Abstract
Purpose
Pathologic response assessment of tumor specimens from patients receiving systemic treatment provide an early indication of therapeutic efficacy and predict long-term survival. Grading systems for pathologic response were first developed for chemotherapy in select tumor types. Immunotherapeutic agents have a mechanism of action distinct from chemotherapy and are being used across a broad array of tumor types. A standardized, universal scoring system for pathologic response that encompasses features characteristic for immunotherapy and spans tumor types is needed.
Experimental Design
Hematoxylin and eosin-stained slides from neoadjuvant surgical resections and on-treatment biopsies were assessed for features of immune-related pathologic response (irPR). 258 specimens from patients with 11 tumor types as part of ongoing clinical trials for anti-PD-1 were evaluated. An additional 98 specimens from patients receiving anti-PD-(L)1 in combination with other treatments were also reviewed, including those from three additional tumor types.
Results
Common irPR features (immune activation, cell death, tissue repair, regression bed) were present in all tumor types reviewed, including melanoma, non-small cell lung, head and neck squamous cell, Merkel cell, and renal cell carcinoma, amongst others. Features were consistent across primary tumors, lymph nodes, and distant metastases. Specimens from patients treated with anti-PD-(L)1 in combination with another agent also exhibited irPR features.
Conclusion
irPR features are consistent across tumor types and treatment settings. Standardized, pan-tumor immune-mediated pathologic response criteria (irPRC) are defined and associated specimen-handling considerations are described. Future, prospective studies are merited to validate irPRC in larger datasets and to associate pathologic features with long-term patient outcomes.
Keywords: irPRC, PD-1, PD-L1, neoadjuvant, pathologic response, pan-tumor, MPR, MPRbx
Introduction
There are over 100 active clinical trials testing neoadjuvant immune checkpoint blockade in cancer. It will be another 5–10 years before definitive long-term survival data are available for the majority of these cohorts. As such, there is great interest in developing earlier measures of therapeutic efficacy. The definitive surgical resection specimen of treated tumor provides a unique window at an early timepoint to assess treatment effect and potentially predict disease-free and overall survival. Specimens are typically obtained following 4–12 weeks of treatment, meaning that information regarding the treatment’s effect on the tumor is potentially available in a few months, rather than years. Approaches to scoring pathologic response in the tumor resection specimen and an association with long-term survival were first reported in the context of cytotoxic chemotherapy. Separate approaches emphasizing different histologic features were developed for each tumor type, Table 1, and even within a given tumor type.1–14 Despite these differences, the broad theme has been consistent, which is that the amount of residual tumor after neoadjuvant treatment inversely correlates with long-term outcomes..
Table 1.
Representative scoring systems for pathologic response to neoadjuvant therapy.
| Chemotherapy Scoring | irPRC | |||
|---|---|---|---|---|
| Non-small cell lung carcinoma3,10,+ | Breast carcinoma5,27 | Colorectal carcinoma6,33 | Pan-tumor | |
| Histologic features assessed | • Residual viable tumor (RVT) • Necrosis • “Fibroinflammatory stroma” • Granulation tissue • Scarring • Foam cells |
• Primary tumor bed size = span of area containing viable cancer cells • Average cancer cellularity • Percentage in situ disease • Number and largest size of lymph node metastases |
• Residual viable cancer cells • Degree of “tumor regression” scar/fibrosis • Acellular pools of mucin represent regression |
• RVT • Necrosis • Regression bed (lymphoid infiltrates, tertiary lymphoid structures, plasma cells, foamy macrophages, cholesterol clefts, proliferative fibrosis, neovascularization) |
| Pathologic score | %RVT = viable tumor/ (viable tumor + stromal tissue + necrosis) | %RVT = average viable cancer cellularity within primary tumor bed area, averaged across all slides that contain tumor bed |
Tumor Regression Score Score 0: no residual cancer Score 1: single cells/rare groups of cancer cells Score 2: larger groups of residual cancer with evidence of regression Score 3: cancer without regression (i.e., no response) |
|
| Grossing/specimen handling recommendations | 1 section per cm along the largest tumor dimension.+ | Complete submission of largest cross-sectional area.* | Complete submission of largest cross-sectional area. | Complete submission of largest cross-sectional area.* |
Updated guidelines by International Association for the Study of Lung Cancer (IASLC) are anticipated.
If the tumor is large, lesser sampling may be performed. In addition, one section from each additional 1 cm of greatest tumor dimension should be submitted, per standard grossing protocols.
