Abstract
Background:
Neuromyelitis optica (NMO) is an autoimmune demyelinating disease of the central nervous system, characterized by optic neuritis and longitudinally extensive transverse myelitis. Magnetic resonance imaging abnormalities may be observed in various brain regions of NMO patients. Only a few studies have addressed the cognitive function in NMOSD, but none among Egyptian patients.
Objective:
To investigate cognitive performance in a cohort of 20 Egyptian patients with NMOSD.
Design:
Observational, prospective study.
Patients:
We studied 20 Egyptian patients with NMOSD and compared them with 18 healthy Egyptian controls matched for age, sex, and educational level.
Main Outcome Measure:
We applied an Arabic translation of MOCA and BICAMS Tests for Multiple Sclerosis.
Results:
Cognitive performance was significantly worse in the NMOSD group than in healthy controls for CVLT (P=0.0099), SDMT (P = 0.0112), BVSMT (P = 0.019) and BICAMS in total (P= 0.0014). Patients with a later disease onset performed worse in MOCA and BVSMT.
Conclusions:
This study confirms the concept of cognitive involvement in NMOSD among Egyptian patients. Information processing speed was the function most commonly impaired.
Keywords: Neuromyelitis optica spectrum disorder, cognitive function, optic neuritis, transverse myelitis, Egypt
Introduction.
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune demyelinating central nervous system disease. It bears resemblance to multiple sclerosis (MS) and for a long time was considered as a subtype of MS. The detection of serum aquaporin-4 antibodies (AQP4) has reliably distinguished NMOSD from MS (1-3), and is detected in more than 70% of NMOSD patients.(4) A subset of AQP4-seronegative NMOSD patients test positive for another antibody, myelin oligodendrocyte glycoprotein (MOG), with a similar phenotype but, a milder disease course (5, 6) and possibly less cognitive impairment.(7)
Although NMOSD primarily targets the optic nerves and spinal cord, recent studies showed that the brain can be affected in specific areas that might aid in the distinction from MS.(1, 2) In NMOSD, cognitive impairment may result from focal reduction in white matter volume, gray matter volume (GMV) or cortical thinning due to immune mediated damage.(8, 9) However, it remains unknown whether this anatomic pathology is associated with cognitive impairment.(10, 11)
Cognitive function in NMOSD has been studied in small case series (12-16). Most of these studies suggest that NMOSD patients have impairment in varied cognitive aspects. The purpose of this study is to study cognitive function in a series of Egyptian NMOSD patients and compare the findings with those previously published in other international NMOSD populations.
Patients and Methods.
In this series, we enrolled the first twenty NMOSD patients who were seen in the neuroimmunology clinic, Elhadara Hospital, University of Alexandria, Egypt, from January 2017 to January 2018. Only patients who fulfilled the 2015 diagnostic criteria of NMOSD were considered. Testing for AQP4-IgG was performed using ELISA technique (ElisaRSR AQP4 Ab Version2) and results were recorded as seropositive or seronegative. We excluded all patients with a history of psychiatric illness, drug, alcohol abuse, and uncooperative patients. Eighteen healthy controls with matching age, sex, median years of education were recruited from hospital staff and patient’s companions.
Enrolled NMOSD patients completed a battery of validated neurocognitive tests listed below.(17-20) Scores were compared by calculating the mean and standard deviation.(21) Any score two or more standard deviations from the control was considered abnormal. We judged as cognitively impaired patients those whose scores were below the mean by 2 or more standard deviations in two or more cognitive domains. For two patients who could not perform tests due to vision, motor, or proprioceptive impairment, their scores were included as sensory deprivation is a determinant of cognitive function. The study was reviewed and approved by the IRB of the University of Alexandria School of Medicine.
Cognitive testing.
The MOCA (Montreal Cognitive Assessment) test and the BICAMS (Brief International Cognitive Assessment in MS) were performed for all patients during the same session, which was at least a month after a relapse.
