Abstract
We have demonstrated that small, modular, tetrameric peptides featuring a Lewis-basic residue, β-dimethylaminoalanine (Dmaa), are capable of atroposelectively coupling naphthols and ester-bearing quinones, yielding non-C2-symmetric BINOL-type scaffolds with good yields and enantioselectivities. The study culminates in the asymmetric synthesis of backbone-substituted scaffolds similar to 3,3'-disubstituted BINOLs, such as (R)-TRIP, with good (94:6 e.r.) to excellent (>99.9:0.1 e.r.) enantioselectivity after recrystallization, and a diastereoselective net arylation of a minimally modified, nonsteroidal anti-inflammatory drug (NSAID), naproxen.
Keywords: Atropisomerism, Biaryl synthesis, Chirality, Peptide catalysis, Quinones
Graphical Abstract

Peptide-catalyzed atroposelective naphthol-quinone couplings: A peptide-based catalytic approach for atroposelective couplings of naphthols and ester-bearing quinones to access non-C2-symmetric BINOL-type scaffolds is presented, achieving good yields and enantioselectivities. A diastereoselective functionalization of a naproxen analog highlights the utility of this system.
Approaches for the synthesis of enantioenriched biaryl atropisomers are often divided into several categories.[1] Perhaps the most well-developed method is direct atroposelective coupling of two arene units.[2] A second method involves a preformed, but stereochemically ambiguous, axis that is kinetically resolved, as pioneered by Bringmann,[3] and for which a number of catalytic instances have now been demonstrated,[4] including several peptide-catalyzed atroposelective bromination reactions.[5] Indeed, a now-burgeoning series of chiral axis-forming bond constructions exploit a range of chiral catalysis approaches.[6] Atroposelective biaryl construction via coupling of one arene to a second partner, which then aromatizes to reveal a newly-formed axis of chirality is also a contemporary approach.[7] Recently, our group has contributed to this category with a peptide-catalyzed cyclodehydration, transforming a pre-existing C─N bond into an axis of chirality.[8] Also within this third category is the innovative work of Tan and co-workers who demonstrated that BINOL-derived chiral phosphoric acids (BINOL-CPAs), such as (R)-TRIP, can catalyze the addition of naphthols to quinones, affording enantioenriched non-C2-symmetric biaryls in good yields and selectivities (Scheme 1A).[9] Complementary to this approach is the excellent work by Salvio and Bella who showed that cinchona alkaloids were also effective in atroposelectively coupling naphthols to halogenated quinones (Scheme 1B).[10] Inspired by these studies, and realizing the potential for site- and diastereo-selective arylation of phenol-containing natural products, we wondered if we could effect a similar transformation utilizing a tetrameric peptide featuring a Lewis-basic catalytic residue as shown in Scheme 1 (bottom). Herein, we present our work on the first peptide-catalyzed,[11] atroposelective carbon-carbon bond-forming reaction between two independent fragments: an ester-bearing quinone and a naphthol, yielding non-C2-symmetric BINOL-type scaffolds.[12]
Scheme 1.

Approaches toward construction of enantioenriched atropisomers via naphthol-quinone coupling.
Notably, related to the pioneering work of Tan, and Salvio and Bella,[9-10] few other examples exist for the organocatalyzed construction of non-C2-symmetric BINOL-type catalysts or ligands with substituents decorating the backbone, a feature that often imparts high enantioselectivity.[13] Important examples include the 3,3ʹ-disubstituted BINOL carboxylic acids developed by Terada and co-workers for hetero-Diels–Alder reactions,[14] H8-BINOL-CPAs developed by Krische and co-workers for the enantioselective C–H crotylation of alcohols,[15] and approaches preparing racemic NOBIN- and BINOL-type biaryls by Gao et. al. and Kamitanaka et. al. using quinone monoacetals as coupling partners.[16]
As we will detail below, these important examples are now complemented by the chemistry presented herein, including the backbone-substituted adducts shown in Scheme 2: we successfully synthesized a highly enantioenriched non-C2-symmetric 3-bromo-substituted biaryl (>99.5% ee post-recrystallization, Scheme 3A) and a 3,3ʹ-dibromo-substituted biaryl with good e.r., which affords the opportunity for their use as ligands or for further derivatization. The demonstration that peptide-based catalysts may chaperone highly atroposelective coupling of fragments represents a significant advance for this emerging class of catalysts, with ramifications for complex molecule diversification.
