Abstract
Owing to the marked sexual dimorphism of hepatocellular carcinoma (HCC), sex hormone receptor signaling has been implicated in numerous aspects of liver cancer pathogenesis. We sought to reconcile the clear contribution of androgen receptor (AR) activity that has been established in preclinical models of HCC with the clinical failure of AR antagonists in advanced HCC patients by evaluating potential resistance mechanisms to AR-targeted therapy. The AR locus was interrogated for resistance-causing genomic modifications using publicly available primary HCC data sets (1090 samples). Analysis of HCC tumor and cell line RNA-Seq data revealed enriched expression of constitutively active, treatment refractory AR splice variants (AR-SVs). HCC cell lines expressed C-terminal-truncated AR-SV; 28 primary HCC samples abundantly expressed AR-SV. Low molecular weight AR species were nuclear localized, and constitutively active. Furthermore, AR/AR-SV signaling promoted AR-mediated HCC cell progression, and conferred resistance to androgen receptor antagonists. Ligand-dependent and independent AR signaling mediated HCC epithelial-mesenchymal transition by regulating the transcription factor SNAI2. These data suggest that AR-SV expression in HCC drives HCC progression and resistance to traditional AR antagonists. Novel therapeutic approaches that successfully target AR-SVs may be therapeutically beneficial for HCC.
Keywords: Primary Liver Cancer, Nuclear Hormone Receptor, Androgen Receptor C-terminal Truncated Isoforms, Antiandrogens therapy, Epithelial–Mesenchymal Transition
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer mortality globally and represents the most prevalent form of primary liver cancer. Chronic viral hepatitis—Hepatitis B virus (HBV) and hepatitis C virus (HCV), non-alcoholic steatohepatitis (NASH), and heavy alcohol intake are major etiological contributors to hepatocarcinogenesis (1). HCC incidence and mortality rates are rapidly outpacing other malignancies in the US, highlighting a substantial unmet medical need (2). Although early diagnosed tumors can be treated with liver resection, transplantation and ablation, HCC is a profoundly invasive tumor and challenging to diagnose at early stages, bringing about restricted treatment choices and low survival rates. The multi-tyrosine kinase inhibitors sorafenib and lenvatinib are the only approved first-line systemic therapy for advanced HCC, yet they have limited efficacy and severe side effects (3). Recently, regorafenib, cabozantinib, nivolumab, and ramucirumab were approved as second-line therapy in patients previously treated with sorafenib (4). The availability of molecular targeted therapy and immune checkpoint inhibitors is a welcome step toward improving systemic therapy for HCC. However, this will bring the challenge of patients’ stratification and drug selection.
Hepatic carcinogenesis is a complex process characterized by dysregulation of both genetic and epigenetic signaling cascades occurring in the context of significant genetic heterogeneity (5). Despite this, HCC is a consistently sexually dimorphic cancer with men having 2- to 4-fold higher likelihood of developing HCC compared to women. Several reports have implicated androgen receptor (AR) in HCC to explain in part sexual dimorphism in HCC (6). The AR, a ligand-activated transcription factor, canonically exerts various physiological and pathological functions in response to binding androgens (7). AR’s effects in prostate tissue are best characterized and include prostate cancer (PCa) initiation and progression (7). In addition, several reports have demonstrated increased levels of AR expression in tumor versus adjacent non-tumor tissue in HCC (8,9). The AR has been shown to modulate cellular oxidative stress and DNA damage repairing systems leading to genomic instability (10) and high levels of AR expression is correlated with high metastatic potential in vitro in HCC cell models (11). In spite of compelling evidence supporting the role of AR signaling in HCC initiation and progression, no survival advantage was found following antiandrogen therapy in HCC patients (12,13).
Notably, strong recent evidence of AR-axis activation in advanced HCC was provided in a report by Zhang et al. that found nuclear and likely active AR, as opposed to total cellular AR, was an independent predictor of overall survival in HCC patients (9). This work showed that AR antagonism in HCC cells was associated with rebound activation of the AKT-mTOR pathway which further contributed to nuclear localized AR protein and AR transcriptional activity. In an effort to reconcile the failure of AR-targeted therapy in HCC with the continued evidence of AR activation in primary disease we evaluated therapeutic resistance mechanisms well-known to the prostate cancer field. In PCa, persistent activation of the AR-axis in the presence of once effective AR-targeted therapy is intensively studied (14). The failure of AR-targeted therapy in PCa has been explained by multiple mechanisms including but not limited to AR amplification, AR point mutations altering steroid and anti-androgen binding, and expression of alternative AR splice variants that lack the C-terminal ligand-binding domain but retain transcriptional capacity (15). In each case, treatment-refractory, ligand-independent, and constitutively-active AR signaling can result. In this study, we report the expression of AR and its truncated splice variants in HCC primary patient samples and cell lines. Our data suggest the expression of truncated AR splice variants in HCC can mediate constitutive AR signaling and these AR-SVs are capable of driving AR signaling in the presence of antiandrogen therapy. Moreover, we characterize both androgen-mediated and androgen-independent AR transcriptional activity in diverse HCC models. Finally, we expand the understanding of AR’s biological contribution in HCC progression by demonstrating its modulation of epithelial-mesenchymal transition (EMT) effector proteins.
Materials and methods
Cell culture, reagents and transfections
Human HCC and PCa cell lines HepG2, PLC/PRF/5, SNU-423, VCaP, 22Rv1, DU145 and the immortalized normal liver cell line THLE2 were obtained from American Type Cell Culture Collection (ATCC, Manassas, VA). For reproducibility experiments, HCC cells panel (HepG2/C3A, PLC/PRF/5, SNU-423, SNU-475) was obtained from ATCC (ATCC® TCP-1011). The read pairs covering exon 4–8 of the AR locus from SNU-475 cell whole genome sequencing are summarized in Supplementary Figure 1A (details in Supplementary Methods). HCCLM3 cells were kindly provided by Thomas Schmittgen, University of Florida. Cell lines have been tested and authenticated by Genetica Cell Line Testing (Burlington, NC) using short tandem repeat DNA profiling. All cells were tested for mycoplasma periodically using MycoAlert™ PLUS Mycoplasma Detection Kit (LT07–705, Lonza, Allendale, NJ) and were negative. Cells were sub-cultured in ATCC recommended medium, DMEM or RPMI-1640 (11995–065, 11875–093, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (F0926–500ML, Sigma-Aldrich, St. Louis, MO) at 37°C in a humidified atmosphere with 5% CO2. Phenol-red free media containing 5% charcoal-stripped fetal bovine serum (csFBS) were used for studies involving steroid hormone signaling (21063–029, 11835–030, Invitrogen, Carlsbad, CA).
Patients and specimens
Freshly frozen primary HCC samples were acquired from two independent cohorts comprising 32 HCC patients who underwent surgical resection. In cohort 1, HCC tumor specimens were acquired from 16 patients (12 tumor only samples used in qRT-PCR analyses and 4 tumor and adjacent normal samples used in western blot analyses) who were diagnosed with HCV-mediated HCC at The James Cancer Center – The Ohio State University (Columbus, Ohio) and collected through the Total Cancer Care (TCC) Protocol. In cohort 2, total RNA samples were collected from 16 patients who were diagnosed with HCV-mediated HCC at National Liver Institute - Menoufia University (Menoufia, Egypt). Both studies were conducted according to the guidelines of the Declaration of Helsinki and approved by local review boards, and written informed consent was obtained from all patients (IRB approval No: 2016E0496). Patient characteristics are provided in supplementary table 1.
Results
Androgen Receptor Splice Variants as a Possible Mechanism of Resistance for Antiandrogen Therapy in HCC
We searched 1019 HCC primary samples from 6 studies in cBioPortal database (16) for AR gene alterations potentially capable of explaining anti-androgen resistance in HCC. Genomic AR alterations were excluded as a potential cause as we determined somatic mutation frequency to be less than 1.5% in all cases, and there were no copy number variations detectable within the AR gene in these cohorts (Supplementary Figure 1B–C). We next considered non-genomic potential mechanisms of resistance, such as the expression of C-terminal truncated AR splice variants that result from alternative splicing of AR pre-mRNA. Several constitutively-active, C-terminal truncated splice variants capable of mediating resistance to anti-androgen therapy have been described in prostate cancer cells and patients (Supplementary Figure 1D) (17). In PCa, approximately 20 AR splice variants have been described, and among these, variant 7 (AR-v7) is best characterized and thought to be the most clinically relevant. To the best of our knowledge, the expression and biological relevance of androgen receptor splice variants (AR-SVs) in HCC had not been previously examined in detail. To explore AR-SV expression in primary liver cancer, we surveyed HCC patient data in TCGA for non-canonical AR mRNA expression by re-analyzing raw RNA-seq data. To our surprise, AR-SV expression was observed in 290/372 (78%) of patients. Notably AR-SVs represented more than one quarter of total AR transcripts in the 100 patients with the highest total levels of AR message (Figure 1A, left). AR-SV expression was detected in both male and female patients with overall higher levels in males (Figure 1B, right and Supplementary Figure 2A), but no obvious expression differences exist when the TCGA data are considered by ethnicity (Supplementary Figure 2B) or tumor stage (Supplementary Figure 2C). Similar to prostate cancer (Supplementary Figure 2D), a well characterized AR-dependent cancer, AR-total expression in the TCGA HCC cohort was not associated with effects on overall survival (Supplementary Figure 2E). We also found no overt effects of HCC AR-SV expression on overall survival. We then hypothesized that AR-SV mediated AR signaling in HCC could explain in part resistance to anti-androgen therapy in HCC patients.
