Abstract
Oxidative stress and inflammation promotes vaso-occlusion in sickle cell disease (SCD). CD33-related Sialic acid-binding immunoglobulin-type lectins (CD33rSiglecs) are cell surface proteins that recognize sialic acids inhibit innate immune cell functions. We have shown that Siglec-9 on human neutrophils interact with erythrocyte sialic acids (prominently glycophorin-A (GYPA) to suppress neutrophil reactive oxygen species (ROS). We hypothesized altered sickle erythrocyte membrane sialic acid leads to decreased Siglec-9 binding capability, and thus a decreased neutrophil oxidative burst. SS erythrocytes express significantly more sialic acid than AA erythrocytes (p=0.02). SS erythrocytes displayed significantly less Siglec-9-Fc binding 39% ± 11 (mean ± SEM) compared to AA erythrocytes 78% ± 5 (p=0.009). Treatment of AA erythrocytes with sialidase to remove sialic acid decreased binding to 3% ± 7.9 (p<0.001). When freshly isolated neutrophils were incubated with AA erythrocytes, neutrophils achieved 16%± 6 of the oxidative burst exhibited by a stimulated neutrophil without erythrocytes. In contrast, neutrophils incubated with SS erythrocytes achieved 47%± 6 of the oxidative burst (AA versus SS, p=0.03). Stimulated neutrophils incubated with AA erythrocytes showed minimal NET formation while with SS erythrocytes NETs increased. SS erythrocytes are deficient in binding to neutrophil Siglec-9 which may contribute to the increased oxidative stress in SCD.
Keywords: Sickle cell disease, Siglec-9, Oxidative stress, Sialic acid
1. Introduction
Sickle cell disease (SCD) pathophysiology involves increased oxidative stress, hemolysis and inflammation. These effects promote downstream complications such as vaso-occlusion, ischemia-reperfusion physiology and organ damage [1–3]. Erythrocytes exert both pro-oxidative and anti-oxidative effects on disease-associated inflammation through iron-driven free radical production and endogenous anti-oxidants, respectively. In SCD, erythrocyte dysfunction caused by accelerated auto-oxidation and iron de-compartmentalization drive this oxidative stress [4–6]. Pro-inflammatory molecules and oxidants derived from immune cells, such as neutrophils, also contribute to elevated oxidative stress and inflammation.
Neutrophils are the most abundant immune cells in circulation [7, 8]. In normal physiology, intravascular neutrophils remain quiescent until challenged by a pathogen or other injury. However, in SCD circulating neutrophils struggle to retain quiescence, and once activated, are a powerful promoter of inflammation and disease progression in SCD [8, 9]. This is accomplished through release of reactive oxygen species (ROS) and proteases, neutrophil extracellular trap (NET) production, and cell adhesion to the vascular wall and other blood cells participating in vaso-occlusion [1, 9–11]. Past studies have sought to elucidate the mechanisms by which neutrophils remain quiescent in the bloodstream until they leave the circulation either to travel to a site of infection or for invader surveillance to eventually undergo apoptosis [8, 10, 12, 13].
One recently discovered mechanism involves CD33-related sialic acid-binding immunoglobulin-type lectins (CD33rSiglecs) present on immune cells, which can recognize host cell-associated sialic acids in the form of “self-associated molecular patterns” (SAMPs) to downregulate immune responses or suppress immune functions [14, 15]. Siglec-9, the most abundant CD33rSiglec found on neutrophils, has been shown to interact with sialylated SAMPs on the erythrocyte membrane (prominently glycophorin-A (GYPA)) leading to neutrophil quiescence and activation. This action can be disrupted through removal or modification of sialic acids on the erythrocyte membrane [16–18]. Given the 1000-fold excess of erythrocytes over neutrophils in whole blood, this finding can also explain the well-known technical problem of spontaneous activation of freshly isolated neutrophils in vitro.
Erythrocyte aging leads to a natural alteration of membrane topography, including a loss of membrane bound sialic acid [19–21]. In SCD, erythrocyte aging and accumulation of damage is accelerated [22, 23]. However, previous studies are in disagreement in regard to comparative concentrations of SS erythrocyte membrane sialic acid [24, 25]. We endeavored to explore the possible interaction of sickle erythrocyte associated sialic acid, neutrophil siglec-9 and how that interaction may contribute to the systemic oxidative stress and inflammation present in sickle cell disease.
