Abstract
Background
Kefiran is a useful polysaccharide made of branched glucogalactose which is produced by microorganisms. Here the anti-MCF-7 breast cancer cells activity of kefiran and cytokine productions (IL-6) of peripheral blood mononuclear cells (PBMC) treated by kefiran was studied. Also, the effect of using kefiran as a useful and cost-effective scaffold in neural stem cell culture (PC12 cell culture) was investigated.
Material and Methods
Kefiran was produced from raw milk with 0.5% fat and 10 g of kefir grains. After incubation for 48 hrs at room temperature, the solvent collected (crude kefiran). These samples were kept at 100°C for 1 hr (boiled kefiran) and the supernatant was precipitated by ethanol (pure kefiran). Then, the electrospun nanofibers, pure polyacrylonitrile (PAN), PAN/kefiran 5%, and PAN/kefiran 10% were fabricated and used as scaffolds in the cell culture. The structure of fabricated was studied by SEM and the cytokine production (IL-6) in vitro in the cell culture supernatant of PBMC line after treatment with kefiran (1mg/mL, 5 mg/mL) and kefiran-PAN 5% and 10% were carried out by enzyme-linked immunosorbent assay (ELISA). The attachment of PC12 cells was examined by inverted microscope. Also, cytotoxicity of kefiran for PC12 and MCF7 cells and morphological changes of PC12 cells were evaluated by MTT and Cresyl violet staining (Nissl staining) respectively.
Results
The mean diameter of fabricated PAN/kefiran 5% and 10% nanofibers were 310.2±43.97 nm. The contact angle measurement results (26.9± 1.9 for the pure PAN scaffold vs 12.3± 1.13 for the PAN/kefiran) revealed enhanced hydrophilicity of scaffolds upon the incorporation of kefiran and PAN. Seeding of PC12 cells on the scaffolds showed that fabrication of kefiran into PAN led to the enhancement of cell attachment, proliferation, and morphological changes. Also, the promotion of PBMC growth and decreasing of MCF7 cell lines viability were shown through MTT assay. No significant changes were measured for the level of IL-6 in PAN/kefiran 5% treated cells compared to the control (p ≥ 0.05).
Conclusion
These results suggest superior properties of kefiran/PAN nanofibrous scaffolds for the neural stem cell culture especially for repairing injured spinal cord. Also, the pure kefiran could be used for the enhancement of PBMC growth and reducing the MCF7 cancerous cells growth. So, using biocompatible, anti-bacterial, and anti-tumor kefiran/PAN nanofibers for regenerative medicine seems promising.
Keywords: kefiran, polyacrylonitrile nanofibers, electrospinning, tissue engineering, PC12 cells
Introduction
The scaffold is a three-dimensional structure that provides cell attachment, proliferation, differentiation, and tissue formation.1,2 Electrospinning is one of the methods for scaffold fabrication with diameters ranging from microns to few nanometers that attracted a great deal of attention.3,4 The scaffolds produced by electrospinning offer a high surface area to volume ratio, high length/diameter ratio which would improve mechanical performance and flexibility in surface functionalities.4,5 There has been much interest in extending this technique to prepare uniform fibers with novel compositions and morphologies for several applications.4 Also, nanofibers have several advantages, including their ability to form ligands, enhancement of differentiation, increasing cell viability, and similarity to the extracellular matrix.6 As such, fabrication of biocompatible, biodegradable, and bioactive scaffolds is desired.1,2 Wide range of polymers were used for making nanofibers. The scaffolds used in tissue engineering are natural polymers like collagen, chitosan and synthetic polymers like poly (ɛ- caprolactone) (PCL),7 poly (lactic acid) (PLA), poly (glycolic acid) (PGA), poly (3 hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), and poly (lactic-co-glycolic acid) (PLGA). They have been used in electrospinning for tissue engineering.8–11 Due to the similarity of the polysaccharides to the extracellular matrix, they have more applications in the cell culture. So the electrospun nanofibers have attracted a lot of attention in tissue engineering.9,12 Many microorganisms containing lactic acid bacteria, algae, fungi, and plants can produce extracellular polysaccharide that could be applied in food industries, chemical and pharmaceutical industries, and drug delivery.13 Lactic acid bacteria producing polysaccharides in the past decade have been extensively studied. Physical properties that make these materials suitable to be used are having viscosity, stability, and emulsifier factors.13
Kefir contains lactic acid bacteria (Lactobacillus, Lactococcus, Leuconostoc, Acetobacter, and Streptococcus) and yeasts (Klyvrvmayss, Trola, Candida, and Saccharomyces). A polysaccharide-protein matrix surrounded both bacteria and yeast.14 The polysaccharide of kefir called kefiran is water-soluble branched glucogalactan. Kefiran has antibacterial, antifungal, and anti-tumor properties.14,15 Antimicrobial and anti-inflammatory properties of kefir for the treatment of patients infected with one or more strains of resistant microorganism were used.16 Kefir helps to regulate the immune system of the gastrointestinal tract (GIT). Also, it protects epithelial cells against Bacillus cereus extracellular factors.13,17 Moreover, it increases mitochondrial dehydrogenase activity.17 Consumption of kefir improves lactose digestion, better immune responses to the pathogenes, and enhancement of antitumor activity.17 Using Microbial polysaccharide as the natural polysaccharide has several advantages such as cost-effectivity, availability, biodegradability, similarity to the extracellular matrix and cell attachment properties.
Cytokines are proteins with a specific role in human immune responses such as cell-mediated, antibody-mediated immunity, the inflammatory responses, immunity to cancerous cells, autoimmunity, and hypersensitivity.18 Measurement of cytokines in the serum has difficulties such as the presence of soluble receptors, anti-cytokine antibodies, and receptor antagonist. So, it prefers to measure the cytokine stimulation in vitro in cell culture supernatant.18
Interleukin −6 (IL-6) is a cytokine that has a wide range of biological functions.19 It is an essential stimulant for the production of acute-phase proteins. Also, it induces terminal B lymphocyte differentiation into antibody-secreting plasma cells.19 It enables proliferation and differentiation of cytotoxic T-lymphocytes.19,20 Infectious stimulants are the most important interleukin synthesis stimuli. IL-6 increases the growth of myeloma cell lines, plasmacytomas, and hybridomas.18 Furuno and Nakanishi in 2012 showed that kefiran suppresses mast cell degranulation and cytokine production. So kefiran shows anti-inflammatory properties.21
PCl2 cells derived from the rat pheochromocytoma, are suitable for studying the neuronal system. They were used in this study to examine the advantages of the 5% and 10% kefiran-polyacrylonitrile nanofibers which are economic and useful scaffolds for cell attachment, proliferation, and differentiation of the cells. Moreover, in this study, the effect of the scaffold on the production of IL-6 in cultures of PBMC from the adult person was evaluated.19 To the best of our knowledge, this is the first time to be manufactured nanofibers from kefiran/PAN to be used for regenerative medicine.