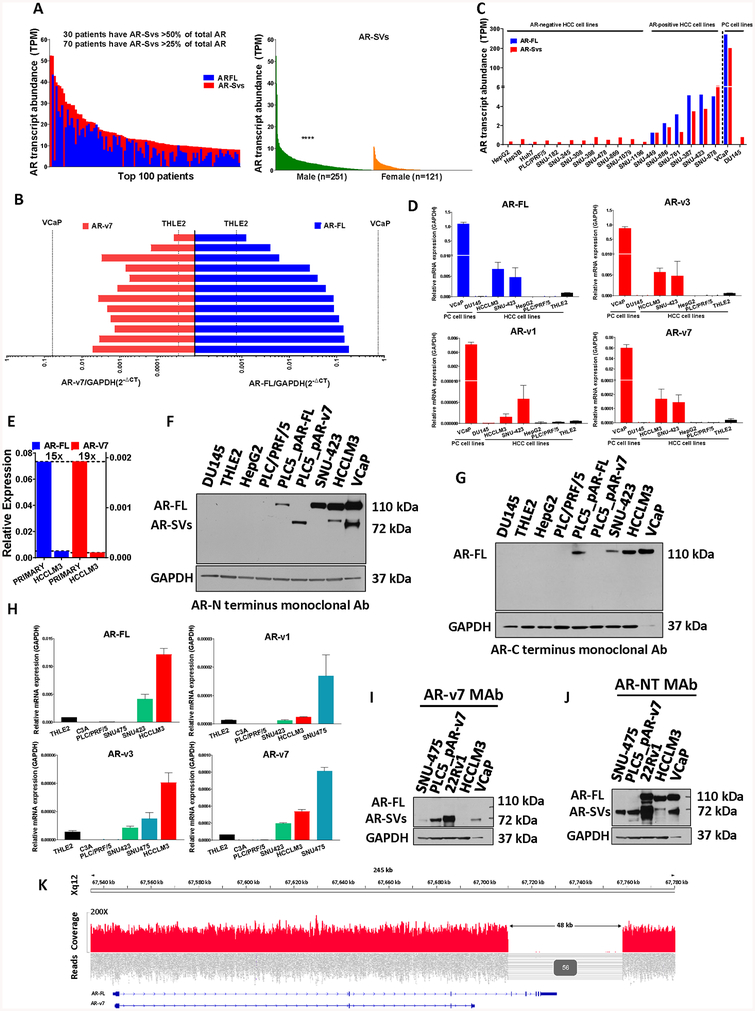
Figure 1. AR-FL and AR-SVs expression in HCC primary samples and cell lines.
(a, left) Top 100 (of 372) combined per-patient (x-axis) AR-FL (blue) and ligand-independent AR-SVs (red, as described in Supplementary Table 2). Numbers of patients with abundant AR-SV expression noted (inset) (a, right) RNA-Seq data from TCGA LIHC cohort were interrogated for AR-SVs transcript expression in female (n=121) and male (n=251). Statistical significance for AR-Svs expression in males vs females were evaluated using Mann-Whitney test **** p<0.0001 versus female. (b) Analyses of tumor RNA from 12 HCC majority cirrhotic and chronic hepatitis infected patients who underwent liver resection (male=10, female=2). Levels are compared to negative control THLE-2, normal liver cells, and positive control VCaP, PCa cells, to show abundant patient AR-FL and AR-v7 expression. Bars represent average technical duplicates and are matched for each patient. (c) Transcript abundance in transcript per million (TPM) of protein coding androgen receptor transcripts in 2 prostate cancer and 18 HCC cell lines from Cancer Cell Line Encyclopedia (CCLE) database. AR-FL (blue) and AR-SVs (red), as in Figure 1A, are presented. HCCLM3 cell data are not present in the CCLE. HCC cell AR transcript and protein expression were further validated by RT-PCR (d, h) and Western Blot (f, g), respectively. (d). RT-PCR analyses of AR-FL and AR-SVs transcripts in AR-positive prostate cancer (VCaP), AR-negative prostate cancer (DU145), AR-positive HCC (HCCLM3, SNU-423), AR-negative HCC (HepG2, PLC/PRF/5) and immortalized normal liver (THLE2) cell lines. (n=3, geometric mean ± SD). ARv4 and ARv12 were undetectable (supplementary Figure 5A). (e) Comparison of mean AR-FL and AR-v7 mRNA in primary samples as compared to the most abundant AR-SV expressing AR-positive HCC cells, HCCLM3, demonstrating robust AR isoform expression in primary HCC. (f) Western blot analysis with an N-terminal directed monoclonal AR antibody shows abundant AR-FL protein in HCCLM3 and SNU-423 cells and low molecular weight (MW) AR species in HCCLM3 cells migrating similarly to known AR-SVs in VCaP PCa cells. No AR-FL or lower MW species of AR were detected in HepG2, PLC/PRF/5, DU145, or THLE-2 cells. AR-negative HCC cell line, PLC/PRF/5, was transfected with either AR-FL expressing plasmid (PLC5_pAR-FL) or AR-v7 expressing plasmid (PLC5_pAR-v7) as positive controls for AR-FL and AR-v7, respectively. (g) Western blot analysis with a C-terminal directed monoclonal AR antibody shows abundant AR-FL protein in HCCLM3, SNU-423 and PLC5_pAR-FL cells. However, N-terminal directed monoclonal AR antibody detectable AR-SVs in VCaP and HCCLM3 cells are not detectable with c-terminal directed monoclonal AR antibody. WB performed with 35μg total protein lysate for all liver cell lines and 10μg for VCaP and DU145 and with primary N-terminal (CS#5153, Cell Signaling) or C-terminal AR mAb (ab52615, Abcam). (h). RT-PCR analyses of AR-FL and AR-SVs transcripts namely AR-v1, v3, and v7 in AR-positive HCC (HCCLM3, SNU-423, SNU475), AR-negative HCC (PLC/PRF/5) and immortalized normal liver (THLE2) cell lines (performed on low passage cells from ATCC Liver Cancer Panel TCP-1011, n=3, geometric mean ± SD). (i) Western blot analysis with an AR-v7 specific monoclonal AR antibody shows AR-v7 protein in 22Rv1, PLC5_pAR-v7, VCaP and SNU-475 cells. No AR-v7 reactive lower MW species of AR were detected in HCCLM3 cells. No AR-FL protein was detected in any of these cells. (j) To further confirm that the low molecular weight species that were detected by an AR-v7 specific AR mAb are C-terminal truncated splice variants, the blot presented in Figure 1I performed with a C-terminal targeting AR mAb was stripped, blocked and incubated with an N-terminal targeting AR mAb revealing abundant AR-FL in 22Rv1, VCaP and HCCLM3. The GAPDH blot from (i) is presented again here for convenience. No AR-FL isoform was detected in SNU-475 or PLC5_pAR-v7. However, low molecular weight AR species were detected in HCCLM3. (k) WGS of SNU-475 cells revealed a large ~48-kb hemizygous deletion in the AR-locus which included exons 4–8 of the AR-FL gene. This deletion is consistent with AR-v7 but not AR-FL expression and is strongly supported by sequencing data which included 56 read pairs with split reads and/or discordant pair alignments.
To further evaluate HCC patient AR-SV expression, we designed isoform-specific primers that anneal to a unique exon-exon junction in each previously characterized AR isoform (Supplementary Figure 3A) and validated them using well-characterized AR-positive and AR-SV-positive prostate cancer cell lines as positive controls (Supplementary Figure 3B–C). We next evaluated AR mRNA expression in total RNA from 12 primary HCV-mediated HCC samples acquired through the James Cancer Center at The Ohio State University. We quantified the expression of AR-FL and v7 using robust, specific RT-PCR assays (Supplementary Figure 4A–B). VCaP and THLE2, immortalized normal human liver cells, were used as positive and negative controls, respectively. Our results showed heterogeneity in total AR expression among patients but, surprisingly, the expression of AR-FL and v7 in some patients approached the levels of AR-FL and v7 in VCaP, an AR-positive PCa cell line that is known to express abundant AR relative to other PCa cell lines (Figure 1B) (18). In the second cohort, we evaluated 16 primary HCV-mediated HCC samples obtained from the national liver institute in Menoufiya University, Egypt using a more variable reference gene (β-actin, Supplementary Figure 4C) which resulted in similar AR-FL and v7 expression levels as compared to the first cohort (Supplementary Figure 4D). These findings substantiated our in silico analyses demonstrating AR-SV expression in a significant subset of HCC patients and further revealed that in addition to AR-FL, the C-terminally truncated AR-v7 mRNA is also abundantly expressed.
To extend our findings in primary HCCs to more readily studied HCC cell lines, we utilized CCLE mRNA data to quantify protein coding AR transcripts in multiple immortalized HCC cell models (19). Specifically, AR transcript data from 18 HCC and 2 PCa cell lines were evaluated (Figure 1C). In our analysis, HCC cell lines were considered AR-positive or AR-negative based on CCLE-determined transcript abundance, our own western blots, and published data (11). Though more than 20 AR isoforms have been described, we focused our in silico and subsequent analyses on experimentally confirmed or potentially constitutively-active isoforms (Supplementary Table 2). We next determined the expression of AR-FL and well-described AR variants in PCa namely, AR-v7, v1, v3, v4 and v12, in an initial panel of HCC cell lines using real-time PCR. These results showed that both AR-FL and AR-v7 expression were elevated in HCC cell lines with mesenchymal morphology derived from advanced disease (HCCLM3 and SNU-423) compared with morphologically epithelial HCC cell lines representing early disease (HepG2/C3A, and PLC/PRF/5) and the immortalized normal liver cell line (THLE2) (Figure 1D and supplementary Figure 5A). Furthermore, despite HCCLM3 cells demonstrating the most abundant AR-FL and AR-v7 expression among HCC cells, primary HCC samples exhibited much greater AR-FL and AR-v7 transcript on average (Figure 1E). Similar to AR-v7, AR-v1 and v3 were more abundant in HCC cells relative to normal liver cells but AR-v12 and v4 were undetectable in evaluated HCC cell lines.