2. Methods
2.1. Human Samples
Human blood samples were obtained from healthy nonpregnant adult volunteers and steady-state and crisis SCD patients by protocols approved by the Institutional Review Board of the University of Minnesota. The study was conducted in accordance with the Declaration of Helsinki.
2.2. Erythrocyte Collection and Ghost Production
Blood was collected directly into BD Vacutainer tubes containing EDTA. The collected blood was centrifuged at 2000 × g for 10 minutes at 4C to separate the cells and plasma. After centrifugation the plasma and buffy coat layers were removed. The collected erythrocytes were placed into 15 ml centrifuge tubes and washed with ice-cold PBS (pH 7.4) twice. After removing the PBS supernatant, 10 ml of ice-cold 10mM Tris-HCl (pH 7.5) containing 1 mM EDTA was added to the washed erythrocytes. After inverting the tube several times to mix, the mixture was transferred to 50 ml tubes and placed on ice for 10 minutes. After incubation the cells were centrifuged at 10,000 × g for 30 minutes with the brake off. After 30 minutes the supernatant was aspirated off. This process was repeated until the membrane pellet became a pale white color. At that time the supernatant was removed, and the membrane pellet was washed with ice-cold de-ionized water.
2.3. HPLC Sialic Acid Quantification
The DMB reagent, as per the conventional method was made with the following recipe and hydrolyzing conditions: 14 mM DMB (1,2-Diamino-4,5-methylenedioxybenzene), 18 mM sodium hydrosulfite, 0.75 M 2-mercaptoethanol, and 1.6 M acetic acid, and incubated at 50°C for 2.5 h. The DMB-derivatized samples were analyzed on a Dionex Ultra3000 HPLC System using a Phenomenex Gemini 5μ C18 250 × 4.6-mm HPLC column at room temperature. The fluorescence was detected at 448 nm using excitation at 373 nm. The data collection time was expanded to 90 min. Standard samples were run with each experiment to account for slight variations in elution time. Data are expressed as picomoles of sialic acid (Neu5Ac)/microgram (μg) RBC ghost protein or RBC.
2.4. Erythrocyte Density Centrifugation
Fractionation of erythrocytes was accomplished using Optiprep Density Media (Sigma Aldrich). Four gradient solutions of 1.077, 1.087, 1.097 and 1.107 g/ml were formulated according to the supplier’s instructions and used to create a discontinuous gradient. 1ml of whole blood was layered on top of the density gradient and centrifuged at 1000 g for 30 minutes at room temperature. Following centrifugation, each layer was collected separately, washed with 1X PBS and prepared for future use.
2.5. Siglec-9-Fc Binding Assay
Approximately 3 ml of venous blood (AA and SS) was collected in EDTA, centrifuged, and erythrocytes isolated. Approximately 2 million erythrocytes were incubated with 10μg of rhSiglec-9 protein (R&D Systems) for 30 minutes on ice. Erythrocytes were then stained with either anti-CD235a (Biolegend) or anti-IgG (Biolegend) on ice for 30 minutes and analyzed by flow cytometry. One set of AA erythrocytes were pretreated with 20mU of Arthrobacter ureafaciens sialidase (AUS) (Sigma-Aldrich) for 15 minutes at RT followed by protein incubation and staining [16].
2.6. Neutrophil Oxidative Burst Assay
Neutrophils were isolated from AA volunteers using a combination of Histopaque 1077 (Sigma-Aldrich, USA) and Hespan 0.09% (Supplier) according to instructions from the manufacturers. Healthy neutrophils were incubated with AA or SS erythrocytes (50:1 ratio) or plasma, followed by measurement of stimulated neutrophil oxidative burst (100nM phorbol myristate acetate, PMA) as previously described [16].
2.7. In Vitro NET assay
Isolated neutrophils from AA volunteers as stated previously and plated on Poly-L-Lysine coated 24 well plate (Greiner). Cells attached for 30 minutes, followed by incubation with phorbol myristate acetate, PMA (100nM) (Sigma-Aldrich), AA/SS plasma or AA/SS erythrocytes. Neutrophils were then stained with DNA dye SYTOX orange (Life-Technologies) and cell permeable dye SYTO13 (Life Technologies) and observed using confocal microscopy as previously described [10].