Materials and Methods
Extraction of Kefiran from Kefir Grains
10 mL pasteurized milk containing 0.5% fat was heated and cooled down at room temperature. Then, 10 g of kefir grains CIDCA AGK1 obtained from a household in Moscow, Russia were added and kept for 48 hrs at room temperature. After incubation, kefir grains were separated from the fermented product by a plastic sieve, and then the solvent collected (crude kefiran).16 Samples were kept in sterilized distilled water at 100 ºC for an hour to inactivate hydrolyzing enzymes and dissolve the polysaccharide. Then, samples were centrifuged at 1180g in 20ºC for 15 mins to remove all the cells from the sample (boiled kefiran). The supernatant, which contained polysaccharide, was precipitated by adding two ethanol volumes in 96% cold ethanol. It was kept 24 hrs at −20 ºC. Then, samples were centrifuged at 1180 g for 15 mins at 4ºC. The pellet was dissolved in the hot distilled water. This step was repeated twice. Finally, the obtained sample was lyophilized after dissolving the sediment in hot distilled water (pure kefiran).15,16,22 This sample also was stored for the quantitation of IL-6.
Fabrication of PAN and Kefiran/PAN Nanofibers
Polyacrylonitrile polymer was purchased from Polyacryl Company in Isfahan, Iran. N-N dimethylformamide solvent was purchased from Merck, USA. Homogenized polyacrylonitrile-dimethylformamide (PAN-DMF) solution was prepared at a concentration of 13% (weight/volume) by dissolving PAN in DMF using a magnetic stirrer for 12 hrs. Kefiran with 5% and 10% (weight/volume) was added to PAN-DMF. In order to prevent aggregation and dispersion of kefiran/PAN, it was exposed to ultrasound for 5 mins. This matrix was electrospun using a 1 mL syringe, and a mass flow rate of 0.5 mL/h. High voltage (14 kV) was applied to the tip of the needle attached to the syringe until a fluid jet was ejected. The needle tip to collector distance was 13 cm. Also, PAN-DMF was solely electrospun as PAN nanofiber using a 1 mL syringe, and a mass flow of rate of 0.5 mL/h with high voltage (12 kV). The needle tip to collector distance was 13 cm.
Characterization of Fiber
The structural morphology of fabricated nanofibers was examined using a scanning electron microscope (SEM; S-4160, Hitachi, Tokyo, Japan) at an operating voltage of 5.0 kV. Scaffold sections were mounted onto sample holders and coated with gold using a sputter-coater (Technics).23,24 Then, the surface morphology of the electrospun nanofibers was investigated by SEM. The SEM micrographs measured the average fiber diameters of the electrospun fibers.
The video contact angle system (VCA Optima, AST products INC, Billerica, MA) was used to measure the contact angle of the electrospun scaffolds. The hydrophilicity of the scaffolds can be determined through the contact angel of the scaffolds. The droplet size was 0.5µL.24 The mean values were reported with the standard deviation (±SD).
FTIR Analysis of Nanofiber
Kefiran and electrospun kefiran/PAN and PAN were studied by using attenuated total reflectance-fourier transform infrared (FTIR) spectroscopy. FTIR identified major structural groups of kefiran, electrospun kefiran/PAN and PAN. The Fourier transform infrared spectra were recorded on a Bruker Vector 22 instrument (Germany) in the region of 4000–400 cm−1, at a resolution of 4 cm−1 and processed by Bruker OPUS software. The dried polysaccharide and the electrospun Kefiran/PAN and PAN nanofibers were used for analysis by FTIR.
For examination of in vitro structural changes of electrospun nanofibres, they were placed in a 24-well plate containing 1 mL of phosphate-buffered saline (PBS; pH 7.4) in each well and incubated at 37°C for 7 days. After this period, the samples were washed and dried. SEM of the scaffolds was performed to show the changes in nanofibers morphology during this period.
PC12 Cell Culture
PC12 cells used in these experiments are as precursors of the dopaminergic neural cells. PC12 cells were purchased from Royan Institute, Isfahan, Iran. These cells were cultured in RPMI medium enriched with heat-inactivated 10% horse serum, 5% fetal bovine serum, 1% penicillin (50 U/mL), and streptomycin (50 U/mL) (all purchased from GIBCO, Grand Island, NY). Cells were grown in 75 cm2 tissue culture flasks and maintained in a humid atmosphere containing 5% CO2 at 37°C; the medium was replaced every day throughout the experiments. Three treatments were examined PAN-kefiran 5%, PAN-kefiran 10%, and PAN scaffolds. The electrospun nanofiber scaffolds (PAN, PAN-kefiran 5% and 10%) sterilized using formaline gas for both top and bottom surfaces for 24 hrs. A density of 10,000 cells/cm2 was seeded onto the nanofibers and untreated sample as a negative control was used as well (Promega, Madison, WI). Cell viability was determined using the3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4 sulfophenyl) −2H-tetrazolium (MTS) cytotoxicity assay. After 1, 2, 4, and 6 days of incubation with PAN, PAN-kefiran 5% and 10%, PC12 cells were incubated with 20% MTT solution. After three h of incubation at 37°C in an atmosphere containing 5% CO2, aliquots were pipetted into 96-well plates. The absorbance at 492 nm was measured using the spectrophotometric plate reader (BioTek, Winooski, VT).
Cresyl Violet Staining (Nissl Staining)
The cresyl violet method was used to show the Nissl substance as an important characteristic of neurons. In the neural differentiation, the maturation grade of neuronal cells is reflected by formational changes in the neuroplasm. The Nissl bodies are rough endoplasmic reticulum with the ability to synthesize protein. PC12 cells were stained with 0.1% cresyl violet solution for 4–15 mins and washed three times with PBS to remove excess stain. PC12 cells were photographed by inverted microscope.25
PBMC Cell Cultures
Whole peripheral blood was collected from three healthy donors approved by ethic committee (Reference code: IR.UI.REC.1398.055). It is confirmed that before collecting samples singed informed statements were received. Sample of peripheral blood was collected into heparinized syringes. Isolation of PBMC was through density gradient centrifugation on Ficoll-Hypaque (D-1,077). Followed by 30 mins of centrifugation at 800 g, cells were collected from interphase. Then, it was washed with physiologic serum and suspended in 1 mL of RPMI. RPMI 1640 supplemented with 5% fetal bovine serum and 1% pencilin/streptomycin mixture (Royan institute, Isfahan, Iran). The cells were cultured in RPMI in a final volume of 150 µL, treated with kefiran (5mg/mL and 1 mg/mL), kefiran nanofiber (kefiran –PAN 5% and 10%) and PAN nanofiber. Also, negative control without kefiran was cultured. The cells were maintained for 48 hrs at 37ºC in a humid atmosphere of 5% CO2 in the air. Following culture, supernatants were collected and kept at −70ºC to be used for measuring the level of IL-6.19
Evaluation of IL-6 Concentration in Kefiran and Cell Culture with Kefiran Nanofibers
The IL-6 concentration was examined by ELISA kit (Roche, Germany) in the crude kefiran, the boiled kefiran, and the pure kefiran. Also, it was evaluated in the supernatant of PBMC cell culture with pure kefiran at concentrations of 5mg/mL and 1 mg/mL, and supernatant of PBMC cell culture with kefiran –PAN 5% and kefiran-PAN 10%. All assays and calculations were performed according to the manufacturer’s instructions.