Many ongoing clinical trials for neoadjuvant immunotherapy plan to use pathologic response as a surrogate endpoint for clinical efficacy. Some trials are adopting scoring systems developed for chemotherapy. There are several limitations to this approach. First, there are well-established mechanistic differences between immunotherapy and cytotoxic chemotherapy, and the microscopic appearance of treatment effect will likely not translate directly. Either under-estimation or over-estimation of pathologic response can adversely affect a patient’s care with an unnecessary change in therapy made in the former scenario and a potential missed opportunity to switch to a more efficacious regimen in the latter scenario. It is also possible that additional, more extensive surgery can be avoided in certain patients who achieve pathologic response following neoadjuvant immunotherapy, and thus it is essential that the pathologic response evaluation captures immunotherapy-related histologic changes. Another limitation is that scoring systems for pathologic response to neoadjuvant chemotherapy are generally limited to the primary tumor only. Scoring of associated disease in regional lymph nodes for most tumor types is limited to the number of nodes involved and the largest tumor deposit, with no assessment of treatment response for these lesions. Additionally, there are tumor types such as melanoma or Merkel cell carcinoma (MCC) where the primary tumor has already been resected and response to therapy must be assessed in regional lymph node metastatic deposits. Lastly, chemotherapy scoring systems were only developed for a few select tumor types, and there is a lack of consistency both within and across tumor types, Table 1. Numerous other tumor types are currently being treated with neoadjuvant immunotherapy, which do not have a pre-existing chemotherapy precedent for assessing pathologic response.
Proposed scoring approaches for pathologic response assessment for non-small cell lung cancer (NSCLC) and melanoma treated with neoadjuvant immunotherapy were recently reported by Cottrell, et al and Tetzlaff, et al, respectively.15,16 These proposals have many similarities, but also key differences including the scoring increments used (e.g. partial pathologic response definition), whether a primary vs. metastatic lesion was assessed, whether necrosis is scored as therapeutic response, and the assessment of tumoral melanosis as therapeutic effect in the case of melanoma. Here, we propose a pan-tumor approach to scoring pathologic response to immunotherapy that takes into account the common histologic features associated with treatment response for this class of agents. Importantly, these features are not limited to primary tumors but may also be assessed in lymph nodes (Figure 1) and advanced metastatic disease, where initial studies have shown they correlate with 5-year survival.17 A universal approach has many advantages; it, 1) allows direct comparisons of relative therapeutic efficacy across different trials and tumor types; 2) supports assessment of response for pan-tumor therapeutic indications such as mismatch-repair deficient cancers; 3) provides a scoring system for tumor types not previously treated in the neoadjuvant setting; 4) allows for assessment of response in cases where the primary tumor is no longer intact, and also provides the opportunity to assess for response in organs involved by clinically-occult disease; 5) avoids the development of multiple scoring systems that are potentially confusing to pathologists once they enter the clinical arena (e.g., as seen with PD-L1 immunohistochemical assays); and 6) provides a standardized assessment of specimens to facilitate expanded biomarker discovery efforts.
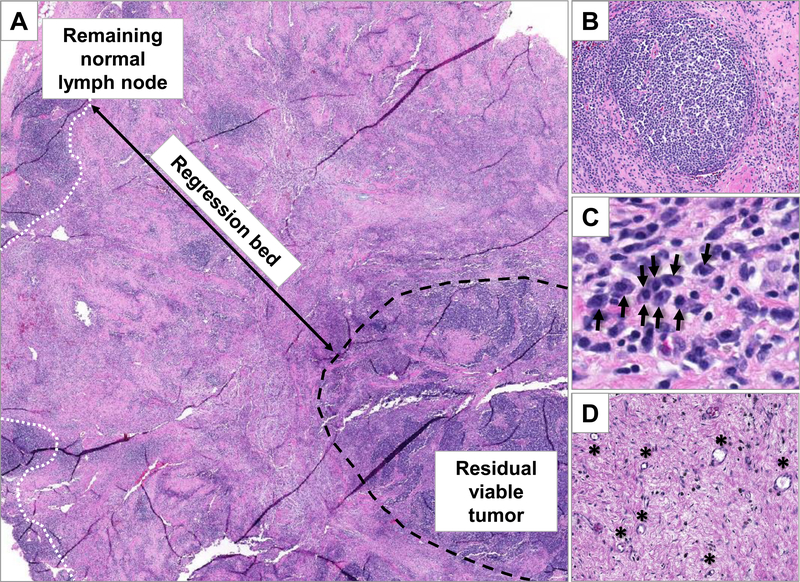
Figure 1. Features of immune-related pathologic response (irPR) in a lymph node dissection specimen from a patient with metastatic Merkel cell carcinoma who received neoadjuvant anti-PD-1 therapy.
