Abstract
Neuron-derived estrogens are synthesized by aromatase and act through membrane receptors to modulate neuronal physiology. In many systems, long-lasting hormone treatments can alter sensory-evoked neuronal activation. However, the significance of acute neuroestrogen production is less understood. Both sexes of zebra finches can synthesize estrogens rapidly in the auditory cortex, yet it is unclear how this modulates neuronal cell signaling. We examined whether acute estrogen synthesis blockade attenuates auditory-induced expression of early growth response 1 (Egr-1) in the auditory cortex of both sexes. cAMP response element-binding protein phosphorylation (pCREB) induction by song stimuli and acute estrogen synthesis was also examined. We administered the aromatase inhibitor fadrozole prior to song exposure and measured Egr-1 across several auditory regions. Fadrozole attenuated Egr-1 in the auditory cortex greater in males than females. Females had greater expression and clustering of aromatase cells than males in high vocal center (HVC) shelf. Auditory-induced Egr-1 expression exhibited a large sex difference following fadrozole treatment. We did not observe changes in pCREB expression with song presentation or aromatase blockade. These findings are consistent with the hypothesis that acute neuroestrogen synthesis can drive downstream transcriptional responses in several cortical auditory regions, and that this mechanism is more prominent in males.
Keywords: estrogens, aromatase, Egr-1, zebra finch, sex differences
Introduction
Estradiol is a potent hormone and neurochemical that mediates sexual differentiation of many brain areas. Estradiol can bind to nuclear receptors to regulate transcriptional events, leading to long-lasting effects on brain and behavior. By contrast, the rapid, non-canonical actions of estradiol and other steroids occur through membrane receptors and intracellular signaling cascades to rapidly change neuronal firing states (Kelly et al. 1976; Teyler et al. 1980; Remage-Healey et al. 2012; Vierk et al. 2012). What is sometimes overlooked is that these non-canonical, activational mechanisms can also enact long-term changes, specifically through activating kinase cascades that target transcriptional mechanisms to change protein expression (Boulware et al. 2005; Park et al. 2011; Micevych et al. 2015). Here, we investigate the relationship between rapid, dynamic changes in endogenous estrogens and auditory-induced immediate-early gene expression in zebra finch auditory cortex. We are particularly interested in understanding how sex operates as a biological variable in auditory activation as well as estrogen production, since both sexes exhibit neuroestrogen synthesis and fluctuation (Callard et al. 1978; Saldanha et al. 2000; Forlano et al. 2005; Peterson et al. 2005; Forlano et al. 2006; Cohen and Wade 2012; Remage-Healey et al. 2012; Chao et al. 2015; Ikeda et al. 2017; Cornil 2018; Kokras et al. 2018).
Neuromodulatory actions of estradiol have been uncovered in many brain regions, such as in the hypothalamus where it controls reproductive behaviors (Balthazart et al. 2009; Micevych et al. 2015) and in hippocampal-dependent memory and neuronal activity in rodents (Woolley 2007; Fernandez et al. 2008; Frick 2013; Tuscher et al. 2016). In zebra finches, estradiol infused into the caudal medial nidopallium (NCM; songbird secondary auditory cortex) enhances auditory evoked neuronal activity in both sexes (Remage-Healey et al. 2010a; Remage-Healey et al. 2012), and estradiol levels rapidly increase when birds hear conspecific song (Remage-Healey et al. 2008). Conversely, inhibition of estrogen synthesis with the aromatase inhibitor fadrozole decreases auditory evoked firing and song preference (Remage-Healey et al. 2010a) further supporting the role of endogenous neuroestrogen synthesis on auditory processing. Together, this work indicates a neuromodulatory role for estradiol in zebra finch audition, and that endogenous estradiol synthesis is likely shaping auditory events.
One marker for auditory-induced neuronal activation of the auditory subregions and memory is the immediate early gene early growth response (Egr-1, also known as ZENK in the songbird literature (Mello et al. 1992)). There has been indirect evidence that rapid estradiol actions mediate auditory-induced Egr-1 expression via non-canonical mechanisms. Long-term implants of estradiol can shift song-induced immediate early gene (IEG) expression in zebra finch auditory forebrain (Maney et al. 2006) in a region dependent manner (Sanford et al. 2010). Chronic administration (6 days) of either estradiol or fadrozole can abrogate the sex difference in Egr-1 expression in the NCM (Lampen et al. 2017). There are indications that chronic fadrozole administration also dampens auditory memory formation (Yoder et al. 2012). One proposed mechanism for fadrozole’s effect on memory is the activation of Egr-1 pathway, supported by studies of Egr-1 in other systems (Knapska and Kaczmarek 2004; Moorman et al. 2011). Further evidence suggests that Egr-1 is regulated by estradiol via non-canonical mechanisms. Egr-1 is part of the MEK-ERK (MAPK/ERK kinase) pathway, and this pathway is necessary for in song-induced expression of Egr-1 in birds (Cheng and Clayton 2004) as well as estradiol induced expression in mammalian in vitro assays (Suva et al. 1991; Pratt et al. 1998; de Jager et al. 2001; Chen et al. 2004). In the songbird forebrain, exogenous estradiol also rapidly regulates song-induced phosphorylation patterns of ERK (extracellular receptor kinase) and CREB (cAMP response element binding protein) in the auditory cortex (Heimovics et al. 2012). Phosphorylated CREB (pCREB) can bind to the promoter of Egr-1 through cAMP response element (CRE) sites (Knapska and Kaczmarek 2004); however, its role in the regulation of auditory-induced Egr-1 expression is unknown in songbirds, and more generally unknown in the mammalian cortex. Furthermore, whether CREB’s role in auditory-induced Egr-1 expression is sex-specific is also unknown.
The zebra finch auditory cortex is structurally similar between males and females, in terms of gross anatomical comparisons, although whether local neuroestrogen synthesis and action is sex-specific is unknown. Somatic aromatase expression in NCM is similar in both sexes (Ikeda et al. 2017); however, males have more aromatase positive in fibers and have more terminal aromatase activity in this region (Saldanha et al. 2000; Peterson et al. 2005). This suggests sex differences in the regulation of rapid neuroestrogen synthesis. Previously, we have published that the organization of aromatase cells into clusters is different between males and females in the ventral NCM. Although the function of somatic clustering is unknown, this may be a way for coordination of neuroestrogen release within local networks. Sex is likely a critical biological variable affecting the sex differences in estrogen production pathways (Ikeda et al. 2017). Peripheral estradiol levels are similar between males and females (Adkins-Regan et al. 1990; Prior et al. 2014) despite the peripheral source from the ovaries in females and central source in males and females (Schlinger and Arnold 1992), indicating that alternative sources, such as brain-expressed aromatase, may be involved in compensating estrogen levels in males (De Vries 2004). Peripheral steroid hormones can have dynamic concentrations in other behavioral states such as stress and aggression (Shors et al. 2009; Heimovics et al. 2016), but in zebra finches, peripheral estradiol levels remain stable in acute timeframes when birds hear song despite concurrent robust changes in estradiol levels within the brain (Remage-Healey et al. 2008). Fecal estrogen analysis indicates elevation of peripheral estradiol in females during song playback (Tchernichovski et al. 1998) over a relatively long-term (several days) timescale. In summary, sex differences in the dynamic, neuronal synthesis of estrogens and the local and acute estrogen signaling in the songbird auditory cortex is not well understood.
Difference in neuroestrogen signaling pathways could reflect the sex differences in auditory processing as well (Krentzel and Remage-Healey 2015). We have previously reported that activation of the membrane G-protein coupled estrogen receptor (GPER-1) can account for sex-specific regulation of auditory responsiveness of NCM neurons (Krentzel et al. 2018). In the mammalian hippocampus, sex-specific regulation of glutamatergic neurotransmission is mediated by multiple membrane estrogen receptors, (Oberlander and Woolley 2016) and rapid estradiol-induced phosphorylation of CREB only occurs in females but not males (Abraham and Herbison 2005; Boulware et al. 2005; Meitzen et al. 2012; Oberlander and Woolley 2016). We therefore sought to understand the sex differences in the role of endogenous estrogen synthesis in the regulation of Egr-1 and CREB, which are two molecules in the same signaling pathway.
We set out to test multiple complimentary hypotheses in this study. First, we tested whether acute estrogen synthesis blockade attenuates auditory-induced Egr-1 expression in subregions of the zebra finch auditory cortex. Second, we tested whether phosphorylation of CREB is similarly regulated by acute estrogen synthesis (in parallel with regulation of Egr-1). Third, we tested whether phosphorylation of CREB is itself inducible by song-exposure. Fourth, we examined the extent to which any of these mechanisms (Egr-1 or pCREB) depend on sex as a biological factor, with the explicit prediction that females are less dependent on acute neuroestrogen synthesis. Finally, we examined aromatase-positive cells, their organization into clusters, and determined whether this differed between males and females in several focal regions of the auditory cortex.
Methods
Animals:
Adult (>120days) male and female zebra finches (Taeniopygia guttata) were raised in a breeding aviary (14L:10D hr light cycle) with food and water available ad libitum. After fledging, birds were moved to single sex cages to mature into adulthood. For all experiments, birds were removed from the aviary ~12–24 hours before manipulations.
