Abstract
Importance:
Understanding how dementia risk is impacted by timing of smoking cessation has public health implications for prevention efforts.
Objective:
Investigate the relationship of cigarette smoking and cessation with dementia risk and cognitive decline in the Atherosclerosis Risk in Communities (ARIC) Study.
Design and Setting:
Begun in 1987–1989, ARIC is an ongoing prospective cohort study conducted in 4 US communities.
Participants:
13002 men and women (25% African American) aged 52–75 years.
Main Outcome and Measures:
All-cause dementia was defined using standardized algorithms incorporating longitudinal cognitive data, proxy report and hospital and death certificate dementia codes. Cognitive decline was measured using a composite cognitive score created from 3 tests measured at 2 time points (1996–1998 and 2011–2013). Smoking and cessation status were defined by self-report using data from 1987–1989 (visit 1) and 1996–1998 (visit 4). Incident dementia risk and differences in cognitive change by smoking status were estimated with Cox proportional hazards and linear regression models, respectively. To address smoking-related attrition, cognitive scores were imputed for living participants with incomplete cognitive testing.
Results:
The proportion of never, former and current smokers was 44%, 41% and 14%; 79% of former smokers quit ≥ 9 years prior to baseline. A total of 1347 participants developed dementia. After adjustment, compared to never smoking, the hazard ratio for all-cause dementia for current smoking was 1.33 (95% CI: 1.12, 1.59), and for recent quitting (<9 years prior to baseline) was 1.24 (95% CI: 1.01, 1.52). Quitting ≥ 9 years prior to baseline was not associated with dementia. We found no differences in rates of cognitive decline by smoking status.
Conclusions and Relevance:
Although quitting at any time suggested benefit, dementia risk depended on time since smoking cessation. Our study highlights the importance of early midlife cessation to decrease dementia risk.
Keywords: smoking, cessation, cognitive decline, dementia
Cigarette smoking, even in low doses,1 increases risk for vascular disease2 and stroke,3,4 providing a strong biological rationale for a relationship between smoking and dementia and cognitive decline. Current smoking is a modifiable cause of dementia5,6 and cognitive decline,7,8 although studies of the impact of smoking cessation, particularly timing of cessation, on dementia risk are lacking.9,10 Observational data supporting an association with current smoking and dementia is generally consistent,5 however evidence for an association between current smoking and cognitive decline is less so.11,12 In many studies, the extent of the contribution of smoking to dementia and cognitive decline may be underestimated given methodological limitations, including selective loss of susceptible smokers due to death or dropout from a study over time,10,13,14 particularly if participants are required to attend clinic visits during follow-up in order to have cognition measured.
We used data from the Atherosclerosis Risk in Communities (ARIC) Study, a large, prospective cohort, to estimate the association of midlife cigarette smoking status and time since smoking cessation on subsequent 12-year risk for dementia and cognitive decline using methods to address informative attrition.
METHODS
Study population
ARIC is a population-based study of 15792 predominantly white or black adults aged 45–64 years in 1987–89 randomly sampled from 4 US communities: Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi (all black participants); and suburbs of Minneapolis, Minnesota.15 Informed consent was obtained from all participants at each visit. Study procedures were approved by the Institutional Review Board for each field center.
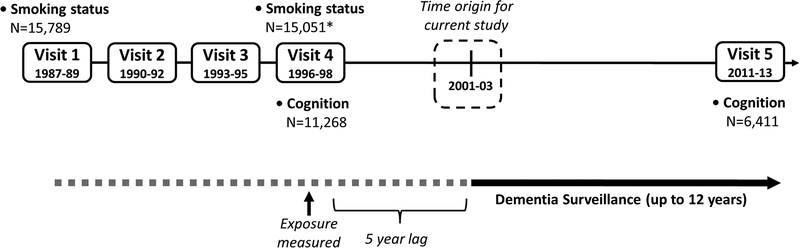
ARIC participants were seen for 4 clinic visits ~3 years apart from 1987–89 to 1996–98, with a 15-year gap between visits 4 (1996–98) and 5 (2011–13). Given this design, baseline for this study was defined as visit 4 (1996–98) in order to capture smoking patterns in midlife. For both the smoking-dementia and the smoking-cognitive decline analyses, participants were excluded if race was other than black or white (N=48), or if non-white from Minneapolis or Washington County field sites (N=55).
For the smoking-dementia analysis, participants were excluded if they had a dementia diagnosis (N=213) or censor date (N=2422) on or before the time origin for the study, or were missing smoking status (N=52), yielding an analytic sample of 13002.
For the smoking-cognitive decline analysis, participants with incomplete cognitive data at the first ARIC testing occasion (visit 2, N=2429) were excluded in order to aid in the imputation of missing cognitive scores at later visits. Participants were also excluded if they were missing cognitive test scores at both visit 4 (1996–98) and 5 (2011–13) (N=1923), as were participants missing education (N=15), resulting in an analytic sample of 11322.
Dementia diagnosis
Dementia was ascertained for all participants including those who died or were lost to follow-up.16,17 For participants who attended visit 5 (2011–13), diagnosis used standardized algorithms incorporating longitudinal cognitive data and a neuropsychological battery administered at that visit, with algorithmic diagnoses confirmed by expert panel review. Diagnoses for participants who did not attend visit 5 were based on a Modified Telephone Interview for Cognitive Status-Modified (TICS) interview with the participant, on modified Clinical Dementia Rating (CDR) interviews with informants, or on hospital or death certificate dementia codes alone.16,17 Active surveillance for dementia was through Sept 1, 2013. For diagnoses ascertained by codes, the date of dementia onset was set 6 months prior to death or hospitalization.
