Abstract
Purpose
To describe a modified technique of endoscopic orbital decompression for dysthyroid optic neuropathy nonresponsive to pulsed corticosteroids.
Methods
Retrospective, interventional single centre case series included 17 consecutive patients with dysthyroid optic neuropathy (DON) who were refractory to pulse corticosteroids. Removal of the posteromedial floor and the orbital process of palatine bone (OPPB) was performed in addition to the endoscopic transethmoidal medial orbital wall decompression (ETMOWD), to achieve maximal orbital apex decompression. Main outcome measures were change in visual acuity (VA), color vision, degree of proptosis reduction, incidence of new-onset diplopia, and any complications.
Results
Seventeen eyes (100%) had a statistically significant improvement in VA from 1.0 ± 0.44 LogMAR to 0.0 ± 0.15, with an average improvement of 0.41 ± 0.30 LogMAR (p 0.05, paired t-test). Fourteen out of 16 eyes had a complete improvement in color vision and two eyes had partial recovery. Afferent pupillary defect (76.5%) resolved in all cases. Five out of 10 cases with preoperative visual field defects demonstrated no residual field defects following surgery. The range of proptosis reduction was 0–5 mm (mean 2.7 ± 1.3 mm). No patients with diplopia (12/17) had worsening or developed new-onset diplopia following surgery.
Conclusion
Combined removal of the posterior medial floor including the OPPB with ETMOWD may be a viable alternative in the surgical management of DON.
Subject terms: Optic nerve diseases, Eye abnormalities
Introduction
Dysthyroid optic neuropathy (DON) occurs in 3–5% of patients with thyroid orbitopathy, and the postulated mechanism involves crowding of the orbital apex with hypertrophic extraocular muscles (EOMs) and/or enlarged retrobulbar fat tissue [1, 2]. Surgical decompression of the orbital apex is usually reserved as an adjuvant to medical therapy or in cases refractory to medical therapy. Traditionally, combined medial wall and inferomedial orbital floor decompression is performed either externally or endoscopically to relieve pressure exerted on the optic nerve, with improvement or stabilization of visual acuity (VA) in 89–100% of cases [3–9]. An endoscopic transethmoidal approach has gained popularity over the last decade as a result of advances in direct endoscopic visualization and instrumentation [5–8]. Modifications in this technique were introduced in the form of preservation of the inferomedial strut, and a periorbital sling technique to reduce the risk of new-onset diplopia and globe dystopia [10–12].
The orbital apex is the narrowest area of the orbit, and the optic nerve lies in close proximity to the medial and superior recti in the area of annulus of Zinn [13]. The orbital process of palatine bone (OPPB) forms the posteromedial portion of the orbital floor abutting the posterior most part of inferomedial strut. Our technique involves removing the OPPB, minor wing of sphenoid (MWS) and posteromedial floor as far as the inferior orbital fissure, along with the medial wall of the orbit, to provide a wider decompression of the apex (Figs. 1 and 2).
Fig. 1.
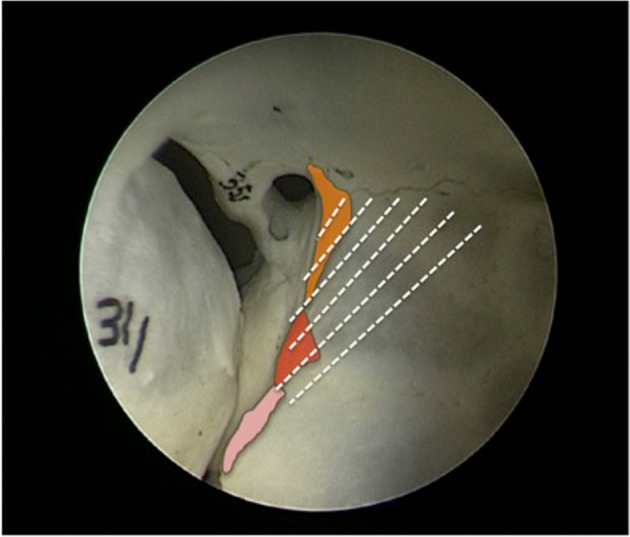
Endoscopic view of the orbital apex of skull model highlights the removed areas with dashed lines—minor wing of sphenoid (yellow, near optic foramen), the orbital process of palatine bone (red triangle), and the lateral portion of posterosuperior aspect of posterior maxillary sinus wall (pink).