Playback design:
Study 1:
Males (n=8) and females (n=8) were isolated from the colony into sound-attenuation chambers (Eckel Industries) for at minimum 12 hr. One hour before playback an oral dose (30μL) of either saline or the aromatase inhibitor fadrozole (1mg/mL (4.47mM); n=4/sex by treatment group) was administered and the animal and a playback speaker were positioned back inside the chamber. Fadrozole and other similar aromatase inhibitors have been used successfully to reduce aromatase activity and neuronal activity (Wade et al. 1994; Saldanha et al. 2004; Alward et al. 2016; Van der Linden and Balthazart 2018) and this dosing through oral administration has been successfully used in prior study to r impair estrogen-dependent behaviors within an acute time course (Rensel et al. 2013). Retrodialysis of fadrozole directly into the auditory cortical subregions has shown that 100 μM of fadrozole is sufficient to reduce estradiol content for up to 1-hour after washout (Remage-Healey et al. 2008). We waited one hour before playback because this is an established timeframe for oral fadrozole pharmacokinetics (Kochak et al. 1990). Previous studies have shown that chronic administration of aromatase inhibitors can change aromatase expression (Foidart et al. 1995); however, given the acute time course between fadrozole bioavailability and sacrifice, we do not expect changes to overall aromatase protein expression. Playback stimuli consisted of novel triplicate song played for 30 minutes (three conspecific songs played back to back (interstimulus interval (ISI): 5 seconds) for ~30 seconds and then 30 seconds of silence, repeated across 30 minutes; ~70dB), which has been validated and replicated to drive elevated Egr-1 expression (Mello et al. 1992). We selected novel songs to avoid influences of song preference. For both sexes, this period was followed by 30 minutes of silence in the dark to avoid male singing and keep song exposure consistent between the sexes (Fig. 1C). Although male singing significantly induces Egr-1 expression in motor nuclei rather than auditory regions which we analyze (Jarvis and Nottebohm 1997), we used this method in attempt to keep this period consistent between sexes. Motor regions such as HVC (high vocal center, new proper name: HVC, Reiner et al. 2004) consistently were absent of Egr-1 expression in all animals, indicating that this method was successful at suppressing singing.
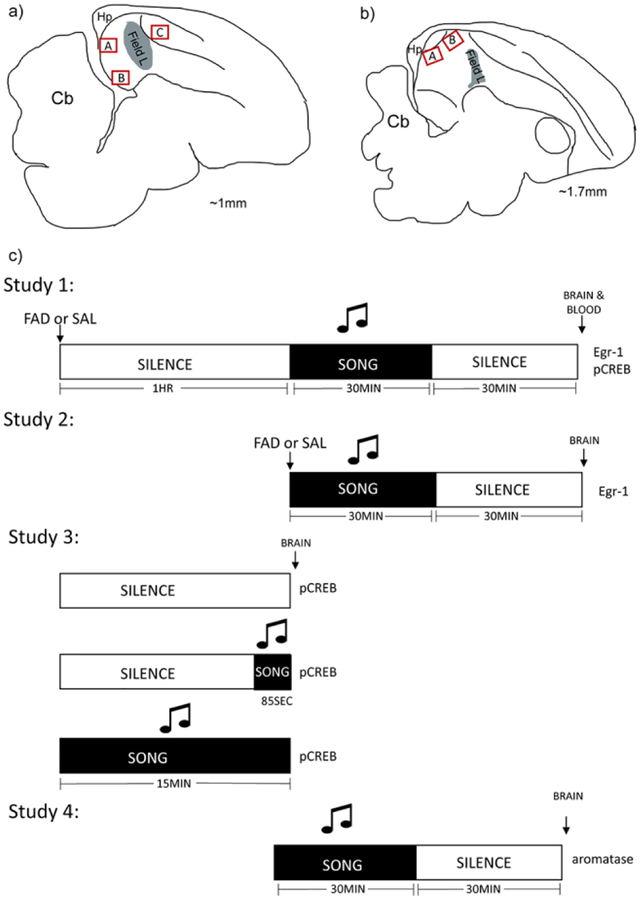
Fig. 1.
Anatomical landmarks and study designs. a) Sagittal schematic of zebra finch brain ~1mm from midline. Red boxes indicate regions of interest that were analyzed across studies. A=dNCM, B=vNCM, C=CMM. b) Sagittal schematic of zebra finch brain ~1.7mm from midline. Red boxes indicate regions of interest that were analyzed across studies. A=pHVCS, B=aHVCS. Red boxes are not representative of size of images. Grey zone is primary auditory cortical region Field L. Abbreviations: cerebellum (Cb) and hippocampus (Hp). c) Experimental design for song exposure. Animals from all studies were exposed to various designs of song exposure. White boxes indicate periods of silence and black boxes indicate periods of triplicate song. At the end of each timeline the protein analyzed is included for that study.
Study 2:
One interpretation of the results of Study 1 is that fadrozole has a toxic and non-specific degradation effect directly on Egr-1 expression. Rather than exposing birds to song playback during the timeframe of decreased aromatase activity and estradiol production (~1 hour after administration in Study 1 (Kochak et al. 1990)), we followed the same design as Study 1 with males and females (n=3 per treatment and sex; total=12), with the exception that we administered the song playback paradigm immediately after fadrozole administration. In this experiment, therefore, song playback occurred before fadrozole pharmacokinetics begin to suppress brain aromatase activity (see prior citations), yet fadrozole would be bioavailable and active in the brain during the peak induction response of Egr-1 protein (Fig. 1c).
Study 3:
Males (n=9) and females (n=10) were collected by two separate experimenters in two separate studies so the sexes were not directly compared. We were interested in whether phosphorylation of CREB was song-responsive. For each study, there were three stimulus groups: no song, 85 seconds of song or 15 minutes of song. The stimuli durations were selected based on previous publications: 85 seconds was chosen because it is the ideal length for induction of phosphorylated ERK, an upstream kinase known to be responsive to song in males (Cheng and Clayton 2004), while 15 minutes was selected because prior work has used this playback design for other phosphorylation studies (Heimovics et al. 2012). Song groups consisted of a triplicate song playback protocol. Since we had a no song control, to avoid any self-stimulation effects all subjects were kept in the dark ~2 hours before the experiment began and throughout playback (Jarvis and Nottebohm 1997; Bailey et al. 2002). The playback speaker was placed inside the chamber ~ 5 minutes before the start of each playback session. For the 15-minute group, triplicate song was played back for the entirety of the session. For the 85 second group, birds sat in silence for 13 minutes and 35 seconds and the song paradigm was played at the end. For the silence group, birds sat in silence for the entirety of the 15 minutes (Fig. 1c).
Study 4:
Although there is no direct evidence that song playback can change aromatase expression in this relatively acute timeframe, we exposed all birds (n=6 males and n=6 females) to the same stimuli paradigm described in Study 2, however no drugs were administered (Fig. 1c). This was to ensure comparisons with other studies under the same conditions.
Hormone Assay:
Whole blood was collected from animals at time of brain extraction for Study 1. We performed a solid phase extraction using C-18 columns (3M, Eagan, MN, USA) on the whole blood and then measured 17β-estradiol using an enzyme-linked immunoassay (EIA; Cayman). Four out of sixteen samples yielded low volume during extraction leading to non-detectable estradiol levels above assay blank controls, so these samples were removed from the analysis. In the remaining samples which were all above the detectability limit of the assay we did not detect any differences in peripheral estradiol levels regardless of drug treatment.
Immunostaining:
After stimuli presentations (Study 1–3), brains were immediately extracted following rapid decapitation and drop-fixed into a 5% acrolein solution made in 0.1M phosphate buffer (PB). Brains sat in fixation overnight at room temperature. The following morning, brains were transferred to a 20% sucrose in 0.1M phosphate buffer/0.9% saline (PBS) solution at 4 °C for at least two days. Brains were sectioned sagittally using a cryostat (Leica, Germany) at 45μm in serial sections (3 series) and stored in cryoprotectant at −20°C. For each study, all subjects were processed at once in the same free-floating immunostaining run. Sections were washed using 0.1M PB and then treated with 0.5% sodium borohydride (NaBH4) in PBS. After 3×-5minute washes in 0.1M PB and 3× 15-minute washes in 0.1M PB, sections were incubated in 10% normal goat serum (S-1000, Vector Labs) made in 0.3% Triton-x/0.1M PBS (PBT) for 1 hour. Sections were then incubated with either anti-Egr-1 rabbit polyclonal antibody (1:10,000, sc-189, Santa Cruz, (Avey et al. 2005; Bailey and Wade 2005; Maney et al. 2006) or anti-pCREB rabbit polyclonal antibody (1:5,000, Ser133, sc-101663, Santa Cruz; same epitope as antibody used in sparrow brain (Heimovics et al. 2012)) made in 0.3% PBT for 1 hour at room temperature followed by ~48 hours at 4 °C. Following washes in 0.1% PBT, sections were incubated in the secondary antibody biotinylated goat anti-rabbit (BA-1000, Vector Labs) made in 0.3% PBT at a 1:200 dilution for 1 hour. Sections were washed and then incubated in Vector A:B (1:500 dilution; Vectastain Elite ABC Kit PK 6100, Vector Labs) solution for 90 minutes followed by washes. For development, we used the Vector SG hydrogen peroxide and chromatin (Vector SG Peroxidase (HRP Substrate Kit, SK-4700, Vector Labs) kit, dropping one drop of each into the wells and the sections developed for 10 minutes. After mounting, sections were dehydrated in ethanol washes followed by HemeD. Sections were coverslipped using permount. We validated the pCREB antibody by first reserving some sections before the experiment, following this same process but incubating the primary in the respective blocking peptide (Ser133, Cell Signaling Technology). We observed total adsorption of the antibody and observed no non-specific binding (Fig. S1).
For comparisons of aromatase expression, we followed a similar protocol. A separate set of animals (males n=6 and females n=6) were anesthetized with isoflurane and then transcardially perfused using 4% paraformaldehyde (PFA) in PBS. After perfusion and a post-fixation of 2 hours with 4% PFA, brains were switched to a 30% sucrose/saline solution. Brains were sectioned at 35 μm. The immunostaining protocol used here is different than that for IEGs since it has been optimized for this primary antibody and using fluorescent secondary antibodies (Ikeda et al. 2017). After sectioning and storage in cryoprotectant, sections were washed with 0.1M PB 3× for 15 minutes each. A specific zebra finch anti-aromatase rabbit antibody (supplied by Colin Saldanha (Saldanha et al. 2000)) was used at 1:2000 dilution and incubated for one hour at room temperature and then ~48 hours at 4 °C. After washing in 0.1% PBT 3 times for 15 minutes, we incubated the sections in secondaries raised in goat anti-rabbit antibodies conjugated to Alexa 488 and 594. To preserve fluorescence, sections were mounted with Pro Diamond Anti-Fade with 4’,6-diamidino-2-phenylindole (DAPI, Thermofisher).