Cognitive decline
Three cognitive tests were administered in 1996–98 (visit 4) and 2011–13 (visit 5): Delayed Word Recall (memory), Word Fluency (language) and the Digit Symbol Substitution (executive function/attention). Scores at each testing occasion were standardized as z-scores and then scaled to the mean and SD on first testing (1996–98). The primary measure for inference was a more reliable Composite Cognitive Score at each visit, which was created by averaging the three test-specific z-scores at that visit, and scaling to the SD of the composite score in 1996–98.18,19 Secondary analyses modeled cognitive change for each individual cognitive test. Cognitive change from visits 4–5 was calculated by subtracting the z-score at visit 4 from that at visit 5.
Cigarette smoking
Self-reported cigarette smoking status was obtained at each visit (never, former, current) and during annual follow-up telephone calls (“Do you now smoke cigarettes?”) beginning in 1998. For the primary analysis, we defined smoking status using data from visit 4 (1996–98), or for those participants who did not attend visit 4, from an annual phone call within 6 months of the mean visit 4 date (N=857). Using smoking data from visit 1 (1987–89), former smokers at visit 4 were categorized as having quit ≥ 9 years earlier if they also reported former smoking at visit 1, because 9 years was the average difference between visits 1 and 4 (Figure 1). Former smokers at visit 4 who were current smokers at visit 1 were categorized as having quit more recently (<9 years prior to baseline).
Figure 1. Study design of the Atherosclerosis Risk in Communities (ARIC) Study, 1987–89 to 2011–13.
* N=11,656 participants attended Visit 4. Missing smoking values at visit 4 were completed using self-report smoking status from annual follow-up phone calls
Additional covariates
Demographic information was collected at visit 1 (1987–89), including age, sex, race and education (<high school, high school or equivalent, or >high school). Covariates measured at baseline (1996–98) included body mass index (kg/m2); pre-hypertension, defined in persons not taking antihypertensive medication as diastolic blood pressure (DBP) 80–89 mmHg or systolic blood pressure (SBP) 120–139 mmHg; hypertension, defined as DBP ≥90 mmHg, SBP ≥140 mmHg, or antihypertensive medication use; diabetes defined as fasting blood glucose level ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, self-reported physician-diagnosed diabetes, or use of diabetes medication; and prevalent stroke, defined as self-reported history of stroke diagnosed by a physician at visit 1 and adjudicated stroke through visit 4. Chronic obstructive pulmonary disease (COPD) was defined as self-reported physician-diagnosed bronchitis or emphysema. Self-reported health compared to other persons of the same age (excellent, good, fair, or poor) was collected at each annual follow-up call beginning in 1989.
Statistical analysis
Baseline covariate distributions by smoking status were compared using the Pearson chi‐square test for categorical values and the Kruskal‐Wallis test for continuous variables.
Cox proportional hazard models were used to estimate hazard ratios (HR) and 95% confidence intervals of incident dementia by smoking status; ties were handled using the Efron method. The proportional hazards assumption was verified by assessing correlation between scaled Schoenfeld residuals and transformed survival times.20 Because poor health related to smoking may influence quitting but may also be related to subsequent dementia risk, the time origin for the dementia analysis was defined as 5 years post-baseline. The timing of this lag was varied in sensitivity analyses (1 year, N=13984; 8 years, N=12118; and 10 years, N=11416). Analyses were adjusted for age (linear and quadratic terms), sex, education, a combination variable of race and study site, BMI, diabetes and hypertension. Adjustment factors were chosen a priori based on known relationships with both exposure and outcome. Because BMI, diabetes hypertension could possibly result from smoking, and therefore mediate the association between smoking and dementia/cognitive decline, models that adjust only for demographic variables are also presented. To account for smoking cessation that may be related to poor health due to smoking, we additionally adjusted for a composite variable of COPD, CHD, or ever self-reporting poor or fair health prior to baseline, and alternatively, we restricted the analysis to participants with self-reported good or excellent health, no CHD and no COPD. We also explored for potential smoking and sex, and smoking and race interactions, by including interaction terms in the models.
Missing covariate data were imputed using multiple imputation using chained equations (MICE) with 10 sets of imputations.21 The imputation model included all covariates in the final, fully-adjusted model, as well as apolipoprotein ε4 genotype, cognitive function and history of stroke.
Two sensitivity analyses were conducted. To address possible bias in ascertaining cases using only hospital codes, we censored individuals after a hospitalization for comorbidities related to cigarette smoking, including cerebrovascular (ICD-9 430–438) or ischemic heart disease (410–414). Also, because current smokers had lower cognitive scores at baseline, they may have been more likely than non-smokers to be diagnosed with dementia assessed at a single point in time (the hospital visit) if that assessment did not include accurate knowledge of prior cognitive performance. To address this possible diagnostic bias, we adjusted for midlife cognitive test scores (visit 2, 1990–92).
Given the strong association of current smoking with mortality, and to aid in the interpretation of the estimated cause-specific hazards of the relationship between smoking and dementia, we conducted a secondary competing risks analysis of smoking and non-dementia deaths using Cox proportional hazards models, treating dementia prior to death as a censoring event.22
Linear regression was used to model the association between smoking status and cognitive change from 1996–98 to 2011–13. Analyses were adjusted for age (linear and quadratic terms), sex, education, a combination variable of race and study site, BMI, history of stroke, diabetes, hypertension and COPD.
Because attrition was strongly related both to smoking status and to cognitive performance, to address potential selection bias for the smoking-cognitive decline analysis, we used MICE to impute missing covariate and cognitive scores. Cognitive scores were imputed at the median follow-up time for participants who were alive at the time of the visit, but did not complete cognitive testing. MICE models included all covariates in the fully-adjusted final model, as well as apolipoprotein ε4 genotype, the TICS,23 suspect dementia status,21 and CDR24. This method has been described and validated in this cohort.21
Analyses were conducted in Stata 15 (StataCorp. 2013. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP).