Next, we evaluated AR protein expression in HCC cell lines utilizing two antibodies, an antibody against an N-terminal AR epitope which detects both AR-FL and C-terminal truncated SVs (AR-NT) and an antibody against a C-terminal AR epitope which detects only AR isoforms containing the C-terminal portion of the protein, but not C-terminal truncated splice variants (AR-CT). Consistent with our mRNA data we detected both AR-FL and lower molecular weight (MW) splice variants in the AR-positive HCC cell line, HCCLM3, when an AR N-terminus directed reagent was used (Figure 1F). However the low MW species in AR-positive HCCLM3, and PCa cell line, VCaP, were undetectable when using the AR C-terminal antibody (Figure 1G). To verify our approach to detecting C-terminal truncated AR-isoforms, we transfected the AR-negative HCC cell, PLC/PRF/5 with either an AR-FL or AR-v7 expressing plasmid (PLC_pARFL and PLC_pAR-v7, respectively). PCa models, VCaP and DU145, were included as AR-positive and AR-negative controls, respectively. As expected, the AR N-terminus directed antibody detected both AR-FL in PLC/PRF/5 overexpressing AR-FL and AR-v7 in PLC/PRF/5 overexpressing AR-v7, whereas the C-terminus directed antibody detected only AR-FL in PLC/PRF/5 overexpressing AR-FL. This was also the case when the western blot was first performed with the C-terminal targeted antibody and then stripped and re-blotted with the N-terminal targeting reagent (Supplementary Figure 5B). We confirmed AR-SV mRNA levels with fresh, lower passage cells acquired as part of an HCC cell panel available from American Type Culture Collection (Figure 1H and Supplementary Figure 5C). This analysis revealed the AR-SV expressing HCC cell line SNU-475 which had abundant AR-v7 expression but undetectable levels of AR-FL mRNA. Unlike HCCLM3, SNU-475 cells produce an AR species detectable with an AR-v7 specific antibody similar to AR-v7 expressing prostate cancer cells (VCaP and 22Rv1) and transfected HCC controls (Figure 1I). Evaluation with an N-terminal AR targeting antibody showed SNU-475 to exclusively express ARv7 protein (Figure 1J). To better understand the origins of AR-v7 expression in the absence of AR-FL message or protein in this model we performed WGS on SNU-475 cells (Supplementary Table 3). This experiment revealed a large 46 kb deletion in the AR locus, consistent with loss of C-terminal AR exons but retention of the N-terminal and cryptic exons required for AR-v7 (Figure 1K). In total, these findings confirm the expression of C-terminal truncated AR-SVs in a subset of HCC tumors.
Androgen Receptor Cellular Localization in HCC
In the absence of endogenous androgen (i.e., testosterone, 5α-dihdyrotesterone), the AR’s location is predominantly cytoplasmic. Upon ligand binding, the AR translocates to the nucleus, interacts with androgen-responsive DNA elements, and recruits the transcriptional apparatus resulting in AR-target gene modulation (7). Breaking from canonical AR action, C-terminal truncated AR splice variants are found predominately in the nucleus, even in the absence of ligand (20). To determine AR subcellular localization and it’s trafficking in response to ligand, we compared nuclear and cytoplasmic AR abundance in SNU-423 and HCCLM3 HCC cell lines using western blot (WB) and immunofluorescence. Cell fractionation showed that under hormone-depleted conditions, AR expression in SNU-423 was mainly cytoplasmic but became nuclear upon presentation with a potent androgen agonist (R1881) whereas in hormone depleted conditions HCCLM3 AR was predominately nuclear (Figure 2A).
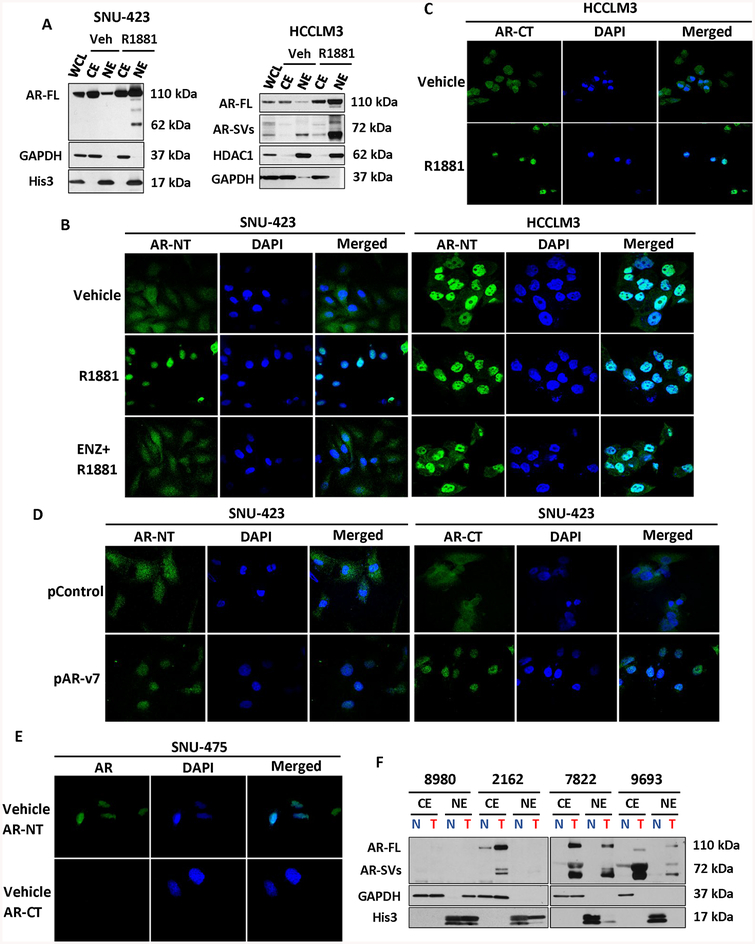
Figure 2. Androgen receptor splice variants are predominately nuclear and ligand-independent.
(a) Western blot of AR expression in SNU-423 (left) and HCCLM3 (right) cytoplasmic or nuclear fractions using an N-terminal targeting AR anti-body. Whole cell lysate (WCL), cytoplasmic extract (CE) and nuclear extract (NE) fractions were assayed after vehicle or 1 nM R1881 treatment for 24hours. In vehicle treated SNU-423 cells, the AR is mainly cytoplasmic but becomes predominantly nuclear following treatment with R1881. In contrast with SNU-423 cells, nuclear localized AR-SVs can be detected in untreated HCCLM3 cells. Following treatment with R1881, the expression of all nuclear localized AR species increases. GAPDH and Histone 3 or HDAC1 serve as cytoplasmic and nuclear controls, respectively. (b) Immunofluorescence analysis of AR in HCCLM3 performed using an N-terminal AR antibody (AR-NT, green) with DAPI nuclear counter stain visualized by confocal microscopy (60×). SNU-423 (left) were treated with either vehicle, 1 nM R1881, or androgen antagonist 10 μM enzalutamide (ENZ) with 1 nM R1881 for 24 hours. Matching nuclear fractionation in Figure 2A, AR stain was primarily diffuse and cytoplasmic in untreated SNU-423 but became nuclear following treatment with R1881. HCCLM3 (right) were similarly treated but revealed intense nuclear staining in the absence of androgen. R1881 treatment reduced the minimal cytoplasmic staining that was apparent in untreated cells but co-treatment with ENZ resulted in considerable residual nuclear localized AR. (c) Immunofluorescence analysis of AR in HCCLM3 with a C-terminal AR (AR-CT, green) antibody. In contrast with N-terminal staining in Figure 2B, C-terminal reactive AR is primarily cytoplasmic in the absence of the ligand but becomes nuclear localized when cells were treated with R1881 for 24 hours. (d) Immunofluorescence analysis of SNU-423 cells was performed after transfection of expression vectors encoding AR-v7 (pAR-v7), or plasmid control (pControl). An N-terminal AR monoclonal antibody was used to detect AR localization (green) as in Figure 2. B. Consistent with Figure 2B, AR localization as determined by AR-NT or AR-CT is predominantly cytoplasmic in untreated control plasmid transfected cells. Whereas transfection with pAR-v7, resulted in strong, predominantly nuclear staining with both AR-NT and AR-CT.(e) Immunofluorescence analysis of SNU-475 cells, consistent with Figure 1H–I, AR localization as determined by AR-NT is predominantly nuclear. Whereas AR was undetectable by AR-CT antibody. (f) CE and NE fractions of four representative, primary HCC samples analyzed for AR expression using an N-terminal reactive AR antibody. Tumor (T) AR expression is greater than patient matched, adjacent non-tumor (N) samples and multiple patients demonstrate expression of nuclear localized low molecular weight AR species. For Immunofluorescence experiments, AR localization was analyzed using the Olympus FluoView 4.2 program on Olympus FV 1000 spectral confocal microscope (panels B-D). DAPI staining (blue) indicates nuclei. All experiments were carried out in triplicate with representative fields presented.
Consistent with WB results, immunofluorescence microscopy showed that AR staining was primarily cytoplasmic in untreated, hormone-depleted SNU-423 cells. Likewise, following stimulation with a potent androgen agonist, a strong fluorescence staining of AR protein occurred in the nuclei (Figure 2B). In contrast, in hormone-depleted untreated HCCLM3 cells, AR protein staining was predominately nuclear irrespective of androgen treatment. Notably, treatment with the antiandrogen enzalutamide did not substantially alter AR nuclear localization in HCCLM3 cells (Figure 2B). Subcellular location of HCCLM3 AR closely mimicked what we observed in VCaP, an AR-SV-positive PCa cell line utilized as a positive control (Supplementary Figure 5D). As in our western blots (Figure 1F and Figure 1G), to distinguish between AR-FL and AR-SV subcellular localization, we used a C-terminal targeted AR antibody. In hormone-depleted HCCLM3, C-terminal reactive AR was mainly cytoplasmic, whereas when cells were treated with androgen, the AR became predominately nuclear suggesting some amount of AR in HCCLM3 cells remains androgen responsive (Figure 2C). To confirm the default nuclear localization of C-terminal truncated splice variants in HCC cells, we overexpressed AR-v7 in the AR-positive HCC cell line, SNU-423. As expected, in the absence of ligand, N-terminal reactive AR is predominantly nuclear in AR-v7-overexpressing SNU-423 relative to SNU-423 vector only control cells. (Figure 2D). Notably, C-terminal reactive AR species in SNU-423s were also predominantly nuclear in the presence of exogenous AR-v7. As with other AR-SV-positive HCC cells in the basal state, the AR in SNU-475 was predominantly nuclear and no AR was detectable with a C-terminal targeting reagent providing additional evidence that these cells lack AR-FL (Figure 2E). Consistent with our detection of AR-SV mRNA expression in primary HCCs (Figure 1B) and the predominantly nuclear localization of AR-SVs in HCC cell lines, we detected low MW AR species in limited nuclear fractions prepared from primary HCC tissues as well (Figure 2F). Together, these data support the constitutive activity of AR-SVs detected in primary HCC samples and HCC cells.