The tumor regression bed is positioned between residual normal lymph node (white dotted line) and residual viable tumor (black dashed line) (A; 20x). Features of irPR include the presence of organized lymphoid aggregates (B; 150x, original magnification) and dense plasma cell collections (C; black arrows point to individual plasma cells; 200x) in a background of proliferative fibrosis, dense tumor infiltrating lymphocytes, and neovascularization (D; black asterisks indicate small blood vessels; 150x). This field contains 30% residual viable tumor.
Features of pathologic response following immune checkpoint blockade
Cottrell, et al., analyzed specimens from the first published anti-PD-1 neoadjuvant lung cancer trial18 to characterize immune-related pathologic response (irPR) in patients with NSCLC. Key findings on routine hematoxylin and eosin staining included: 1) immune activation, as indicated by lymphoid infiltrates, tertiary lymphoid structures, and plasma cells (the last two suggesting an orchestrated B-cell contribution); 2) cell death, signified by foamy macrophages and cholesterol clefts; and 3) the identification and histologic description of a tumor regression bed (i.e., original tumor site that has now been cleared), including features of tissue repair, in particular proliferative fibrosis (high fibroblast nuclei:collagen ratio) and neovascularization. The regression bed is often peripheral to the residual tumor (Figure 1).15
We have now reviewed over 250 anti-PD-1-treated specimens from 11 tumor types and have observed similar features. Specifically, irPR was seen in definitive surgical specimens from patients with cervical carcinoma, vulvovaginal carcinoma, head and neck squamous cell carcinoma, melanoma, MCC, NSCLC, and renal cell carcinoma receiving neoadjuvant anti-PD-1 (Table 2, Figure 2). On-treatment biopsies (core, punch, incisional, or excisional) from patients with these same seven types of advanced cancer as well as patients with advanced basal cell carcinoma, cutaneous squamous cell carcinoma, MSI-high colorectal carcinoma, and nasopharyngeal carcinoma exhibited the same pathologic features (Table 2, Supplemental Figure S1). Biopsies from patients with advanced cancer were taken from a broad array of locations within the body, including skin and soft tissue, lung, lymph nodes, liver, adrenal glands, pancreas, kidney, and mucosa. Taken together, these findings support pan-tumor features of pathologic response to immune checkpoint blockade.
Table 2.
Tumor types and associated anti-PD-1 trials of on-treatment specimens studied for immune-related pathologic response (irPR).
| Treatment Setting | Tumor type | Treatment | NCT* |
|---|---|---|---|
| Neoadjuvant | Cervical cancer | Nivolumab | |
| Head and neck squamous cell carcinoma | Nivolumab | 34 | |
| Melanoma | Nivolumab | 16,35 | |
| Merkel cell carcinoma | Nivolumab | 36 | |
| Non-small-cell lung carcinoma | Nivolumab | 15,18 | |
| Renal cell carcinoma | Nivolumab | ||
| Vulvovaginal carcinoma | Nivolumab | ||
| Advanced Unresectable Disease | Basal cell carcinoma | Nivolumab | |
| Cervical cancer | Nivolumab | ||
| Cutaneous squamous cell carcinoma | Pembrolizumab; nivolumab | Off-label | |
| Head and neck squamous cell carcinoma | Nivolumab | ||
| Melanoma | Nivolumab; pembrolizumab | 17; standard of care | |
| Merkel cell carcinoma | Nivolumab | ||
| Microsatellite instability-high colorectal cancer | Pembrolizumab | 37 | |
| Nasopharyngeal carcinoma | Nivolumab | ||
| Non-small-cell lung carcinoma | Nivolumab; pembrolizumab | Standard of care | |
| Renal cell carcinoma | Nivolumab | ||
| Vulvovaginal carcinoma | Nivolumab |
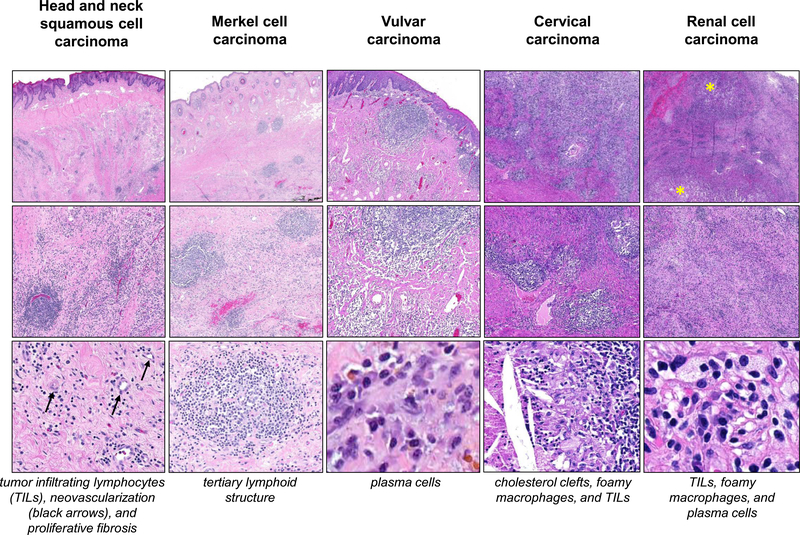
Figure 2. Consistent histologic features of immune-related pathologic response (irPR) following neoadjuvant anti-PD-1 therapy in different tumor types.