Scheme 2.

Showcase examples of BINOL-type brominated biaryls synthesized via peptide-catalyzed quinone arylation.
Scheme 3.
Substrate Scope.[a] [a] Isolated yield; e.r. determined by CSP-HPLC (254 nm, uncorrected). Average of two trials. Absolute configuration shown as (S) and assigned in analogy to the data reported by Tan and co-workers[9] (see Section 6.5 in Supporting Information for details). A. Variations in quinone coupling partner. B. Variations in naphthol coupling partner. Numbering is shown on the naphthol partner for convenience. C. Variations in both coupling partners. Additional coupling partners are boxed. PMB = p-methoxybenzyl.
Our investigation commenced with interrogation of a peptide sequence best suited for inducing enantioselectivity in the model reaction shown in Table 1. We believe that the amine on β-dimethylaminoalanine (Dmaa), the catalytic residue, interacts initially with the naphthol –OH group to promote a close interaction between the substrate and catalyst. While the i+1 residue is generally a proline of either stereochemistry to encourage the formation of a β-turn via the indicated cross-strand hydrogen bonds,[17] amino acids at i+2 and i+3 are often quite diverse. Positing that an aromatic residue at i+3 would enforce potential π-π interactions between catalyst and substrate, we initially evaluated catalysts with a phenylalanine at this position and chose to vary residues at i+2 (Table 1, entries 1–5). Our group previously demonstrated in several enantioselective bromination reactions that this position is particularly important in inducing enantioselectivity,[11e] however in this reaction it proved not to be influential. Replacing phenylalanine with non-aromatic leucine at i+3 and exchanging d-proline for l-proline at i+1 resulted in significantly lower enantioselectivity (Table 1, entry 6); reinstalling the d-proline residue in catalyst P7 did not rescue the enantioselectivity (Table 1, entry 7). We suspected that a tertiary amide at the C-terminus (Table 1, entries 8–9) might enhance the donor capabilities of the i+3 carbonyl, thus increasing the strength of the cross-strand hydrogen bond and affording a more rigid solution-phase conformation. Using identical conditions from Table 1, entry 8, but with an additional phenylalanine at i+2 (P9) resulted in higher yield, but only a marginal decrease in enantioselectivity (Table 1, entry 9). Considering our hypothesis regarding π-π interactions, we incorporated naphthylalanine (Nal) (Table 1, entries 10–12) and O-benzyltyrosine (Table 1, entry 13) at i+3. Retaining the C-terminal dimethylamide (Scheme 4, entries 10–13), we found that P10 provided the highest enantioselectivity observed thus far with 92:8 e.r. (Table 1, entry 10). Using 2Nal at i+3, we again probed the effect that substituents at the C-terminus have on selectivity while varying i+2 between aminoisobutyric acid (Aib) and aminocyclopropane carboxylic acid (Acpc) (Table 1, entries 14–19). Catalyst P16 with a C-terminal methyl ester afforded product in similar enantioenrichment as the dimethylamide (P10) and diethylamide (P13) analogs, but with consistently higher yield over three trials and three hour reaction time. Further attempts to increase the enantioselectivity by incorporating α-methyl valine at i+2 (P19) failed, as did appending substituents at the 4-position of the proline (P20 and P21). Catalyst P22 displays a l-proline/d-valine-induced β-turn and a chiral C-terminal substituent which reversed the enantioselectivity (Table 1, entry 23). Interestingly, this reversal is not observed using catalysts P6 and P21 where l-proline is positioned at i+2 (Table 1, entries 6 and 22).
Table 1.
Dmaa[a] Catalyst Screen.