RESULTS
Compared to participants included in the analysis, excluded participants were older (66 vs. 63 years); more likely to be black (34% vs. 25%), male (54% vs. 43%), an ever smoker (61% vs. 56%) and to die during follow-up (86% vs. 20%); and more likely to have less than a high school education (36% vs. 21%), diabetes (58% vs. 19%), hypertension (83% vs. 51%), and chronic obstructive pulmonary disease (28% vs. 9%) (Supplementary Table S1).
Of 13002 participants, 5784 (44%), 5346 (41%) and 1872 (14%) were never, former and current cigarette smokers at baseline (visit 4), respectively (Table 1). 70% of never smokers were female compared to 46% of ever smokers. Most former smokers (79%) quit ≥ 9 years prior to baseline. Current smokers and former smokers who quit recently (<9 years prior to baseline) were more likely to have less than a high school education (30% and 26%, respectively, vs. 19% for never smokers) and to have COPD (15% and 14% respectively, vs. 6% for never smokers). Consistent with the hypothesis that health concerns may be associated with cessation, compared to participants who recently quit (<9 years prior to baseline), a somewhat lower proportion of current smokers had hypertension (50% vs. 52%) and diabetes (17% vs. 23%); current smokers also had a lower BMI (27±5 vs 29±6 kg/m2). 20% of participants died during follow-up; current smokers (32%) and former smokers who quit recently (29%) were more likely to die than participants who were never smokers (15%) or who quit ≥9 years prior to baseline (20%) (Table 1).
Table 1.
Participant Characteristics by Smoking Status (visit 4, 1996–98), Atherosclerosis Risk in Communities (ARIC) Neurocognitive Studya
| Total Cohort (N=13002) | Smoking Status | P-valueb | ||||
|---|---|---|---|---|---|---|
| Never (N=5784) | Former (N=5898) | Current (N=1872) | ||||
| Quit ≥ 9 years ago (N=4235) | Quit < 9 years ago (N=1111) | |||||
| Age (years), mean (SD) | 63.0 (5.7) | 63.1 (5.7) | 63.6 (5.7) | 62.9 (5.4) | 61.7 (5.4) | <0.0001 |
| Black race, N(%) | 3304 (25) | 1659 (29) | 783 (18) | 312 (28) | 550 (29) | <0.0001 |
| Female, N(%) | 7427 (57) | 4071 (70) | 1697 (40) | 622 (56) | 1037 (55) | <0.0001 |
| Education, N(%) | ||||||
| < High school | 2773 (21) | 1111 (19) | 819 (19) | 292 (26) | 551 (30) | <0.0001 |
| High school or equivalent | 5364 (41) | 2440 (42) | 1676 (40) | 478 (43) | 770 (41) | |
| > High school | 4847 (37) | 2224 (39) | 1736 (41) | 340 (31) | 547 (29) | |
| BMI (kg/m2) | 28.8 (5.9) | 29.2 (5.8) | 30.0 (5.3) | 29.2 (5.6) | 27.0 (5.2) | <0.0001 |
| Diabetes, N(%) | 2111 (19) | 945 (19) | 718 (19) | 204 (23) | 244 (17) | 0.007 |
| Hypertensive Status, N(%) | ||||||
| Normotensive | 5315 (45) | 2391 (44) | 1719 (44) | 431 (44) | 774 (49) | <0.0001 |
| Prehypertension | 429 (4) | 180 (3) | 183 (5) | 42 (4) | 24 (2) | |
| Hypertension | 6085 (51) | 2805 (52) | 1987 (51) | 509 (52) | 784 (50) | |
| COPD, N(%) | 928 (9) | 294 (6) | 303 (8) | 123 (14) | 208 (15) | <0.0001 |
| ≥1 APOE ε4 allele | 3837 (31) | 1686 (30) | 1216 (30) | 355 (33) | 580 (32) | 0.081 |
| Follow-up Status, N(%) | ||||||
| Died during follow-up | 2644 (20) | 878 (15) | 855 (20) | 320 (29) | 591 (32) | <0.0001 |
| Living, did not attend Visit 5 | 4492 (35) | 2021 (35) | 1412 (33) | 381 (34) | 678 (36) | |
| Attended Visit 5 | 5866 (45) | 2885 (50) | 1968 (46) | 410 (37) | 603 (32) | |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; APOE, apolipoprotein
Restricted to participants with complete covariate data. Analysis includes imputed exposure data: education, N=18; diabetes, N=1892; hypertension, N=1173; APOE ε4, N=435
P-values from Kruskal-Wallis test (continuous variables) or Pearson’s chi2 test (categorical variables)
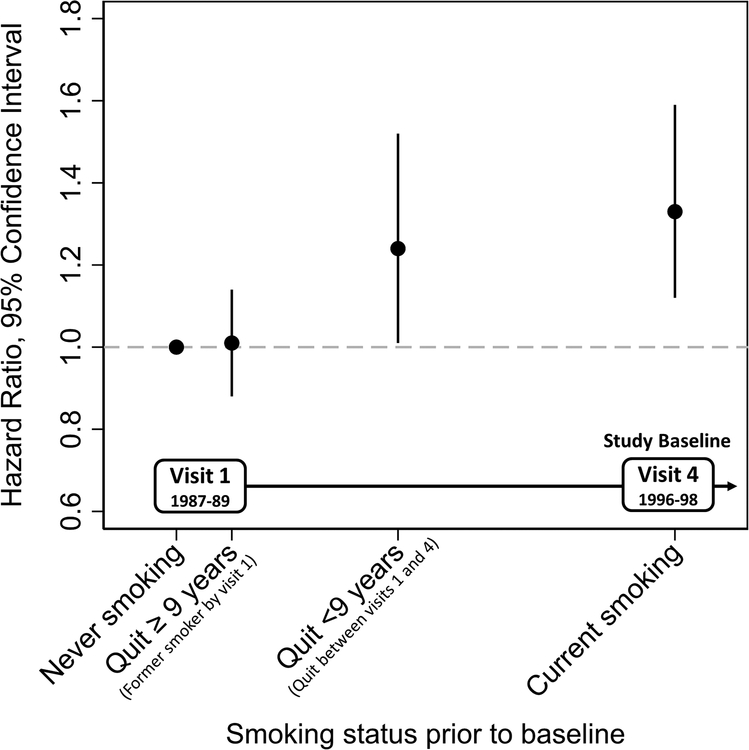