Surveying Anti-Cancerous Effect of Kefiran
MCF7 cells used in these experiments are commonly used in breast cancer cell lines. MCF7 cells were purchased from Royan Institute, Isfahan, Iran. The MCF7 cells were verified and counted using inverted microscope (3×104 cells/well) in 96-well culture plates. The freshly crude kefiran was sterilized using 0.22 μm filter and added to each sample in the final concentration of 0.5,1, 2 and 4mg/mL and untreated sample as negative control was also evaluated. Cell viability was determined after 48, 72 and 96 hrs of incubation using the 3-(4,5-dimethylthiazol-2-il)-2,5-diphenyltetrazolium bromide (MTT) reduction assay (0.5 mg/mL). It was done as previously described.
Role of Kefiran in Proliferation of PBMC
The PBMC were verified and counted using an inverted microscope (3×104 cells/well) in 96-well culture plates. The freshly crude kefiran was sterilized using 0.22 μm filter and added to each sample in the final concentration of 0.5, 1, 2, and 4mg/mL, and untreated sample as a negative control was also evaluated. Cell viability was determined after 24, 48, 72 and 96 hrs of incubation using the 3-(4,5-dimethilthiazol-2-il)-2, 5- diphenyl tetrazolium bromide (MTT) reduction assay (0.5 mg/mL).
Statistical Analysis
All data were presented as mean ±SD. The two-way ANOVA test and unpaired t test were used to determine the significant differences between the two means evaluated at p ≤ 0.05. Statistical analysis was performed using GraphPad prism 6 Demo. All experiments were done in triplicate. Charts were drawn by GraphPad prism 6 Demo.
Results
Pure kefiran was used for the production of nanofiber in 5% and 10% concentration. Higher concentration of kefiran in the matrix causes higher viscosity which leads to prevent fabrication.
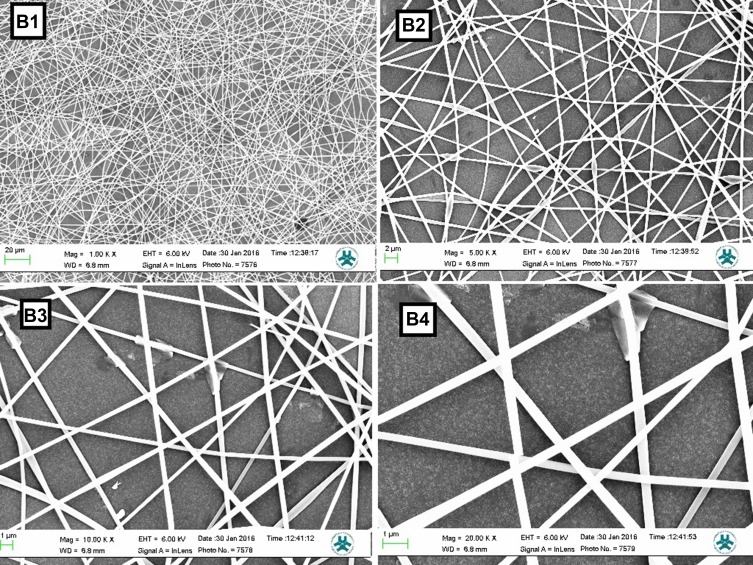
Nanofibers morphology is one of the crucial parameters for attachment and cell proliferation. The nanofibers were fabricated regularly and particles uniformly distributed throughout the fiber. Accumulation of nanoparticles can be seen only in few locations (Figure 1). SEM data showed that the mean diameters of PAN-kefiran nanofibers were 310.2±43.9 (Figure 2).
Figure 1.
SEM images of nanofibers with different magnifying (B1-B4). B1: SEM image of nanofiber with magnifying 1000 X. B2: SEM image of nanofiber with magnifying 5000 X. B3: SEM image of nanofiber with magnifying 10,000 X. B4: SEM image of nanofiber with magnifying 20,000 X.
Figure 2.
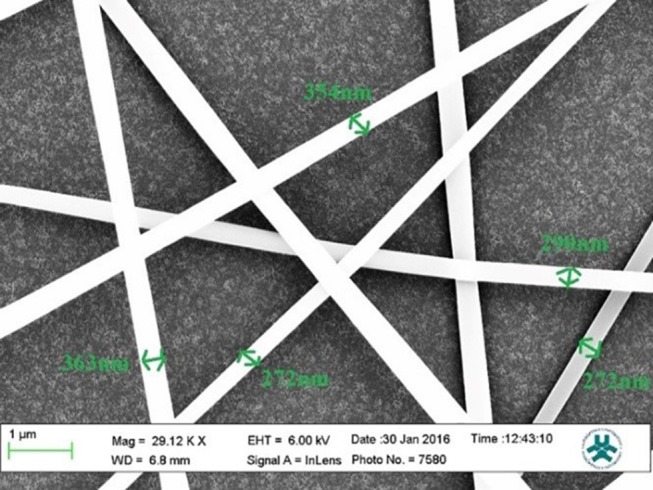
Mean diameters of PAN-kefiran nanofiber by SEM with magnifying 29,120 X.
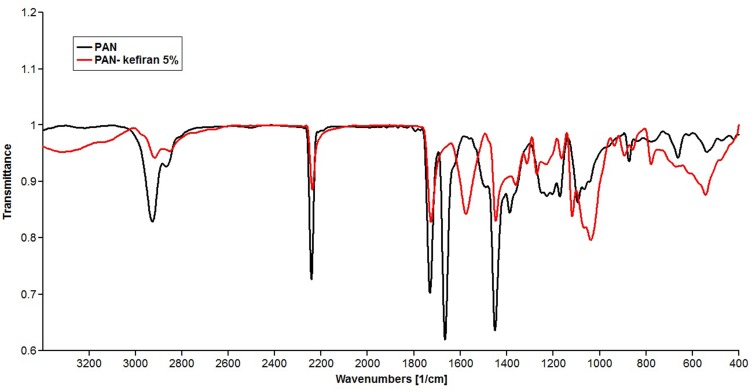
ATR-FTIR analysis of the PAN nanofibrous scaffold revealed sharp peaks at 2923 cm−1 (related to -CH2-), 2242 cm−1 (related to C≡N), 1662 cm−1 (related to C≡N) and 1451 cm−1 (related to CH2 and CH3) (Figure 3), which is in accordance with formula of polyacrylonitrile (C3H3N) n. In the PAN-kefiran nanofiber, two sharp peaks were observed at 1038 cm−1 and 1118 cm−1 in which peaks related to C-O-C, C-O of carbohydrates in kefiran structures.26 1576 cm−1 is associated with NH2.