Imaging:
All regions of interest were determined from established aromatase expression patterns in the zebra finch brain (Shen et al. 1995; Saldanha et al. 2000; Peterson et al. 2005; Saldanha and Coomaralingam 2005; Rohmann et al. 2007; Ikeda et al. 2017) as well as regional and sex differences reported in co-expression patterns with neuronal-subtype markers (Ikeda et al. 2017). We selected the caudal medial nidopallium (NCM) because of its aromatase fiber and activity sex difference (Saldanha et al. 2000; Peterson et al. 2005). Other auditory brain regions, Field L, nucleus interfacialis of nidopallium (Nif), and HVC are all absent of Egr-1 as well as somatic aromatase expression so we did not select these regions for analysis. We divided the NCM region into dorsal (dNCM) and ventral (vNCM) subregions. We defined NCM sections by the thickness of the hippocampus and the absence of nucleus taenia (Tn) which comprises medial-most sections ~ 0.2–1.0 mm from the midline. We selected HVC shelf as another region of interested because it is known to express aromatase and is auditory responsive. We divided the shelf into anterior (aHVCS) and posterior (pHVCS) and used the absence of Egr-1 in HVC to set the bounds of HVC shelf. Sections were selected from ~1.7–2.5mm from the midline. Fig. 1a and 1b depict sagittal sketches with subregions marked. For females, HVC location was difficult due to its small size. In some subjects HVC was not always reliably visible, so we selected sections that appeared similar based on other anatomical markers such as architecture of the overlying hippocampus and presence of the arcopallium and took images of dorsal caudolateral nidopallium. Because pCREB does not have noticeable distribution differences between HVC and HVC shelf as is the case with Egr-1, for Study 1 we used the Egr-1 immunoreactivity to guide the region of interest for HVC shelf for the pCREB immunoreactivity in adjacent sections (Egr-1 and pCREB were stained on serial sections from the same study cohort). For Egr-1, we also quantified cells in caudomedial mesopallium (CMM) as a negative control. CMM is an auditory region that does not have somatic aromatase expression,(Saldanha et al. 2000; Ikeda et al. 2017) and therefore, Egr-1 expression is not expected to change with aromatase activity inhibition. For Studies 2 and 3, we collected images only from the dorsal and ventral NCM.
For Studies 1–3, images were taken at 20× using brightfield microscopy (Zeiss Axio Lab A.1) and the Zeiss software ZEN 2012 blue edition. We took 3–4 images per region per animal indiscriminate of hemisphere. Images were quantified by an experimenter blinded to treatment and sex. The experimenter counted cells in the entire image. Since both Egr-1 and pCREB are nuclear stains, discrete nuclei (dark black, clear edge) were counted manually using Image J. The coefficient of variation for duplicate sections was 10.5±3.6% for Egr-1 and 7.6±4.6% for pCREB.
For study 4, we used a laser confocal microscope (NA1, Nikon, Tokyo, Japan) with NIS-Elements imaging software (Ar) to take pictures at 60× with z-slices that ranged from 9–15 μm. Images were selected to match the regions outlined in the above studies. We could only unequivocally identify HVC shelf reliably in 6 males and 3 females. Aromatase counts are represented as a percentage of DAPI. For cluster analysis, detailed methods can be found in a previous publication which includes analysis validations (Ikeda et al. 2017). Briefly, aromatase clusters were identified as two or more fluorescent-stained somas physically touching (0μm). Clusters were carefully identified by examining each stack of the z-projection image (~1μm/stack). Once cluster sizes were quantified, percentage of clusters of increasing sizes were normalized to the total amount of aromatase within an image so that comparisons within and between groups could be made.
Data Analysis:
We averaged sections from subject replicates per regions for one representative count of that region for each study. As is standard in the Egr-1 literature, we represent the cell counts within an area of mm2. Aromatase cell densities are depicted as percent of DAPI staining. All statistics were performed using Origin 2017. We originally ran a three-way analysis of variance (ANOVA) to determine significant interactions to further analyzed. We fixed the variable region for analyses of Egr-1 cell counts and performed individual two-way between subject ANOVA tests for sex and treatment. Although we detect significant main effects, we did not have the power to detect significant interactions because of a small sample size and therefore did not run post-hocs for multiple comparisons. Because our a priori hypothesis was that males would have a stronger response to fadrozole and we wanted to avoid Type I Error by running multiple t-test comparisons and Type II Error, we calculated Cohen’s d and the 95% confidence interval (Cohen 1994) based on the mean differences and standard deviations of saline vs. fadrozole for each sex and in each region. Effect sizes can be directly compared both within studies and in meta-analyses, making this measure the most ideal to test our hypothesis of robustness comparing the sexes (Beltz et al. 2019; Ho et al. 2019). We also performed two-way between subject ANOVA tests fixing for region for the pCREB analysis. For Study 3, since males and females were collected in two different studies, we did not run them together in a statistical model and instead performed a one-way within-subject ANOVA for each sex and region. For Study 4, sex differences in aromatase cell counts were fixed by region and one-way ANOVAs were performed for sex comparisons. For cluster analysis, a two-way mixed factor ANOVA with cluster size as the repeated variable was performed for each region comparing the sexes. Post hocs were performed when appropriate using Tukey’s honestly significant difference (HSD) tests. Cohen’s d (d) is reported for effect size of posthocs reported and male-female comparisons for aromatase comparisons. For F-tests, generalized eta squared (η2) is reported for effect size. Significance threshold for all analyses was p<0.05.
Results
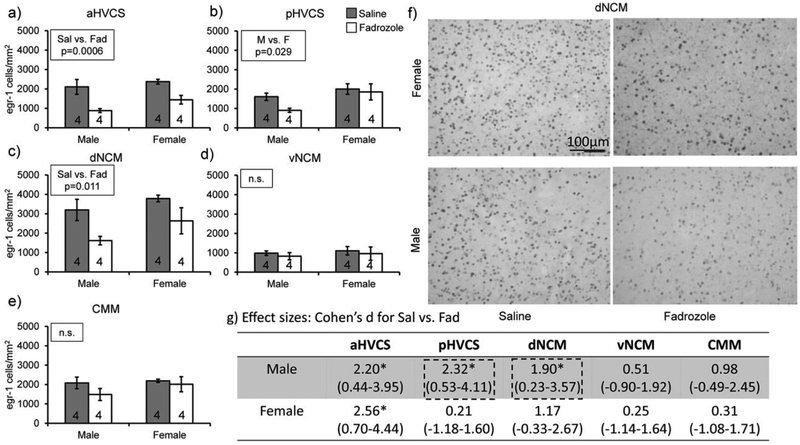
We tested the hypotheses that endogenous estrogen synthesis is important for the auditory-evoked IEG induction in auditory regions on an acute timescale. First, we found a decrease in auditory-induced Egr-1 expression by fadrozole pre-treatment (F(1,14)=7.44, p=0.016) and that this change in expression depended on brain region (region: F(4,56)=34.17, p<.0001; region*treatment: F(4,560)=5.08, p=.0015). We did not detect a main effect of sex (F(1,14)=2.73, p=0.12). Because of the significant treatment by region interaction, we performed two-way between subjects’ ANOVA analyses fixing for region, comparing treatment and sex. The a priori hypothesis was that Egr-1 expression would be more reliant on estrogen synthesis in males than females; therefore, the decrease in Egr-1 induction would be stronger. We report effect sizes (Cohen’s d) for mean differences between saline and fadrozole treatments for each sex and region to test the strength of treatment mean differences. For aHVCS, in both males and females, fadrozole decreased Egr-1 expression compared to saline control (Treatment: F(1,12)=21.0, p=0.0006; Sex: F(1,12)=3.11, p=0.10; Treatment*Sex (F(1,12)=0.38, p=0.55 Fig. 2a; Male Sal vs. Fad d=2.20 (0.44–3.95) and Female Sal vs. Fad d=2.56 (0.70–4.44); Fig. 2g). For pHVCS, females had more Egr-1-positive cells than males (Treatment: F(1,12)=2.47, p=0.14; Sex: F(1,12)=6.17, p=0.03; Treatment*Sex: F(1,12)=1.05, p=0.33, Fig. 2b). This sex difference was primarily driven by the low induction of Egr-1 in males treated with fadrozole, which when compared to saline controls had a 10-fold bigger effect size vs. the female treatment groups (Males d=2.32 (0.53–4.11) and Females d=0.21 (−1.18–1.60), Fig. 2g). For dNCM, both males and females treated with fadrozole had a decrease in Egr-1-positive cell counts compared to saline controls and this effect was stronger in males (Treatment: F(1,12)=8.96, p=0.01; Sex: F(1,12)=3.10, p=0.10; Treatment*Sex: F(1,12)=0.22, p=0.64, Fig. 2c; Males d=1.90 (0.23–3.57) and Females d=1.17 (−0.33–2.66), Fig. 2f&g). In both vNCM and CMM there were no significant main effects or interactions detected (vNCM: Treatment p=0.52, Sex p=0.59, Treatment*Sex p=0.98, Males d=0.51(−.90–1.92) and Females d=0.25 (−1.14–1.64), Fig. 2d&g; CMM: Treatment p=0.21, Sex p=0.30, Treatment*Sex p=0.47, Males d=0.98 (−0.49–2.45) and Females d=0.31 (−1.08–1.71), Fig. 2e&g). These findings indicate that more regions in males depend on acute estrogen synthesis for Egr-1 auditory induction than in females. This decrease in Egr-1 expression also is region-specific in both sexes.
Fig. 2.
Fadrozole treatment decreases auditory-induced Egr-1 expression in secondary auditory regions more robustly in males compared to females. All bar graphs represent means and error bars standard error of the mean across each group (n=4/sex/treatment) per region a) aHVCS, b) pHVCS, c) dNCM, d) vNCM, and e) CMM. Data from Study 1. p<0.05*. f) Egr-1 expression decreases in dorsal NCM of males administered fadrozole but not female zebra finches. Images were taken with a bright field microscope at 20× magnification. Top row includes example images from females exposed to saline (left) and fadrozole (right). Bottom row includes example images from males exposed to saline (left) and fadrozole (right). Images are from Study 1. g) Asterisks next to effect sizes indicate mean differences with 95% confidence intervals (C.I.) that do not contain 0. Dotted-line boxes are around effect sizes that pass the C.I. threshold in males but not females.