2.8. Statistics
Analyses were performed with Prism 8 for Mac OSX (Prism). Comparison of multiple treatment groups were made using 1-way analysis of variance (Tukey’s Multiple Comparisons Test). An unpaired t test was used for 2 groups.
3. Results and Discussion
3.1. Erythrocyte Membrane Sialic Acid Content
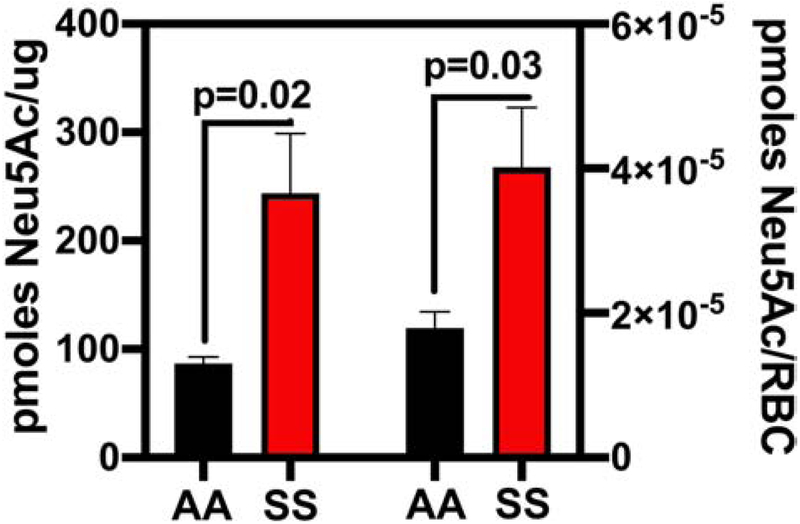
Using modern HPLC techniques AA and SS erythrocytes were analyzed to determine their respective membrane sialic acid (Neu5Ac) concentrations. SS (n=10) erythrocytes express significantly more sialic acid than AA (n=8) erythrocytes per μg/membrane protein (p=0.02) and per individual erythrocyte (p=0.03) (Figure 1). These results are surprising given that erythrocytes are believed to lose sialic acid as they age or undergo damage.
Figure 1. Sickle Erythrocytes Contain More Sialic Acid than AA Erythrocytes.
Expressed as pmoles Neu5ac/μg SS or AA erythrocyte membrane protein or red blood cells (RBC).
3.2. Erythrocyte Siglec-9Fc Binding
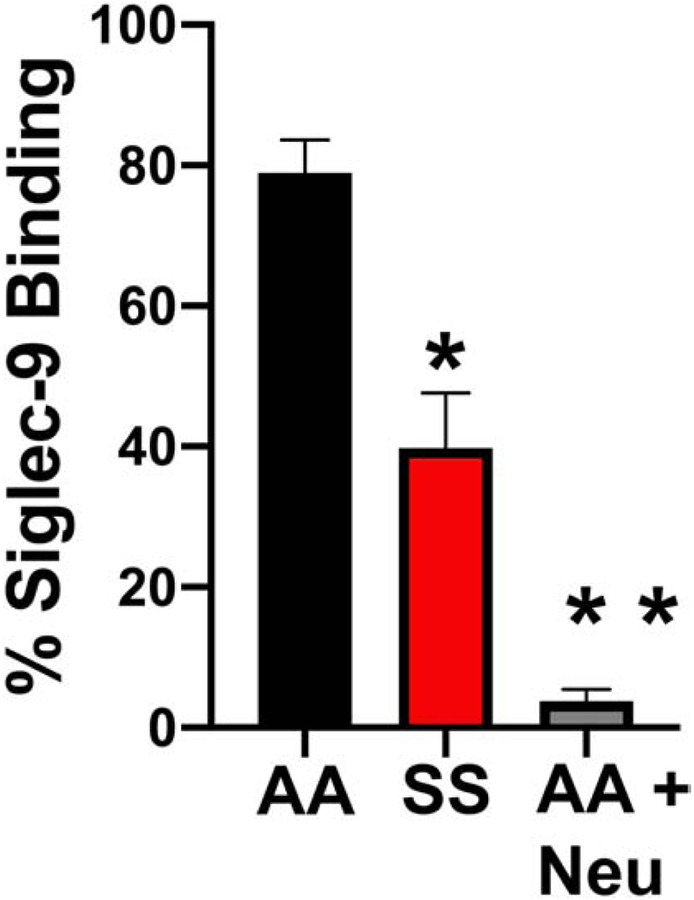
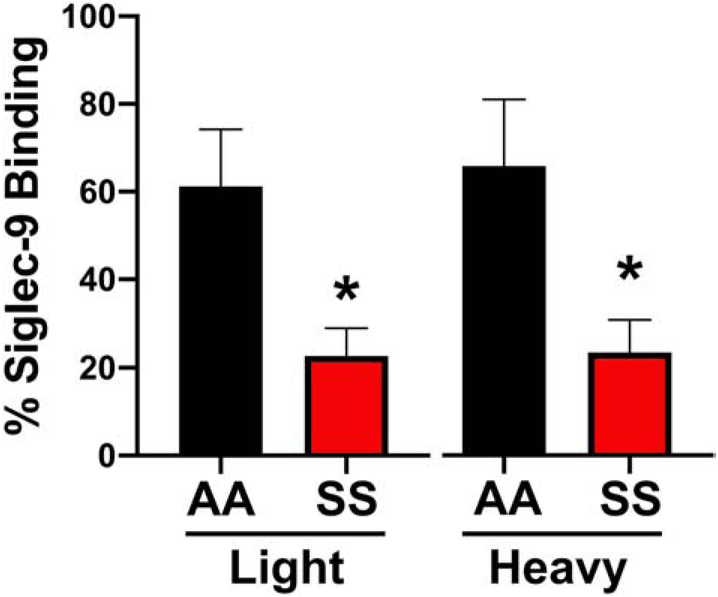
Based on our observations above, we explored whether the altered SS erythrocyte sialome would impact the sialic acid/Siglec-9 mediated interaction with neutrophils. Initial efforts involved determining whether the altered SS erythrocyte sialome would bind recombinant soluble Siglec-9-Fc