Figure 3.
FTIR spectra of Polyacrylonitrile (PAN) scaffold and polyacrylonitrile-kefiran scaffold. 2 peaks (1038 and 1118 cm−1) revealed in FTIR of PAN-kefiran related to polysaccharide region. One peak (1576 cm−1) revealed in FTIR of PAN-kefiran related to NH2.
The contact angle was measured to evaluate the hydrophilicity of the nanofibers. The droplet size was 0.5 μL. Five samples were used for each test. The hydrophilicity of the kefiran-PAN nanofiber was higher than the hydrophilicity of the PAN nanofiber. So, the hydrophilicity increases with the addition of kefiran. It could be due to the presence of hydrophilic groups on the surface of the scaffold. Hydrophilic groups belong to carboxyl groups and amide groups related to polysaccharide and protein of kefir, respectively.
In vitro Degradation Analysis of Samples
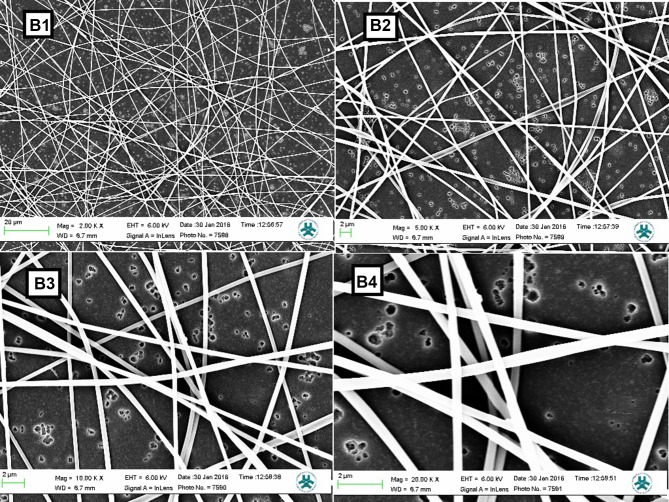
The results of the in vitro degradation of PAN/kefiran were confirmed by SEM images taken after 7 days in the PBS medium. No significant morphological changes in the PAN/kefiran during 1 week period were observed (Figure 4).
Figure 4.
SEM images of nanofibers. PAN-kefiran sample after 7 days in the PBS medium with different magnifying. B1: SEM image of PAN-kefiran after 7 days in the PBS medium with magnifying 2000 X. B2: SEM image of PAN-kefiran after 7 days in the PBS medium with magnifying 5000X. B3: SEM image of PAN-kefiran after 7 days in the PBS medium with magnifying 10,000 X. B4: SEM image of PAN-kefiran after 7 days in the PBS medium with magnifying 20000X.
PC12 Cell Viability
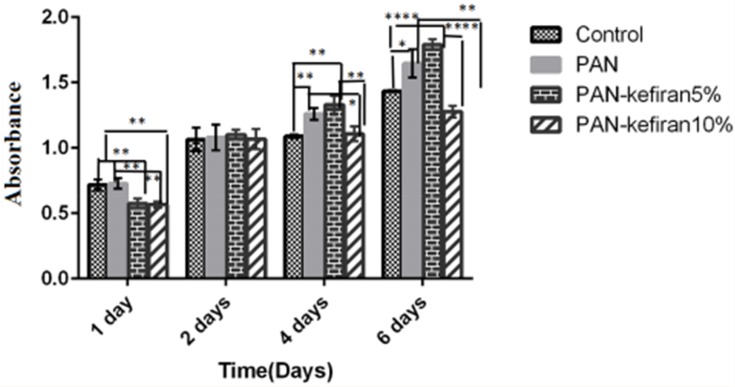
The MTT assay showed that PAN and PAN–kefiran 5% and 10% scaffolds did not have any toxicity effect in PC12 cells because the viability of the cells was demonstrated during 6 days after seeding (Figure 5). Treatment of cells with PAN-kefiran 5% and PAN-kefiran 10% had less viability compared to the control after 24 hrs incubation, but PAN nanofibers showed no significant difference in cell viability compared to the control. However, after 48 hrs, no significant differences were shown in all samples. The viability of PC12 cells in PAN-kefiran 5% and PAN nanofibers were more than the cell viability in the control sample after 96 hrs. But the viability of PC12 cells in PAN-kefiran 10% nanofibers had no significant difference compared to the control. Noteworthy topic in this process that occurred was the morphological changes of PC12 cells. The morphological changes were shown only in PAN-kefiran 10% (Figure 6) and were not shown in PAN-kefiran 5%, PAN treatment and the control. Results showed that the elongation and protuberance of PC12 cells were observed. This kind of elongation of PC12 cells could improve and initiate the neural regeneration process. After 144 hrs, the attachment and morphology of PC12 cells cultured on the nanofiber samples were examined by inverted microscope (Figure 7). Also, after 6 days significantly, the viability of the cells in PAN-kefiran 5% and PAN scaffolds was more than the control (Figure 5). But the viability of the cells in PAN-kefiran 10% was significantly less than the control.
Figure 5.
MTT assay of PC12 cells was performed to evaluate the toxicity of PAN-kefiran scaffolds after 1, 2, 4, and 6 days. Results confirmed that the PAN–kefiran scaffolds %5 and %10 do not have any toxicity effect on the PC12 cells. Treatment with PAN-kefiran 5% shows significant increase in cell number (compared to control) in 4 days and 6 days after seeding (p≤0.05), but treatment with PAN-kefiran 10% shows no significant increase in cell number compared to control. This increase is indicated by (*). The significant scale is that *p≤0.05, **p≤0.01, ****p≤0.0001.The two-way ANOVA test was used to determine the significant difference between the two means.
Figure 6.
Staining: the Nissl substance (rough endoplasmic reticulum) with Cresyl violet. The Nissle bodies appeared dark blue due to the staining of ribosomal RNA. Magnification ×400.
Figure 7.
The morphology of PC12 cells cultured on the nanofiber samples (A) PAN-kefiran 5% after 6 days of cell seeding (magnifying×400). (B) PAN-kefiran 10% after 6 days (magnifying×400). (C) PAN nanofiber sample after 6 days (magnifying×400). (D) The control sample (without any treatment) after 6 days (magnifying×400).