To determine whether fadrozole administration reduced estradiol systemically, we measured estradiol content from whole blood collected at sacrifice. We did not detect any differences in peripheral estradiol levels regardless of drug treatment. (F(1,8)=0.56, p=0.475; Sal 11.2±4.37pg/mL and Fad 6.10±5.22pg/mL, d=0.434 (−0.72–1.59)) or sex (F(1,8)=.099, p=.72; Male 7.57±4.68pg/mL and Female 9.71±4.96pg/mL, d=0.39 (−0.75–1.54)), indicating that the 60-min treatment with fadrozole did not significantly shift peripheral estradiol levels, and that changes to Egr-1 expression from treatment are likely due to the active synthesis of estradiol locally. This finding is consistent with prior work (Prior et al. 2014; Lampen et al. 2017), however due to the loss of samples (see Methods), these measurements are underpowered and should be interpreted cautiously (Power is 10%).
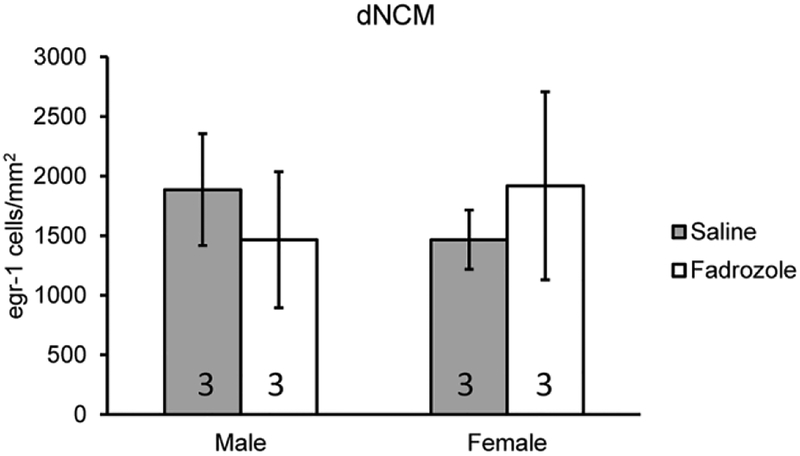
The suppression of Egr-1 induction by fadrozole could be explained by decrease in estrogen-dependent synthesis of the IEG or alternatively a nonspecific ‘toxic’ protein degradation as a consequence of the drug treatment with fadrozole. To distinguish between these possibilities, we administered fadrozole or saline orally immediately prior to song playback in a new set of animals (Study 2). In principle, this treatment time course is not pharmacokinetically sufficient to suppress brain aromatase activity during the immediate 30-min song exposure. However, song-induced Egr-1 protein made during the following 30 min silence period could be affected by the drug as oral fadrozole should be systematically available. We did not observe a difference between saline and fadrozole treated birds (dNCM: F(1,8)=0.021, p=0.89, Male Sal vs. Fad d=0.68 (−0.97–2.32), Female Sal vs. Fad d=0.45 (−2.07–1.17); vNCM: F(1,8)=0.24, p=0.64, Male Sal vs. Fad d=1.05 (−0.66–2.76), Female Sal vs. Fad d=0.89 (−2.57–0.79); Fig. 3), indicating that fadrozole is not sufficient to degrade Egr-1 induction at this time point.
Fig. 3.
Egr-1 expression does not degrade with fadrozole in either sex. Bar graph represents means and error bars standard error of the mean for each group (n=3/sex/treatment). Data is from Study 2 testing whether fadrozole administration degrades Egr-1 expression. No statistical significance detected.
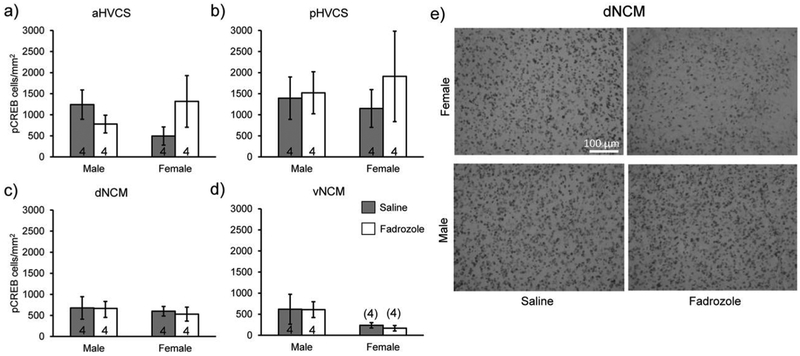
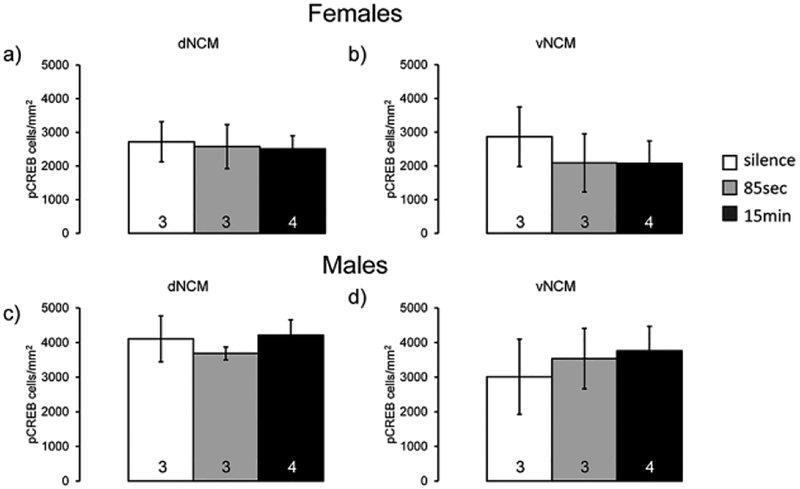
We next examined expression of pCREB in serial sections from the same experiment in order to determine if pCREB expression has similar fadrozole and sex specific changes as it is a potential transcription factor that targets Egr-1 (Study 1). There was a significant difference in expression across regions (F(3,42)=7,84, p=0.00029; significant posthocs: dNCM vs. pHVCS: t(42)=5.15, p=0.0040, d=0.95, vNCM vs. pHVCS: t(42)=6.39, p=0.00028, d=1.16); however there was not a detectable difference overall within sex (F(1,14)=0.25, p=0.62, η2=0.0063) or a detectable change with fadrozole administration (F(1,14)=0.25, p=0.63, η2=0.0062). We did not observe any detectable main effects or interactions when comparing sex and treatment fixing for region (aHVCS: Treatment p=0.65/η2=0.015, Sex p=0.79/η2=0.0049, Treatment*Sex p=0.12/η2=0.19; pHVCS: Treatment p=0.53/η2=0.034, Sex p=0.92/η2=0.00088, Sex*Treatment p=0.65/η2=0.017; dNCM: Treatment p=0.85/η2=0.0032, Sex p=0.60/η2=0.024, Treatment*Sex p=0.89/η2=0.0017; vNCM: Treatment p=0.85/η2=0.0023, Sex p=0.07/η2=0.25, Treatment*Sex p=0.89/η2=0.0013, Fig. 4a–e), indicating that phosphorylation of CREB is not regulated in tandem with Egr-1 in either sex or acute neuroestrogen synthesis comparisons. To interpret these findings, we reasoned that either 1) pCREB is not itself song inducible in the NCM or 2) that the time course necessary for Egr-1 induction is different than that for pCREB induction in the NCM. Therefore, we tested in a new experiment (Study 3) whether shorter song-exposure could initiate induction of pCREB. We observed that pCREB expression did not change in either dorsal or ventral NCM across the conditions of silence, 85 secs, and 15 min song exposures (Males: dNCM F(2,4)=0.46, p=0.66, η2=0.19, vNCM F(2,4)=0.14, p=0.88, η2=0.064; Females: dNCM F(2,4)=0.035, p=0.97, η2=0.017, vNCM F(2,4)=0.23, p=0.80, η2=0.10; Fig. 5a–d). Males and females were examined in two separate studies so no direct sex comparisons were possible.
Fig. 4.
Fadrozole has no effect on pCREB expression in secondary auditory regions of either sex. All bar graphs represent means and error bars standard error of the mean across each group (n=4/sex/treatment). a) aHVCS, b) pHVCS, c) dNCM, and d) vNCM. Data from Study 1. No significance detected. d) pCREB expression does not decrease in dorsal NCM of either sex administered fadrozole. Images were taken with a brightfield microscope at 20× magnification. Top row includes example images from females exposed to saline (left) and fadrozole (right). Bottom row includes example images from males exposed to saline (left) and fadrozole (right). Images are from Study 1.
Fig. 5.
pCREB expression does not change with song exposure. All bar graphs represent means and error bars standard error of the mean across each group (n=3 silence and 85 seconds, n=4 for 15 minutes). Data for females of dNCM (a) and vNCM (b) and males of dNCM (c) and vNCM (d) from Study 3. White bars are silence group, gray bars short exposure (85 seconds), and black bars longer exposure (15 minutes). No significance detected.