protein. When measured by flow cytometry (Figure 2), SS (n=15) erythrocytes displayed significantly less Siglec-9-Fc binding 39% ± 11.9 (mean ± SEM) compared to AA (n=14) erythrocytes 78% ± 5.4 (p=0.009). Treatment of AA erythrocytes with neuraminidase to remove sialic acid decreased binding to 3% ± 7.9 (p≤0.001). Given the significant difference in Siglec-9Fc binding percentage of AA vs. SS erythrocytes, we used a density centrifugation method to separate the erythrocytes into light (x<1.100 g/ml) and heavy (x>1.100 g/ml). When measured by flow cytometry heavy SS erythrocytes (n=7) displayed significantly less Siglec-9Fc binding (23.43%±7.42) compared to AA heavy erythrocytes (n=4) (65.80%±15.17) (p=0.04). The light SS erythrocytes (n=7) also displayed significantly less Siglec-9FC binding (22.70%± 6.33) compared to light AA erythrocytes (n=4) (61.20%± 13) (p=0.012) (Figure 3).
Figure 2. Sickle Erythrocytes Bind Less Siglec-9Fc than AA Erythrocytes.
AA erythrocytes, SS erythrocytes or AA erythrocytes treated with neuraminidase (AA NEU Treated) were assess by flow cytometry as in Methods for Siglec-9Fc binding.
Figure 3. Light or Heavy Fractions of SS Erythrocytes Bound Less Siglec-9 Fc Than AA Erythrocytes.
Density gradient erythrocytes from light and dense fraction of SS and AA erythrocytes were assessed for binding Siglec-9Fc by flow cytometry.