Ligand-Dependent and Independent AR Transcriptional Activity in HCC
To evaluate the transcriptional activity of ligand-responsive AR-FL in HCC, we transfected HCC cells with either a non-inducible basal promoter (pGL4.24) or an AR responsive fire-fly luciferase vector (MMTV-LUC). Transfected cells were then treated with vehicle, R1881, or a combination of R1881 and antagonist (enzalutamide) with VCaP and DU145 serving as AR-positive and AR-negative controls, respectively. In cells transfected with the AR responsive vector, there was a significant androgen mediated-, antagonist-reversible, induction in SNU-423 (AR+) and VCaP (AR+) transcriptional signal whereas AR-negative cell lines, DU145 and HepG2, did not respond to androgen. Notably, similar to AR-negative control cell lines, HCCLM3 (AR+) HCC cells did not show significant ligand-inducible transcriptional activity (Figure 3A), despite being readily transfected according to internal transfection controls (Supplementary Figure 5E). Under identical conditions, cells transfected with a non-inducible basal vector did not respond to any treatment (Supplementary Figure 6A).
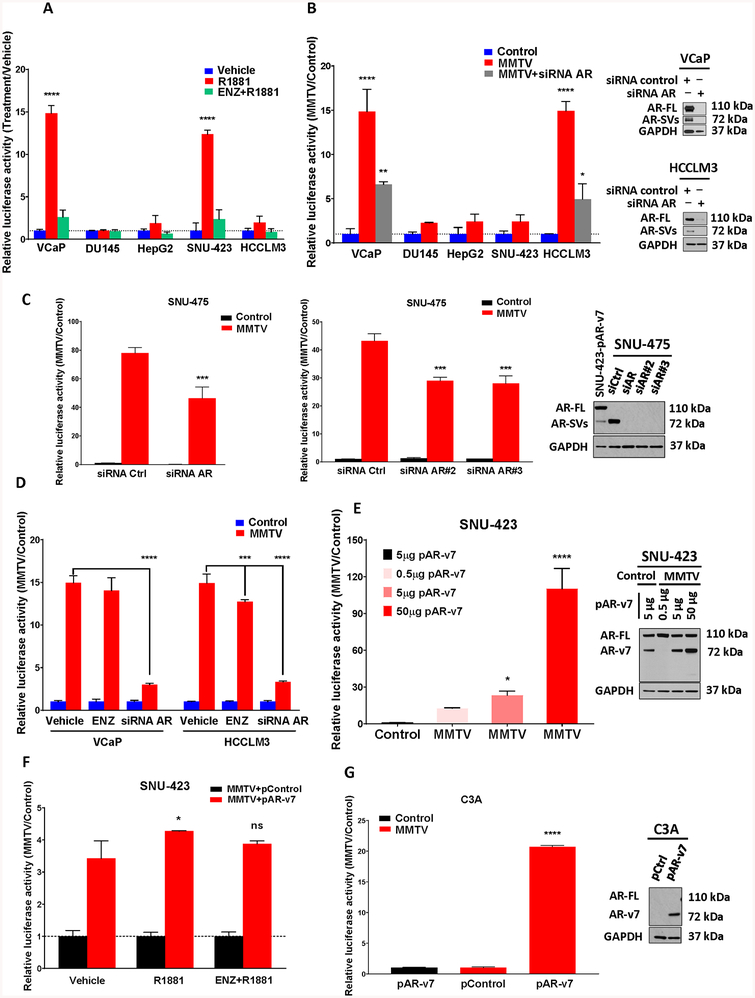
Figure 3. AR-FL and AR-SVs are Transcriptionally Active in HCC Cells.
(a) HCC (HepG2, SNU-423 and HCCLM3) and PCa (VCaP and DU145) cells were transiently transfected with an androgen responsive inducible reporter construct (MMTV-LUC) along with constitutively active renilla luciferase (RN-LUC) transfection control. Cells were maintained for 24 hours in charcoal-stripped FBS containing media (csFBS) then treated with vehicle, 1 nM R1881 or 10 μM enzalutamide (ENZ) with 1 nM R1881 for 24 hours. In SNU-423 and VCaP cells there was significant promoter and androgen-dependent induction of transcriptional activation in R1881-treated cells that was also reversible by co-treatment with ENZ. By contrast, there was no significant activation in HCCLM3, HepG2 and DU145 cells. (b) Comparing basal MMTV-LUC activity to pGL4.24 controls (in the absence of ligand) revealed a constitutive, ligand-independent transcriptional response for VCaP and HCCLM3 cells (left). This activity was significantly reduced by siRNA targeting AR-FL and AR-SV isoforms (AR exons 3 and 7). Successful AR knockdown was confirmed by WB in VCaP and HCCLM3 using N-terminal AR mAb (right). (c) AR-SV expressing HCC cells SNU-475 shows constitutive transcriptional activity similar to HCCLM3 (as determined in Figure 3B). This activity was significantly reduced by 3 different siRNA targeting AR-FL and AR-SV isoforms (left). Successful knock down of AR-v7 in SNU-475 was confirmed by WB with an N-terminal AR mAb (right). (d) Constitutive transcriptional activity in VCaP and HCCLM3 cells (as determined in Figure 3B) was insensitive or only weakly sensitive, respectively, to 24 hour 10 μM ENZ treatment. However, the AR-dependence of the transcriptional signal was demonstrated by knockdown of AR using siRNA targeting AR-FL and AR-SV isoforms (as in Figure 3B, 24 hours). (e) AR expressing SNU-423 HCC cells were transiently transfected with pGL4.24 LUC control or MMTV-LUC and an increasing amount of AR-v7 expressing plasmid (left). Successful overexpression of AR-v7 in SNU-423 was confirmed by WB with an N-terminal AR mAb (right). Exogenous AR-v7 expression in SNU-423 cells demonstrated a concentration dependent ability to increase constitutive MMTV-LUC activation. (f) SNU-423 cells were transiently cotransfected with MMTV-LUC and 10 μg pAR-v7 or empty expression vector control (pcw107) and treated as indicated for 24 hours. Relative to the control construct (pcw107), cells demonstrated increased AR-v7-dependent transcriptional activity (red bars) that was only weakly responsive to treatment with R1881 and insensitive to antagonism with ENZ. (g) C3A cells were transiently cotransfected with pGL4.24 LUC control (black bar) or MMTV-LUC (red bars) and 10 μg pAR-v7 or empty expression vector control (pcw107) for 24 hours. Relative to the control construct (pcw107), cells demonstrated a significant promotor and AR-v7-dependent transcriptional activity. All panels: Dual Luciferase Assay (Promega) with triplicate FF/RN values reported as fold versus vehicle treated control (a, d, f), basal promoter control (b, c, e), or expression vector control (g) as mean+STD. One-way ANOVA with Dunnett’s multiple comparisons test. * p<0.05, ** p<0.01, *** p<0.001, and **** p<0.0001 versus vehicle treated cells (a, d, f), basal promoter transfected cells (b,e) siRNA controls (c) and empty expression vector controls (g), respectively.
To assess potential ligand-independent AR transcriptional activity in the same models, ligand-independent, basal luciferase activity was determined by comparing luciferase signal between vehicle treated pGL4.24 control and MMTV-LUC transfected cells. Consistent with constitutive transcriptional activity, we detected a 15-fold induction in luciferase signal in MMTV-LUC transfected HCCLM3 and VCaP cells relative to corresponding pGL4.24 controls (Figure 3B). This induction was significantly reversed with multiple siRNA targeting the AR N-terminus which resulted in both AR-FL and AR-SV knockdown and suppressed the MMTV-LUC signal supporting AR’s contribution to this ligand-independent transcriptional effect (Figure 3B, inset and Supplementary Figure 6B). By contrast, using the same analysis, the androgen-responsive HCC cell line SNU-423 demonstrated no constitutive transcriptional activity which further mirrored results from AR-negative DU145 and HepG2 cells. Instead, SNU-423’s MMTV-LUC activity was both ligand (Figure 3B) and receptor dependent (Supplementary Figure 6C). As with other AR-SV positive cells, SNU-475 demonstrated strong constitutive transcriptional activity sensitive to AR knock down (Figure 3C).
To further interrogate AR transcriptional activity in HCCLM3 and VCaP cells, we attempted to modulate constitutive transcriptional activity with enzalutamide (ENZ) which is not expected to impact C-terminal truncated AR-SV mediated transcription (Figure 3D). This experiment was performed both alone or in the presence of siRNA targeting all AR species (as in Figure 3C) revealing the relative inability of ENZ treatment to suppress transcription as compared to AR knockdown in HCCLM3 and VCaP cells.
To further demonstrate the ability of AR-SVs to drive constitutive AR activity in HCC cells, we recapitulated a ligand-independent MMTV-LUC signal in AR-SV-negative SNU-423 cells by introducing an AR-v7-expression vector. As in HCCLM3 cells, the presence of AR-SV in SNU-423 cells boosted basal transcriptional activation (Figure 3E) and muted response to R1881 (Figure 3F). To demonstrate that AR-FL is not required for constitutive activation of the MMTV-LUC reporter in HCC cells, the AR(–) HCC cell line HepG2/C3A was similarly transfected with AR-v7 resulting in robust activation (Figure 3G). Given the apparent movement of cytoplasmic AR-FL species in HCCLM3 to the nucleus following treatment with R1881 (Figure 2C), we also tested the ability of R1881 to further increase the already high, constitutive MMTV-LUC signal in these cells (Figure 3B). No additional stimulation was apparent (Supplementary Figure 6D). When considered together, these results demonstrate ligand-independent and ligand-dependent AR-mediated transcription in HCC cells.