 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Cat | i+1 | i+2 | i+3 – PG | Yield (%)[b] | e.r. | Entry | Cat | i+1 | i+2 | i+3 – PG | Yield (%)[b] |
e.r. |
| 1 | P1 | d-Pro | Aib | Phe-OMe | 47 | 88:12 | 13[c] | P12 | d-Pro | Aib | Tyr(Bn)-NMe2 | 53 | 88:12 |
| 2 | P2 | d-Pro | Acpc | Phe-OMe | 51 | 89:11 | 14[e] | P13 | d-Pro | Aib | 2Nal-NEt2 | 62 | 91:9 |
| 3 | P3 | d-Pro | Gly | Phe-OMe | 48 | 84:16 | 15 | P14 | d-Pro | Acpc | 2Nal-NMe2 | 92 | 83:17 |
| 4 | P4 | d-Pro | Ach | Phe-OMe | 81 | 83:17 | 16 | P15 | d-Pro | Acpc | 2Nal-(R)-αMBA | 70 | 83:17 |
| 5 | P5 | d-Pro | Aic | Phe-OMe | 71 | 82:18 | 17[f] | P16 | d-Pro | Aib | 2Nal-OMe | 76 | 92:8 |
| 6 | P6 | l-Pro | Aib | Leu-OMe | 65 | 63:37 | 18 | P17 | d-Pro | Acpc | 2Nal-NBn2 | 57 | 74:26 |
| 7[c] | P7 | d-Pro | Phe | Leu-OMe | 51 | 65:35 | 19 | P18 | d-Pro | Aib | 2Nal-pyrrolidine | 67 | 85:15 |
| 8[c] | P8 | d-Pro | Aic | Phe-NMe2 | 54 | 88:12 | 20 | P19 | d-Pro | (αMe)-Val | 2Nal-OMe | 76 | 86:14 |
| 9[c] | P9 | d-Pro | Phe | Phe-NMe2 | 99 | 84:16 | 21 | P20 | d-Pro (4S-NHCOPh) | Acpc | 2Nal-NMe2 | 77 | 80:20 |
| 10 | P10 | d-Pro | Aib | 2Nal-NMe2 | 64 | 92:8 | 22 | P21 | Pro (4S-NHCOCyMe) | Aib | 2Nal-OMe | 99 | 63:37 |
| 11[d] | P10 | d-Pro | Aib | 2Nal-NMe2 | 55 | 91:9 | 23[f] | P22 | l-Pro | d-Val | 2Nal-(R)-αMBA | 80 | 40:60 |
| 12[c] | P11 | d-Pro | Aib | 1Nal-NMe2 | 48 | 88:12 | |||||||
A general β-turn catalyst is shown at top left with peptide nomenclature denoted.
Boc-Dmaa is the ith residue in all catalysts.
Isolated yield of product.
2 hour reaction time.
2 hour reaction time, 20 mol% catalyst.
Yield and e.r. are the average of two trials.
Yield and e.r. are the average of three trials.
Uncommon amino acids are highlighted in red within the table and depicted below.

Scheme 4.
Applications of this method. [a] 1 equiv of quinone and 1 equiv of naphthol were used in the reaction. Reported e.r. and yields are from one trial). [b] X-ray crystal structures verifying the connectivity of products 3s and 3t (crystallographic data gathered on racemic crystals). [c] Average of two trials; d.r. determined by 1H NMR integrations (see Section 7.6 of the Supporting Information).
With optimal catalyst P16 in hand, we addressed the reaction conditions to increase enantioselectivity and yield. Our standard catalyst screening conditions resulted in the highest enantioselectivity observed (Table 2, entry 1) but with moderate yield. Increasing the catalyst loading to 10 mol% increased the yield, but lowered enantioselectivity (Table 2, entry 2). Further increasing the catalyst loading resulted in decreased enantioselectivity without a substantial change in yield (Table 2, entries 2–4). Running the reaction at a lower concentration markedly improved the yield, perhaps by attenuating side reactivity (Table 2, entries 5–6); however, the enantioenrichment was not restored to the initial result in entry 1. Extended reaction time using 5 mol% catalyst over 24 hours afforded product in good yield, but lower selectivity once again (Table 2, entry 7), and lowering the catalyst loading to 2 mol% did not meaningfully alter these results (Table 2, entry 8). Over the course of optimizing the catalyst and the reaction conditions, we were able in some instances to detect by LC-MS the presence of two side products, one originating from oxidation of the desired hydroquinone product (3) to quinone, and the other from intramolecular cyclization, presumably leading to a Bringmann-type lactone (see Supporting Information for details).[1a, 3b] We suspected that perhaps dissolved oxygen in the reaction solvent was responsible for the oxidation and residual acid from dichloromethane was catalyzing the reaction non-selectively or promoting the cyclization. For this reason, we employed anhydrous, oxygen-free dichloromethane that had been filtered through basic alumina prior to use. These efforts, as shown in Table 2, entry 9, did not increase the enantioselectivity, though we were able to isolate product nearly quantitatively after borohydride reduction. In this case, while the formation of lactone was not detected, we still observed the presence of oxidized 3 prior to reductive work up; attempts to isolate this product were unsuccessful. Rather than exogenous oxygen, we propose, in agreement with Salvio and Bella, that in some cases, coupling is followed by rapid oxidation of 3 by 2.[10] To ensure that all coupled material was in the hydroquinone form, we opted to employ a sodium borohydride reduction as a standard procedure in the work-up of all entries in Table 2.