3.3. Neutrophil Oxidative Burst Suppression
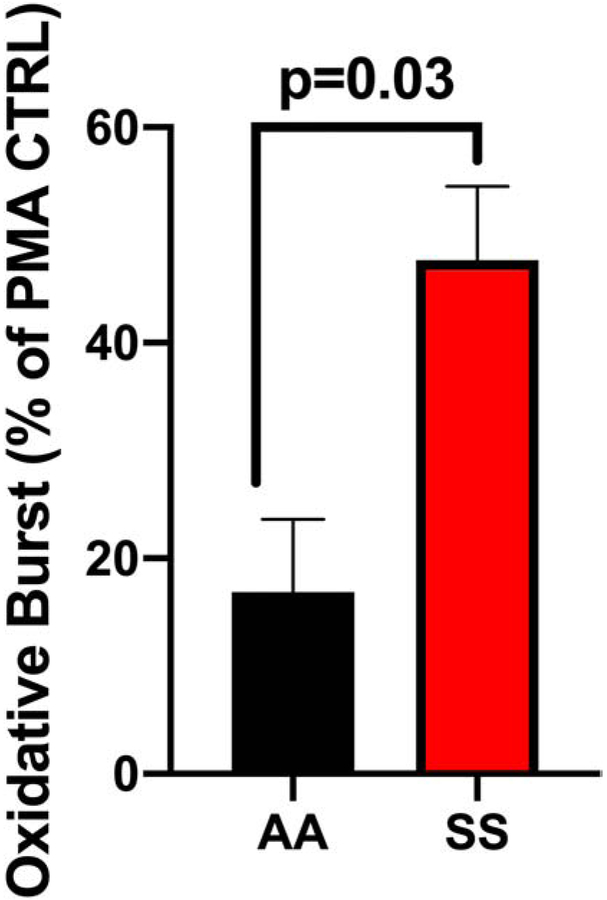
Neutrophil ROS production was measured by flow cytometry using dihydrorhodamine 123 (DHR123) (Figure 4). When freshly isolated neutrophils were incubated with AA (n=4) erythrocytes, they were only able to achieve 16.8%± 6.7 of the oxidative burst exhibited by a PMA (100nM) stimulated neutrophil without erythrocyte addition. In contrast, neutrophils incubated with SS erythrocytes (n=12) achieved 47.6%± 6.8 of the oxidative burst (AA versus SS, p=0.03). Thus, SS erythrocytes showed significantly reduced ability to suppress neutrophil activation when compared with AA erythrocytes.
Figure 4. AA RBCs inhibit Neutrophil Oxidative Burst More than SS RBCs.
AA and SS RBC added to neutrophils stimulated with PMA and oxidative burst measured presented a percent of control stimulated neutrophils (% of PMA CTRL).
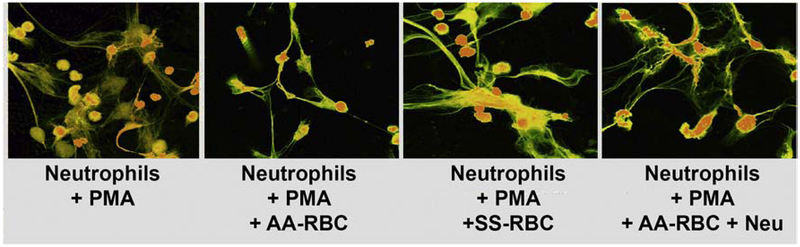
Neutrophil oxidative burst can lead to NETosis, which further contributes to the heightened inflammatory state. We show a drastic visible reduction of NETosis of neutrophils when incubated with AA erythrocytes compared with only slight visible reduction when incubated with SS erythrocytes or sialidase treated AA erythrocytes (Figure 5). Removal of sialic acid with neuraminidase SS RBC also inhibit neutrophil NETosis. These data are consistent with a decreased ability of SS erythrocytes to suppress neutrophil oxidative burst and activation.
Figure 5. AA RBC inhibit Neutrophil NETosis more than SS RBCs.
Neutrophils stimulated with PMA in presence of AA or SS or AA RBC treated with neuraminidase and NETosis assessed with SYTOX orange. Intracellular DNA stains green and extracellular and membrane-compromised PMN DNA stains yellow- orange. AA erythrocytes demonstrated significantly increased ability to suppress NETosis when compared with SS erythrocytes. Neuraminidase treatment reduces the ability of the AA erythrocytes to suppress NETosis.
4. Conclusion
A constant disease state of oxidative stress and inflammation underlies SCD pathophysiology. These results suggest that improper erythrocyte binding of neutrophil Siglec-9 may contribute to SCD pathophysiology. Previous literature has shown that GYPA, the primary Siglec-9 ligand on erythrocytes, demonstrates significant clumping on the SS erythrocyte [23]. We hypothesize that CYPA clumping might inhibit Siglec-9 binding to erythrocytes; however this hypothesis requires further testing. As noted, previous studies have shown both decreased and increased levels of sialic acid on sickle erythrocytes [26–31]. Maturation of reticulocytes has been shown to decrease GYPA on erythrocyte membranes [32], however our studies with light and heavy erythrocytes showed similar Siglec-9 binding to light and heavy sickle erythrocytes, suggesting other mechanisms might be involved.