MOCA is a rapid screening test, which measures many aspects of the cognition: attention and concentration, executive functions, memory, language, visuoconstructional skills, conceptual thinking, calculations, and orientation. Its score is calculated out of 30, with one point added for 12 years or less of education. BICAMS is a battery of tests used to assess the cognitive function of multiple sclerosis patients and consists of 3 different tests:
The symbol-digit modalities test (SDMT): assesses information processing speed. Participants had to match as quickly as possible as many digits as they can to their corresponding symbols according to a key provided. Performance was assessed by the number of digits correctly paired during the 90 seconds test.
California verbal learning test (CVLT-II): assesses immediate verbal recall. Patients listened to a list of 16 words, then were asked to recall them in no specific order, over 5 trials. Each time the list would be read again. The results were calculated by the total number of words recalled over the 5 trials.
Brief visuospatial memory test (BVSMT-R): tests immediate visual recall. The test was performed by showing the participant 6 abstract designs for 10 seconds, over 3 trials. The participants were asked to recall the designs via paper and pencil and each design receives a score from 0-2 based on accuracy and location. Results were calculated by summing up the score of the 3 trials.
Results.
We enrolled the first 20 NMOSD patients, who met eligibility criteria for this study. The mean age of the cohort was 34.4 years, while the mean age at onset was 27.8 years with an average disease duration of 80 months, range (3 - 216 months). There was an expected strong female predominance with a ratio of 5.6:1. Optic neuritis was the most frequent initial presentation among patients, followed by transverse myelitis. Most relapses were transverse myelitis, and the average relapse rate was 1.6 attacks per year.
Brain MRIs were abnormal in 12 patients during the course of the disease with non-specific white matter changes being the most common – observed in 8 patients, followed by area postrema involvement in four patients. One patient showed an ADEM-like (acute disseminated encephalomyelitis) picture on initial brain MRI.