Table 2.
Conditions Optimization.[a]
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Solvent | T [°C] | t [h] | Cat loading[b] |
Yield [%] | e.r. |
| 1 | DCM | −78 | 3 | 5 | 55 | 93:7 |
| 2 | DCM | −78 | 3 | 10 | 74 | 91:9 |
| 3 | DCM | −78 | 3 | 20 | 64 | 91:9 |
| 4 | DCM | −78 | 3 | 30 | 71 | 89:11 |
| 5[c] | DCM | −78 | 3 | 30 | 99 | 88:12 |
| 6[c] | DCM | −78 | 3 | 5 | 99 | 89:11 |
| 7 | DCM | −78 | 24 | 5 | 89 | 90:10 |
| 8 | DCM | −78 | 24 | 2 | 92 | 90:10 |
| 9[d] | DCM (dry) | −78 | 24 | 5 | 99 | 88:12 |
| 10[d] | CHCl3 | −60 | 3 | 5 | 99 | 74:26 |
| 11 | DCE | rt | 3 | 5 | 52 | 50:50 |
| 12 | DCE | −10 | 24 | 5 | 51 | 69:31 |
| 13 | PhMe (dry) | −78 | 24 | 5 | 49 | 71:29 |
| 14 | CCl4 | −10 | 24 | 5 | 52 | 61:39 |
| 15 | Et2O | −78 | 24 | 5 | 45 | 76:24 |
| 16 | EtCN | −78 | 16 | 5 | 51 | 93:7 |
| 17 | MeOH | −78 | 16 | 5 | 8 | 58:42 |
| 18 | DCM | −78 | 18 | 5 | 99 | 89:11 |
Isolated yield. Employed 1 equiv of naphthol and 1 equiv of quinone in DCM (0.06 M in naphthol). NaBH4 (5 equiv) used in work-up.
Catalyst (P16) loading in mol%.
DCM (0.02 M in naphthol).
Solvent filtered through basic alumina prior to use.
While evaluating other chlorinated solvents, we observed that lower temperatures favored higher enantioselectivity (Table 2 entries 10–12). Toluene provided lower yield and enantioselectivity (Table 2, entry 13), lending evidence to productive π-π substrate-catalyst interactions. Similarly, carbon tetrachloride and diethyl ether both furnished the product in lower e.r. and yield (Table 2, entries 14 and 15); however, it should be noted that the solubility of 1a in these solvents was poor. Despite presenting the opportunity to competitively hydrogen bond with the catalyst, propionitrile afforded desired product in good e.r. albeit with low yield (Table 2, entry 16). With H-bonding donor capabilities, methanol as a solvent resulted in severe depletion of enantioselectivity and yield (Table 2, entry 17). We ultimately chose to pursue the conditions in Table 2, entry 18, which allows the use of more concentrated reaction conditions, no special treatment of the solvent, and slightly reduced reaction time.
Intrigued by the lower selectivity observed when running the reaction in toluene (Table 2, entry 13) we wondered if appending an extended aromatic system to the quinone substrate would enhance catalyst-substrate recognition (Scheme 3A, 2b). Indeed, we were able to increase the selectivity slightly, relative to our standard benzylester-p-quinone coupling partner, 2a. Investigating a potential correlation between enantioselectivity and electronics on the phenyl group of the quinone, we synthesized more electron rich quinone 2c and electron deficient quinone 2d. Both substrates gave similar e.r. values, and the trifluoromethyl group afforded the highest e.r. to date at 93:7 e.r (Scheme 3A, 3d). Unfortunately, quinone 2c was a poor coupling partner in the reaction, and we isolated significant quantities of starting naphthol and hydroquinone after work-up. The reaction is also tolerant of aromatic heterocycles on the quinone coupling partner (Scheme 3A, 2e), affording product 3e in good e.r.. In addition, both selectivity and yield remained high when assessing alkoxycarbonyl quinones (Scheme 3A, 2f–h).