The plasma sialome (the sum total of plasma glycoproteins) might have a separate inhibitory effect via Siglec-9. Sialic acid levels in plasma can be elevated in inflammatory states such as rheumatoid arthritis [33] and could possibly play a role in modulating inflammation. However, plasma from SS and AA individuals equally inhibited ROS formation. This is a complex issue that requires further investigation, but so far there does not appear to be a difference between AA and SS plasma.
In conclusion, this work demonstrates that despite increased sialic aid content, SS erythrocytes display a decreased ability to bind Siglec-9 and modulate neutrophil activation. This defective interaction with Siglec-9 may be a novel source of inflammation and oxidative stress in SCD. The development of neutrophil Siglec-9 agonists may prove a promising target for drugs seeking to decrease oxidation and inflammation in SCD.
Funding
Dr. Kiser was supported by the Hematology Research Training Grant T32 HL007062/HL/NHLBI This research was supported by National Institute of Health grants 5R01HL114567 and RO1GM3273 (AV)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Drs Belcher and Vercellotti receive research funding to their laboratory from CSL Behring, Hillhurst and Astellas. Other authors have no conflicts of interest.
References
- [1].Hebbel RP, Vercellotti GM, Chapter 41 - Pathobiology of Sickle Cell Disease, in: Hoffman R, Benz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, Salama ME, Abutalib SA (Eds.) Hematology (Seventh Edition), Elsevier; 2018, pp. 571–583. [Google Scholar]
- [2].Conran N, Belcher JD, Inflammation in sickle cell disease, Clin. Hemorheol. Microcirc, 68 (2018) 263–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kato GJ, Steinberg MH, Gladwin MT, Intravascular hemolysis and the pathophysiology of sickle cell disease, J. Clin. Invest, 127 (2017) 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Silva DGH, Belini Junior E, de Almeida EA, Bonini-Domingos CR, Oxidative stress in sickle cell disease: an overview of erythrocyte redox metabolism and current antioxidant therapeutic strategies, Free Radic. Biol. Med, 65 (2013) 1101–1109. [DOI] [PubMed] [Google Scholar]
- [5].Hebbel RP, Ischemia-reperfusion injury in sickle cell anemia: relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain, Hematol. Oncol. Clin. North Am, 28 (2014) 181–198. [DOI] [PubMed] [Google Scholar]
- [6].Hebbel RP, Morgan WT, Eaton JW, Hedlund BE, Accelerated autoxidation and heme loss due to instability of sickle hemoglobin, Proc. Natl. Acad. Sci. U. S. A, 85 (1988) 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Voskou S, Aslan M, Fanis P, Phylactides M, Kleanthous M, Oxidative stress in beta-thalassaemia and sickle cell disease, Redox biology, 6 (2015) 226–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang D, Xu C, Manwani D, Frenette PS, Neutrophils, platelets, and inflammatory pathways, at the nexus of sickle cell disease pathophysiology, Blood, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nauseef WM, Borregaard N, Neutrophils at work, Nat. Immunol, 15 (2014) 602–611. [DOI] [PubMed] [Google Scholar]
- [10].Chen G, Zhang D, Fuchs TA, Manwani D, Wagner DD, Frenette PS, Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease, Blood, 123 (2014) 3818–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lard LR, Mul FP, de Haas M, Roos D, Duits AJ, Neutrophil activation in sickle cell disease, J. Leukoc. Biol, 66 (1999) 411–415. [DOI] [PubMed] [Google Scholar]
- [12].Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K, IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice, J. Immunol, 179 (2007) 8274–8279. [DOI] [PubMed] [Google Scholar]
