stress as a component of patient evaluation and management in AF. Further studies to investigate how stress may contribute towards adverse clinical events associated with AF, patient-reported outcomes, or self-care behaviors such as medication adherence are essential.
Strengths and limitations
Strengths of our study include its moderate size, use of a disease-specific quality of life instrument with domains pertinent to the patient experience of AF, and assessment of psychological stress with a validated measure as a complementary patient-reported outcome. We would note some important limitations of our work. First, we recruited a convenience cohort from a single, regional health center, and consequently recognize the inherent selection bias in establishing such a cohort. Selection for participation here required that individuals have access to health care and the social resources to present for an ambulatory appointment. We consider that a range of issues may limit access to care and adherence to visits, and as such express caution about the generalizability of our findings. However, we would qualify that albeit not representative, we consider our findings valid and expect that other settings will similarly demonstrate a strong association between depression and AF-specific quality of life and psychological stress. Second, our cohort had limited racial and ethnic diversity, which may further limit the generalizability of our findings. Third, we conducted a cross-sectional study, and are therefore not able to exclude reverse causality. It is possible that individuals with worse disease-specific quality of life may have greater general depressive symptoms, and the same may be true for those with greater psychological stress. We determined not to examine the reciprocal associations between either the AFEQT or the psychological stress measures employed here, as we sought to focus on depression as our independent variable. Fourth, we are not able to account for residual confounding, as multiple factors may influence depression, quality of life, and psychological stress. For example, our study does not account for the contributions of the social environment or lifestyle factors that may influence the associations examined here. Likewise, we did not include assessments of treatment or medication adherence. It is possible that individuals with depression had decreased adherence to treatments, thereby influencing their quality of life, specific to AF as measured by the AFEQT. Fifth, we did not include a history of treatment for depression or other mental health disorders. A more robust study of psychological health and mental health in individuals with AF would be informative to delineate how such factors affect the patient experience of this common, chronic arrhythmia.
Conclusion
In conclusion, in a moderate-sized cohort of individuals with AF, we identified a significant association between depressive symptoms and disease-specific quality of life and psychological stress. Our findings suggest the relevance for consideration of depressive symptoms when evaluating patients with AF. Treatment of depression has shown benefit to individuals with other cardiovascular conditions, such as heart failure and coronary disease, and our findings indicate the necessity for further evaluation and treatment of depression as part of AF management.
Supplementary Material
Highlights.
Depression is a frequent comorbid condition in individuals with atrial fibrillation (AF).
Quality of life and psychological stress are relevant to patients’ experience of AF.
We identified strong associations between depression and these patient-reported outcomes.
Addressing depression may improve patient-reported outcomes in AF.
Acknowledgments
Sources of Funding: This work was supported by NIH grant R61HL144669 and by grant 2015084 from the Doris Duke Charitable Foundation.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the u.S. Adult population. Am J Cardiol. 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 2.Park LT, Zarate CA Jr. Depression in the primary care setting. N Engl J Med. 2019;380:559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jani BD, Mair FS, Roger VL, Weston SA, Jiang R, Chamberlain AM. Comorbid depression and heart failure: A community cohort study. PLoS One. 2016;11:e0158570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frasure-Smith N, Lesperance F, Habra M, Talajic M, Khairy P, Dorian P, Roy D, Atrial F, Congestive Heart Failure I. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120:134–140, 133p following 140. [DOI] [PubMed] [Google Scholar]
- 5.Christensen AV, Juel K, Ekholm O, Thrysoe L, Thorup CB, Borregaard B, Mols RE, Rasmussen TB, Berg SK. Significantly increased risk of all-cause mortality among cardiac patients feeling lonely. Heart. 2019 [DOI] [PubMed] [Google Scholar]