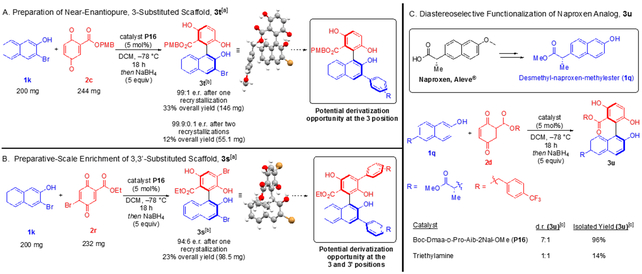
Variations on the naphthol are also well tolerated (Scheme 3B). The bulky 7-isopropoxy group (Scheme 3B, 1i) affected neither the yield nor selectivity, unlike the strongly withdrawing cyano group which did not affect selectivity, but lowered the yield, perhaps due to decreased nucleophilicity of the naphthol (Scheme 3B, 3j). Substitution at the 3 position of the naphthol significantly reduced the enantioselectivity, presumably through sterically inhibiting interactions between the catalyst and the naphthol substrate (Scheme 3B, 3k). Appending the bromine to a position more distal from the naphthol –OH restored the enantioselectivity (Scheme 3B, 3l–m). Unsubstituted 2-naphthol also performed well in the reaction, retaining good selectivity and yield (Scheme 3B, 3n). Investigations into the ramifications of substituents at the 6 position of the naphthol revealed that even sterically encumbered groups, such as 4-tert-butyl phenyl (Scheme 3B, 1o), do not seriously affect the enantioselectivity or coupling efficiency (Scheme 3B, 3o); however, substituting methoxy at this position drastically reduced the reactivity (Scheme 3C, 1p). Substituents on the quinone ring itself lowered the enantioselectivity, once again indicating that perhaps productive catalyst-substrate interactions are inhibited by steric bulk at positions near the phenols on the substrates (Scheme 3C, 3q). Product 3r further corroborates this hypothesis, as placement of the bromine substituent even more proximal to the BINOL-type phenol further reduced enantioselectivity. In the case of 3,3ʹ-disubstitution (Scheme 3C, 3s), enantioenrichment is almost entirely ablated. Product 3t served as a springboard for our foray into preparative scale synthesis and recrystallization of enantioenriched backbone-substituted biaryls, as it achieved higher selectivity than the benzylester congener (3k).
As shown in Scheme 4A, we succeeded in preparing near enantiopure 3t on preparative scale after two recrystallizations. It should be noted that significant product enantioenrichment was obtained after only one recrystallization (99:1 er). Furthermore, we were able to obtain 3s in 94:6 er after one recrystallization (Scheme 4B). We believe these examples represent a significant advancement towards obtaining optically pure scaffolds of this type with the opportunity for myriad diversification, particularly using 3s to modulate the 3 and 3ʹ positions of both arenes.[18]
Concurrently, we explored the possibility of derivatizing a medicinally interesting compound using our catalytic system. To that end, we succeeded in diastereoselectively functionalizing a naproxen analog, as shown in Scheme 4C. Following minimal modification of naproxen, we obtained 1q, and under our optimized reaction conditions using quinone 2d and catalyst P16, compound 3u was afforded in excellent yield with 7:1 d.r.; notable is the lower yield and lack of inherent selectivity when triethylamine is used as a catalyst.
In conclusion, we have demonstrated that a tetrameric peptide featuring a Lewis-basic catalytic residue is capable of efficient and enantioselective fragment coupling to establish an atropisomeric axis. The reaction scope includes backbone-substituted adducts (3 and 3ʹ positions), as well as the arylation of a naproxen analog. Intrinsic selectivity may be enhanced with recrystallization, and the products possess handles for myriad functionalizations. The chemistry seems well-poised for appending an appropriately configured bioactive scaffold to either reaction component.
Experimental Section
Experimental details can be found in the Supporting Information. X-Ray crystallographic data for compounds 3s (1906659) and 3t (1900403) are available free of charge from the Cambridge Crystallographic Data Centre.