In addition to previously reported findings in non-small-cell lung carcinoma15 and melanoma,16 features of irPR are seen here in five other tumor types. The tumor regression bed appears similar in head and neck squamous cell carcinoma, Merkel cell carcinoma, vulvar, cervical, and renal cell carcinomas (top and middle rows; 10x-100x, original magnification). Residual tumor is marked with yellow asterisks (other tumors show pCR). Individual features of tissue repair and immune activation, as displayed in Figure 1, as well as tumor cell clearance (e.g. foamy macrophages and cholesterol clefts), are shown at higher magnification here (bottom row; 200x-400x, original magnification). H&E staining, all panels.
Scoring specimens for percent residual viable tumor
Percent residual viable tumor (%RVT) is calculated by:
where the total tumor bed surface area includes RVT, tumor-associated stroma, necrosis, and the area where viable tumor used to be before treatment but is no longer, i.e., the regression bed. We recommend that specimens are scored as 0%, 0< and<10%; 10% RVT, and increasing 10% increments. This same calculation is applied to lymph nodes, or other any other organ involved by tumor. Scoring treatment effect beyond the primary tumor is not currently detailed in most chemotherapy pathologic scoring, or when mentioned, requires pre-treatment pathologic confirmation.19 (One exception is breast carcinoma.2) In addition to being applicable to multiple tumor types, irPRC has the advantage that it may be applied to all organs involved by tumor. Further, the features are characteristic enough that regressed tumor may be recognized, even if not pathologically-confirmed or even clinically-suspected pre-treatment.
Information regarding the degree of pathologic response can be gathered and reported for the patient as a whole or for a given anatomic location. An example case report form is provided in Supplemental Table 1. An overall irPRC score can be assigned, e.g. a major pathologic response (MPR) including both the primary tumor and associated lymph node metastases. In this scenario, the calculation of (RVT surface area/total tumor bed surface area) x 100 combines the relevant surface areas for the primary tumor and lymph node deposits. Alternatively, irPRC for each anatomic location may be determined. For example, it is possible to have a pathologic complete response (pCR) in the primary tumor, and no evidence of pathologic response in the lymph nodes.18 Information gained from both of these approaches is likely to have prognostic value and potentially different clinical implications.
If features of an irPR regression bed are not appropriately recognized, the therapeutic efficacy is underestimated. Similarly, if certain pathologic features are erroneously considered signs of treatment response, there is a risk of overestimating pathologic response in a patient. For example, there are conflicting reports as to whether necrosis is a feature of response to immunotherapy.15–17,20 Tetzlaff, et al, score necrosis as representing treatment effect. Necrosis was not a defining feature of pathologic response in the specimens reviewed here, or in studies examining paired pre- and on-treatment specimens from patients with melanoma and NSCLC demonstrating response to anti-PD-1 therapy. 15,17 Necrosis was also not associated with long-term benefit in patients with advanced melanoma receiving anti-CTLA-4.21 More recently, necrosis has been specifically identified as inversely correlated with response to anti-PD-1 in patients with renal cell carcinoma.22 It is possible that necrosis may not specifically be a sign of response to immune checkpoint blockade, but may represent therapeutic response to other agents. It is also possible that the histologic type or context of necrosis may differ in importance. For example, central necrosis may be observed in high-grade tumor deposits when the tumor outstrips its blood supply, and such tumors may be more unlikely to respond to immunotherapy. In this specific scenario, the surface area for residual ‘viable’ tumor should include the area of central necrosis. In contrast, necrosis that directly interfaces with stroma characterized by proliferative fibrosis, histiocytes and less chromatin debris, may signify treatment efficacy. Further study is required to define the types of necrosis that may be clinically meaningful.