- 6.Axon RN, Gebregziabher M, Hunt KJ, Lynch CP, Payne E, Walker RJ, Egede LE. Comorbid depression is differentially associated with longitudinal medication nonadherence by race/ethnicity in patients with type 2 diabetes. Medicine (Baltimore). 2016;95:e3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: Findings from the heart and soul study. Arch Intern Med. 2005;165:2508–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jha MK, Qamar A, Vaduganathan M, Charney DS, Murrough JW. Screening and management of depression in patients with cardiovascular disease: Jacc state-of-the-art review. J Am Coll Cardiol. 2019;73:1827–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wandell P, Carlsson AC, Gasevic D, Wahlstrom L, Sundquist J, Sundquist K. Depression or anxiety and all-cause mortality in adults with atrial fibrillation--a cohort study in swedish primary care. Annals of medicine. 2016;48:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polikandrioti M, Koutelekos I, Vasilopoulos G, Gerogianni G, Gourni M, Zyga S, Panoutsopoulos G. Anxiety and depression in patients with permanent atrial fibrillation: Prevalence and associated factors. Cardiol Res Pract. 2018;2018:7408129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange HW, Herrmann-Lingen C. Depressive symptoms predict recurrence of atrial fibrillation after cardioversion. J Psychosom Res. 2007;63:509–513. [DOI] [PubMed] [Google Scholar]
- 12.Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: A systematic review. Am J Med. 2006;119:448 e441–419. [DOI] [PubMed] [Google Scholar]
- 13.Wandell P, Carlsson AC, Holzmann MJ, Arnlov J, Sundquist J, Sundquist K. Mortality in patients with atrial fibrillation and common co-morbidities - a cohort study in primary care. Annals of medicine. 2018;50:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehi AK, Sears S, Goli N, Walker TJ, Chung E, Schwartz J, Wood KA, Guise K, Mounsey JP. Psychopathology and symptoms of atrial fibrillation: Implications for therapy. Journal of cardiovascular electrophysiology. 2012;23:473–478. [DOI] [PubMed] [Google Scholar]
- 15.Paradise HT, Berlowitz DR, Ozonoff A, Miller DR, Hylek EM, Ash AS, Jasuja GK, Zhao S, Reisman JI, Rose AJ. Outcomes of anticoagulation therapy in patients with mental health conditions. Journal of general internal medicine. 2014;29:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker GA, Heidenreich PA, Phibbs CS, Go AS, Chiu VY, Schmitt SK, Ananth L, Frayne SM. Mental illness and warfarin use in atrial fibrillation. The American journal of managed care. 2011;17:617–624. [PubMed] [Google Scholar]
- 17.Emren SV, Senoz O, Bilgin M, Beton O, Aslan A, Taskin U, Aciksari G, Asarcikli LD, Cakir H, Bekar L, Bolat I, Yayla C, Celebi B, Dalgic O, Celik O, Safak O, Akyel S, Gungor H, Duzel B, Zoghi M. Drug adherence in patients with nonvalvular atrial fibrillation taking non-vitamin k antagonist oral anticoagulants in turkey: Noac-tr. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2018;24:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson C, Inglis SC, Newton PJ, Middleton S, Macdonald PS, Davidson PM. Barriers and enablers to adherence to anticoagulation in heart failure with atrial fibrillation: Patient and provider perspectives. J Clin Nurs. 2017;26:4325–4334. [DOI] [PubMed] [Google Scholar]
- 19.Sears SF, Serber ER, Alvarez LG, Schwartzman DS, Hoyt RH, Ujhelyi MR. Understanding atrial symptom reports: Objective versus subjective predictors. Pacing Clin Electrophysiol. 2005;28:801–807. [DOI] [PubMed] [Google Scholar]
- 20.Randolph TC, Simon DN, Thomas L, Allen LA, Fonarow GC, Gersh BJ, Kowey PR, Reiffel JA, Naccarelli GV, Chan PS, Spertus JA, Peterson ED, Piccini JP, Investigators OA, Patients. Patient factors associated with quality of life in atrial fibrillation. Am Heart J. 2016;182:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman JV, Simon DN, Go AS, Spertus J, Fonarow GC, Gersh BJ, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Chang P, Peterson ED, Piccini JP, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation I, Patients. Association between atrial fibrillation symptoms, quality of life, and patient outcomes: Results from the outcomes registry for better informed treatment of atrial fibrillation (orbit-af). Circ Cardiovasc Qual Outcomes. 2015;8:393–402. [DOI] [PubMed] [Google Scholar]
- 22.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 aha/acc/hrs guideline for the management of patients with atrial fibrillation: A report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- 23.Walters TE, Wick K, Tan G, Mearns M, Joseph SA, Morton JB, Sanders P, Bryant C, Kistler PM, Kalman JM. Symptom severity and quality of life in patients with atrial fibrillation: Psychological function outweighs clinical predictors. International journal of cardiology. 2019;279:84–89. [DOI] [PubMed] [Google Scholar]
- 24.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 25.Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient education and counseling. 1999;38:33–42. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K, Spitzer RL, Williams JB. The phq-9: Validity of a brief depression severity measure. Journal of general internal medicine. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, Lakkireddy DR, Wimmer AP, Bhandari A, Burk C. Development and validation of the atrial fibrillation effect on quality-of-life (afeqt) questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:15–25. [DOI] [PubMed] [Google Scholar]
- 28.Payne TJ, Wyatt SB, Mosley TH, Dubbert PM, Guiterrez-Mohammed ML, Calvin RL, Taylor HA Jr., Williams DR. Sociocultural methods in the jackson heart study: Conceptual and descriptive overview. Ethnicity and Disease. 2005;15:S6–38–48. [PubMed] [Google Scholar]