Aquaporin-4 antibody was tested for all patients. Ten had a positive test result with an average titer of 19.6 U/L (4.9 - 36.9 U/L) and a median of 16 U/L.
Cognitive testing was performed for all 20 patients and 18 healthy controls using MOCA and BICAMS. No significant differences in age, sex, or years of education were noted between the two groups. The control group performed significantly better in the following tests: CVLT (P= 0.0099), SDMT (P= 0.0112), BVSMT (P = 0.019) and BICAMS (P= 0.0014) in total. They also performed non-significantly better in the MOCA as well (P=0.054).
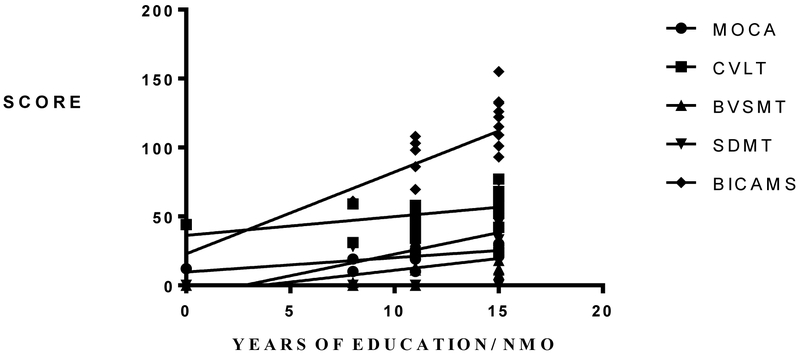
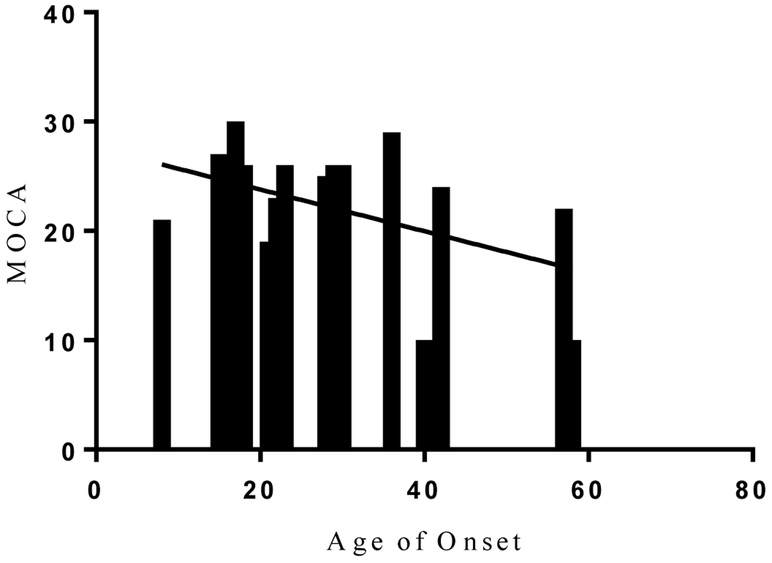
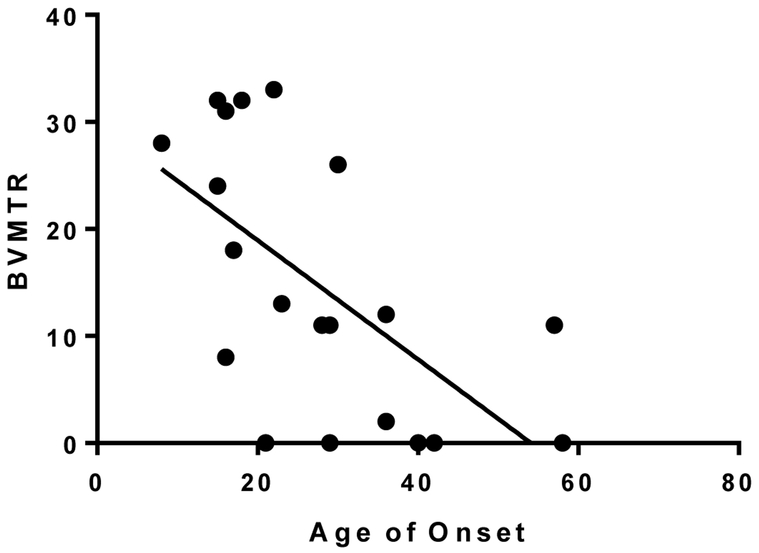
Overall, 75% of the patients had cognitive impairment in two or more of the studied cognitive domains (Table 1). The most frequently impaired function among NMOSD patients was information processing speed (SDMT), where 15 out of 20 patients (75%) had abnormal test results, followed by CVLT (70%), and BVSMT (55%). We found an expected correlation, among all patients, between the median years of education and the cognitive performance in all tests (Figure 1A). Patients with later disease onset had a significantly worse cognitive performance in MOCA and BVSMT (Figure 1B, 1C).
Table 1:
Cognitive testing scores in NMOSD vs controls.
| NMOSD n=20 |
Controls n=18 |
P value | |
|---|---|---|---|
| MOCA | |||
| Mean ± SD | 22.30 ± 1.3 | 25.44 ± 0.8332 | 0.054 |
| Median | 24 | 25 | |
| CVLT | |||
| Mean ± SD | 52.80 ± 2.494 | 61.78 ± 2.093 | 0.0099* |
| Median | 53 | 59 | |
| BVSMT | |||
| Mean ± SD | 14.6 ± 2.781 | 23.06 ±1.899 | 0.019* |
| Median | 11.5 | 26 | |
| SDMT | |||
| Mean ± SD | 29.35 ± 4.424 | 45.78 ±4.214 | 0.0112* |
| Median | 32.5 | 45 | |
| BICAMS | |||
| Mean ± SD | 95.35 ± 6.977 | 1 3 0 .1 ± 7.181 | 0.0014* |
| Median | 99.5 | 131.5 |
Figure 1.
A. Relationship between median years of education and cognitive performance in NMOSD. Patients with a higher median number of years of education performed significantly better in MOCA; P= 0.0032, R2= 0.4611, CVLT; P= 0.021, R2= 0.2144, BVSMT; P = 0.0157, R2= 0.2713, SDMT; P= 0.0016, R2= 0.3588 and total BICAMS score; P= 0.0001, R2 = 0.5113.