Pathologic complete response (pCR) and major pathologic response (MPR)
Some clinical trials are using pCR (no residual tumor) and MPR (<10% residual viable tumor) following neoadjuvant immunotherapy as endpoints. Only a minority of patients achieve a pCR to single agent neoadjuvant anti-PD-(L)1, e.g., 5–15% in NSCLC18,23,24 and 19–43% in melanoma.25,26 It is likely that patients do not need complete pathologic resolution of tumor burden to experience clinical benefit, since a major mechanism of clinical benefit for neoadjuvant immunotherapy is the priming of anti-tumor immune responses that will systemically seek out and destroy microscopic tumor deposits that would otherwise cause relapse. If a threshold of <10% RVT (MPR) is used instead, a significantly larger proportion of patients are captured (19–45% and 30–60%, respectively). Chemotherapy studies with long-term follow-up have shown that MPR is predictive of long-term survival, and preliminary data suggest this will also be the case for patients treated with immunotherapy.17,25 It is possible that other clinically relevant thresholds will emerge if pathologic response can be refined and standardized, as is proposed here.
Reproducibility) and extension of scoring to include additional treatment regimens
When applied to immunotherapy-treated specimens, chemotherapy grading is not as reproducible among pathologists as grading using irPRC.15 Reproducible scoring will be necessary to test a continuum of pathologic response, which may enable more refined survival predictions than MPR or pCR alone. Inter-reader agreement assessments between four pathologists using irPRC at 10% scoring intervals on specimens from patients treated with neoadjuvant immunotherapy showed an intraclass correlation coefficient of 0.982, 95% CI [0.965, 0.992]. The reproducibility observed with irPRC may be attributed to: 1) a refined description of the character of features to count, e.g. rather than simply “inflammation”, the types of inflammatory cells to include have been detailed; 2) a distinction made between tumor cellularity,27 fibrotic tumor-associated stroma, and the fibrosis associated with immune-mediated tumor regression; 3) an estimation of surface area for the calculation of %RVT that is summed across slides, rather than weighing each slide equally, irrespective of the amount of tumor present;3,28 and 4) an explicit recognition and description of how to score the regression bed.
It is possible that the approach to scoring described here could be extended to other treatment regimens. Preliminary results also show irPR in patients receiving anti-PD-(L)1 combined with other agents, such as anti-CTLA-4, cytotoxic chemotherapy, and tyrosine kinase inhibitors (Supplemental Figure S2). There is evidence to suggest that patients treated with targeted therapy, neoadjuvant chemotherapy, and immunotherapeutic regimens beyond checkpoint blockade, e.g. GVAX plus chemotherapy, show similar histologic features of immune-mediated tumor clearance.10,29–32 If a specimen from a patient with NSCLC treated with neoadjuvant chemotherapy does demonstrate a component of immune-mediated regression, the utility of using irPRC is evident. That is, if a specimen does not clearly display an immune-mediated regression bed, regression bed = 0% surface area, and the calculation for %RVT becomes: total area involved by tumor / (+ RVT + tumor-associated stroma + necrosis surface areas) x 100, i.e. essentially the basic calculation used in scoring approaches for chemotherapy (Figure 3).3,28 Thus, a single scoring system could be used for both chemotherapy and immunotherapy-treated patients. This has great appeal, given that there are ongoing trials that include both chemotherapy and immunotherapy-treated arms, as well as arms that include chemotherapy + immunotherapy. It would be impractical to use different scoring systems for each arm. Further, it would be impossible to have a pathologist blinded to treatment arm while conducting the scoring if each required their own scoring approach.