PC12 Cells Differentiation
By using cresyl violet staining, the Nissl substance (rough endoplasmic reticulum) appeared dark blue due to the staining of ribosomal RNA (Figure 6). So, it was shown that PC12 cells treated with PAN-kefiran 10% nanofiber were differentiated without nerve growth factor (NGF).
Quantitation of IL-6
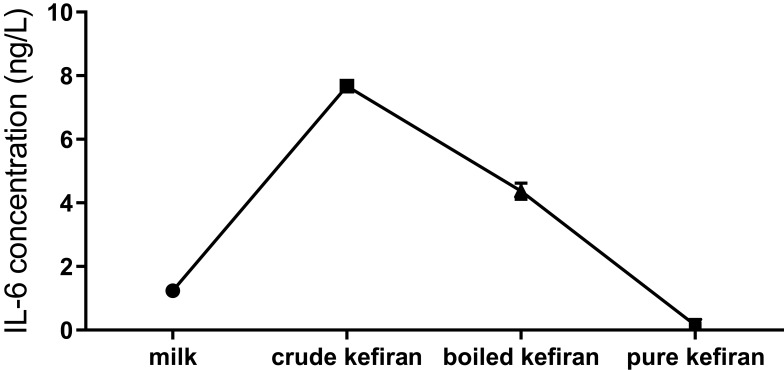
The measurement of IL-6, in crude kefiran and boiled kefiran, was significantly higher than the pure kefiran (p<0.05) (Figure 8).
Figure 8.
The concentration of IL-6 in kefiran samples.
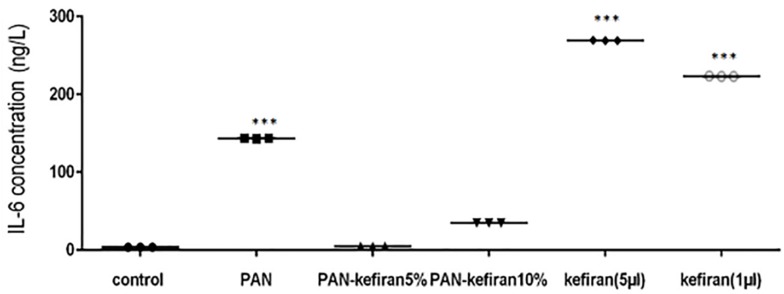
IL-6 in PBMC cell culture stimulated with PAN was significantly higher than PBMC cell culture stimulated with PAN-kefiran 5% and 10% nanofibers. IL-6 in PAN-kefiran 5% was shown no significant differences compared to the control. IL-6 in PBMC cell culture stimulated with kefiran (1µL, 5 µL) was increased significantly compared to the others (p<0.05) (Figure 9).
Figure 9.
Level of IL-6 in PBMC cell culture stimulated with different types and concentration of kefir. The significant increase in the IL-6 concentration was observed in some treatment (p≤0.05). Unpaired t test was used to determine the significant difference between the two mean. ***P≤0.001.
Cell Viability
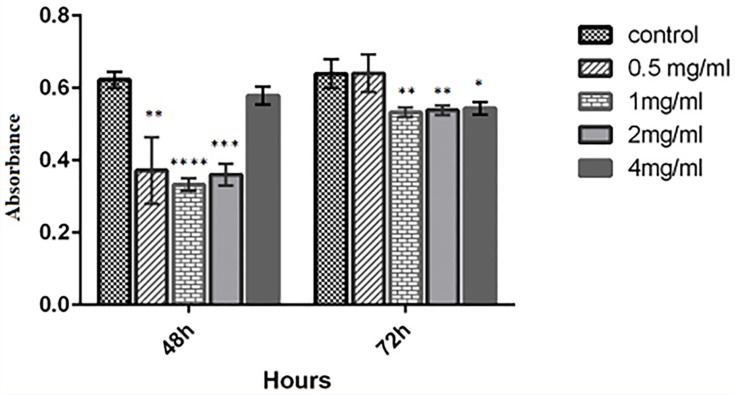
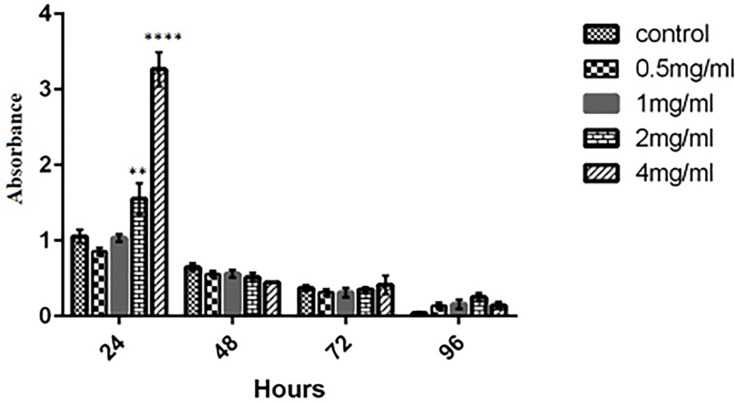
Cell viability was studied by exposure of PBMC and MCF7 to different concentration of the kefiran. As shown in Figure 10, the viability of the treated MCF7 was decreased significantly in 0.5, 1, and 2 mg/mL of kefiran compared to the control after 48 hrs (p<0.05). Also, it was significantly reduced in 1mg/mL and 4 mg/mL of kefiran after 72 hrs (p<0.05). The viability of the treated PBMC increased considerably in 2 mg/mL and 4 mg/mL of kefiran in 24 hrs of seeding (p<0.05). Too much growth of PBMC caused unfavorable condition to grow the cells due to the higher waste materials in the medium. Hence, the cells entered the death phase in a shorter time (Figure 11).
Figure 10.
MTT assay of MCF7 cells was performed to evaluate the toxicity of kefiran after 2 and 4 days. Results confirmed that the viability of MCF7 cells decreases when treated by kefiran. Treatment with kefiran showed the significant decline in cancerous cell number (compared to control) in 0.5 mg/mL, 1 mg/mL, 2 mg/mL of kefiran after 48 hrs and in 2 mg/mL and 4 mg/mL of kefiran in 72 hrs (p≤0.05). The two-way ANOVA test was used to determine the significant difference between the two means This decrease is indicated by (*). The significant scale is that *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001.
Figure 11.
MTT assay of PBMC was performed to evaluate the toxicity of kefiran after 2, 4, and 3 days. Results confirmed that the viability of PBMC increases when treated by kefiran. Treatment with kefiran showed a significant promotion in cell number (compared to control) in 2 mg/mL and 4 mg/mL after 24 hrs (p≤0.05). The two-way ANOVA test was used to determine the significant difference between the two means. **P≤0.01, ****P≤0.0001.