Fig. 2.
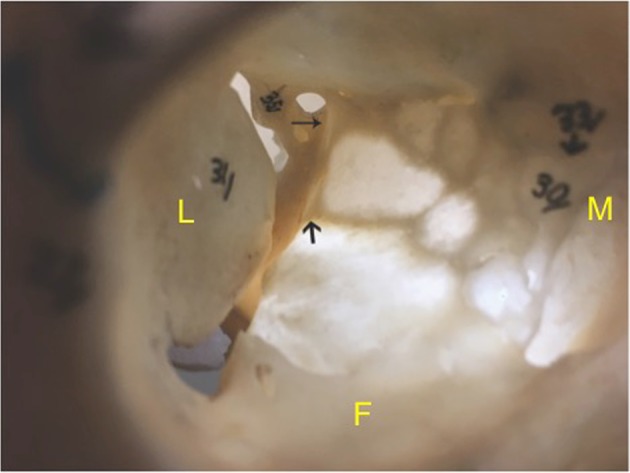
Transilluminated orbital floor and medial orbital wall show the thick areas in the orbital apex represented by OPPB (bold arrow) and MWS (smaller arrow); M medial orbital wall; L lateral orbital wall; F floor of the orbit
In this study, the authors describe an augmented endoscopic transethmoidal medial orbital wall decompression (ETMOWD), combined with OPPB removal, and its outcomes in a series of patients with DON.
Methods
All consecutive patients managed by a single surgeon between 2011 and 2018, who underwent endoscopic orbital apex decompression for DON refractory to corticosteroid therapy were recruited. The study adhered to tenets of the declaration of Helsinki and was approved by the hospital’s ethics committees. All surgical procedures were performed by a single surgeon (DS) or his fellows under supervision. Written consent was obtained from all patients before the surgery in accordance with the institution’s policies.
Included patients had Graves ophthalmopathy with DON (based on the EUGOGO classification), which was defined as reduced VA with or without dyschromatopsia (on Ishihara color plates), and evidence of orbital apex crowding on computed tomography (CT) or optic disc edema, or an afferent pupillary defect. Optic nerve dysfunction alone was responsible for the reduced vision (excluding glaucomatous optic neuropathy, diabetic maculopathy, and exposure keratopathy). Non-responsiveness to or deterioration despite corticosteroid therapy, was defined as an optic neuropathy persisting despite three consecutive days of 1000 mg of intravenous methylprednisolone followed by 500 mg weekly therapy for 6 weeks and another weekly therapy with 250 mg for 6 weeks. All patients underwent CT of orbits and sinuses. Navigation guidance was used as a teaching aid in cases where a fellow was one of the surgeons.
Clinical data including patients’ demographics (gender, age at the time of surgery), past medical history, including previous treatment (thyroidectomy, radioactive iodine, thyroid status), details of complete ophthalmologic examination (best corrected visual acuity (BCVA), pupillary responses, color vision, optic disc findings), extraocular motility, diplopia scoring (Gorman’s score), visual fields, length of follow-up, and nasal endoscopy findings (septal deviation, sinusitis) were recorded. Degree of proptosis was assessed using Hertel’s exophthalmometry, and visual fields were conducted using 30–2 SITA-standard threshold strategy. Assessed preoperative and postoperative parameters included amount of proptosis, BCVA, degree of diplopia, any new-onset diplopia, motility disturbances, and visual field changes. Any intraoperative and postoperative complications were also recorded. Patients were reviewed at 2 weeks, 3 months, and 6-monthly thereafter. Exclusion criteria included, patients with less than 6 months of follow-up and patients in whom lateral wall or external floor decompression was also performed.
Surgical technique
The technique is described below.
Positioning and anesthesia
The procedure was carried out under total intravenous anesthesia with controlled hypotension. Nasal vasoconstriction and decongestion were achieved using co-phenylcaine (5% lignocaine/0.5% phenylephrine) saturated cottonoid pledgets and mucosal injections of 1% lidocaine (1:100,000 epinephrine). The head is positioned in slight extension and turned towards the operating side.
Nasal phase
A combination of 0° and 30° rigid endoscopes (18 cm length, 4 mm diameter, Karl Storz®, Tuttlingen, Germany) were used. Where necessary, a septoplasty and/or middle turbinectomy was performed for access. A swing-door uncinectomy was performed enabling visualization of the natural ostium of the maxillary sinus. The ostium was enlarged using a through biting Blakesley forceps to the posterior maxillary wall and superiorly to the floor of the orbit. A standard anterior and posterior ethmoidectomy and sphenoidotomy was then performed to expose the lamina papyracea and the MWS [6, 7]. The sphenoidotomy was extended laterally to the lateral wall of the sphenoid sinus.