1347 (10%) participants developed dementia during follow-up (Table 2, Model 3). After full adjustment, recent quitting and current smoking were both associated with increased risk of all-cause dementia. In models using imputed covariate data, the hazard ratio (HR) comparing recent quitting to never smoking was 1.24 [95% Confidence Interval (CI): 1.01, 1.52] and comparing current to never smokers was 1.33 (95% CI: 1.12, 1.59) (Table 2, Model 3, Figure 2). In analyses restricted to participants with complete covariate data (Model 2), estimates were similar in magnitude. To account for smoking cessation related to poor health due to smoking, we adjusted for COPD, CHD and self-reported health. Estimates in these models were attenuated slightly (Model 4). When the analysis was restricted to participants without CHD and COPD who self-reported good or excellent health (Model 5), compared to never smokers, participants who quit recently had 1.34 times the hazard of developing dementia (95% CI: 0.91, 1.99) and current smokers had 1.40 times the hazard (95% CI: 1.02, 1.92) of developing dementia. Estimates were similar when the time of the lag between smoking status and start of dementia follow-up was varied (Supplementary Table S2). Interaction terms for race and sex were not statistically significant.
Table 2.
Multivariable-adjusted Hazard Ratios and 95% Confidence Intervals (CIs) of Dementia (2001–2013) by Smoking Status (1996–98), Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study, N=13002
| Smoking Status | ||||||
|---|---|---|---|---|---|---|
| Never | Former | Current | ||||
| Quit ≥ 9 years ago | Quit < 9 years ago | |||||
| Primary Analysis | MODEL 1 | N dementia / N total | 501 / 4893 | 363 / 3564 | 80 / 857 | 127 / 1362 |
| HR (95% CI) | Ref | 1.05 (0.91, 1.20) | 1.18 (0.93, 1.49) | 1.37 (1.12, 1.67) | ||
| P-value | -- | 0.535 | 0.183 | 0.002 | ||
| MODEL 2 | N dementia / N total | 501 / 4893 | 363 / 3564 | 80 / 857 | 127 / 1362 | |
| HR (95% CI) | Ref | 1.05 (0.91, 1.21) | 1.19 (0.94, 1.51) | 1.40 (1.15, 1.72) | ||
| P-value | -- | 0.486 | 0.148 | 0.001 | ||
| MODEL 3 | N dementia / N total | 632 / 5784 | 432 / 4235 | 112 / 1111 | 171 / 1872 | |
| HR (95% CI) | Ref | 1.01 (0.88, 1.14) | 1.24 (1.01, 1.52) | 1.33 (1.12, 1.59) | ||
| P-value | -- | 0.938 | 0.037 | 0.001 | ||
| MODEL 4 | N dementia / N total | 632 / 5784 | 432 / 4235 | 112 / 1111 | 171 / 1872 | |
| HR (95% CI) | Ref | 0.99 (0.87, 1.13) | 1.19 (0.97, 1.46) | 1.29 (1.08, 1.54) | ||
| P-value | -- | 0.908 | 0.089 | 0.005 | ||
| MODEL 5 | N dementia / N total | 212 / 2816 | 167 / 1996 | 29 /392 | 49 / 654 | |
| HR (95% CI) | Ref | 1.13 (0.91, 1.39) | 1.34 (0.91, 1.99) | 1.40 (1.02, 1.92) | ||
| P-value | -- | 0.271 | 0.139 | 0.039 | ||
| Sensitivity Analyses | MODEL 6 Ascertainment Bias | N dementia / N total | 186 / 3500 | 124 / 2373 | 37 / 603 | 52 / 1126 |
| HR (95% CI) | Ref | 1.06 (0.84, 1.35) | 1.51 (1.05, 2.16) | 1.27 (0.92, 1.75) | ||
| P-value | -- | 0.625 | 0.026 | 0.143 | ||
| MODEL 7 Diagnostic Bias | N dementia / N total | 632 / 5784 | 432 / 4235 | 112 / 1111 | 171 / 1872 | |
| HR (95% CI) | Ref | 1.00 (0.88, 1.14) | 1.23 (1.00, 1.51) | 1.27 (1.07, 1.52) | ||
| P-value | -- | 0.958 | 0.045 | 0.008 | ||
Model 1, N=10676, Available case (restricted to participants with complete data). Adjusted for demographic factors, including age (years), age2 (years2), sex, education, and race*center interaction.
Model 2, N=10676, Available case (restricted to participants with complete data). Adjusted forModel 1 covariates as well as BMI, diabetes, hypertensive status; time-varying covariates measured in 1996–98.
Model 3, N=13002, Imputation model. Adjusted for Model 2 covariates. Model includes imputed covariate data (BMI, N=2196; diabetes, N=1892; hypertension, 24N=1173; and COPD, N=2178).