Androgen receptor’s biological role in hepatocellular cancer progression
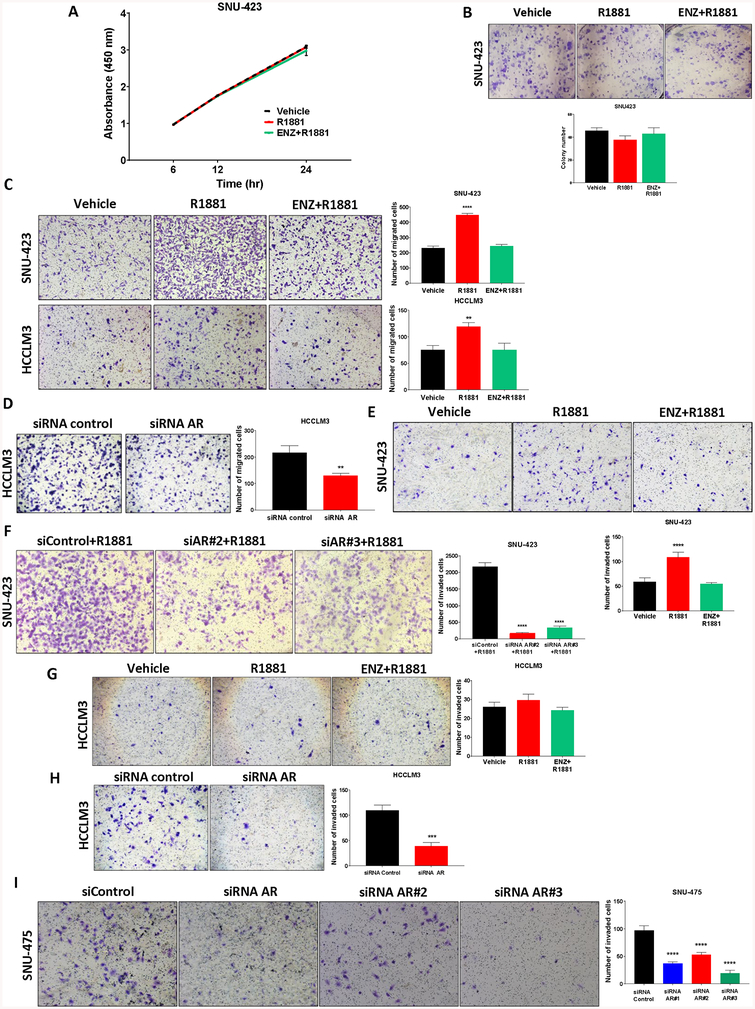
To assess the potential biological contribution of AR signaling in HCC, we performed cell proliferation assays over 24 hours comparing vehicle, R1881 or ENZ and R1881 in androgen responsive SNU-423 cells. Short term androgen treatment did not increase cell proliferation (Figure 4A) or increase colony numbers (Figure 4B). As no effects on proliferation were apparent, we further assessed the potential role of AR in HCC cell migration and invasion given that androgens and the androgen receptor have been reported to promote cell invasion and metastasis in HCC (11). Under similar conditions, R1881 treatment significantly increased SNU-423 and, surprisingly, HCCLM3 cell migration relative to vehicle in an effect that was reversible by co-treatment with ENZ (Figure 4C). To further evaluate the contribution of AR to HCC cell migration, HCCLM3 cells were transfected with either siRNA targeting AR-FL and AR-SV species (as in Figure 3C) or control siRNA. Basal HCCLM3 cell migration was significantly suppressed following AR knockdown consistent with the contribution of constitutively-active AR signaling to HCCLM3 migration (Figure 4D). Next, we investigated the role of AR in invasion in HCC using the Matrigel invasion assay, in which SNU-423 demonstrated androgen-dependent (Figure 4E) and androgen-receptor dependent effects (Figure 4F) but, unlike migration, HCCLM3 showed no difference in invasion when cells were treated with R1881 (Figure 4G). However, matching migration, treatment with siRNA targeting AR-FL and AR-SVs reduced basal HCCLM3 cell invasion (Figure 4H). Likewise, multiple siRNAs knocking down AR-v7 in SNU-475 cells significantly suppressed invasion (Figure 4I). Additionally, overexpression of AR-v7 in AR(–) HepG2/C3A cells resulted in elevated invasion, despite the limited invasive capacity of these epithelial cells (Supplementary Figure 7A). Taken together, these data suggest that both ligand-independent and ligand-dependent AR signaling are capable of modulating HCC cell migration and invasion.
Figure 4. Androgen receptor’s role in hepatocellular carcinoma cell migration and invasion.
(a) The androgen dependence of SNU-423 cell proliferation was assayed using BrdU incorporation following treatment with vehicle, 1 nM R1881 or 10 μM enzalutamide with 1 nM R1881 for the indicated times. There were no significant androgen-dependent effects on SNU-423 cell proliferation. (b) The androgen dependence of SNU-423 cell colony formation was determined following cell seeding in 6-well plates (5% csFBS) and treatment with vehicle, 1nM R1881 or 10 μM enzalutamide with 1nM R1881 for two weeks. Manual determination of colony numbers revealed no significant androgen-dependence determined in SNU-423 colony formation groups. (c) The androgen dependence of SNU-423 and HCCLM3 cell transwell migration was determined following seeding into the upper chamber of transwell inserts in serum and phenol-red free media. Phenol-red free medium containing 5% csFBS was added to the bottom well as chemoattractant along with the indicated treatments. After 48 hours of incubation, cells were fixed and stained with 0.1% crystal violet (left) and migrating cells were quantified manually (right). Both SNU-423 and HCCLM3 cells demonstrated androgen-dependent transwell migration that was reversed by treatment with 10 μM enzalutamide. (d) The androgen receptor dependence of basal HCCLM3 cell migration was determined using siRNA targeting AR-FL and AR-Svs (as in Figure 3B) in phenol-red free medium containing 5% csFBS. 48 hours HCCLM3 cell migration was reduced following AR knockdown. (e) To determine the androgen dependence of SNU-423 cell invasion, the Matrigel invasion assay was performed following resuspension in serum-free phenol-red free medium and seeding into the upper chamber of transwell inserts covered with Matrigel. Phenol-red free medium containing 5% csFBS was added to the bottom well as chemoattractant with treatments as indicated. After 48 hours, cells were fixed and stained with 0.1% crystal violet (top) and the number of invaded cells was quantified manually (bottom) revealing androgen-dependent invasion in SNU-423 cells. (f) The androgen receptor dependence of SNU-423 cell invasion was determined using 2 different siRNA targeting AR-FL and AR-Svs in phenol-red free medium containing 5% csFBS. 48 hour SNU-423 cell invasion was reduced following AR knockdown. (g) The androgen dependence of HCCLM3 cell invasion was determined as in (e). Unlike SNU-423 cells, androgen treatment did not increase HCCLM3 invasion. (h) siRNA knockdown of the AR (as in panel D) resulted in reduced invasion supporting an androgen-receptor dependence to HCCLM3 invasion. (i) siRNA knock down of AR in SNU-475 cells, which only express AR-SVs, resulted in reduced invasion, supporting the androgen-receptor dependence of SNU-475 cell invasion. All panels: data are expressed as the mean ± SD; n=3 for each group. One-way ANOVA with Dunnett’s multiple comparisons test. **** p<0.0001, *** p<0.001, ** p<0.01 and, * p<0.05 versus vehicle treated cells (c, e, g) or control siRNA transfected cells (d, f, h, i).
Androgen receptor modulates EMT via upregulation of slug-encoding gene, SNAI2
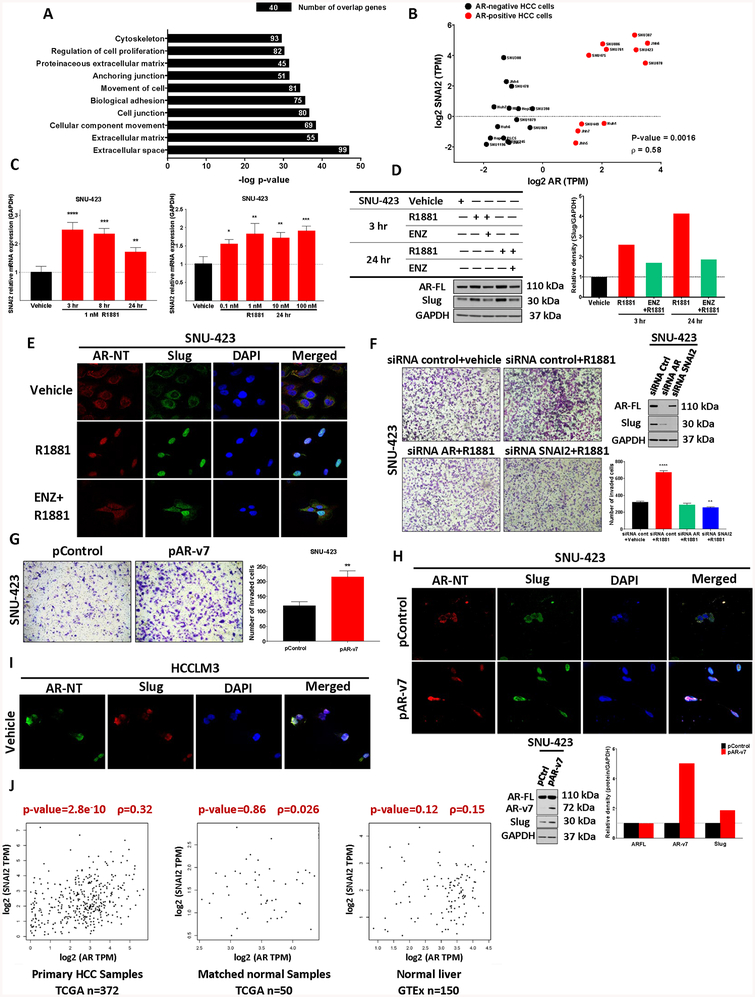
In the context of prostate cancer, the AR has been shown to play a crucial role in cell migration and invasion through modulating expression of epithelial to mesenchymal transition (EMT) effector proteins (21). However, the precise role that AR plays in HCC development remains poorly understood. To further elucidate how AR signaling might contribute to the EMT in HCC, we analyzed whole transcriptome data in 24 HCC cell lines from the CCLE database. Interestingly, multidimensional scaling of entire transcriptomes successfully separated HCC cell lines according to AR-expression (positive or negative, as characterized above in Figure 1C), supporting the biological relevance of AR-dependent transcriptional programs in AR-positive HCC cells (Supplementary Figure 8A). A differential gene expression (DGE) analysis of AR-positive relative to AR-negative HCC cells returned 1058 differentially expressed genes (Supplementary Table 4) which were further evaluated for potential biological function by gene set enrichment analysis (GSEA) using the molecular signature database (MSigDB) (22). GSEA revealed significant enrichment of gene sets involved in the EMT pathway, including extracellular matrix and cell adhesion genes, among HCC AR-positive cells (Figure 5A).