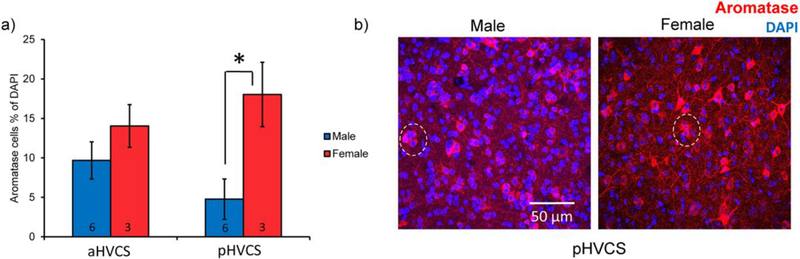
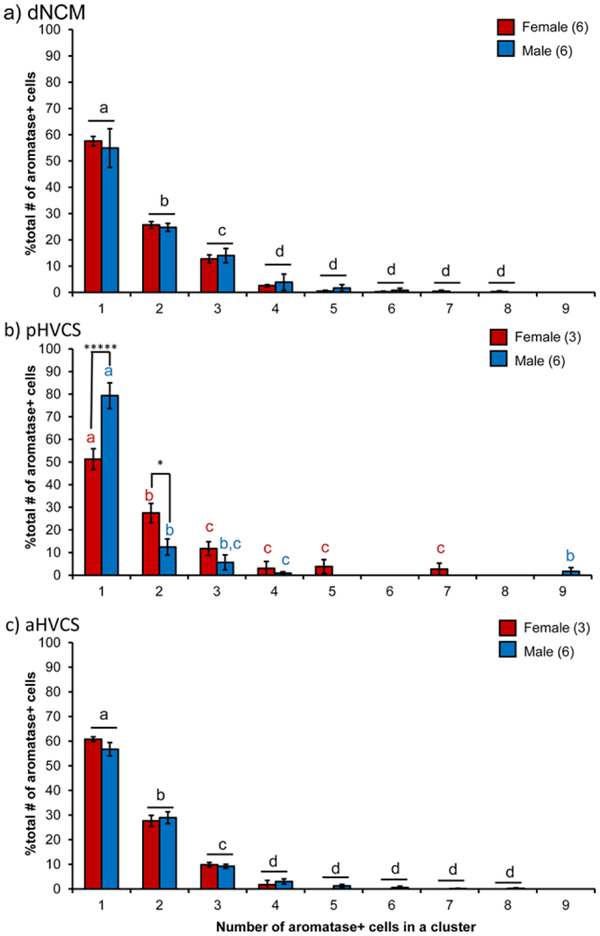
As there were region and sex-specific decreases in auditory-induced Egr-1 expression with aromatase inhibition, we reasoned that local aromatase expression in these regions may differ between the sexes by region. In a separate cohort of males and females, we quantified both the cell density of somatic aromatase expression as well as the organization of aromatase cells into soma-somatic clusters. We have previously published on this cohort, outlining regional differences and coexpression patterns of aromatase with inhibitory neuron markers parvalbumin and calbindin. Sex comparisons within regions were only conducted on dNCM and vNCM and there were no statistical differences detected (Ikeda et al. 2017). In the earlier report, we also found that in the ventral NCM (vNCM), aromatase somas cluster together sex-specifically, in which males had more clustered somas than females despite a similar number of overall aromatase cells (Ikeda et al. 2017). For the current study, we returned to this cohort and performed sex comparisons for the subregion HVC shelf, i.e., the same region in which we observed the biggest difference in responsiveness between the sexes here with Egr-1. We therefore quantified aromatase clusters in dNCM, aHVCS, and pHVCS. We detected a sex difference in cell density of the pHVCS in which females had more aromatase expression (F(1,8)=8.33, p=.023, d=2.04; Fig. 6a&b). By contrast, in aHVCS, there was not a sex difference (F(1,8)=1.27, p=.30, d=0.79; Fig. 6a). We did not find a sex by cluster size interaction in either dNCM (F(8,72)=0.18, p=0.99, η2=0.0011; Fig. 7a) or aHVCS (F(8,56)=0.59, p=0.78, η2=0.0015; Fig. 7c), indicating that both males and females have a similar distribution ‘decay’ of aromatase clusters in these brain areas (dNCM: F(8,72)=161.34, p<0.0001, η2=0.93; aHVCS: F(8,56)=343.58, p<0.0001, η2=1.91; Fig. 7a&c). However, in pHVCS we detected a significant sex by cluster interaction (F(8,56)=6.78, p<0.0001, η2=0.052; Fig. 7b), in which females had fewer isolated (i.e., non-clustered) aromatase cells (t(56)=10.70, p<0.0001, d=2.23) and more cells found in clusters of two (t(56)=5.72, p=0.02, d=1.78). Females and males had similar overall cell densities (i.e., DAPI quantity) meaning the differences in clustering cannot be explained by quantity of cells (DAPI t(7)=−1.29, p=0.24, d=0.91) For the remaining cluster sizes, both sexes showed a similar distribution ‘decay’ (F(8,56)=91.49, p<0.0001, η2=0.70). Therefore, the same region in which we observed sex-specific, rapid suppressive effects of aromatase inhibition on Egr-1 induction (pHVCS) we also observed a sex difference in expression of aromatase protein in terms of aromatase density and organization.
Fig. 6.
Females express more somatic aromatase in pHVCS than males. a) All bar graphs represent means and error bars standard error of the mean across each group (n=6 for males and n=3 for females. b) Example images of aromatase (red) and DAPI (blue) in male (left) and female (right) pHVCS. Dotted white circle shows an example of a 2-cell cluster in the female. Images taken with a confocal microscope at 60× magnification as z-stacks (~9–15um thick). Images are maximal projection images. p<0.05*
Fig. 7.
Aromatase organization into clusters differs by sex in pHVCS. Percentage of aromatase cells found in increasing cluster sizes are normalized to the total amount of aromatase quantified for individual images. All bar graphs represent means and error bars standard error of the mean for each sex (dNCM: n=6 males and n=5 females; anterior and posterior HVCS: n=6 males and n=3 females). Letters indicate posthoc tests for a significant main effect of cluster size. Black letters indicate there is no sex*cluster size interaction and red and blue letters indicate posthocs within each sex for cluster size. Asterisks in b) are between subject post hoc comparisons comparing male and female subjects for each cluster size. p<0.05*, p<0.0001*****.
Discussion
Auditory-induced responsiveness depends on aromatase activity
Here, we report that acute estrogen synthesis blockade attenuates auditory-induced Egr-1 expression of cells within aromatase rich auditory regions of the zebra finch forebrain. This study therefore establishes a direct link between acute endogenous estrogen synthesis and auditory-evoked immediate early gene expression in the brain. Prior work has shown that exogenous estradiol application can change auditory-induced Egr-1 expression (Maney et al. 2006; Sanford et al. 2010) demonstrating sufficiency of estradiol for induction over a longer timeframe. Chronic week-long fadrozole exposure eliminates the sex difference in Egr-1 expression in NCM (Lampen et al. 2017) which is similar to the effects we observed in dNCM. The current study builds on these findings to show that endogenous estrogen synthesis is, at least in part, necessary for maximal Egr-1 induction within an acute time frame. This indicates that aromatase activity likely influences auditory neuronal activity dynamically. Our findings also reveal that males have more regions of the auditory forebrain that depend on endogenous neuroestrogen synthesis than females. Some sex differences have been described relating to brain derived estrogens and how they may impact audition (Saldanha et al. 2000; Peterson et al. 2005; Rohmann et al. 2007; Chao et al. 2015), and this study now reports that sex differences in auditory-induced Egr-1 gene expression is likely due to differences in brain-derived estrogen production.
We found that depending on brain region, males and females had differing degrees of suppressed auditory-induced Egr-1 expression when pre-treated with an aromatase inhibitor. In dNCM, aHVCS, and pHVCS, males have reduced Egr-1 expression when administered fadrozole as opposed to saline. However, this effect only exists in aHVCS for females, indicating that in dNCM and pHVCS, there is a sex difference in dependency of estrogen synthesis on auditory activation. This reduction in Egr-1 expression is maximally 50% of vehicle expression depending on brain region, indicating that this is not a total elimination of Egr-1 expression but rather an attenuation of the song-induced response. Aromatase inhibition may have effects on basal expression of Egr-1 however because all animals in this study were exposed to song, we cannot rule this out. Although, no study to date has tested aromatase blockade on animals not exposed to songs or tones.
Three non-competing hypotheses can explain the sex-specific effects of aromatase inhibition on auditory-induced Egr-1 expression across the auditory cortex. The first hypothesis is that auditory-induced Egr-1 expression is dependent on neuroestrogens and that sex differences in Egr-1 expression are related to how estrogens are synthesized and metabolized locally within brain regions. The second hypothesis is that due to ovarian estradiol in females, peripheral estradiol levels are maintaining estrogen-mediated neural responses to songs in females. A third, non-exclusive alternative is that EGR1-driven cellular responses are both regulated by neuroestrogens in males and females, but to a differing degree, perhaps set by differing estrogen receptor expression levels/binding affinities in NCM neurons between the sexes. For the first hypothesis we would predict that differential aromatase expression and/or activity would be accounting for the sex difference. For the second hypothesis, we would predict that brain vs. plasma estradiol levels would differ between the sexes. And for the third hypothesis, we would predict that receptor expression and/or identity would differ between the sexes for NCM neurons.
Hypothesis 1: Sex and brain regional differences of neuroestrogens
For the first hypothesis, we have several pieces of evidence that the effects observed are due to differences in neuronal sources of estradiol. Because of the region-specific differences we observed in decreased Egr-1 expression in fadrozole treated groups, these effects may be due to brain-derived aromatase activity. For example, regions such as CMM that contain estrogen receptors but no somatic aromatase (Saldanha et al. 2000; Ikeda et al. 2017) did not exhibit altered Egr-1 induction in response to fadrozole administration. In males, fadrozole treatment decreased auditory induced Egr-1 expression in three auditory regions (dNCM, aHVCS, pHVCS) while, in females, only one (aHVCS), and all of these regions contain significant local aromatase. These observations are consistent with the idea that males are more reliant on acute, local synthesis of estrogens than females. We hypothesized that this effect was due to a difference in aromatase expression in these regions and observed that, indeed, pHVCS exhibits a larger somatic aromatase expression in female pHVCS as compared to that in males, as well as more clustering in females compared to males. However, for dNCM, we previously found that males and females have similar somatic aromatase expression (Ikeda et al. 2017) in agreement with prior findings (Saldanha et al. 2000). We did not find a sex-specific difference in clustering of aromatase cells in dNCM, indicating that somatic expression and organization alone does not explain the sex-specific differences in the sensitivity of Egr-1 induction to fadrozole. However, a sex difference has been described in aromatase terminal activity (Peterson et al. 2005; Rohmann et al. 2007) and aromatase fiber expression (Saldanha et al. 2000) within NCM. Our findings along with those of others indicate that aromatase cell organization and expression in different cellular compartments may have functional consequences for auditory activity. There is likely a functional difference in somatic vs. terminal expression of aromatase. Somatic aromatase may be more relevant for paracrine signaling of estradiol, in which estradiol is released locally within the brain but not targeted to specific synapses. By contrast, terminal expression is likely involved in neuromodulator/neurotransmitter like functions (Remage-Healey et al. 2010b; Remage-Healey et al. 2011; Cornil et al. 2012). Fiber expression and terminal activity have not been described in detail in the shelf region surrounding HVC, so it is unclear if there is a similar relationship in this region. More somatic aromatase in pHVCS may protect auditory responsiveness in females via higher neuroestrogen concentrations that have not been metabolized within the timescale of our fadrozole administration. Direct measurement of estradiol within HVC shelf would need to be conducted to confirm this. One surprising region where we did not see an effect was ventral NCM which is known to have robust aromatase expression and a sex difference in clustering (Ikeda et al. 2017); however, we did not see an effect of aromatase inhibition in either sex on Egr-1 auditory evoked expression. Ventral NCM had the lowest Egr-1 expression of the regions analyzed, so it is possible that further decreases were less detectable due to a floor effect.