B. Relationship between age of onset and MOCA in NMOSD. Patients with a later disease onset performed worse in MOCA testing; P= 0.0467, R2 = 0.202.
C. Relationship between age of onset and BVSMT in NMOSD. Patients with later disease onset performed worse in BVSMT; P= 0.0041, R2 = 0.3753.
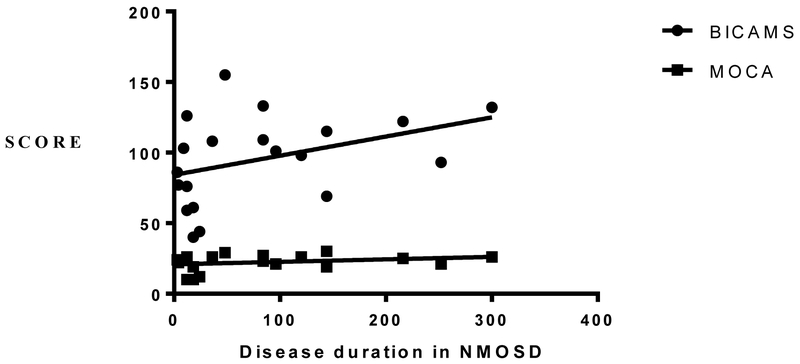
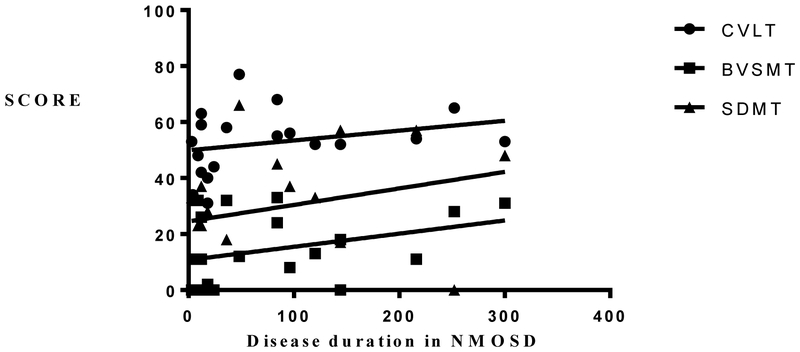
There was also a significant negative correlation between disability level as measured by the Expanded Disability Status Scale (EDSS) and MOCA scores, but not with any other test. There was no significant difference in cognitive performance between those who had evidence of brain disease and those who had none. Despite the wide range of disease duration, there was no significant correlation with cognitive performance (Figure 2A and 2B). When accounting for the serostatus, seropositive NMOSD performed significantly worse in MOCA, BVSMT and BICAMS, compared to the seronegative group (Table 2).
Figure 2.
A. Relationship between disease duration, BICAMS and MOCA in NMOSD. There was no significant correlation between disease duration and BICAMS; P= 0.095, R2 = 0.1506 or MOCA; P = 0.0371, R2 = 0.0729.
2.B. Relationship between disease duration and CVLT, BVSMT and SDMT in NMOSD. There was no significant correlation between disease duration and CVLT; P= 0.182, R2 = 0.0787, BVSMT; P = 0.242, R2 = 0.1135 or SDMT; P= 0.1887, R2 = 0.0704.
Table 2:
Cognitive test scores of seropositive and seronegative NMOSD.
| AQP4-Seropositive n=10 |
AQP4-Seronegative n=10 |
P value | |
|---|---|---|---|
| MOCA | |||
| Mean ± SD | 19.4 ± 2.088 | 25.2 ± 0.9522 | 0.021* |
| Median | 20.5 | 26 | |
| CVLT | |||
| Mean ± SD | 48.7 ± 3.612 | 56.9 ± 3.078 | 0.1011 |
| Median | 52 | 55 | |
| BVSMT | |||
| Mean ± SD | 6.1 ± 2.622 | 23.1 ± 3.118 | 0.0006* |
| Median | 1 | 27 | |
| SDMT | |||
| Mean ± SD | 23.3 ± 5.948 | 35.4 ± 6.256 | 0.1568 |
| Median | 30 | 37 | |
| BICAMS | |||
| Mean ± SD | 75.77 ± 8.636 | 114.2 ± 7.243 | 0.0031* |
| Median | 69.33 | 111.5 |
Discussion.