Discussion
Characterization of the Kefiran Nanofiber
SEM micrographs showed that kefiran was spread uniformly on the sheath. Addition of kefiran to PAN produced higher viscosity for PAN. However, Mehrasa et al 2015 reported that the addition of gelatin to Poly lactic-co-glycolic acid revealed lower viscosity and lower fiber mean diameter.24 Hydrophilicity of the scaffolds is a major factor for cell culture scaffolds. The greater protein absorption and cell adhesion were observed in high hydrophilic scaffold. The contact angle measurement results (26.9± 1.9 for the pure PAN scaffold vs 12.3± 1.13 for the PAN/kefiran) revealed enhanced hydrophilicity of scaffolds upon the incorporation of kefiran and PAN. The enhanced hydrophilicity of the electrospun kefiran-PAN is presumably due to the presence of amine and carboxylic functional groups in the kefiran structure. Mehrasa et al 2015 reported that hydrophilicity and mean diameter of aligned PLGA/gelatin/10 wt% mesoporous silica nanoparticles (MSNPs) were 18 ±8.7 and 267±58, respectively.24 The hydrophilicity of PAN-kefiran was better than the hydrophilicity of aligned PLGA/gelatin/10 wt% mesoporous. Fabrication of kefiran-PAN was more straightforward, faster, and cost-effective with high hydrophilicity, so it could be suggested to be applied in scaffolds especially for PC12 cells.
One of the important factors for the selection of materials used for the fabrication of neural stem cell is low degradation rate of material as scaffold and appropriate mechanical properties.27 PAN-kefiran scaffolds might be suitable for nerve tissue engineering applications due to their low degradation rates compared to the Mehrasa et al report. Our findings are not in accordance with the results of Mehrasa et al (2015). Because it was reported that aligned PLGA/gelatin scaffolds were not used for neural tissue engineering due to the high degradation rates of used scaffolds.24
PAN-kefiran nanofiber showed peaks at 1038 cm−1 and 1118 cm−1 which were related to C-O-C, C-O of carbohydrates in kefiran structures.26 But the peak 1576 cm−1 was related to NH2 that showed protein structure in nanofiber.
Cell–Scaffolds Interaction and Metabolic Activities
This study demonstrated that PAN and PAN –kefiran 5% and 10% scaffolds did not have any toxicity effect in PC12 cells (Figure 5). The viability of the PC12 cells was shown in PAN-kefiran 5%, PAN-kefiran 10%, PAN scaffolds, and the control during 1 and 2 days after cell seeding. The viability of the PC12 cells in PAN, PAN-kefiran 5%, and PAN-kefiran 10% nanofiber was similar to the cell viability in control only 2 days after seeding. It was confirmed that the conditions were suitable for the cell viability in all types of nanofibers. After 4 days of seeding, the viability of PC12 cells in PAN-kefiran 5% was more than PAN-kefiran 10% and the control. The viability of PC12 cells in PAN-kefiran 10% showed no significant difference compared to the control. However, morphological changes were shown only in PAN-kefiran 10% (Figure 6). It seems that the morphological changes were due to the higher existence of kefiran (containing polysaccharide and peptide) in PAN-kefiran 10%. However, PAN-kefiran 5% induced higher growth of the PC12 cells compared to the control. It might be due to the higher hydrophilicity of kefiran-PAN scaffolds. Recently, there are a lot of studies regarding electrospun polysaccharides, which are potentially useful for regenerative medicine. However, so far there are only two reports about kefiran electrospun nanofibers.16,23 Esnaashari et al (2014) showed fabrication of kefiran electrospun nanofiber by using water as the solvent.23 But, production of this fiber in water was impossible in our experiment. Perhaps, water was not a good solvent.16 Also, Jenab et al (2017) reported the manufacturing of the electrospun antimicrobial kefiran/polyethylene oxide (PEO) nanofibers as a biocontrol agent in food packaging as well as food preservation.16 But, this study is the first report about the manufacturing of anti-microbial, anti-tumor, biocompatible and cost-effective kefiran-polyacrylonitrile nanofibers which are potentially useful for regenerative medicine with the ability to differentiate PC12 cells that seems promising for neural stem cell culture.
On the other hand, there are a lot of studies lately regarding electrospun scaffolds which are useful for regenerative medicine like Mehrasa et al (2015), and Binulal et al (2012) reported that there were the better adhesion and more growth of PC12 cells on the gelatin nanoparticles-PCL scaffolds and aligned forms of PLGA/MSNPs and PLGA/gelatin/MSNPs scaffolds, respectively.24,28 These results are in accordance with the current study. Babolmorad et al (2015) reported that the viability of PC12 cells on the PLO 9% S-layer scaffold showed higher growth than the control (PLO-laminin) only after 24 hrs of cell seeding.29 They could not do their experiments for more than 24 hrs. It may be due to the S-layer was not sterilized by formalin gas, so the scaffold might be contaminated. Also, scaffold must join to PLO, but kefiran-PAN scaffold can be used separately without PLO-laminin. Morphological changes and protuberance of PC12 cells were revealed only in PAN-kefiran 10% nanofiber after 4 days incubation. This observation might be due to the high concentration of kefiran in this scaffold. So, kefiran could probably induce more elongation of the fiber and more obvious morphological changes of PC12 cells. Cheng et al (2003) reported that chitosan with gelatin could increase differentiation and proliferation of PC12 cells, but chitosan is immunogene.30 So, this scaffold may not be used for in vivo experiments. The results of the present study showed that during 6 days incubation, the cell viability in PAN-kefiran 5% and PAN scaffolds were more compared to the control sample significantly. However, the cell viability in PAN-kefiran 10% was less compared to the control significantly. It is related to the differentiation time of PC12 cells (4 days) in the PAN-kefiran 10% scaffold because the PC12 cells will enter the death phase after differentiation.
Measuring of IL-6 in Kefiran and Cell Culture with Kefiran Nanofiber
Chellat et al in 2006 reported that chitosan-DNA nanoparticles did not induce pro-inflammatory cytokines like IL-6, IL1β, even in the presence of high amount of chitosan nanoparticles.31 But, in our study, the level of IL-6 in PBMC cell culture stimulated with PAN was significantly higher than the PBMC cell culture stimulated with PAN-kefiran 5% and 10% nanofibers. So, PAN solely could be immunogenic. The level of IL-6 in PAN-kefiran 5% and PAN-kefiran scaffolds 10% have not shown significant difference compared to the control. So these scaffolds probably did not affect the inflammatory processes and could be used in vivo, especially in neural stem cell culture and tissue engineering in the future.