Supplementary Material
Acknowledgements
The authors are grateful to Aaron L. Featherston, Dr. Anna E. Hurtley, Dr. Anthony J. Metrano, Dr. Jonathan M. Ryss, Dr. Christopher R. Shugrue, and Elizabeth A. Stone for preparation of catalysts. We also acknowledge E.A.S. for invaluable assistance in manuscript preparation. We also thank Dr. Brandon Q. Mercado for solving our X-ray crystal structures, and Dr. Eric K. Paulson for helpful NMR discussions. This work is supported by the National Institute of General Medical Sciences of the National Institutes of Health (R35 GM132092). G.C. acknowledges the support of the NSF Graduate Research Fellowship Program.
References
- [1]a).Bringmann G, Price Mortimer AJ, Keller PA, Gresser MJ, Garner J, Breuning M, Angew. Chem. Int. Ed 2005, 44, 5384–5427; [DOI] [PubMed] [Google Scholar]; b) Wang Y-B, Tan B, Acc. Chem. Res 2018, 51, 534–547. [DOI] [PubMed] [Google Scholar]
- [2]a).Brussee J, Jansen ACA, Tetrahedron Lett. 1983, 24, 3261–3262; [Google Scholar]; b) Lloyd-Williams P, Giralt E, Chem. Soc. Rev 2001, 30, 145–157; [Google Scholar]; c) Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M, Chem. Rev 2002, 102, 1359–1470; [DOI] [PubMed] [Google Scholar]; d) Nelson TD, Crouch RD, Organic Reactions 2004, 63, 265–555; [Google Scholar]; e) Wencel-Delord J, Panossian A, Leroux FR, Colobert F, Chem. Soc. Rev 2015, 44, 3418–3430; [DOI] [PubMed] [Google Scholar]; f) Liao G, Zhou T, Yao Q-J, Shi B-F, Chem. Commun 2019, 55, 8514–8523. [DOI] [PubMed] [Google Scholar]
- [3]a).Bringmann G, Breuning M, Tasler S, Synthesis 1999, 1999, 525–558; [Google Scholar]; b) Bringmann G, Menche D, Acc. Chem. Res 2001, 34, 615–624; [DOI] [PubMed] [Google Scholar]; c) Bringmann G, Breuning M, Pfeifer R-M, Schenk WA, Kamikawa K, Uemura M, J. Organomet. Chem 2002, 661, 31–47; [Google Scholar]; d) Bringmann G, Tasler S, Pfeifer R-M, Breuning M, J. Organomet. Chem 2002, 661, 49–65; [Google Scholar]; Bringmann G, Scharl H, Maksimenka K, Radacki K, Braunschweig H, Wich P, Schmuck C, Eur. J. Org. Chem 2006, 2006, 4349–4361. [Google Scholar]
- [4]a).Kakiuchi F, Le Gendre P, Yamada A, Ohtaki H, Murai S, Tetrahedron: Asymmetry 2000, 11, 2647–2651; [Google Scholar]; b) Miyaji R, Asano K, Matsubara S, J. Am. Chem. Soc 2015, 137, 6766–6769; [DOI] [PubMed] [Google Scholar]; c) Yu C, Huang H, Li X, Zhang Y, Wang W, J. Am. Chem. Soc 2016, 138, 6956–6959. [DOI] [PubMed] [Google Scholar]
- [5]a).Gustafson JL, Lim D, Miller SJ, Science 2010, 328, 1251–1255; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Barrett KT, Miller SJ, J. Am. Chem. Soc 2013, 135, 2963–2966; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Diener ME, Metrano AJ, Kusano S, Miller SJ, J. Am. Chem. Soc 2015, 137, 12369–12377; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Metrano AJ, Abascal NC, Mercado BQ, Paulson EK, Miller SJ, Chem. Commun 2016, 52, 4816–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zilate B, Castrogiovanni A, Sparr C, ACS Catal. 2018, 2981–2988. [Google Scholar]