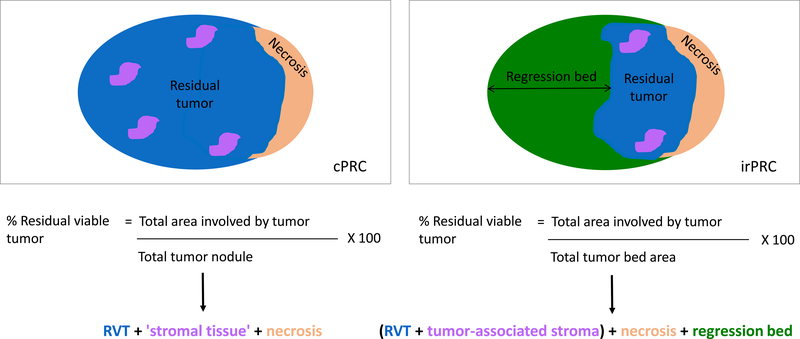
Figure 3. Calculation of percent residual viable tumor using (A) chemotherapy pathologic response criteria (cPRC), and (B) immune-related pathologic response criteria (irPRC).
Representative schematics of calculating percent residual viable tumor (RVT) using cPRC and irPRC are shown. The main difference between the calculation shown in (A) and (B) is the assessment of the regression bed when scoring with irPRC. If a specimen does not exhibit any features of immune-mediated regression, then the ‘regression bed’ value seen in the equation for percent RVT in irPRC (B) falls to zero, and both methods (A) and (B) fundamentally become the same calculation. However, the reverse is not true, i.e., cPRC does not immediately translate to patients treated with immunotherapy.
Gross examination and specimen handling
We recommend that one complete cross-section of tumor bed from the longest dimension of the tumor is submitted for paraffin embedding and made into slides, with an additional one section per centimeter taken for the remaining specimen. For larger tumors (e.g., >5 cm), a modified approach of submitting every-other section or even every third section across the largest cross-sectional diameter is reasonable. This is in keeping with existing grossing recommendations for breast carcinoma, rectal carcinoma, and osteosarcoma specimens from patients treated with neoadjuvant chemotherapy, Table 1.2,4 Complete cross-sections of grossly-evident lymph node tumor deposit(s) should also be handled similarly.
Conclusions and future directions
In summary, pathologic features of response to immune checkpoint blockade are remarkably consistent across tumor types, indicating that a universal, standardized scoring system may be possible. The proposed scoring system described here is supported by routine surgical pathology workflows and is fast as well as widely and immediately available. Utilization of a standardized, H&E-based scoring system across many institutions will be needed to assess neoadjuvant immunotherapies in ongoing large phase 3 trials, and in routine clinical practice if these treatments eventually become standard of care. Our findings suggest that developing independent pathologic response scoring systems within the context of individual clinical trials is unnecessary and will hinder comparisons across trials in different tumor types.
The gold standard comparison for the development of a surrogate endpoint is its association with overall survival. Long-term survival data are not yet available from neoadjuvant immunotherapy-treated cohorts, but importantly, irPR has already been shown to predict 5-year survival in patients with advanced, unresectable melanoma receiving these drugs.17 Collection of data, e.g., percent residual viable tumor, in a standardized fashion will allow for future association with long-term patient outcomes when disease-free and overall survival data from these studies mature.
Supplementary Material
Statement of translational relevance.
As immunotherapies are being tested in the neoadjuvant setting, a standardized system for grading pathologic response is highly desirable. Such a system will allow for comparison of treatment efficacy between studies and different tumor types. The current report presents features of immune-related pathologic response (irPR) and shows that these features are consistent across more than ten different tumor types from patients treated with anti-PD-(L)1 agents. Similar features are also seen in patients treated with regimens combined with anti-PD-(L)1 agents. These findings indicate that a universal approach to scoring pathologic response to immunotherapy may be possible. Many clinical trial endpoints for neoadjuvant immunotherapy include determinations of pathologic response, and the irPR criteria (irPRC) described here will be of value in that setting. irPRC may also ultimately be used to help guide use of adjuvant therapy as well as treatment decisions for patients with advanced disease.
Acknowledgements
The authors would like to thank Robin Edwards, MD from Bristol-Myers Squibb for helpful discussions. We would also like to thank Daphne Wang and Elizabeth Engle for assistance with specimen management and slide scanning. This work was supported by The Bloomberg~Kimmel Institute for Cancer Immunotherapy (JMT, RAA, EMJ, SLT, DMP); Sidney Kimmel Cancer Center Core Grant P30 CA006973 (JMT); National Cancer Institute R01 CA142779 (JMT, SLT, DMP); Melanoma Research Alliance (JMT), The Mark Foundation for Cancer Research (JMT, RAA, EMJ, EJL, MY, DMP), NIH T32 CA193145 (JES); The National Cancer Institute Specialized Program of Research Excellence (SPORE) in Gastrointestinal Cancers P50 CA062924 (MY), and the NIH Center Core Grant P30 CA006973 (MY).