Model 4, N=13002, Imputation model. Adjusted for Model 2 covariates plus a composite variable of ever reporting fair or poor health prior to visit 4, presence of COPD or CHD at visit 4. Model includes imputed covariate data.
Model 5, N=5858, Imputation model. Restricted to participants with good health (did not ever self-report fair or poor health prior to baseline, did not have COPD or CHD at visit 4). Adjusted for Model 2 covariates. Model includes imputed covariate data.
Model 6, Ascertainment bias, N=7602, Participants censored following a hospitalization with an ischemic heart disease or cerebrovascular disease code. Adjusted for Model 2 covariates. Model includes imputed covariate data.
Model 7, Diagnostic bias, N=13002, Adjusted for Model 2 covariates and for Visit 2 cognition. Model includes imputed covariate data.
Figure 2. Hazard Ratios and 95% Confidence Intervals (CIs) of Dementia (2001–2013) by Smoking Status (1996–98), Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study, N=13002.
Adjusted for age (years), age2 (years2), sex, education, race*center interaction, BMI, diabetes, hypertensive status; time-varying covariates measured in 1996–98.
In a sensitivity analysis that censored participants following a hospitalization for cerebrovascular or ischemic heart disease, the HR for incident dementia associated with recent quitting was 1.51 (95% CI: 1.05, 2.16) and with current smoking, 1.27 (95% CI: 0.92, 1.75) (Table 2, Model 6). When models were adjusted for midlife cognition (1990–92), the relationship between recent quitting (vs. never smoking) and current smoking (vs. never smoking) was 1.23 (95% CI: 1.00, 1.51) and 1.27 (95% CI: 1.07, 1.52), respectively (Table 2, Model 7).
17% (N=2266) of participants died during follow-up. In the competing risks analysis, compared to never smoking, the HR for mortality for quitting ≥9 years prior to baseline was 1.18 (95% CI: 1.06, 1.33), for more recent quitting was 1.96 (95% CI: 1.72, 2.24), and for current smoking was 2.61 (95% CI: 2.35, 2.93) (Supplemenatry Table S3, Model 2).
In analyses restricted to participants with complete data (N=5434), rates of 15-year global cognitive decline did not differ by smoking status (Table 3, Available case). Estimated absolute rates of change were greater in models accounting for informatively missing cognitive data (N=11322) in all exposure groups, but differences between groups remained small and non-significant (Table 3, Imputation model). Results were similar and also not statistically significant for each cognitive test (Supplementary Table S4).
Table 3.
Multivariable-adjusteda Estimates and Differences in Estimates in the 15-Year Rates of Cognitive Change in Global Function (1996–98 to 2011–13) by Smoking Status (1996–98), Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study, N=11322
| Smoking Status | AVAILABLE CASE (N=5434) | IMPUTATION MODEL (N=11322) | |||
|---|---|---|---|---|---|
| Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | ||
| Average Rates of Change over Time by Smoking Status Category | |||||
| Never (N=5763) | −0.563 (−0.646, −0.480) | <0.0001 | −0.704 (−0.815, −0.592) | <0.0001 | |
| Former (N=5809) | Quit ≥ 9 years ago (N=4356) | −0.596 (−0.676, −0.515) | <0.0001 | −0.699 (−0.802, −0.596) | <0.0001 |
| Quit < 9 years ago (N=1128) | −0.578 (−0.681, −0.475) | <0.0001 | −0.723 (−0.853, −0.594) | <0.0001 | |
| Current (N=1853) | −0.613 (−0.708, −0.520) | <0.0001 | −0.746 (−0.867, −0.626) | <0.0001 | |
| Difference in Average Rates of Change over Time Compared to Never Smoking | |||||
| Difference comparing Former (Quit ≥ 9 years ago) to Never | −0.032 (−0.076, 0.011) | 0.144 | 0.005 (−0.040, 0.050) | 0.826 | |
| Difference comparing Former (Quit <9 years ago) to Never | −0.015 (−0.091, 0.061) | 0.698 | −0.020 (−0.106, 0.067) | 0.650 | |
| Difference comparing Current to Never | −0.050 (−0.117, 0.016) | 0.139 | −0.043 (−0.129, 0.043) | 0.318 | |
Available case, Analysis includes only participants with complete data, N=5434: includes only participants with available covariate data
Imputation model, Analysis with imputed values for missing cognitive outcome and covariate data, N=11322. Imputed covariate data includes BMI, N=1883; history of stroke, N=1869; diabetes, N=1499; hypertension, N=1899; and COPD, N=1830.
ARIC visit 4 and visit 5 global z-scores were imputed for participants with an observed Visit 2 global z-score who were missing a global z-score at visit 4 or 5 (but not at both visits) but who were alive at the start of data collection for the visit.
Adjusted for age (years), age2 (years2), sex, education, race*center, BMI, history of stroke, diabetes, hypertension, and COPD; all time-varying covariates measured in 1996–98.
DISCUSSION
In 13002 men and women (aged 52–75 years, 25% African American), current cigarette smoking and recent cessation (<9 years) were associated with increased risk of all-cause dementia over 12 years in a dose-dependent manner: 33% and 24%, respectively. There was no increased dementia risk in persons who quit ≥ 9 years prior to when smoking was measured (at a mean age of 54 years). Associations with quitting were robust to adjustment for diagnoses of CHD, COPD and self-reported poor or fair health. We did not find that the smoking-dementia association varied by sex or race but may have been underpowered to detect these associations.