- [7]a).Bao J, Wulff WD, Fumo MJ, Grant EB, Heller DP, Whitcomb MC, Yeung S-M, J. Am. Chem. Soc 1996, 118, 2166–2181; [Google Scholar]; b) Hattori T, Date M, Sakurai K, Morohashi N, Kosugi H, Miyano S, Tetrahedron Lett. 2001, 42, 8035–8038; [Google Scholar]; c) Vorogushin AV, Wulff WD, Hansen H-J, J. Am. Chem. Soc 2002, 124, 6512–6513; [DOI] [PubMed] [Google Scholar]; d) Anderson JC, Cran JW, King NP, Tetrahedron Lett. 2003, 44, 7771–7774; [Google Scholar]; e) Shibata T, Fujimoto T, Yokota K, Takagi K, J. Am. Chem. Soc 2004, 126, 8382–8383; [DOI] [PubMed] [Google Scholar]; f) Nishii Y, Wakasugi K, Koga K, Tanabe Y, J. Am. Chem. Soc 2004, 126, 5358–5359; [DOI] [PubMed] [Google Scholar]; g) Wanjohi JM, Yenesew A, Midiwo JO, Heydenreich M, Peter MG, Dreyer M, Reichert M, Bringmann G, Tetrahedron 2005, 61, 2667–2674. [Google Scholar]
- [8].Kwon Y, Li J, Reid JP, Crawford JM, Jacob R, Sigman MS, Toste FD, Miller SJ, J. Am. Chem. Soc 2019, 141, 6698–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen Y-H, Cheng D-J, Zhang J, Wang Y, Liu X-Y, Tan B, J. Am. Chem. Soc 2015, 137, 15062–15065. [DOI] [PubMed] [Google Scholar]
- [10].Moliterno M, Cari R, Puglisi A, Antenucci A, Sperandio C, Moretti E, Di Sabato A, Salvio R, Bella M, Angew. Chem. Int. Ed 2016, 55, 6525–6529. [DOI] [PubMed] [Google Scholar]
- [11]a).Davie EAC, Mennen SM, Xu Y, Miller SJ, Chem. Rev 2007, 107, 5759–5812; [DOI] [PubMed] [Google Scholar]; b) Wennemers H, Chem. Commun 2011, 47, 12036–12041; [DOI] [PubMed] [Google Scholar]; c) Wende RC, Schreiner PR, Green Chem. 2012, 14, 1821–1849; [Google Scholar]; d) Akagawa K, Kudo K, Acc. Chem. Res 2017, 50, 2429–2439; [DOI] [PubMed] [Google Scholar]; e) Metrano AJ, Miller SJ, Acc. Chem. Res 2019, 52, 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].For peptide catalysis establishing axial chirality, see: Mbofana CT, Miller SJ, J. Am. Chem. Soc 2014, 136, 3285–3292. [DOI] [PubMed] [Google Scholar]
- [13]a).Kočovský P, Vyskočil Š, Smrčina M, Chem. Rev 2003, 103, 3213–3246; [DOI] [PubMed] [Google Scholar]; b) Shibasaki M, Matsunaga S, Chem. Soc. Rev 2006, 35, 269–279. [DOI] [PubMed] [Google Scholar]
- [14].Momiyama N, Tabuse H, Noda H, Yamanaka M, Fujinami T, Yamanishi K, Izumiseki A, Funayama K, Egawa F, Okada S, Adachi H, Terada M, J. Am. Chem. Soc 2016, 138, 11353–11359. [DOI] [PubMed] [Google Scholar]
- [15].Zbieg JR, Yamaguchi E, McInturff EL, Krische MJ, Science 2012, 336, 324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]a).Gao H, Xu Q-L, Keene C, Yousufuddin M, Ess DH, Kürti L, Angew. Chem. Int. Ed 2016, 55, 566–571; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kamitanaka T, Morimoto K, Tsuboshima K, Koseki D, Takamuro H, Dohi T, Kita Y, Angew. Chem. Int. Ed 2016, 55, 15535–15538. [DOI] [PubMed] [Google Scholar]
- [17]a).MacArthur MW, Thornton JM, J. Mol. Biol 1991, 218, 397–412; [DOI] [PubMed] [Google Scholar]; b) Haque TS, Little JC, Gellman SH, J. Am. Chem. Soc 1994, 116, 4105–4106; [Google Scholar]; c) Haque TS, Little JC, Gellman SH, J. Am. Chem. Soc 1996, 118, 6975–6985; [Google Scholar]; d) Stanger HE, Gellman SH, J. Am. Chem. Soc 1998, 120, 4236–4237; [Google Scholar]; e) Rao Raghothama S, Kumar Awasthi S, Balaram P, J. Chem. Soc., Perk. T 2 1998, 137–144. [Google Scholar]
- [18].Important examples with scaffolds substituted at the 3- and 3’-positions have also been reported by Bella and coworkers in Ref. 10.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.