Author Disclosures:
Dr. Stein has no relevant disclosures. Dr. Lipson reports consulting or advisory role for Bristol-Myers Squibb, Novartis, EMD Serono, Array BioPharma, Regeneron/Sanofi Genzyme, Macrogenics, Merck, Millennium; research funding from Bristol-Myers Squibb, Merck, Sysmex; and patents, royalties, other intellectual property regarding a method of preventing organ transplantation rejections using agonists to the PD-1 checkpoint pathway. Dr. Cottrell has no relevant disclosures. Dr. Forde is a consultant/advisory board member of Abbvie, AstraZeneca, Bristol-Myers Squibb, Boehringer lngelheim, EMD Serono, lnivata, Janssen, Lilly, Merck, Novartis; he received grant/research support from AstraZeneca, Bristol-Meyers Squibb, Corvus, Kyowa, Novartis. Dr. Anders receives grant funding from FLX Bio and Five Prime Therapeutics, and is a consultant for Bristol-Myers Squibb, Merck, and AstraZeneca. Dr. Cimino-Mathews receives grant funding from Bristol-Myers Squibb. Dr. Thompson receives grant funding from Bristol-Myers Squibb. Dr. Allaf has no relevant disclosures. Dr. Yarchoan reports receiving a commercial research grant from Bristol-Myers Squibb, Exelixis, and Merck & Co, and is a consultant/advisory board member for Eisai and Exelixis. Dr. Feliciano is a consultant/advisory board member of AstraZeneca, Merck, Genentech, and Eli Lilly, and has provided expert testimony for legal review. Dr. Wang has no relevant disclosures. Dr. Jaffee reports receiving a commercial research grant from Bristol-Myers Squibb, Aduro Biotech, and Amgen, has ownership interest (including stock, patents, etc.) in Aduro Biotech, and is a consultant/advisory board member for CStone, Dragonfly, Genocea, and Adaptive Biotechnologies. Dr. Pardoll reports other support from Aduro Biotech, Amgen, Bayer, Camden Partners, DNAtrix, Dracen, Dynavax, Five Prime, FLX Bio, Immunomic, Janssen, Merck, Rock Springs Capital, Potenza, Tizona, Trieza, and WindMil; grants from Astra Zeneca, Medimmune/Amplimmune, and Compugen; grants and other support from ERvaxx and Potenza. Dr. Topalian reports stock and other ownership interests in Aduro Biotech, Compugen, Potenza Therapeutics, Jounce Therapeutics, Five Prime Therapeutics, Tizona Therapeutics, DNAtrix, FLX Bio, WindMIL, Dragonfly Therapeutics, ERVAXX; consulting or advisory role for Five Prime Therapeutics, Amgen, MedImmune, Merck, Abbvie, Compugen, DNAtrix, FLX Bio, Tizona Therapeutics, WindMIL, Dragonfly Therapeutics, Bayer, Dynavax, ERVAXX, Immunomic Therapeutics, Janssen Oncology; research funding from Bristol-Myers Squibb, Compugen, Potenza Therapeutics; patents, royalties, other intellectual property from Aduro Biotech, Bristol-Myers Squibb, Immunonomic Therapeutics; travel, accommodations, and expenses: Bristol-Myers Squibb, Five Prime Therapeutics. Dr. Taube reports nonfinancial support from Akoya during the conduct of the study; grants and personal fees from Bristol-Myers Squibb, personal fees from Merck, Astra Zeneca, and Amgen outside the submitted work; equipment and reagents from Akoya Biosciences, and a patent pending related to image processing of mIF/IHC images.