We characterized the cognitive function of NMOSD in a cohort of Egyptian patients living in Alexandria and nearby governorates (ELbehera, Kafr elsheikh and Matrouh) using a battery of standardized, validated tests. To our knowledge, this is the first cognitive study of NMOSD in Egypt.
Cognitive performance among NMOSD patients in other countries was observed to be less than adequate in many previous studies. One study concluded that both MS and NMO patients performed significantly worse than healthy controls in information processing speed testing as measured by the symbol digit modalities test of Wechsler Adult Intelligence Scale –Revised.(15) In another study in China, NMOSD patients were significantly impaired in the following aspects of cognition; processing speed, verbal and visual memory with the use of the following tests: MOCA, SDMT, CVLT II, and BVSMT.(13)
According to a recent review and meta-analysis of cognitive dysfunction in NMOSD (7), Six studies, which included 213 NMO patients and 171 healthy subjects, demonstrated that NMO patients performed significantly worse on verbal memory testing (CVLT-II) compared with healthy subjects, which is compatible with our results. The same meta-analysis reported that the seven studies, which included 249 NMO patients and 207 healthy subjects, demonstrated that NMO patients were significantly impaired in the information processing speed measured by the SDMT compared with healthy subjects.
The only determinants of cognitive performance among our cohort were the years of education, later disease onset and EDSS. Similar to what was reported by Moore et al, higher EDSS was associated with worse cognitive performance,(12) but that was only for the MOCA scores in our cohort. In contrast, the Chinese study found no correlation between EDSS and cognition. On the other hand, higher median years of education accounted for the better performance among Egyptian and Chinese NMOSD cohort. Disease duration along with brain involvement did not have any significant impact on the cognitive function neither in our study, nor in some previous studies.(12, 13) Patients who had a later onset of their disease tended to perform worse in MOCA and visual memory testing (BVSMT) in our cohort, similar to what was reported by Zhang et al.(13) Unlike what was observed in many previous studies (12, 13), the AQP-4-seropositive patients in our cohort, performed significantly poorer in MOCA, BVSMT and BICAMS than their seronegative peers, but that could be explained by the higher level of education observed coincidentally among the seronegative group.
The most affected domain in our cohort was information processing speed (75%) followed by memory (70%). This finding differ from what was reported by the Chinese study, where memory was the most affected domain, observed in 55.6%, followed by processing speed in 38.9%.(13)
Conclusion.
This is the first cognitive NMOSD study in Egypt. Despite limitations in antibody testing and sample size, our results are consistent with the literature. Our patients exhibited lower cognitive abilities than controls, with decline in information processing speed and memory. Educational level and later disease onset were most relevant with cognition. Further studies with a larger sample size and advanced imaging modalities are recommended to characterize determinants of cognitive impairment among NMOSD patients.
The main limitations of the study are the retrospective design, small number of patients, and the fact that it was conducted at a single center. Further multicenter studies using different testing batteries as well as advanced imaging techniques might aid in understanding the anatomical correlates of cognitive dysfunction in NMOSD patients.
Supplementary Material
Highlights.
Neuromyelitis optica spectrum disorder is understudied in Egypt and North Africa.
High disability and resemblance to multiple sclerosis triggered this study.