The concentration of IL-6 in PBMC cell culture stimulated with kefiran (1µL, 5µL) was increased significantly in comparison with other cell cultures and control (p<0.05) (Figure 9). This study is in agreement with previous studies, for example: the products derived from milk fermentation by kefir microflora (PMFKM) up-regulated IL-6 secretion in vivo. It is necessary for B-cell terminal differentiation to IgA secreting cells in the gut lamina propria. Thereby, using of PMFKM enhances immune responses.32 Hong et al 2009 reported that Lactobacillus kefiranofaciens M1 had strong potential to induce in vitro production of IL-6 in RAW264.7 cells through TLR-2 (Toll-Like Receptor-2). It would potentially have beneficial effects on the promotion of cell-mediated immune responses against tumors and intracellular pathogenic infections.33 Vinderola et al (2006) reported that kefir induces up-regulation of TNFα and IL-6 on macrophages.34 But, de LeBlanc et al (2006) reported that kefir decreased the level of IL-6 (+) cells in cancerous mammary glands. It caused delay in tumor growth.35 So, different components of kefir can stimulate the immune cells of the innate immune system. It can down-regulate the Th2 immune phenotype or to promote cell-mediated immune responses against tumors and intracellular pathogenes. So, kefir has the in vivo role as oral bio therapeutic substances.
Anti-Cancerous Activities of Kefiran
The results of this study showed that the viability of the treated MCF7 cells decreased significantly in 0.5, 1, and 2 mg/mL of kefiran concentration compared to the control after 48 hrs incubation. But the viability of the treated MCF7 cells in 4 mg/mL of kefiran concentration was not a significant difference compared to the control. It might be due to the presence of higher concentration of polysaccharide in 4mg/mL of kefiran. Also, it was decreased significantly in 2mg/mL and 4 mg/mL of kefiran after 72 hrs. This study is in accordance with many studies that demonstrated that kefir acts against different cancers such as colorectal cancer, malignant T lymphocytes, lung cancer and breast cancer.36,37 But Rizk et al (2014) reported that kefir treatment showed no effect on the motility of colorectal, as well as the breast (MCF-7 and MB-MDA-231) cancerous cells. But they mentioned that kefir could inhibit the proliferation and induction of apoptosis in HT-29 and Caco-2 CRC cells.38
Conclusion
In this study, PAN-kefiran 5% and 10% (w/v) nanofibres were manufactured using the electrospinning method. The results show that during 4 days of incubation, the viability of PC12 cells in PAN-kefiran 5% was more than PAN-kefiran 10% and the control. The viability of PC12 cells in PAN-kefiran 10% had no significant difference compared to the control, but the morphological changes were shown only in PAN-kefiran 10% treatment. This is due to the existence of more kefiran (containing polysaccharide and peptide) in PAN-kefiran 10%. The existence of this level of kefiran may induce the differentiation of PC12 cells. So, the use of PAN-kefiran 10% nanofiber for neural stem cell culture seems promising, although, PAN-kefiran 5% and PAN induce more growth. It can increase cell viability compared to the control. Kefiran-PAN scaffold is cost-effective with superior properties such as high hydrophilicity that might be a good alternative for neural stem cell culture and tissue engineering. However, more studies need to be done like Real-Time PCR and Immunocytochemistry for the differentiation of PC12 cells in PAN-kefiran 10%. Also, the pure kefiran may be used for increasing the number of the PBMC and decreasing of the MCF7 cancerous cells. Moreover, IL-6 in PAN-kefiran 5% and PAN-kefiran scaffolds 10% were shown no significant difference compared to the control and did not affect on the concentration of IL-6. Overall in this study, it was shown that electrospinning of kefiran as a natural polymer would be anti-microbial, anti-tumor, biocompatible, and cost-effective for regenerative medicine. For using in vitro especially in neural stem cell culture seems promising, but however it needs more researches to be done.
Acknowledgment
We would like to give many thanks to Doctor Mohsen Golabi who assisted in this study.
Ethics Statement
The approval of National Committee on Ethics in Biomedical Research of the University of Isfahan (reference code: IR.UI.REC.1398.055) was obtained.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–2953. doi: 10.1016/j.biomaterials.2008.04.023 [DOI] [PubMed] [Google Scholar]
- 2.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12:107–124. doi: 10.1163/156856201744489 [DOI] [PubMed] [Google Scholar]
- 3.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering application: a review. Tissue Eng. 2006;12(5):1197–1211. doi: 10.1089/ten.2006.12.1197 [DOI] [PubMed] [Google Scholar]
- 4.Mehrasa M, Anarkoli AO, Rafienia M, et al. Incorporation of zeolite and silica nanoparticles into electrospun PVA/collagen nanofibrous scaffolds: the influence on the physical, chemical properties and cell behavior. Int J Polym Mater. 2016;65:457–465. doi: 10.1080/00914037.2015.1129958 [DOI] [Google Scholar]
- 5.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11:101–109. doi: 10.1089/ten.2005.11.101 [DOI] [PubMed] [Google Scholar]
- 6.Hong S, Kim G. Fabrication of electrospun polycaprolactone biocomposites reinforced with chitosan for the proliferation of mesenchymal stem cells. Carbohydr Polym. 2011;83(2):940–946. doi: 10.1016/j.carbpol.2010.09.002 [DOI] [Google Scholar]
- 7.Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application. A review. Int J Polym Sci. 2011;2011:1–19. [Google Scholar]
- 8.Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59:207–233. doi: 10.1016/j.addr.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Roether JA, Boccaccini AR. Tissue engineering scaffolds from bioactive glass and composite materials. Top Tissue Engi. 2008;4:1–27. Eds. N.Ashammakhi. [Google Scholar]
- 10.Mourin˜o V, Boccaccini AR. Bone tissue engineering therapeutics: controlled drug delivery in three- dimensional scaffolds. J R Soc Interface. 2010;7:209–227. doi: 10.1098/rsif.2009.0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63(15):2223–2253. doi: 10.1016/S0266-3538(03)00178-7 [DOI] [Google Scholar]
- 12.Gomes M, Azevedo H, Malafaya P, et al. Natural polymers in tissue engineering applications In: Blitterswijk CV, Boer JD, et al editors. Tissue Engineering. Academic Press; 2008. January 1:145–192. [Google Scholar]