Figure 5. Androgen receptor modulates EMT pathway via upregulation of the EMT effector protein, slug.
(a) We performed differential gene expression (DGE) analysis of 8 AR-positive relative to 14 AR-negative HCC cell lines (as listed in Figure 1C) and obtained 1058 differentially expressed genes. Gene set enrichment analysis (GSEA) on this set of genes using molecular signature database (MSigDB) revealed significant enrichment of the EMT pathway among the top 10 molecular pathways in AR-positive HCC cells. P-value < 0.01 (Fisher exact test). (b) Transcript abundance from CCLE data show a positive correlation (Spearman correlation coefficient) between SNAI2 and AR expression in AR-positive (red) relative to AR-negative (black) cell lines suggesting a putative role for AR:SNAI2(Slug) mediated migration and invasion in HCC. (c) RT-PCR of SNAI2 mRNA in SNU-423 cells treated with 1 nM R1881 for 3, 8 and 24 hours (left) as well as by dose response at 24 hours (right). SNAI2 mRNA demonstrated both time- and concentration-dependent, androgen-dependent regulation. (d) SNU-423 cells were treated with vehicle, 1 nM R1881 or 10 μM enzalutamide with 1 nM R1881 for 3 and 24hours. AR and slug protein were assessed by western blot (left) revealing androgen-dependent slug regulation (densitometry, right). (e) The cellular localization of AR and slug in SNU-423 cells were determined by immunofluorescence in the presence of 1 nM R1881 alone and in combination with 10 μM enzalutamide for 24 hours. AR and slug are cytoplasmic in the absence of androgen, but both became predominantly nuclear upon stimulation with 1 nM R1881 for 24 hours. This androgen-mediated nuclear translocation of slug was inhibited in part upon co-treatment with enzalutamide. (f) Androgen treatment with 1 nM R1881 for 48 hours promoted invasion that was both AR- and SNAI2-dependent as demonstrated by the Matrigel invasion assay (performed and analyzed as described in Figure 4E, quantification bottom right). Both AR and SNAI2 were successfully knocked down using siRNA targeting AR (as in Figure 3B) or SNAI2 (western blot inset, top right). (g) 48 hours Matrigel invasion assays were performed on SNU-423 cells transfected with either 10 μg AR-v7 expressing plasmid (pAR-v7) or control (pcw107, pControl) demonstrating increased invasive capacity for AR-v7 expressing cells.(h) 48 hours post transfection, immunofluorescence analysis of AR-v7 or control transfected cells showed AR (anti-AR mAb targeting N-terminal region of AR, red) and slug (green) were cytoplasmic in the presence of control plasmid. Upon the addition of exogenous, constitutively active AR-v7, both AR and slug staining became predominantly nuclear. Cells were also harvested and analyzed for AR and slug protein content by western blot (inset bottom left) revealing an AR-v7 mediated increase in slug protein (western blot inset, bottom). (i) Immunofluorescence analysis of HCCLM3 cells shows AR and slug co-nuclearization in the absence of androgen stimulation. (j) Correlation analysis of AR and SNAI2 expression in the HCC cohort in TCGA demonstrates a relationship between AR and SNAI2 mRNA levels in liver cancer tissue (TCGA) but not normal tissue (TCGA and GTEx). Spearman correlation analyses showed statistically significant positive correlations between AR and SNAI2 in primary samples (372 patients, left) but no correlation in matched normal samples (50 patients, middle) or normal liver tissues (150 donors, right) (43). One-way ANOVA with Dunnett’s multiple comparisons test. All panels: * p<0.05, ** p<0.01, *** p<0.001, and **** p<0.0001 versus vehicle (c, f), and expression plasmid controls (g)
To identify candidate direct AR-target genes, the promoter regions of significantly enriched genes within the EMT pathway were searched for consensus androgen-responsive elements. Of the 8 EMT genes evaluated, we determined only SNAI2 had a strong putative ARE in its proximal promoter region (Supplementary Figure 8B). Subsequent analyses of CCLE RNA-seq data revealed a moderate positive correlation between SNAI2 and AR expression in AR-positive cell lines as compared to AR-negative cell lines further supporting putative AR:SNAI2 regulatory interactions in AR-dependent HCC migration and invasion (Figure 5B). In agreement with our in silico findings, we determined SNAI2 mRNA levels were transiently induced by androgen treatment in androgen-responsive SNU-423 cells (Figure 5C) and androgen-dependent effects on slug protein levels (the product of SNAI2 mRNA) where more pronounced with nearly 4-fold induction apparent 24 hours after androgen stimulation (Figure 5D). Consistent with consequential slug induction, CDH1 (E-cadherin) mRNA was also responsive to short-term androgen treatment whereas other common EMT regulators were not (Supplementary Figure 8C and Supplementary Figure 8D, respectively). Like AR, slug is a transcription factor that is nuclear localized when activated (23). Congruent with androgen-dependent slug activation, 24 hour androgen treatment in SNU-423 cells results in the re-localization of slug and AR protein to the nucleus in an effect partially reversible by ENZ co-administration (Figure 5E).
The role of AR-dependent SNAI2 regulation in AR-mediated HCC cell invasion was further interrogated by AR or SNAI2 siRNA knockdown in SNU-423 cells. Androgen treatment promoted SNU-423 invasion (as in Figure 4D) which was prevented by AR or SNAI2 knockdown (Figure 5F). Notably, AR knockdown in either SNU-423 (Figure 5F, inset) or HCCLM3 cells (Supplementary Figure 8E) resulted in reduced levels of Slug and the EMT protein ZEB-1 (24). However, whereas AR and SNAI2 knock-down in SNU-423 resulted in an expected rebound in E-cadherin protein levels (Supplementary Figure 8F), E-cadherin levels were not impacted by AR status in HCCLM3 (Supplementary Figure 8E).
To evaluate the potential ability of ligand-independent AR-SV activity to modulate HCC cell invasion, we overexpressed AR-v7 in SNU-423 cells resulting in increased invasion (Figure 5G) and primarily nuclear AR staining (Figure 5H). Exogenous AR-v7 expression in SNU-423 cells also elevated slug protein levels and promoted slug nuclear translocation relative to controls. Constitutively active, nuclear localized AR-SVs in HCCLM3 also co-localized with nuclear slug (Figure 5I). As with other AR-SV-positive HCC cells, siRNA-mediated knockdown of AR-v7 in SNU-475 cells resulted in suppression of slug protein (Supplementary Figure 8G).
To verify the clinical relevance of AR driven SNAI2 mRNA expression we evaluated both TCGA and GTEx liver tissue expression data (25). Similar to the relationship in HCC cell lines, our analyses revealed a statistically significant positive correlation between AR and SNAI2 in primary HCC samples (TCGA, 372 patients). Notably, we failed to detect a similar correlation between AR and SNAI2 in matched normal samples (TCGA, 50 patients) and normal liver tissues (GTEx, 150 donors) suggesting AR-mediated slug activation is liver cancer specific (Figure 5J). Taken together, these data suggest that both ligand-independent and ligand-dependent AR regulation of SNAI2/Slug can modulate HCC cell EMT signaling.
mTOR signaling in AR-SV-positive HCC cells
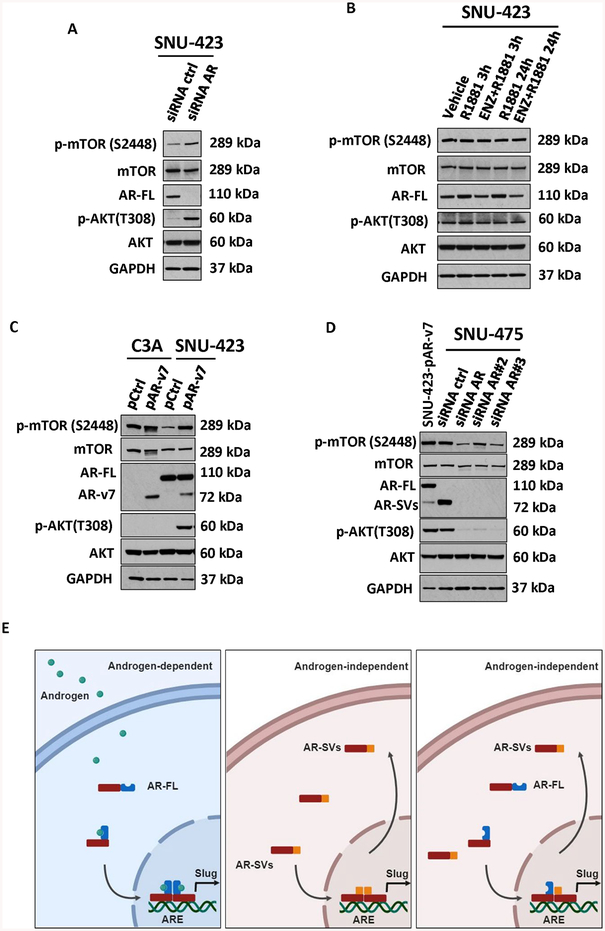
Given the findings of Zhang et. al (9) that demonstrated antagonism of AR in HCC results in activation of the AKT-mTOR pathway we evaluated the role of AR-SV signaling in this cross talk. As shown by Zhang et. al, AR knockdown in SNU-423 cells resulted in activation of both p-AKT and p-mTOR (Figure 6A) but agonist stimulation alone or in combination with ENZ had no effect on either protein (Figure 6B). Interestingly, ectopic expression of AR-v7 resulted in p-AKT and p-mTOR induction in AR-positive SNU-423 but not AR-negative HepG2/C3A cells (Figure 6C). Basal p-AKT and p-mTOR levels were also suppressed by knock-down of AR-v7 in SNU-475 cells (Figure 6D). Taken together, these data suggest that, similar to AR-antagonism with ENZ alone, AR-SV expression in mesenchymal like HCC cells supports AKT-mTOR pathway activation.
Figure 6. Androgen-dependent and Androgen-independent signaling in HCC.