Hypothesis 2: Ovarian-sourced estradiol compensatory for females
For the second hypothesis, there is sufficient evidence that there is compensation in males vs. females in their overall, systemic estradiol levels. Males and females do not differ in measurable peripheral levels of estradiol (Adkins-Regan et al. 1990; Prior et al. 2014), despite there being a significant amount of estradiol synthesized from female ovaries (Schlinger and Arnold 1991; Schlinger and Arnold 1992). There is also evidence that for both sexes there are sources of estradiol that are non-gonadal; specifically when zebra finches are gonadectomized, there is an increase in peripheral estradiol (Adkins-Regan et al. 1990). A major source of this estradiol is likely the brain as there is detectable and robust production of neuroestradiol than can be measured in the periphery, particularly in males (Schlinger and Arnold 1992). One conclusion that can be drawn from this study is that males are more reliant on aromatase activity within various subregions which may indicate that their brains produce more estradiol end-product. Indeed, aromatase activity is elevated in male NCM (Rohmann et al. 2007). Our previous findings did not indicate robust difference in somatic aromatase expression throughout the forebrain (Ikeda et al. 2017) with the exception of pHVCS reported here – however, we did not compare the sexes for activity of these subregions, which may vary greatly. Aromatase expression can be changed by both castration (Saldanha et al. 2000) and chronic administration of nonsteroidal aromatase inhibitors (Foidart et al. 1995) (such as the one used in this study); however, given the acute time course between fadrozole administration and sacrifice, it is unlikely that our treatment significantly altered aromatase protein expression, although this cannot be ruled out. There has not been a detectable difference in estradiol content between the sexes in adulthood when sampling via microdialysis in gonadally intact males and females (Remage-Healey et al. 2012). This indicates that brain-derived estradiol is equitable between the sexes; however, without performing this study in gonadectomized animals, it is unclear how peripheral estrogens contribute to this finding (Maney 2016). Furthermore, future testing in other brain areas for aromatase activity would also be necessary to assess sex differences in estradiol production.
Hypothesis 3: Estrogen receptor specific mechanisms for auditory-responsiveness
For the third hypothesis, there is some indication that male and female auditory responsiveness may be mediated by different estrogen receptors. Although both males and females increase auditory-induced activation of NCM neurons (Remage-Healey et al. 2010a; Remage-Healey et al. 2012), to date, estrogen receptor agonists cannot increase auditory-induced activation alone (Remage-Healey et al. 2013; Krentzel et al. 2018). However, when a GPER-1 antagonist is administered directly into NCM, males have a cell-type specific decrease in auditory activation where females do not (Krentzel et al. 2018). This finding indicates that the estrogen receptors mediating increases to auditory-induced neuronal activation by estradiol are not only sex-specific, but also may have sex specific expression on certain cell types. This hypothesis can be further pursued through sex comparisons of estrogen receptor expression on different cell types.
A role for peripheral sourced estrogens in auditory responsiveness
In this study, there is reasonable evidence to suggest that acute oral fadrozole treatment does not significantly decrease peripheral sources of estradiol in either sex. We found that fadrozole did not alter peripheral estradiol levels in either sex within this 60–90 min timeframe. Prior evidence suggests that acute exposure to aromatase inhibitors is not sufficient to decrease peripheral estradiol in either zebra finch sex (Prior et al. 2014) although in sheep it decreases peripheral estradiol by 2–8 hours (Benoit et al. 1992). Likely, peripheral steroids are not rapidly changing to song exposure. Peripheral estradiol fluctuations in response to song exposure in females have been measured in the feces, and it takes several days to detect changes (Tchernichovski et al. 1998). When measuring plasma testosterone and estradiol after song playback, male zebra finches do not show changes in the peripheral estradiol levels despite changes in steroid content in the brain (Remage-Healey et al. 2008). These results suggest that in zebra finches, in the timeframe of this study, song is not sufficient to dynamically change peripheral estrogens, though this would need to be more systematically explored. Given this evidence, it is possible that gonadal estradiol in females has not yet been metabolized and decreased significantly by fadrozole. This provides another mechanism by which the aromatase inhibitor suppression of auditory-induced Egr-1 in auditory regions is blunted in females as opposed to males.
Future work will need to determine the role that brain-derived estradiol and gonadal-derived estradiol plays in auditory physiology, particularly in females. Experiments directly testing the necessity of ovaries in auditory responsiveness are needed to confirm or refute the sufficiency of brain-derived estradiol to maintain auditory-induced gene expression. Conversely, targeting specific brain regions, such as dNCM or pHVCS for aromatase inhibition can determine whether auditory responsiveness is maintained as seen in this study.
HVC shelf: sexually differentiated neural locus?
Sex differences in estrogen dependent Egr-1 and aromatase expression in HVCshelf indicate that the shelf is a sexually differentiated nucleus. There is little known about the shelf of HVC other than it is auditory and sends projections into HVC and RA cup (Vates et al. 1996), which are sexually dimorphic motor nuclei that are critical for production of birdsong in males. Since the sex difference we observe here is dependent on the anterior-posterior axis of the shelf, in which anterior shelf has a similar response between the sexes and the posterior shelf is differential, this may indicate a true sex difference in shelf neurochemical functioning or a sex difference in size and shape which affects what region was sampled. HVC is smaller in females than in males (Nottebohm and Arnold 1976; Hamaide et al. 2017), and shelf is defined by the parameters of this nucleus. Although we were careful in the identification of shelf, there is a lack of thorough anatomical description of this region, particularly in females. This difference might represent portions of the dorsal caudolateral nidopallium (NCL).
Potential cellular-signaling mechanisms for estradiol on auditory-responsiveness
We also reported that pCREB is not song-inducible, nor is it regulated in parallel with Egr-1 expression changes in either sex. We proposed pCREB to be a likely candidate as a transcription factor to target Egr-1 and which is sensitive to estradiol signaling via the MEK-ERK pathway. However, unlike the phosphorylation of ERK (Cheng and Clayton 2004), we did not see changes in pCREB expression after short song exposure in dorsal or ventral NCM. Phosphorylation of CREB also has sex-specific induction of estradiol in the mammalian hippocampus and striatum (Boulware et al. 2005; Grove-Strawser et al. 2010), which made it another enticing candidate for testing sex differences of downstream targets of estradiol. However, we did not observe any sex or treatment specific effects in the study. This indicates that pCREB may not be regulated in the same pathway as pERK and Egr-1 in audition, though there are still open questions about its sensitivity to acute estradiol application. These findings now turn attention toward other transcription factors that can target the Egr-1 promotor such as Elk-1, which itself is also a target of the MEK-ERK pathway (Chen et al. 2004; Knapska and Kaczmarek 2004).
Although we did not detect changes in pCREB due to auditory or endogenous estrogen manipulations, this does not rule out that sex differences in Egr-1 expression may be due to sex differences in cell signaling mechanisms. We have previously shown that auditory-evoked neuronal activity decreases in male but not female NCM neurons when administered an antagonist for the membrane estrogen receptor – GPER1; however, expression of GPER1 did not differ between the sexes (Krentzel et al. 2018). Phosphorylation of ERK and its role in audition has exclusively been characterized in male but not female zebra finches (Cheng and Clayton 2004), as well as the rapid effect of estradiol on pCREB, pTH, and pERK in male song sparrows (Heimovics et al. 2012). Although pCREB seemed a potential candidate from the mammalian literature (Abraham and Herbison 2005), our study indicates that the role of pCREB in sexually differentiated auditory responsiveness is not as robust as Egr-1 expression changes. Nevertheless, these findings do not necessarily indicate that males and females utilize the same intracellular mechanisms in the auditory cortex. Many ubiquitous kinase cascades such as MEK-ERK are sensitive to perturbations in a sex specific manner (Gresack et al. 2009; Sharma et al. 2009; Armstead et al. 2011). Future studies examining downstream pathways of estradiol regulation of audition will need to competently consider sex as a biological variable to determine how males and females are utilizing estradiol.
Egr-1: neuroestrogens and auditory memory
Although Egr-1 expression is often used as a neuronal activation marker for auditory regions in the songbird, Egr-1 is implicated in learning and memory (Davis et al. 2003; Knapska and Kaczmarek 2004; Veyrac et al. 2013). Egr-1 targets promotor regions of genes involved in synapse formation and maintenance (Knapska and Kaczmarek 2004; Moorman et al. 2011). Egr-1 knockout mice have memory deficits (Han et al. 2014). Within songbirds, Egr-1 has been described as a marker for the “genomic action potential” (Clayton 2000), as part of the essential process that neurons use to encode salient stimuli and translate the signal from an action potential to changes that occur in gene expression, protein expression, and neuronal architecture. Secondary auditory regions such as NCM have been suggested to play a role in song memory and may be a loci for “tutor memory” or a template for male song (Bolhuis and Gahr 2006; Bolhuis and Moorman 2015; Yanagihara and Yazaki-Sugiyama 2016). Egr-1 may be part of the mechanism by which these memories are stored. If this is the case, the sensitivity of Egr-1 to neural estradiol suggests that estradiol may be an important signal in auditory memories and song development.