- 13.Wang Y, Ahmed Z, Feng W, Li C, Song S. Physicochemical properties of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir. Int J Biol Macromol. 2008;43(3):283–288. doi: 10.1016/j.ijbiomac.2008.06.011 [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues KL, Caputo LR, Carvalho JC, Evangelista J, Schneedorf JM. Antimicrobial and healing activity of kefir and kefiran extract. Int J Antimicrob Agents. 2005;25(5):404–408. doi: 10.1016/j.ijantimicag.2004.09.020 [DOI] [PubMed] [Google Scholar]
- 15.Ghasemlou M, Khodaiyan F, Jahanbin K, Gharibzahedi SM, Taheri S. Structural investigation and response surface optimisation for improvement of kefiran production yield from a low-cost culture medium. Food Chem. 2012;133(2):383–389. doi: 10.1016/j.foodchem.2012.01.046 [DOI] [PubMed] [Google Scholar]
- 16.Jenab A, Roghanian R, Emtiazi G, Ghaedi K. Manufacturing and structural analysis of antimicrobial kefiran/polyethylene oxide nanofibers for food packaging. Iran Polym J. 2017;26(1):31–39. doi: 10.1007/s13726-016-0496-7 [DOI] [Google Scholar]
- 17.Medrano M, Pérez PF, Abraham AG. Kefiran antagonizes cytopathic effects of Bacillus cereus extracellular factors. Int J Food Microbiol. 2008;122(1–2):1–7. doi: 10.1016/j.ijfoodmicro.2007.11.046 [DOI] [PubMed] [Google Scholar]
- 18.Katial RK, Sachanandani D, Pinney C, Lieberman MM. Cytokine production in cell culture by peripheral blood mononuclear cells from immunocompetent hosts. Clin Diagn Lab Immunol. 1998;5(1):78–81. doi: 10.1128/CDLI.5.1.78-81.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malavé I, Vethencourt MA, Chacón R, Quiñones D, Rebrij C, Bolívar G. Production of interleukin-6 in cultures of peripheral blood mononuclear cells from children with primary protein-calorie malnutrition and from eutrophic controls. Ann Nutr Metab. 1998;42(5):266–273. doi: 10.1159/000012743 [DOI] [PubMed] [Google Scholar]
- 20.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro-and anti-inflammatory properties of the cytokine interleukin-6. BBA Mol Cell Res. 2011;1813(5):878–888. [DOI] [PubMed] [Google Scholar]
- 21.Furuno T, Nakanishi M. Kefiran suppresses antigen-induced mast cell activation. Biol Pharm Bull. 2012;35(2):178–183. doi: 10.1248/bpb.35.178 [DOI] [PubMed] [Google Scholar]
- 22.Rimada PS, Abraham AG. Comparative study of different methodologies to determine the exopolysaccharide produced by kefir grains in milk and whey. Lait. 2003;83(1):79–87. doi: 10.1051/lait:2002051 [DOI] [Google Scholar]
- 23.Esnaashari SS, Rezaei S, Mirzaei E, Afshari H, Rezayat SM, Faridi-Majidi R. Preparation and characterization of kefiran electrospun nanofibers. Int J Biol Macromol. 2014;70:50–56. doi: 10.1016/j.ijbiomac.2014.06.014 [DOI] [PubMed] [Google Scholar]
- 24.Mehrasa M, Asadollahi MA, Ghaedi K, Salehi H, Arpanaei A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int J Biol Macromol. 2015;79:687–695. doi: 10.1016/j.ijbiomac.2015.05.050 [DOI] [PubMed] [Google Scholar]
- 25.Katebi S, Esmaeili A, Ghaedi K, Zarrabi A. Superparamagnetic iron oxide nanoparticles combined with NGF and quercetin promote neuronal branching morphogenesis of PC12 cells. Int J Nanomedicine. 2019;14:2157. doi: 10.2147/IJN.S191878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis R, Mauer LJ. Fourier transform infrared (FT-IR) spectroscopy: a rapid tool for detection and analysis of foodborne pathogenic bacteria In:Méndez-Vilas A, editor. Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Vol 2 2010:1582–1594. [Google Scholar]
- 27.Ciardelli G, Chiono V. Materials for peripheral nerve regeneration. Macromol Biosci. 2006;6:13–26. doi: 10.1002/(ISSN)1616-5195 [DOI] [PubMed] [Google Scholar]
- 28.Binulal NS, Natarajan A, Menon D, Bhaskaran VK, Mony U, Nair SV. Gelatin nanoparticles loaded poly (ε-caprolactone) nanofibrous semi-synthetic scaffolds for bone tissue engineering. Biomed Mater. 2012;7(6):065001. doi: 10.1088/1748-6041/7/6/065001 [DOI] [PubMed] [Google Scholar]
- 29.Babolmorad G, Emtiazi G, Ghaedi K, Jodeiri M. Enhanced PC12 cells proliferation with self-assembled S-layer proteins scaffolds. Appl Biochem Biotech. 2015;175(1):223–231. doi: 10.1007/s12010-014-1248-9 [DOI] [PubMed] [Google Scholar]
- 30.Cheng M, Deng J, Yang F, Gong Y, Zhao N, Zhang X. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials. 2003;24:2871–2880. doi: 10.1016/S0142-9612(03)00117-0 [DOI] [PubMed] [Google Scholar]
- 31.Chellat F, Grandjean-Laquerriere A, Le Naour R, et al. Metalloproteinase and cytokine production by THP-1 macrophages following exposure to chitosan-DNA nanoparticles. Biomaterials. 2005;26(9):961–970. doi: 10.1016/j.biomaterials.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 32.Vinderola G, Perdigón G, Duarte J, Farnworth E, Matar C. Effects of the oral administration of the products derived from milk fermentation by kefir microflora on immune stimulation. J Dairy Res. 2006;73(4):472–479. doi: 10.1017/S002202990600197X [DOI] [PubMed] [Google Scholar]
- 33.Hong WS, Chen HC, Chen YP, Chen MJ. Effects of kefir supernatant and lactic acid bacteria isolated from kefir grain on cytokine production by macrophage. Int Dairy J. 2009;19(4):244–251. doi: 10.1016/j.idairyj.2008.10.010 [DOI] [Google Scholar]
- 34.Vinderola G, Perdigon G, Duarte J, Thangavel D, Farnworth E, Matar C. Effects of kefir fractions on innate immunity. Immunobiology. 2006;211(3):149–156. doi: 10.1016/j.imbio.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 35.de LeBlanc AD, Matar C, Farnworth E, Perdigon G. Study of cytokines involved in the prevention of a murine experimental breast cancer by kefir. Cytokine. 2006;34(1–2):1–8. doi: 10.1016/j.cyto.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 36.Sharifi M, Moridnia A, Mortazavi D, Salehi M, Bagheri M, Sheikhi A. Kefir: a powerful probiotics with anticancer properties. Med Oncol. 2017;34(11):183. doi: 10.1007/s12032-017-1044-9 [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Chan HM, Kubow S. Kefir extracts suppress in vitro proliferation of estrogen-dependent human breast cancer cells but not normal mammary epithelial cells. J Med Food. 2007;10(3):416–422. doi: 10.1089/jmf.2006.236 [DOI] [PubMed] [Google Scholar]
- 38.Rizk S, El Khoury N, El-Hayek S, Tarras O, El-sibai M, El-Sabban M. Kefir exhibits anti-proliferative and pro-apoptotic effects on colon adenocarcinoma cells with no significant effects on cell migration and invasion (647.24). FASEB J. 2014;28(1):647–24. [DOI] [PubMed] [Google Scholar]