- [13].Furze RC, Rankin SM, The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse, FASEB J, 22 (2008) 3111–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Varki A, Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them, Glycobiology, 21 (2011) 1121–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Crocker PR, Paulson JC, Varki A, Siglecs and their roles in the immune system, Nat. Rev. Immunol, 7 (2007) 255–266. [DOI] [PubMed] [Google Scholar]
- [16].Lizcano A, Secundino I, Dohrmann S, Corriden R, Rohena C, Diaz S, Ghosh P, Deng L, Nizet V, Varki A, Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation, Blood, 129 (2017) 3100–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen Z, Bai FF, Han L, Zhu J, Zheng T, Zhu Z, Zhou LF, Targeting Neutrophils in Severe Asthma via Siglec-9, Int. Arch. Allergy Immunol, 175 (2018) 5–15. [DOI] [PubMed] [Google Scholar]
- [18].Angata T, Varki A, Cloning, characterization, and phylogenetic analysis of siglec-9, a new member of the CD33-related group of siglecs. Evidence for co-evolution with sialic acid synthesis pathways, J. Biol. Chem, 275 (2000) 22127–22135. [DOI] [PubMed] [Google Scholar]
- [19].Jakubowska-Solarska B, Solski J, Sialic acids of young and old red blood cells in healthy subjects, Med. Sci. Monit, 6 (2000) 871–874. [PubMed] [Google Scholar]
- [20].Mehdi MM, Singh P, Rizvi SI, Erythrocyte sialic acid content during aging in humans: correlation with markers of oxidative stress, Dis. Markers, 32 (2012) 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang YX, Wu ZJ, Mehrishi J, Huang BT, Chen XY, Zheng XJ, Liu WJ, Luo M, Human red blood cell aging: correlative changes in surface charge and cell properties, J. Cell. Mol. Med, 15 (2011) 2634–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Waugh SM, Willardson BM, Kannan R, Labotka RJ, Low PS, Heinz bodies induce clustering of band 3, glycophorin, and ankyrin in sickle cell erythrocytes, J. Clin. Invest, 78 (1986) 1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hebbel RP, Yamada O, Moldow CF, Jacob HS, White JG, Eaton JW, Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease, J. Clin. Invest, 65 (1980) 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen Y, Junger WG, Measurement of oxidative burst in neutrophils, Methods Mol. Biol, 844 (2012) 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tran H, Jha R, Nguyen J, Jarrett S, Rodriguez J, Mittal A, Lei J, Gupta K, Hemin-Induced Mast Cell-Extracellular Traps Impart Resistance to Therapy in a Sickle Microenvironment, Blood, 126 (2015) 3385–3385. [Google Scholar]
- [26].Mohandas N, Gallagher PG, Red cell membrane: past, present, and future, Blood, 112 (2008) 3939–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yang Y, Koo S, Heng LT, Meiselman HJ, Neu B, Non-adsorbing macromolecules promote endothelial adhesion of erythrocytes with reduced sialic acids, Biochim. Biophys. Acta, 1840 (2014) 288–293. [DOI] [PubMed] [Google Scholar]
- [28].Ekeke GI, Ibeh GO, Sialic acid in sickle cell disease, Clin. Chem, 34 (1988) 1443–1446. [PubMed] [Google Scholar]
- [29].Aminoff D, Anderson J, Dabich L, Gathmann WD, Sialic acid content of erythrocytes in normal individuals and patients with certain hematologic disorders, Am. J. Hematol, 9 (1980) 381–389. [DOI] [PubMed] [Google Scholar]
- [30].Westerman MP, Diloy-Puray M, Streczyn M, Membrane components in the red cells of patients with sickle cell anemia. Relationship to cell aging and to irreversibility of sickling, Biochim. Biophys. Acta, 557 (1979) 149–155. [DOI] [PubMed] [Google Scholar]
- [31].Onyemelukwe GC, Esievo KA, Kwanashie CN, Kulkarni AG, Obinechie EN, Erythrocyte sialic acid in human sickle-cell disease, J. Comp. Pathol, 97 (1987) 143–147. [DOI] [PubMed] [Google Scholar]
- [32].Liu J, Guo X, Mohandas N, Chasis JA, An X, Membrane remodeling during reticulocyte maturation, Blood, 115 (2010) 2021–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li W, Liu Y, Zheng X, Gao J, Wang L, Li Y, Investigation of the Potential Use of Sialic Acid as a Biomarker for Rheumatoid Arthritis, Ann. Clin. Lab. Sci, 49 (2019) 224–231. [PubMed] [Google Scholar]