(a) SNU-423 were transfected with siRNA control or siRNA against AR (as in 5F). Relative to siControl cells, siRNA AR-transfected cells demonstrated upregulation of both phosphorylated mTOR and AKT with no change in total mTOR and AKT. (b) AR expressing HCC cells SNU-423 were treated with vehicle, 1 nM R1881 or 10uM enzalutamide with 1 nM R1881 for 3 and 24 hours. Relative to vehicle-treated cells, no change in protein expression of total or phosphorylated mTOR or AKT was apparent following treatment. (c) AR-negative, C3A, and AR-expressing SNU-423 HCC cells were transiently transfected with either 10 μg AR-v7 expressing plasmid (pAR-v7) or control (pcw107, pControl). Relative to pControl, AR-v7-overexpressing cells showed an upregulation of phosphorylated mTOR and AKT with no change in the total levels of mTOR and AKT. AR protein levels in C3A also shown in Figure 3G (d) AR-Sv expressing HCC cells SNU-475 were transfected with control siRNA (siControl) or 3 different siRNA against AR and compared to AR-v7 transfected SNU-423 cells. Relative to siControl, siRNA AR-transfected SNU-475 cells showed a downregulation of both phosphorylated mTOR and AKT with no change in the protein levels of total mTOR and AKT. (e) Graphical depiction of potential AR signaling to modulate EMT in HCC, androgen-dependent AR-FL homodimers (left), androgen-independent AR-Svs homodimers (middle) and androgen-independent AR-FL and AR-Svs heterodimers (right).
Discussion
In an attempt to reconcile the discordant observations of the partial AR-dependency of HCC development (10,26) and the total failure of therapies targeting the AR-axis in HCC patients (12,13) we thoroughly evaluated the AR-locus using publicly available primary HCC and HCC cell line data. We found no widespread genomic mutations or amplifications capable of explaining therapeutic resistance (Supplementary Figure 1) but, we uncovered robust expression of AR-SV’s which could serve as potential mediators of persistent AR-signaling in the presence of AR-targeted therapy (Figure 1A). We confirmed AR-SV expression in two cohorts of primary HCCs (Figure 1B and Supplementary Figure 4) and HCC cell lines (Figures 1D and 1H), and further demonstrated that C-terminal truncated AR mRNA species are translated into C-terminal truncated proteins (Figure 1F–G) including AR-v7 (Figure 1I–J) in HCC. Using multiple lines of evidence, we demonstrated AR-SV function in HCC cells is consistent with treatment refractory, constitutive activity including default nuclear localization (Figure 2A–B and E), ligand independent transcriptional activation (Figure 3B–C), and relative insensitivity to anti-androgen treatment (Figure 2B, Figure 3D and Figure 3F). To our knowledge, we are the first to report a detailed characterization of AR-SVs in HCC.
Depending on the experimental context, C-terminal truncated AR-isoforms are indistinguishable from AR-FL. This creates the potential for AR-SVs to have remained effectively “hidden” in previous studies of AR-signaling in HCC and likely contributes to the persistent controversy surrounding the role of the AR in HCC (27,28). Similar to AR-SVs studied in prostate cancer (29), we found multiple low MW AR species that reside in the nucleus (Figure 2A–B, E–F). Nevertheless, our findings are consistent with Zhang et al.(9) who reported the overexpression of nuclear AR in HCC patients and associated nuclear AR expression with tumor progression and poor prognosis. Zhang et al.’s (9) immunohistochemistry was performed with the same N-terminal targeted reagent we utilized and is therefore incapable of distinguishing nuclear C-terminal truncated AR-SVs from AR-FL in this large HCC cohort. Intriguingly, ENZ treatment in prostate cancer cells is associated with both increased AR-FL and AR-SV expression (18). In this sense, unrecognized AR-SV signaling in Zhang et al’s work may contribute to the reported feedback activation of AKT-mTOR signaling and associated resistance to anti-androgen treatment. Irrespective of ENZ treatment, we found both endogenously expressed AR-v7 (Figure 6D) and ectopically expressed AR-v7 (Figure 6C) to activate AKT-mTOR signaling in HCC cells. Our data support further study of how AR-SVs interact with the AKT-mTOR pathway.
AR-SV expression notwithstanding, there exists considerable disagreement in recent literature concerning the expression of AR in common HCC cell models (9,11,30,31). Our survey of AR expression in HCC cells (Figures 1C, 1D and 1H) is in strong agreement with Ao et al. (11) and Zhang et al. (9) but differs from the AR expression in Huh7 and PLC/PRF/5 cells studied by both Feng et al. (31) and Yu et al. (30). In further agreement with Ao et al.(11), we found AR-positive HCC cell migration and invasion to be AR dependent (Figure 4D, 4F, 4H, 4I and 5F, respectively). However, differing from Zhang et al (9), we were unable to show predominantly nuclear AR in the absence of androgens in SNU-423 cells (Figures 2B and 5E) or clear androgen dependent HCC cell proliferation (Figure 4A). In each case, our disparate results may be due to our pre-incubation of cells in hormone depleted, charcoal stripped FBS to minimize basal hormone signaling prior to visualization or treatment. One notable finding from our characterization of AR expression in HCC cells was the discovery that SNU-475 cells express only AR-v7 with no detectable AR-FL expression (Figures 1H–J). WGS revealed a large genomic deletion in the AR locus in these cells that we speculate causes exclusive use of the previously described alternative poly-A site within intron 3 to make AR-v7 (32). To our knowledge, this deletion is a novel source of AR-v7 and the first description of a human cancer cell line expressing solely constitutively active variant androgen receptor protein. These features may make SNU-475 cells uniquely useful in the study of AR-SV signaling.
Building on these in vitro observations, our in silico informatics approach revealed a strong EMT transcriptional signature in AR-positive versus AR-negative HCC cell lines (Figure 5A–B) and identified SNAI2 as a putative novel direct AR target in HCC (Figure 5C–F). SNU423 cell invasion was both AR and SNAI2 dependent (Figure 5F) and androgen stimulation of SNU-423 cells resulted in both increased Slug expression (Figure 5D) and nuclear localization (Figure 5E). Furthermore, in both HCCLM3 and SNU-475 AR-SV-positive cells, AR knock-down was associated with reduced invasion (Figure 4H–I) and Slug protein expression (Supplementary Figures 8E, G). This pattern of Slug regulation is similar to reports in prostate cancer where AR:Slug interactions have been shown to promote the androgen-independent growth of castration resistant disease (23). The positive relationship between AR and SNAI2 expression was substantiated in primary HCCs (Figure 5J) consistent with the previously reported highly invasive character of AR-positive HCC (9,33). Following resection, Nagasue et al. reported the 5-year survival of recurrence-free HCC patients to be 55% and 0% for AR-negative and AR-positive HCC, respectively. The identification of the androgen receptor as a therapeutically targetable driver of EMT in HCC has potentially broad implications for AR-targeted therapy in the adjuvant setting where systemic therapy has failed to demonstrate clinical benefit (34).
Clinically relevant AR-SV expression in prostate cancer is thought to occur in the context of androgen ablative and/or anti-androgen therapy (35,36). Though AR-SVs role as drivers of therapeutic resistance in prostate cancer remains unresolved (17), multiple preclinical studies demonstrate AR-SV-mediated resistance to anti-androgen treatment (37,38) and clinical studies associate AR-SV expression with resistance to hormone therapy (39,40). The activation of AR-SV signaling in response to selective pressures of AR-targeted therapy is a plausible adaptive biological response by hormone addicted prostate cancer cells but raises the critical question of what underlies abundant AR-SV expression in primary HCC (Figure 1A–B and Figure 2F). Unlike prostate cancer, hormone ablative and anti-androgen therapy are not utilized in HCC treatment suggesting AR-SVs in HCC are of disparate origins. One potential explanation are the high rates of hypogonadism, approaching 90% in men, associated in advanced liver disease (41). This well-known phenomenon suggests that “castrate” liver conditions could routinely accompany worsening liver disease, at least in men, and provide similar selective pressures as prostate cancer therapy in promoting AR-SV expression. One limitation of our study is that we have not yet confirmed the sequence of the predominant low MW AR species in HCCLM3 or primary HCC samples. These data as well as future studies identifying drivers of AR-SV expression are key to better understanding the role of AR-SV expression in both diseased and normal liver.
We demonstrate AR-SV expression in primary cells that exceeds even our most abundant expressing HCC cell line (Figure 1E) but it is still an open question as to what may constitute clinically relevant amounts of AR-SV expression. As has been generally shown in PCa (17), AR-SV protein expression was typically accompanied by AR-FL (Figure 1D, F), with SNU-475 being the notable exception (Figure 1H–J). However, we demonstrate that the ligand independent characteristics of AR-SV-positive HCCLM3 and SNU-475 cells can be recapitulated by adding modest amounts of exogenous AR-SVs to AR-SV-negative SNU-423 cells, suggesting the expression of small amounts of AR-SVs may act effectively like a ligand by inducing transcription (Figure 3E), muting response additional AR activation (Figure 3F), and driving residual AR-FL to the nucleus resulting in the engagement of disease relevant AR signaling networks (Figure 5H) including activation of the AKT-mTOR pathway (Figure 6C–D). Our results raise the intriguing possibility that AR-dependent HCC signaling may be readily dissociated from the requirement of circulating androgens. These findings add greatly to the potential complexity of AR-signaling in HCC and support previous claims that targeting the androgen receptor, as opposed to its ligand, may be the most effective approach in HCC (27). We acknowledge that, with the exception of our in silico approaches, we have evaluated a relatively small number of primary HCC samples and expansion to more primary samples is required for a broader interpretation of our findings. Nevertheless, we report several key features of AR-SVs in HCC worthy of further investigation.
Overall, our report is the first to describe the expression, biological relevance, and potential clinical relevance of AR-SVs in HCC. Our findings support the AR’s role in promoting hepatocellular carcinoma migration and invasion via well-characterized EMT effector proteins and implicates AR signaling in HCC progression (Figure 6E). We provide an additional explanation for the failure of traditional approaches to targeting the AR-axis in HCC (i.e. hormone ablation and steroid competitive antagonism) but, importantly, also highlight the potential of effective AR-targeted therapy in AR-positive hepatocellular carcinoma. A growing number of novel therapies designed to target AR-SV-mediated signaling have been described with several currently being evaluated in prostate cancer patients (42). Our data suggest similar targeting of the AR-axis in AR-positive HCC could result in new therapeutic strategies for HCC patients with precious few treatment options.
Supplementary Material
Statement of significance:
Treatment refractory, constitutively active androgen receptor splice variants promote hepatocellular carcinoma progression by regulating the epithelial mesenchymal transition pathway.