Our findings are consistent with previous studies showing a ~30% increase in all-cause dementia risk in older adults associated with current smoking.12,25,26 Meta-analyses of longitudinal studies suggest a stronger association with probable Alzheimer’s disease than for other dementias –a 40–80% increase in risk,5,12,26 and it has been estimated that 5–14% of Alzheimer’s disease worldwide may be attributable to smoking.5,27 Current smoking may also be associated with vascular dementia.26 However, findings from individual studies often are not statistically significant,25 and are often based on a small number of clinically diagnosed vascular dementia cases, a condition which is generally underdiagnosed.28 It is a limitation of our study that etiologic diagnoses were not assigned for all dementia cases, and that we cannot stratify by etiologic subtype.
Most studies fail to show a relationship between former smoking and increased dementia risk,25,26 and a very limited number of longitudinal studies have investigated the impact of smoking cessation on dementia risk. In 46,140 men aged 60 years or older in in the Korean National Health Insurance System - National Health Screening Cohort, nonsmokers and participants who quit four or more years prior to baseline had a 19% and 14% decreased risk of Alzheimer’s disease over 8 years, respectively, and a 29% and 32% decreased risk of vascular dementia, respectively.29 In our study, we found that risk depended on time since smoking cessation. When assessed at a mean of 63 years, recent quitters had a 24% increased dementia risk compared to never smokers, but those who had quit ≥9 years prior to baseline had the same risk of dementia as never smokers, although we did find an 18% increase in mortality for that group. Our study adds to the literature by explicitly testing the relationship between time since quitting and incident dementia.
Smoking is related to hypertension, cardiovascular disease, stroke, incidence and progression of cerebrovascular small vessel lesions, including white matter hyperintensities,30–32 and possibly increased number of neuritic plaques on autopsy.33 A recent systematic review also concluded that cigarette smoking is associated with Alzheimer’s disease specifically, potentially through increased cerebral oxidative stress.34 Because most of the studies included in that review primarily relied on clinical diagnoses of Alzheimer’s disease, concomitant cerebrovascular pathology could potentially help to explain the association. However, the authors also conducted a study showing that a history of smoking was associated with increased cortical amyloid deposition compared to never smoking in 263 cognitively normal older adults.34
Although interventional studies of smoking cessation on dementia risk are limited,10 cardiovascular and cerebrovascular risk is known to decrease rapidly with cessation.35 Cessation seems also to be related to diminishing dementia risk over time, which may help to explain the general lack of an association with former smoking in the literature, particularly when smoking history is assessed in late life (i.e., at a greater time since quitting for former smokers). Our study highlights the importance of early midlife cessation to decrease dementia risk.
For participants who attended visit 5 (2011–13), dementia diagnosis incorporated longitudinal cognitive data. However, only 45% of participants attended visit 5 and importantly, death rates differed by smoking status – 32% of current smokers died compared to only 15% of never smokers. Therefore, for participants who did not attend visit 5, auxiliary cognitive information (TICS, CDR) and community-wide hospital and death certificate surveillance were used to ascertain dementia cases. Although the availability of multiple sources of data and the ability to assign dementia diagnoses for all participants - not just those who survived to visit 5 - is a strength of our study, it should be acknowledged that a potential limitation is that the auxiliary cognitive information (TICS, hospital and death certificate codes) is likely an insensitive measure of dementia diagnosis. There may also be a few false-positive hospital codes.16 Additionally, another possible limitation of our study is that the use of hospitalization codes might overestimate the association between smoking and dementia, simply because smokers are more likely to have the opportunity to be hospitalized,36 and therefore to be diagnosed. Associations were robust to censoring following a hospitalization for ischemic heart disease or cerebrovascular disease, however. Additionally, because current smokers had lower cognitive scores at baseline, they may have been more likely than non-smokers to be diagnosed with dementia at a single (hospital) visit (i.e., a diagnostic bias). However, when we adjusted for midlife cognition (1990–92), although attenuated by ~20%, estimates remained significant. Diagnostic bias could therefore account for a proportion of the risk associated with smoking. Alternatively, smoking-dementia association may be due in part to some cognitive deficits related to smoking already present at study baseline.
To investigate the etiologic association between smoking and dementia, our primary analysis estimated the cause-specific hazard of dementia prior to death by smoking status. However, our estimate cannot be interpreted as the predicted risk of dementia over time, because whether dementia occurs depends both on the risk for dementia as well as for competing events, including death. To aid in the interpretation of our findings, therefore, we conducted a secondary analysis to estimate the cause-specific hazard of death without dementia.22 Associations were stronger than the estimated associations for the dementia analysis, suggesting that that differential survival by smoking status may have had a substantial impact on the time smokers were at-risk for dementia compared to non-smokers.
Contrary to our hypothesis, we found no relationship between smoking and cognitive decline. Cognitive decline is associated with death and dropout, and attrition is also strongly associated with smoking. We therefore expected that available-case analyses would underestimate associations with decline (although not for dementia, as we believe our ascertainment is likely to be nearly complete). When multiple imputation was used to address selection bias due to informative attrition, absolute rates of cognitive decline were greater than in available-case analyses. However, we again found no difference between rates of change by smoking status. MICE imputation strengthened effect estimates of cognitive decline for other exposures in this cohort (e.g., diabetes,19 retinal signs37), yet did not for smoking in this analysis. One possible explanation of this finding is that smoking is analogous to education. Some factors, such as education, are related to dementia risk, but not with rates of cognitive decline.38 Education is strongly related to pre-morbid cognitive ability; thus, even in the absence of an effect on cognitive decline, persons with lower pre-morbid cognitive ability likely reach the threshold for dementia earlier than those with higher pre-morbid cognitive ability. Given the social and cultural concomitants of smoking, it may also be strongly related to premorbid ability. However, when we adjusted for baseline global cognition, we found little attenuation of the relationship between smoking and dementia. A second explanation for this finding could be that MICE methods were insufficient given the strong association of current smoking with attrition. Current smokers who survived had better cognitive health than smokers who died, so that the imputation, which is necessarily based on living, observed cases, may have underestimated the attrition bias.21 This concern may help explain mixed findings in other studies,11, 39, 40 and is supported by a study that found rates of cognitive decline to be strengthened using inverse probably of attrition weighting.13
This study documented a dose-dependent association between cigarette smoking assessed at a mean age 63 years (range 52 to 75) and greater 12-year risk of incident dementia in a biracial cohort of 13002 men and women from four US communities. Although quitting at any time may be beneficial – risk in recent quitters was reduced by 24% compared to current smokers – quitting early in midlife may be especially beneficial, as the risk in persons who quit at least 9 years earlier did not differ from the risk in never smokers. Although there is consensus that smoking cessation is beneficial for brain health, there is a lack of evidence as to when cessation should occur in order to minimize dementia risk. Our findings support current recommendations for smoking cessation for dementia prevention, and inform clinical practice and public health messaging about the importance of early smoking cessation to reduce dementia risk.