Conclusions
This study demonstrates that acute neuroestrogen synthesis blockade attenuates auditory-induced IEG responsiveness in multiple auditory cortical regions of the zebra finch. This is particularly evident in males, supporting the hypothesis that males are more reliant on active estrogen synthesis than females for auditory responsiveness. Future work is necessary to unpack the regional specific regulation of aromatase activity and estradiol sensitive signaling pathways within auditory regions of the forebrain. Direct comparisons between the sexes will help further our understanding of the role estradiol has as a neuromodulator in auditory processing.
Supplementary Material
Acknowledgements:
A.A.K contributed to all the study designs, execution of the experimental studies, data analyses, and manuscript preparation. M.Z.K contributed to Study 4 design, execution of the experimental study and data analysis. T.J.O. contributed execution of the experimental study and data analysis of Study 4. E.K. contributed to the execution of the experimental study and data analysis of Study 3. L.RH. contributed to the study designs and manuscript preparation. We thank Colin Saldanha for the aromatase antibody, Jim Chambers for assistance and training on the confocal microscope, Laura Bernal-Corzo for piloting the Egr-1 study, and Ardara Berry for assistance on quantification. This work was supported by the National Institutes of Health R01NS082179 and the University of Massachusetts Graduate Student Dissertation Grant. All procedures were in accordance with protocols approved by the Institutional Animals Care and Use Committee and euthanasia methods followed guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
List of Abbreviations
- aHVCs
anterior HVC shelf
- ANOVA
analysis of variance
- Cb
cerebellum
- CI
confidence interval
- CMM
caudomedial mesopallium
- CRE
cAMP response element
- CREB
cAMP response element-binding protein
- DAPI
4’,6-diamidino-2-phenylindole
- EIA
enzyme-linked immunoassay
- Egr-1
early grow response 1
- ERK
extracellular receptor kinase
- Fad
fadrozole
- GPER-1
g-protein estrogen receptor 1
- Hp
hippocampus
- HSD
honestly significant difference
- HVC
high vocal center
- HVCS
HVC shelf
- IEG
immediate early gene
- ISI
interstimulus interval
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- NCL
caudal lateral nidopallium
- NCM
caudal medial nidopallium
- NIf
nucleus interfacialis of the nidopallium
- PB/PBS
phosphate buffer (saline)
- pCREB
phosphorylated cAMP response element-binding protein
- pERK
phosphorylated extracellular receptor kinase
- PFA
paraformaldehyde
- pHVCs
posterior HVC shelf
- pTH
phosphorylated tyrosine hydroxylase
- Sal
saline
- Tn
nucleus taenia
- ZENK
zif268, EGR-1, NGFI-A, and krox24
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- Abraham IM, Herbison AE (2005) Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. Neuroscience 131:945–951 doi: 10.1016/j.neuroscience.2004.10.046 [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA (1990) Sex steroid levels in developing and adult male and female zebra finches (poephila guttata). Gen Comp Endocrinol 78:93–109 [DOI] [PubMed] [Google Scholar]
- Alward BA, de Bournonville C, Chan TT, Balthazart J, Cornil CA, Ball GF (2016) Aromatase inhibition rapidly affects in a reversible manner distinct features of birdsong. Sci Rep 6:32344doi: 10.1038/srep32344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead WM, Kiessling JW, Riley J, Kofke WA, Vavilala MS (2011) Phenylephrine infusion prevents impairment of atp- and calcium-sensitive potassium channel-mediated cerebrovasodilation after brain injury in female, but aggravates impairment in male, piglets through modulation of erk mapk upregulation. J Neurotrauma 28:105–111 doi: 10.1089/neu.2010.1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avey MT, Phillmore LS, MacDougall-Shackleton SA (2005) Immediate early gene expression following exposure to acoustic and visual components of courtship in zebra finches. Behav Brain Res 165:247–253 doi: 10.1016/j.bbr.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Rosebush JC, Wade J (2002) The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. J Neurobiol 52:43–51 doi: 10.1002/neu.10070 [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Wade J (2005) Fos and zenk responses in 45-day-old zebra finches vary with auditory stimulus and brain region, but not sex. Behav Brain Res 162:108–115 doi: 10.1016/j.bbr.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Cornil CA, Charlier TD, Taziaux M, Ball GF (2009) Estradiol, a key endocrine signal in the sexual differentiation and activation of reproductive behavior in quail. J Exp Zool A Ecol Genet Physiol 311:323–345 doi: 10.1002/jez.464 [DOI] [PubMed] [Google Scholar]
- Beltz AM, Beery AK, Becker JB (2019) Analysis of sex differences in pre-clinical and clinical data sets. Neuropsychopharmacology doi: 10.1038/s41386-019-0524-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit AM, Inskeep EK, Dailey RA (1992) Effect of a nonsteroidal aromatase inhibitor on in vitro and in vivo secretion of estradiol and on the estrous cycle in ewes. Domest Anim Endocrinol 9:313–327 [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M (2006) Neural mechanisms of birdsong memory. Nat Rev Neurosci 7:347–357 doi: 10.1038/nrn1904 [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Moorman S (2015) Birdsong memory and the brain: In search of the template. Neurosci Biobehav Rev 50:41–55 doi: 10.1016/j.neubiorev.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG (2005) Estradiol activates group i and ii metabotropic glutamate receptor signaling, leading to opposing influences on camp response element-binding protein. J Neurosci 25:5066–5078 doi: 10.1523/JNEUROSCI.1427-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callard GV, Petro Z, Ryan KJ (1978) Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology 103:2283–2290 doi: 10.1210/endo-103-6-2283 [DOI] [PubMed] [Google Scholar]
- Chao A, Paon A, Remage-Healey L (2015) Dynamic variation in forebrain estradiol levels during song learning. Dev Neurobiol 75:271–286 doi: 10.1002/dneu.22228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lee WR, Safe S (2004) Egr-1 is activated by 17beta-estradiol in mcf-7 cells by mitogen-activated protein kinase-dependent phosphorylation of elk-1. J Cell Biochem 93:1063–1074 doi: 10.1002/jcb.20257 [DOI] [PubMed] [Google Scholar]
- Cheng HY, Clayton DF (2004) Activation and habituation of extracellular signal-regulated kinase phosphorylation in zebra finch auditory forebrain during song presentation. J Neurosci 24:7503–7513 doi: 10.1523/JNEUROSCI.1405-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DF (2000) The genomic action potential. Neurobiol Learn Mem 74:185–216 doi: 10.1006/nlme.2000.3967 [DOI] [PubMed] [Google Scholar]
- Cohen J (1994) The earth is round (p<.05). American Psychologist 49:997–1003. [Google Scholar]
- Cohen RE, Wade J (2012) Expression of aromatase and two isozymes of 5alpha-reductase in the developing green anole forebrain. J Neuroendocrinol 24:1213–1221 doi: 10.1111/j.1365-2826.2012.02328.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA (2018) On the role of brain aromatase in females: Why are estrogens produced locally when they are available systemically? J Comp Physiol A Neuroethol Sens Neural Behav Physiol 204:31–49 doi: 10.1007/s00359-017-1224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Leung CH, Pletcher ER, Naranjo KC, Blauman SJ, Saldanha CJ (2012) Acute and specific modulation of presynaptic aromatization in the vertebrate brain. Endocrinology 153:2562–2567 doi: 10.1210/en.2011-2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Bozon B, Laroche S (2003) How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behavioural Brain Research 142:17–30 doi: 10.1016/s0166-4328(02)00421-7 [DOI] [PubMed] [Google Scholar]
- de Jager T, Pelzer T, Muller-Botz S, Imam A, Muck J, Neyses L (2001) Mechanisms of estrogen receptor action in the myocardium. Rapid gene activation via the erk1/2 pathway and serum response elements. J Biol Chem 276:27873–27880 doi: 10.1074/jbc.M010984200 [DOI] [PubMed] [Google Scholar]
- De Vries GJ (2004) Minireview: Sex differences in adult and developing brains: Compensation, compensation, compensation. Endocrinology 145:1063–1068 doi: 10.1210/en.2003-1504 [DOI] [PubMed] [Google Scholar]
- Fernandez SM et al. (2008) Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci 28:8660–8667 doi: 10.1523/JNEUROSCI.1968-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foidart A, Tlemcani O, Harada N, Abe-Dohmae S, Balthazart J (1995) Pre- and post-translational regulation of aromatase by steroidal and non-steroidal aromatase inhibitors. Brain Res 701:267–278 [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH (2005) Distribution of estrogen receptor alpha mrna in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J Comp Neurol 483:91–113 doi: 10.1002/cne.20397 [DOI] [PubMed] [Google Scholar]
- Forlano PM, Schlinger BA, Bass AH (2006) Brain aromatase: New lessons from non-mammalian model systems. Front Neuroendocrinol 27:247–274 doi: 10.1016/j.yfrne.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Frick KM (2013) Epigenetics, oestradiol and hippocampal memory consolidation. J Neuroendocrinol 25:1151–1162 doi: 10.1111/jne.12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Schafe GE, Orr PT, Frick KM (2009) Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience 159:451–467 doi: 10.1016/j.neuroscience.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG (2010) Membrane estrogen receptors activate the metabotropic glutamate receptors mglur5 and mglur3 to bidirectionally regulate creb phosphorylation in female rat striatal neurons. Neuroscience 170:1045–1055 doi: 10.1016/j.neuroscience.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaide J et al. (2017) Exploring sex differences in the adult zebra finch brain: In vivo diffusion tensor imaging and ex vivo super-resolution track density imaging. Neuroimage 146:789–803 doi: 10.1016/j.neuroimage.2016.09.067 [DOI] [PubMed] [Google Scholar]
- Han S et al. (2014) Impaired extinction of learned contextual fear memory in early growth response 1 knockout mice. Mol. Cells 37:24–30 doi: 10.14348/molcells.2014.2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Prior NH, Ma C, Soma KK (2016) Rapid effects of an aggressive interaction on dehydroepiandrosterone, testosterone and oestradiol levels in the male song sparrow brain: A seasonal comparison. J Neuroendocrinol 28:12345doi: 10.1111/jne.12345 [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Prior NH, Maddison CJ, Soma KK (2012) Rapid and widespread effects of 17beta-estradiol on intracellular signaling in the male songbird brain: A seasonal comparison. Endocrinology 153:1364–1376 doi: 10.1210/en.2011-1525 [DOI] [PubMed] [Google Scholar]
- Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A (2019) Moving beyond p values: Data analysis with estimation graphics. Nature Methods 16:565–566 doi: 10.1038/s41592-019-0470-3 [DOI] [PubMed] [Google Scholar]
- Ikeda MZ, Krentzel AA, Oliver TJ, Scarpa GB, Remage-Healey L (2017) Clustered organization and region-specific identities of estrogen-producing neurons in the forebrain of zebra finches (taeniopygia guttata). J Comp Neurol doi: 10.1002/cne.24292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Nottebohm F (1997) Motor-driven gene expression. Proc Natl Acad Sci U S A 94:4097–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA (1976) Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res 114:152–157 [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L (2004) A gene for neuronal plasticity in the mammalian brain: Zif268/egr-1/ngfi-a/krox-24/tis8/zenk? Prog Neurobiol 74:183–211 doi: 10.1016/j.pneurobio.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Kochak GM, Mangat S, Mulagha MT, Entwistle EA, Santen RJ, Lipton A, Demers L (1990) The pharmacodynamic inhibition of estrogen synthesis by fadrozole, an aromatase inhibitor, and its pharmacokinetic disposition. J. Clin. Endocrinol. Metab 71:1349–1355 [DOI] [PubMed] [Google Scholar]
- Kokras N, Pastromas N, Papasava D, de Bournonville C, Cornil CA, Dalla C (2018) Sex differences in behavioral and neurochemical effects of gonadectomy and aromatase inhibition in rats. Psychoneuroendocrinology 87:93–107 doi: 10.1016/j.psyneuen.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Krentzel AA, Macedo-Lima M, Ikeda MZ, Remage-Healey L (2018) A membrane g-protein coupled estrogen receptor is necessary but not sufficient for sex-differences in zebra finch auditory coding. Endocrinology doi: 10.1210/en.2017-03102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Remage-Healey L (2015) Sex differences and rapid estrogen signaling: A look at songbird audition. Front Neuroendocrinol 38:37–49 doi: 10.1016/j.yfrne.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen J, McAuley JD, Chang SE, Wade J (2017) Zenk induction in the zebra finch brain by song: Relationship to hemisphere, rhythm, oestradiol and sex. J Neuroendocrinol 29 doi: 10.1111/jne.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL (2016) Perils and pitfalls of reporting sex differences. Philos Trans R Soc Lond B Biol Sci 371:20150119doi: 10.1098/rstb.2015.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT (2006) Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci 23:1523–1529 doi: 10.1111/j.1460-9568.2006.04673.x [DOI] [PubMed] [Google Scholar]
- Meitzen J, Grove DD, Mermelstein PG (2012) The organizational and aromatization hypotheses apply to rapid, nonclassical hormone action: Neonatal masculinization eliminates rapid estradiol action in female hippocampal neurons. Endocrinology 153:4616–4621 doi: 10.1210/en.2012-1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF (1992) Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A 89:6818–6822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Wong AM, Mittelman-Smith MA (2015) Estradiol membrane-initiated signaling and female reproduction. Compr Physiol 5:1211–1222 doi: 10.1002/cphy.c140056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman S, Mello CV, Bolhuis JJ (2011) From songs to synapses: Molecular mechanisms of birdsong memory. Molecular mechanisms of auditory learning in songbirds involve immediate early genes, including zenk and arc, the erk/mapk pathway and synapsins. Bioessays 33:377–385 doi: 10.1002/bies.201000150 [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP (1976) Sexual dimorphism in vocal control areas of the songbird brain. Science 194:211–213 [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS (2016) 17beta-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci 36:2677–2690 doi: 10.1523/JNEUROSCI.4437-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CJ et al. (2011) Genetic rescue of nonclassical eralpha signaling normalizes energy balance in obese eralpha-null mutant mice. J Clin Invest 121:604–612 doi: 10.1172/JCI41702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ (2005) Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci 272:2089–2096 doi: 10.1098/rspb.2005.3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt MA, Satkunaratnam A, Novosad DM (1998) Estrogen activates raf-1 kinase and induces expression of egr-1 in mcf-7 breast cancer cells. Mol Cell Biochem 189:119–125 [DOI] [PubMed] [Google Scholar]
- Prior NH, Yap KN, Soma KK (2014) Acute and chronic effects of an aromatase inhibitor on pair-maintenance behavior of water-restricted zebra finch pairs. Gen Comp Endocrinol 196:62–71 doi: 10.1016/j.ygcen.2013.10.018 [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Mello CV, & Jarvis ED (2004). Songbirds and the revised avian brain nomenclature. Ann NY Acad Sci, 1016, 77–108. doi: 10.1196/annals.1298.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA (2010a) Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A 107:3852–3857 doi: 10.1073/pnas.0906572107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA (2011) Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J. Neurosci 31:10034–10038 doi: 10.1523/jneurosci.0566-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA (2012) Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol 107:1621–1631 doi: 10.1152/jn.00749.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Jeon SD, Joshi NR (2013) Recent evidence for rapid synthesis and action of oestrogens during auditory processing in a songbird. J Neuroendocrinol 25:1024–1031 doi: 10.1111/jne.12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, London SE, Schlinger BA (2010b) Birdsong and the neural production of steroids. J Chem Neuroanat 39:72–81 doi: 10.1016/j.jchemneu.2009.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA (2008) Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci 11:1327–1334 doi: 10.1038/nn.2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensel MA, Salwiczek L, Roth J, Schlinger BA (2013) Context-specific effects of estradiol on spatial learning and memory in the zebra finch. Neurobiol Learn Mem 100:41–47 doi: 10.1016/j.nlm.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Schlinger BA, Saldanha CJ (2007) Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol 67:1–9 doi: 10.1002/dneu.20303 [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Coomaralingam L (2005) Overlap and co-expression of estrogen synthetic and responsive neurons in the songbird brain--a double-label immunocytochemical study. Gen Comp Endocrinol 141:66–75 doi: 10.1016/j.ygcen.2004.11.013 [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA, Micevych PE, Horvath TL (2004) Presynaptic n-methyl-d-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J Comp Neurol 469:522–534 doi: 10.1002/cne.11035 [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA (2000) Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol 423:619–630 [DOI] [PubMed] [Google Scholar]
- Sanford SE, Lange HS, Maney DL (2010) Topography of estradiol-modulated genomic responses in the songbird auditory forebrain. Dev Neurobiol 70:73–86 doi: 10.1002/dneu.20757 [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP (1991) Brain is the major site of estrogen synthesis in a male songbird. Proc Natl Acad Sci U S A 88:4191–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP (1992) Circulating estrogens in a male songbird originate in the brain. Proc Natl Acad Sci U S A 89:7650–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Mohammad F, Singh P (2009) Gender differences in a drosophila transcriptomic model of chronic pentylenetetrazole induced behavioral deficit. PLoS One 4:e8136doi: 10.1371/journal.pone.0008136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P, Schlinger BA, Campagnoni AT, Arnold AP (1995) An atlas of aromatase mrna expression in the zebra finch brain. J Comp Neurol 360:172–184 doi: 10.1002/cne.903600113 [DOI] [PubMed] [Google Scholar]
- Shors TJ, Pickett J, Wood G, Paczynski M (2009) Acute stress persistently enhances estrogen levels in the female rat. Stress 3:163–171 doi: 10.3109/10253899909001120 [DOI] [PubMed] [Google Scholar]
- Suva LJ, Harm SC, Gardner RM, Thiede MA (1991) In vivo regulation of zif268 messenger rna expression by 17 beta-estradiol in the rat uterus. Mol Endocrinol 5:829–835 doi: 10.1210/mend-5-6-829 [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Schwabl H, Nottebohm F (1998) Context determines the sex appeal of male zebra finch song. Anim Behav 55:1003–1010 doi: 10.1006/anbe.1997.0673 [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Vardaris RM, Lewis D, Rawitch AB (1980) Gonadal steroids: Effects on excitability of hippocampal pyramidal cells. Science 209:1017–1018 [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM (2016) Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm Behav 83:60–67 doi: 10.1016/j.yhbeh.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linden A, Balthazart J (2018) Rapid changes in auditory processing in songbirds following acute aromatase inhibition as assessed by fmri. Horm Behav doi: 10.1016/j.yhbeh.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F (1996) Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (taenopygia guttata). J. Comp. Neurol 366:613–642 doi: [DOI] [PubMed] [Google Scholar]
- Veyrac A, Gros A, Bruel-Jungerman E, Rochefort C, Kleine Borgmann FB, Jessberger S, Laroche S (2013) Zif268/egr1 gene controls the selection, maturation and functional integration of adult hippocampal newborn neurons by learning. Proc Natl Acad Sci U S A 110:7062–7067 doi: 10.1073/pnas.1220558110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierk R et al. (2012) Aromatase inhibition abolishes ltp generation in female but not in male mice. J Neurosci 32:8116–8126 doi: 10.1523/JNEUROSCI.5319-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Schlinger BA, Hodges L, Arnold AP (1994) Fadrozole: A potent and specific inhibitor of aromatase in the zebra finch brain. Gen Comp Endocrinol 94:53–61 doi: 10.1006/gcen.1994.1059 [DOI] [PubMed] [Google Scholar]
- Woolley CS (2007) Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol 47:657–680 doi: 10.1146/annurev.pharmtox.47.120505.105219 [DOI] [PubMed] [Google Scholar]
- Yanagihara S, Yazaki-Sugiyama Y (2016) Auditory experience-dependent cortical circuit shaping for memory formation in bird song learning. Nat Commun 7:11946doi: 10.1038/ncomms11946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Lu K, Vicario DS (2012) Blocking estradiol synthesis affects memory for songs in auditory forebrain of male zebra finches. Neuroreport 23:922–926 doi: 10.1097/WNR.0b013e3283588b61 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.