- 29.Subramanyam MA, Diez-Roux AV, Hickson DA, Sarpong DF, Sims M, Taylor HA Jr., Williams DR, Wyatt SB. Subjective social status and psychosocial and metabolic risk factors for cardiovascular disease among african americans in the jackson heart study. Social Science and Medicine. 2012;74:1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, Lee KL, Packer DL, Investigators C. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: The cabana randomized clinical trial. JAMA. 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes DN, Piccini JP, Allen LA, Fonarow GC, Gersh BJ, Kowey PR, O’Brien EC, Reiffel JA, Naccarelli GV, Ezekowitz MD, Chan PS, Singer DE, Spertus JA, Peterson ED, Thomas L. Defining clinically important difference in the atrial fibrillation effect on quality-of-life score. Circulation. Cardiovascular quality and outcomes. 2019;12:e005358. [DOI] [PubMed] [Google Scholar]
- 32.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 esc guidelines for the management of atrial fibrillation developed in collaboration with eacts. Europace. 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 33.Smolderen KG, Buchanan DM, Gosch K, Whooley M, Chan PS, Vaccarino V, Parashar S, Shah AJ, Ho PM, Spertus JA. Depression treatment and 1-year mortality after acute myocardial infarction: Insights from the triumph registry (translational research investigating underlying disparities in acute myocardial infarction patients’ health status). Circulation. 2017;135:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.May HT, Horne BD, Knight S, Knowlton KU, Bair TL, Lappe DL, Le VT, Muhlestein JB. The association of depression at any time to the risk of death following coronary artery disease diagnosis. European heart journal. Quality of care & clinical outcomes. 2017;3:296–302. [DOI] [PubMed] [Google Scholar]
- 35.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128:e240–327. [DOI] [PubMed] [Google Scholar]
- 36.Wang JT, Hoffman B, Blumenthal JA. Management of depression in patients with coronary heart disease: Association, mechanisms, and treatment implications for depressed cardiac patients. Expert opinion on pharmacotherapy. 2011;12:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bangalore S, Shah R, Pappadopulos E, Deshpande CG, Shelbaya A, Prieto R, Stephens J, McIntyre RS. Cardiovascular hazards of insufficient treatment of depression among patients with known cardiovascular disease: A propensity score adjusted analysis. European heart journal. Quality of care & clinical outcomes. 2018;4:258–266. [DOI] [PubMed] [Google Scholar]
- 38.Lichtman JH, Bigger JT Jr., Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES. Depression and coronary heart disease: Recommendations for screening, referral, and treatment: A science advisory from the american heart association prevention committee of the council on cardiovascular nursing, council on clinical cardiology, council on epidemiology and prevention, and interdisciplinary council on quality of care and outcomes research: Endorsed by the american psychiatric association. Circulation. 2008;118:1768–1775. [DOI] [PubMed] [Google Scholar]
- 39.Malm D, Fridlund B, Ekblad H, Karlstrom P, Hag E, Pakpour AH. Effects of brief mindfulness-based cognitive behavioural therapy on health-related quality of life and sense of coherence in atrial fibrillation patients. Eur J Cardiovasc Nurs 2018:1474515118762796. [DOI] [PubMed] [Google Scholar]
- 40.Meyer T, Belnap BH, Herrmann-Lingen C, He F, Mazumdar S, Rollman BL. Benefits of collaborative care for post-cabg depression are not related to adjustments in antidepressant pharmacotherapy. J Psychosom Res. 2014;76:28–33. [DOI] [PubMed] [Google Scholar]
- 41.Huffman JC, Mastromauro CA, Beach SR, Celano CM, DuBois CM, Healy BC, Suarez L, Rollman BL, Januzzi JL. Collaborative care for depression and anxiety disorders in patients with recent cardiac events: The management of sadness and anxiety in cardiology (mosaic) randomized clinical trial. JAMA Intern Med. 2014;174:927–935. [DOI] [PubMed] [Google Scholar]
- 42.Huffman JC, Beach SR, Suarez L, Mastromauro CA, DuBois CM, Celano CM, Rollman BL, Januzzi JL. Design and baseline data from the management of sadness and anxiety in cardiology (mosaic) randomized controlled trial. Contemp Clin Trials. 2013;36:488–501. [DOI] [PubMed] [Google Scholar]
- 43.O’Neal WT, Qureshi W, Judd SE, Glasser SP, Ghazi L, Pulley L, Howard VJ, Howard G, Soliman EZ. Perceived stress and atrial fibrillation: The reasons for geographic and racial differences in stroke study. Ann Behav Med. 2015;49:802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graff S, Prior A, Fenger-Gron M, Christensen B, Glumer C, Larsen FB, Vestergaard M. Does perceived stress increase the risk of atrial fibrillation? A population-based cohort study in denmark. Am Heart J. 2017;188:26–34. [DOI] [PubMed] [Google Scholar]
- 45.Albert MA, Durazo EM, Slopen N, Zaslavsky AM, Buring JE, Silva T, Chasman D, Williams DR. Cumulative psychological stress and cardiovascular disease risk in middle aged and older women: Rationale, design, and baseline characteristics. Am Heart J. 2017;192:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohlin B, Nilsson PM, Nilsson JA, Berglund G. Chronic psychosocial stress predicts long-term cardiovascular morbidity and mortality in middle-aged men. Eur Heart J. 2004;25:867–873. [DOI] [PubMed] [Google Scholar]
- 47.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S, investigators I. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the interheart study): Case-control study. Lancet. 2004;364:953–962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.