Supplementary Material
Supplementary Table S1. Characteristicsa of Participants Included in and Excluded from the Analytic Sample, Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study, N=15792
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; APOE, apolipoprotein
a Time-varying covariates measured at the ARIC study baseline visit (1987–89) except for COPD, which was measured at visit 2 (1990–92)
b P-values from Kruskal-Wallis test (continuous variables) or Pearson’s chi2 test (categorical variables)
Supplementary Table S2. Multivariable-adjusteda Hazard Ratios and 95% Confidence Intervals (CIs) of Dementia by Smoking Status (1996–98) with 1-year (N=13984), 8-Year (N=12118) and 10-Year (N=11416) Time Lags between Smoking and Start of Dementia Follow-up, Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study
a Adjusted for age (years), age2 (years2), sex, education, race*center interaction, BMI, diabetes, hypertensive status; time-varying covariates measured in 1996–98. Models include imputed covariate data. Except for the timing of the lag post baseline (visit 4), these models are equivalent to Model 3 from Table 3.
Supplementary Table S3. Competing Risks Analysisa: Multivariable-adjusted Hazard Ratios and 95% Confidence Intervals (CIs) of Death (2001–2013) without Dementia by Smoking Status (1996–98), Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study, N=13002
Model 1, N=10673, Available case. Adjusted for demographics, including for age (years), age2 (years2), sex, education and race*center interaction
Model 2, N=10673, Available case. Adjusted Model 1 covariates as well as BMI, diabetes, hypertensive status; time-varying covariates measured in 1996–98.
Model 3, N=13002, Imputation model. Adjusted for Model 2 covariates. Model includes imputed covariate data.
Model 4, N=13002, Imputation model. Adjusted for Model 2 covariates plus a composite variable of ever reporting fair or poor health prior to baseline or presence of COPD at baseline. Model includes imputed covariate data.
Model 5, N=6138, Imputation model. Restricted to participants with good health (did not ever self-report fair or poor health prior to baseline, did not have COPD at baseline). Adjusted for Model 2 covariates. Model includes imputed covariate data.
a Models are equivalent to models in Table 3 except that the outcome is time to dementia-free death. Participants were censored at the time of dementia diagnosis.
Supplementary Table S4. Multivariable-adjusteda Differences in Estimates in the 15-Year Rates of Cognitive Change in Standardized Cognitive Test Scores (1996–98 to 2011–13) by Smoking Status (1996–98), Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study, N=5434
Abbreviations: DSST, Digit Symbol Substitution Test; DWRT, Delayed Word Recall Test; WFT, Word Fluency Test
a Adjusted for age (years), age2 (years2), sex, education, race*center, BMI, history of stroke, diabetes, hypertension, and COPD; all time-varying covariates measured in 1996–98.
ACKNOWLEDGEMENTS
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data is collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. The authors thank the staff and participants of the ARIC study for their important contributions. Dr. Deal was supported by NIH/NIA grant K01AG054693. Dr. Schneider was supported by the NIH/NINDS through an administrative supplement to award R25NS065729. Dr. Palta was supported by NIH/NIA grant K99AG052830.
Sponsor’s Role: The National Institutes of Health funded the study but otherwise did not participate in the study activities, including its design, methods, analysis, interpretation or preparation of the article.
Footnotes
Conflict of Interest: The authors do not have any relevant conflicts of interest.