Acknowledgments
We thank Campus Microscopy & Imaging Facility for their assistance with immunofluorescence as well as Genomic Shared Resource in The Ohio State University Comprehensive Cancer Center (Supported by the OSU CCC Core Grant, P30 CA016058, PI: Dr. Raphael Pollock). We also thank the Molecular Carcinogenesis and Chemoprevention Program (OSU Comprehensive Cancer Center). We are grateful to Dr. Mitch Phelps for the valuable input and review of the manuscript, and Mohamed Badawi, Apollinaire Ngankeu, Shelley Orwick, Hanna Radomska, and Riley Mullins (The Ohio State University, College of Pharmacy) for technical assistance in preparation of tissue samples for analysis. This work was also supported in part by NCI/NIH K12-CA133250-07 (Dr. Coss), American Foundation for Pharmaceutical Education Pre-Doctoral Fellowship (A. Dauki), Pelotonia Idea Award (OSU Comprehensive Cancer Center, Dr. Coss), and the National Institute of General Medical Sciences of the National Institutes of Health under award number 5T32GM068412-12 (E. Kautto). The results shown here are in part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/. We acknowledge our use of the gene set enrichment analysis, GSEA software, and Molecular Signature Database (MSigDB). We would also like to express our sincere gratitude to the participants in TCGA and GTEx for donating cancer specimens and tissues. We also thank the reviewers for their insights and helpful suggestions which resulted in an improved manuscript.
Financial support:
This work was funded in part by the American Cancer Society Grant IRG-67-003-50 (Coss), National Cancer Institute 2 K12CA133250-07 (Coss), Sustainable Sciences Institute (Abdel-Rahman and Ezzat) and The American Foundation of Pharmaceutical Education (AFPE), Pre-doctoral Award in Pharmaceutical Sciences (Dauki).
List of Abbreviations:
- HCC
hepatocellular carcinoma
- AR
androgen receptor
- AR-SVs
androgen receptor splice variants
- CE
cryptic exon
- ARE
androgen response elements
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- NASH
non-alcoholic steatohepatitis
- ENZ
enzalutamide
- NTC
negative template control
- PCa
prostate cancer
- ATCC
American type culture collection
- csFBS
charcoal-stripped fetal bovine serum
- MMTV
mouse mammary tumor virus
- DGE
differential gene expression
- GSEA
gene set enrichment analysis
- MSigDB
molecular signature database
- TCGA
the cancer genome atlas
- GTEx
genotype tissue expression
- EMT
epithelial–mesenchymal transition
- IF
immunofluorescence
- siRNA
small interfering RNA
- ANOVA
analysis of variance
- WGS
whole genome sequencing
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References:
- 1.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (London, England) 2018;391:1301–14 [DOI] [PubMed] [Google Scholar]
- 2.Islami F, Miller KD, Siegel RL, Fedewa SA, Ward EM, Jemal A. Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA: a cancer journal for clinicians 2017;67:273–89 [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet (London, England) 2018;391:1163–73 [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nature reviews Clinical oncology 2018;15:599–616 [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nature reviews Disease primers 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 6.Kanda T, Takahashi K, Nakamura M, Nakamoto S, Wu S, Haga Y, et al. Androgen Receptor Could Be a Potential Therapeutic Target in Patients with Advanced Hepatocellular Carcinoma. Cancers 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelmann EP. Molecular biology of the androgen receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2002;20:3001–15 [DOI] [PubMed] [Google Scholar]
- 8.Barone M, Margiotta M, Scavo MP, Gentile A, Francioso D, Papagni S, et al. Possible involvement of androgen receptor alterations in hepatocarcinogenesis. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 2009;41:665–70 [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Li XX, Yang Y, Zhang Y, Wang HY, Zheng XFS. Significance and mechanism of androgen receptor overexpression and androgen receptor/mechanistic target of rapamycin cross-talk in hepatocellular carcinoma. Hepatology (Baltimore, Md) 2018;67:2271–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, Lai JJ, et al. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology 2008;135:947–55, 55.e1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ao J, Meng J, Zhu L, Nie H, Yang C, Li J, et al. Activation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasion. Mol Oncol 2012;6:507–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimaldi C, Bleiberg H, Gay F, Messner M, Rougier P, Kok TC, et al. Evaluation of antiandrogen therapy in unresectable hepatocellular carcinoma: results of a European Organization for Research and Treatment of Cancer multicentric double-blind trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1998;16:411–7 [DOI] [PubMed] [Google Scholar]
- 13.Randomized trial of leuprorelin and flutamide in male patients with hepatocellular carcinoma treated with tamoxifen. Hepatology (Baltimore, Md) 2004;40:1361–9 [DOI] [PubMed] [Google Scholar]
- 14.Armstrong CM, Gao AC. Adaptive pathways and emerging strategies overcoming treatment resistance in castration resistant prostate cancer. Asian journal of urology 2016;3:185–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farooqi AA, Sarkar FH. Overview on the complexity of androgen receptor-targeted therapy for prostate cancer. Cancer cell international 2015;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo J, Attard G, Balk SP, Bevan C, Burnstein K, Cato L, et al. Role of Androgen Receptor Variants in Prostate Cancer: Report from the 2017 Mission Androgen Receptor Variants Meeting. European urology 2018;73:715–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu LL, Xie N, Sun S, Plymate S, Mostaghel E, Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene 2014;33:3140–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012;483:603–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan SC, Li Y, Dehm SM. Androgen receptor splice variants activate androgen receptor target genes and support aberrant prostate cancer cell growth independent of canonical androgen receptor nuclear localization signal. The Journal of biological chemistry 2012;287:19736–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CY, Jan YJ, Kuo LK, Wang BJ, Huo C, Jiang SS, et al. Elevation of androgen receptor promotes prostate cancer metastasis by induction of epithelial-mesenchymal transition and reduction of KAT5. Cancer science 2018;109:3564–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America 2005;102:15545–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K, Gore C, Yang L, Fazli L, Gleave M, Pong RC, et al. Slug, a unique androgen-regulated transcription factor, coordinates androgen receptor to facilitate castration resistance in prostate cancer. Molecular endocrinology (Baltimore, Md) 2012;26:1496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wels C, Joshi S, Koefinger P, Bergler H, Schaider H. Transcriptional activation of ZEB1 by Slug leads to cooperative regulation of the epithelial-mesenchymal transition-like phenotype in melanoma. J Invest Dermatol 2011;131:1877–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA, et al. A Novel Approach to High-Quality Postmortem Tissue Procurement: The GTEx Project. Biopreservation and biobanking 2015;13:311–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang SH, Yeh SH, Shiau CW, Chen KF, Lin WH, Tsai TF, et al. Sorafenib Action in Hepatitis B Virus X-Activated Oncogenic Androgen Pathway in Liver through SHP-1. J Natl Cancer Inst 2015;107. [DOI] [PubMed] [Google Scholar]
- 27.Ma WL, Lai HC, Yeh S, Cai X, Chang C. Androgen receptor roles in hepatocellular carcinoma, fatty liver, cirrhosis and hepatitis. Endocrine-related cancer 2014;21:R165–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalra M, Mayes J, Assefa S, Kaul AK, Kaul R. Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World journal of gastroenterology 2008;14:5945–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhan Y, Zhang G, Wang X, Qi Y, Bai S, Li D, et al. Interplay between Cytoplasmic and Nuclear Androgen Receptor Splice Variants Mediates Castration Resistance. Molecular cancer research : MCR 2017;15:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Z, Gao YQ, Feng H, Lee YY, Li MS, Tian Y, et al. Cell cycle-related kinase mediates viral-host signalling to promote hepatitis B virus-associated hepatocarcinogenesis. Gut 2014;63:1793–804 [DOI] [PubMed] [Google Scholar]
- 31.Feng H, Yu Z, Tian Y, Lee YY, Li MS, Go MY, et al. A CCRK-EZH2 epigenetic circuitry drives hepatocarcinogenesis and associates with tumor recurrence and poor survival of patients. J Hepatol 2015;62:1100–11 [DOI] [PubMed] [Google Scholar]
- 32.Van Etten JL, Nyquist M, Li Y, Yang R, Ho Y, Johnson R, et al. Targeting a Single Alternative Polyadenylation Site Coordinately Blocks Expression of Androgen Receptor mRNA Splice Variants in Prostate Cancer. Cancer research 2017;77:5228–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagasue N, Chang YC, Hayashi T, Galizia G, Kohno H, Nakamura T, et al. Androgen receptor in hepatocellular carcinoma as a prognostic factor after hepatic resection. Annals of surgery 1989;209:424–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Z, Liang W, Wang D, Schroder PM, Ju W, Wu L, et al. Adjuvant chemotherapy for patients with primary hepatocellular carcinoma: a meta-analysis. International journal of cancer 2015;136:E751–9 [DOI] [PubMed] [Google Scholar]
- 35.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. The New England journal of medicine 2014;371:1028–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu Y, Dai B, Ye D, Kong Y, Chang K, Jia Z, et al. Constitutively active AR-V7 plays an essential role in the development and progression of castration-resistant prostate cancer. Scientific reports 2015;5:7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer research 2013;73:483–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nyquist MD, Li Y, Hwang TH, Manlove LS, Vessella RL, Silverstein KA, et al. TALEN-engineered AR gene rearrangements reveal endocrine uncoupling of androgen receptor in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America 2013;110:17492–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA oncology 2016;2:1441–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scher HI, Graf RP, Schreiber NA, McLaughlin B, Lu D, Louw J, et al. Nuclear-specific AR-V7 Protein Localization is Necessary to Guide Treatment Selection in Metastatic Castration-resistant Prostate Cancer. European urology 2017;71:874–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grossmann M, Hoermann R, Gani L, Chan I, Cheung A, Gow PJ, et al. Low testosterone levels as an independent predictor of mortality in men with chronic liver disease. Clinical endocrinology 2012;77:323–8 [DOI] [PubMed] [Google Scholar]
- 42.Imamura Y, Sadar MD. Androgen receptor targeted therapies in castration-resistant prostate cancer: Bench to clinic. International journal of urology : official journal of the Japanese Urological Association 2016;23:654–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Z, Li C, Kang B, Gao G, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic acids research 2017;45:W98–w102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.