Cognitive function in NMOSD is an interesting area of research
Acknowledgments
Funding: This work was supported by a scholarship from the Egyptian ministry of higher education, JS-3725 (SS), as well as a grant from the National Institute of Neurological Disease and Stroke, NS-078555 (ML).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63(7):964–8. [DOI] [PubMed] [Google Scholar]
- 2.Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130(Pt 5):1194–205. [DOI] [PubMed] [Google Scholar]
- 3.Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69(24):2221–31. [DOI] [PubMed] [Google Scholar]
- 4.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–12. [DOI] [PubMed] [Google Scholar]
- 5.Probstel AK, Dornmair K, Bittner R, Sperl P, Jenne D, Magalhaes S, et al. Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology. 2011;77(6):580–8. [DOI] [PubMed] [Google Scholar]
- 6.Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140(12):3128–38. [DOI] [PubMed] [Google Scholar]
- 7.Meng H, Xu J, Pan C, Cheng J, Hu Y, Hong Y, et al. Cognitive dysfunction in adult patients with neuromyelitis optica: a systematic review and meta-analysis. J Neurol. 2017;264(8):1549–58. [DOI] [PubMed] [Google Scholar]
- 8.Saji E, Arakawa M, Yanagawa K, Toyoshima Y, Yokoseki A, Okamoto K, et al. Cognitive impairment and cortical degeneration in neuromyelitis optica. Ann Neurol. 2013;73(1):65–76. [DOI] [PubMed] [Google Scholar]
- 9.Hyun JW, Park G, Kwak K, Jo HJ, Joung A, Kim JH, et al. Deep gray matter atrophy in neuromyelitis optica spectrum disorder and multiple sclerosis. Eur J Neurol. 2017;24(2):437–45. [DOI] [PubMed] [Google Scholar]
- 10.Kim JE, Kim SM, Ahn SW, Lim BC, Chae JH, Hong YH, et al. Brain abnormalities in neuromyelitis optica. J Neurol Sci. 2011;302(1-2):43–8. [DOI] [PubMed] [Google Scholar]
- 11.Filippi M, Rocca MA, Moiola L, Martinelli V, Ghezzi A, Capra R, et al. MRI and magnetization transfer imaging changes in the brain and cervical cord of patients with Devic's neuromyelitis optica. Neurology. 1999;53(8):1705–10. [DOI] [PubMed] [Google Scholar]
- 12.Moore P, Methley A, Pollard C, Mutch K, Hamid S, Elsone L, et al. Cognitive and psychiatric comorbidities in neuromyelitis optica. J Neurol Sci. 2016;360:4–9. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N, Li YJ, Fu Y, Shao JH, Luo LL, Yang L, et al. Cognitive impairment in Chinese neuromyelitis optica. Mult Scler. 2015;21(14):1839–46. [DOI] [PubMed] [Google Scholar]
- 14.Vanotti S, Cores EV, Eizaguirre B, Melamud L, Rey R, Villa A. Cognitive performance of neuromyelitis optica patients: comparison with multiple sclerosis. Arq Neuropsiquiatr. 2013;71(6):357–61. [DOI] [PubMed] [Google Scholar]
- 15.Blanc F, Zephir H, Lebrun C, Labauge P, Castelnovo G, Fleury M, et al. Cognitive functions in neuromyelitis optica. Arch Neurol. 2008;65(1):84–8. [DOI] [PubMed] [Google Scholar]
- 16.Fujimori J, Nakashima I, Baba T, Meguro Y, Ogawa R, Fujihara K. Cognitive impairment in neuromyelitis optica spectrum disorders: A comparison of the Wechsler Adult Intelligence Scale-III and the Wechsler Memory Scale Revised with the Rao Brief Repeatable Neuropsychological Battery. eNeurologicalSci. 2017;9:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. [DOI] [PubMed] [Google Scholar]
- 18.Rahman TT, El Gaafary MM. Montreal Cognitive Assessment Arabic version: reliability and validity prevalence of mild cognitive impairment among elderly attending geriatric clubs in Cairo. Geriatr Gerontol Int. 2009;9(1):54–61. [DOI] [PubMed] [Google Scholar]
- 19.Langdon DW, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult Scler. 2012;18(6):891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reliability of BICAMS (Arabic version) in Egyptian Multiple Sclerosis patients [Internet]. ECTRIMS Online Library. October 26,2017. [Google Scholar]
- 21.Beier M, Gromisch ES, Hughes AJ, Alschuler KN, Madathil R, Chiaravalloti N, et al. Proposed cut scores for tests of the Brief International Cognitive Assessment of Multiple Sclerosis (BICAMS). J Neurol Sci. 2017;381:110–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.