REFERENCES
- 1.Hackshaw A, Morris JK, Boniface S, Tang JL, Milenkovic D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018;360:j5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. [DOI] [PubMed] [Google Scholar]
- 4.Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of ischemic stroke risk in the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2004;160:259–269. [DOI] [PubMed] [Google Scholar]
- 5.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 6.Deckers K, van Boxtel MP, Schiepers OJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30:234–246. [DOI] [PubMed] [Google Scholar]
- 7.IOM (Institute of Medicine). Cognitive Aging: Progress in Understanding and Opportunites for Action. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 8.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015;11:718–726. [DOI] [PubMed] [Google Scholar]
- 9.Cataldo JK, Glantz SA. Smoking cessation and Alzheimer’s disease: facts, fallacies and promise. Expert Rev Neurother. 2010;10:629–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kane RL, Butler M, Fink HA, et al. Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer’s-Type Dementia. Comparative Effectiveness Review no. 188. (Prepared by the Minnesota Evidence-Based Practice Center Under Contract no. 290-2015-00008-I.). Rockville, MD: Agency for Healthcare Research and Quality: AHRQ Publication No. 17-EHC008-EF; March 2017. [PubMed] [Google Scholar]
- 11.Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36–2318–8–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166:367–378. [DOI] [PubMed] [Google Scholar]
- 13.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabia S, Elbaz A, Dugravot A, et al. Impact of smoking on cognitive decline in early old age: the Whitehall II cohort study. Arch Gen Psychiatry. 2012;69:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 16.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild Cognitive Impairment and Dementia Prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottesman RF, Albert M, Alonso A, et al. Midlife vascular risk factors and incident dementia in the ARIC cohort. JAMA Neurology. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottesman RF, Schneider AL, Albert M, et al. Midlife Hypertension and 20-Year Cognitive Change: The Atherosclerosis Risk in Communities Neurocognitive Study. JAMA Neurol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawlings AM, Sharrett AR, Schneider AL, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81;515–526. [Google Scholar]
- 21.Rawlings AM, Sang Y, Sharrett AR, et al. Multiple imputation of cognitive performance as a repeatedly measured outcome. Eur J Epidemiol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. [DOI] [PubMed] [Google Scholar]
- 23.Brandt J, Spencer M, Folstein MF. The telephone interview for cognitive status. Neuropsychiatry, neuropsychology, and behavioral neurology. 1988;1:111–117. [Google Scholar]
- 24.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 25.Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D. Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology. 2017;61:143–187. [DOI] [PubMed] [Google Scholar]
- 26.Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One. 2015;10:e0118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. [DOI] [PubMed] [Google Scholar]
- 28.Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi D, Choi S, Park SM. Effect of smoking cessation on the risk of dementia: a longitudinal study. Ann Clin Transl Neurol. 2018; 5:1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Power MC, Deal JA, Sharrett AR, et al. Smoking and white matter hyperintensity progression: the ARIC-MRI Study. Neurology. 2015;84:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39:2712–2719. [DOI] [PubMed] [Google Scholar]
- 32.Longstreth WT Jr, Arnold AM, Beauchamp NJ Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. [DOI] [PubMed] [Google Scholar]
- 33.Tyas SL, White LR, Petrovitch H, et al. Mid-life smoking and late-life dementia: the Honolulu-Asia Aging Study. Neurobiol Aging. 2003;24:589–596. [DOI] [PubMed] [Google Scholar]
- 34.Durazzo TC, Mattsson N, Weiner MW, Alzheimer’s Disease Neuroimaging Initiative. Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimer’s & Dementia. 2014;10:S122–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation. 1997;96:1089–1096. [DOI] [PubMed] [Google Scholar]
- 36.Alonso A, Mosley TH Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry. 2009;80:1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deal JA, Sharrett AR, Rawlings AM, et al. Retinal signs and 20-year cognitive decline in the Atherosclerosis Risk in Communities Study. Neurology. 2018;90:e1158–e1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–278. [DOI] [PubMed] [Google Scholar]
- 39.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. [DOI] [PubMed] [Google Scholar]
- 40.Zaninotto P, Batty GD, Allerhand M, Deary IJ. Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English Longitudinal Study of Ageing. J Epidemiol Community Health. 2018; August;72(8):685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Characteristicsa of Participants Included in and Excluded from the Analytic Sample, Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study, N=15792
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; APOE, apolipoprotein
a Time-varying covariates measured at the ARIC study baseline visit (1987–89) except for COPD, which was measured at visit 2 (1990–92)
b P-values from Kruskal-Wallis test (continuous variables) or Pearson’s chi2 test (categorical variables)
Supplementary Table S2. Multivariable-adjusteda Hazard Ratios and 95% Confidence Intervals (CIs) of Dementia by Smoking Status (1996–98) with 1-year (N=13984), 8-Year (N=12118) and 10-Year (N=11416) Time Lags between Smoking and Start of Dementia Follow-up, Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study
a Adjusted for age (years), age2 (years2), sex, education, race*center interaction, BMI, diabetes, hypertensive status; time-varying covariates measured in 1996–98. Models include imputed covariate data. Except for the timing of the lag post baseline (visit 4), these models are equivalent to Model 3 from Table 3.
Supplementary Table S3. Competing Risks Analysisa: Multivariable-adjusted Hazard Ratios and 95% Confidence Intervals (CIs) of Death (2001–2013) without Dementia by Smoking Status (1996–98), Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study, N=13002
Model 1, N=10673, Available case. Adjusted for demographics, including for age (years), age2 (years2), sex, education and race*center interaction
Model 2, N=10673, Available case. Adjusted Model 1 covariates as well as BMI, diabetes, hypertensive status; time-varying covariates measured in 1996–98.
Model 3, N=13002, Imputation model. Adjusted for Model 2 covariates. Model includes imputed covariate data.
Model 4, N=13002, Imputation model. Adjusted for Model 2 covariates plus a composite variable of ever reporting fair or poor health prior to baseline or presence of COPD at baseline. Model includes imputed covariate data.
Model 5, N=6138, Imputation model. Restricted to participants with good health (did not ever self-report fair or poor health prior to baseline, did not have COPD at baseline). Adjusted for Model 2 covariates. Model includes imputed covariate data.
a Models are equivalent to models in Table 3 except that the outcome is time to dementia-free death. Participants were censored at the time of dementia diagnosis.
Supplementary Table S4. Multivariable-adjusteda Differences in Estimates in the 15-Year Rates of Cognitive Change in Standardized Cognitive Test Scores (1996–98 to 2011–13) by Smoking Status (1996–98), Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study, N=5434
Abbreviations: DSST, Digit Symbol Substitution Test; DWRT, Delayed Word Recall Test; WFT, Word Fluency Test
a Adjusted for age (years), age2 (years2), sex, education, race*center, BMI, history of stroke, diabetes, hypertension, and COPD; all time-varying covariates measured in 1996–98.