References
- 1.Hartman DJ, Krasinskas AM. Assessing treatment effect in pancreatic cancer. Arch Pathol Lab Med. 2012;136(1):100–109. [DOI] [PubMed] [Google Scholar]
- 2.Provenzano E, Bossuyt V, Viale G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol. 2015;28(9):1185–1201. [DOI] [PubMed] [Google Scholar]
- 3.Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2012;7(5):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chui MH, Kandel RA, Wong M, et al. Histopathologic Features of Prognostic Significance in High-Grade Osteosarcoma. Arch Pathol Lab Med. 2016. [DOI] [PubMed] [Google Scholar]
- 5.Sahoo S, Lester SC. Pathology of breast carcinomas after neoadjuvant chemotherapy: an overview with recommendations on specimen processing and reporting. Arch Pathol Lab Med. 2009;133(4):633–642. [DOI] [PubMed] [Google Scholar]
- 6.Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47(2):141–146. [DOI] [PubMed] [Google Scholar]
- 7.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127(11):1335–1339. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118(12):3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SM, Katz MH, Liu L, et al. Validation of a Proposed Tumor Regression Grading Scheme for Pancreatic Ductal Adenocarcinoma After Neoadjuvant Therapy as a Prognostic Indicator for Survival. Am J Surg Pathol. 2016;40(12):1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Junker K, Langner K, Klinke F, Bosse U, Thomas M. Grading of tumor regression in non-small cell lung cancer : morphology and prognosis. Chest. 2001;120(5):1584–1591. [DOI] [PubMed] [Google Scholar]
- 11.Betticher DC, Hsu Schmitz SF, Totsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer. 2006;94(8):1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99(6):442–450. [DOI] [PubMed] [Google Scholar]
- 13.Stefani A, Alifano M, Bobbio A, et al. Which patients should be operated on after induction chemotherapy for N2 non-small cell lung cancer? Analysis of a 7-year experience in 175 patients. J Thorac Cardiovasc Surg. 2010;140(2):356–363. [DOI] [PubMed] [Google Scholar]
- 14.Qu Y, Emoto K, Eguchi T, et al. Pathologic Assessment After Neoadjuvant Chemotherapy for NSCLC: Importance and Implications of Distinguishing Adenocarcinoma From Squamous Cell Carcinoma. J Thorac Oncol. 2019;14(3):482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol. 2018;29(8):1853–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tetzlaff MT, Messina JL, Stein JE, et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol. 2018;29(8):1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein JE, Soni A, Danilova L, et al. Major pathologic response on biopsy (MPRbx) in patients with advanced melanoma treated with anti-PD-1: evidence for an early, on-therapy biomarker of response. Ann Oncol. 2019;30(4):589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forde PM, Chaft JE, Pardoll DM. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018;378:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumenthal GM, Bunn PA Jr., Chaft JE, et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J Thorac Oncol. 2018;13(12):1818–1831. [DOI] [PubMed] [Google Scholar]
- 20.Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–1661. [DOI] [PubMed] [Google Scholar]
- 21.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein JE, Signoretti S, Sznol M, et al. Pathologic scoring of pre-treatment H&E biopsies predicts overall survival in patients with metastatic clear cell renal cell carcinoma receiving nivolumab monotherapy. Paper presented at: ESMO 2019 Abstract Book; 27 Sept - 1 Oct, 2019; Barcelona, Spain. [Google Scholar]
- 23.Kwiatkowski DJ, Rusch VW, Chaft JE, et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). Journal of Clinical Oncology. 2019;37(15_suppl):8503–8503. [Google Scholar]
- 24.Cascone T, William WN, Weissferdt A, et al. Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. Journal of Clinical Oncology. 2019;37(15_suppl):8504–8504. [Google Scholar]
- 25.Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948–960. [DOI] [PubMed] [Google Scholar]
- 26.Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–4422. [DOI] [PubMed] [Google Scholar]
- 28.Hellmann MD, Chaft JE, William WN Jr., et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15(1):e42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelekanou V, Carvajal-Hausdorf DE, Altan M, et al. Effect of neoadjuvant chemotherapy on tumor-infiltrating lymphocytes and PD-L1 expression in breast cancer and its clinical significance. Breast Cancer Research. 2017;19(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25(3):611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19(5):1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutz ER, Wu AA, Bigelow E, et al. Immunotherapy Converts Nonimmunogenic Pancreatic Tumors into Immunogenic Foci of Immune Regulation. Cancer Immunology Research. 2014;2(7):616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruo L, Tickoo S, Klimstra DS, et al. Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg. 2002;236(1):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferris RL, Gonçalves A, Baxi SS, et al. LBA46An open-label, multicohort, phase 1/2 study in patients with virus-associated cancers (CheckMate 358): Safety and efficacy of neoadjuvant nivolumab in squamous cell carcinoma of the head and neck (SCCHN). Annals of Oncology. 2017;28(suppl_5). [Google Scholar]
- 35.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topalian SL, Bhatia S, Kudchadkar RR, et al. Nivolumab (Nivo) as neoadjuvant therapy in patients with resectable Merkel cell carcinoma (MCC) in CheckMate 358. Journal of Clinical Oncology. 2018;36(15_suppl):9505–9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.