The posterior medial floor of the orbit was removed to the posterior wall of the maxillary sinus with a combination of drilling and downfracture with an angled curette (e.g., mastoid curette or 90° frontal curette).
The MWS was then drilled to expose the medial apex and entrance to the optic canal. This was done with an irrigating diamond burr (either 20° 2.5 mm DCR burr or skull base diamond burr (Medtronic Inc, Jacksonville, FL, USA)). Drilling was performed in short bursts with irrigation at 15 ml/min in an attempt to minimize the risk of optic nerve injury. The sphenoid bone inferomedial to the optic canal entrance was also drilled until the OPPB is reached at the posterosuperior aspect of the posterior maxillary wall (Fig. 3). The OPPB was then drilled remaining above the level of the sphenopalatine foramen. The maxillary bone of the floor lateral to the OPPB was removed with a combination of drilling and rongeurs until the junction of the posterior and lateral wall of the maxillary sinus was reached. This exposed ~5 mm of periosteum overlying the anterior pterygopalatine fossa as the bone at the junction of the posterior maxillary sinus wall and floor was removed. At the conclusion of this apical bone removal, ~140–160° of the periorbita overlying the orbital apex is exposed.
Fig. 3.
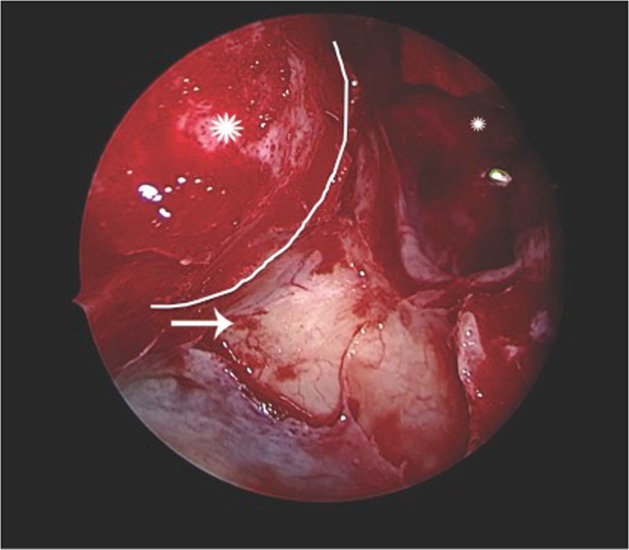
Intraoperative endoscopic view showing the decompressed orbital apex (white curvilinear line) with drilled OPPB (marked with an arrow), the exposed periorbita along the medial wall (marked with an asterisk) and the opened up sphenoid sinus (marked with a small asterisk)
The lamina overlying the medial periorbita was elevated and removed with a combination of a Freer’s elevator, microcurette, and Blakesley forceps. The amount of periorbita exposed was titrated to the amount of proptosis present. In selected cases with no proptosis and in an effort to maintain some symmetry, only the posterior half of the medial wall was removed.
The periorbita was incised with a combination of a sickle knife, cataract side port blade, or spear knife starting just anterior to the annulus of Zinn and extending anteriorly. The first incision was made in the inferior periorbita, allowing prolapse of orbital fat into the posterior maxillary sinus and then two incisions were made in the medial periorbita. The periobita of the medial wall was removed and a periorbital sling was not retained. A pediatric ball probe was used to encourage prolapse of orbital fat into the sinus. If additional proptosis reduction was required following maximal bone removal endonasally then orbital fat was removed with a manual suction cutter or laryngeal skimmer blade [14]. Routine postoperative care in the form of saline spray and oral antibiotics was commenced. Figures 1–3 highlight the areas decompressed using this technique.
Results
A total of 17 patients (6 male and 11 female) (17 orbits) were included in this study. The mean age of the patients was 69 years (range, 43–80 years). Two patients (12%) had a history of previous thyroidectomy and one patient (6%) had undergone prior radioablative iodine treatment. Four patients (24%) had received radiotherapy and two (12%) were treated with Rituximab immunotherapy for DON. Three patients out of 17 had bilateral DON at presentation and all had one eye that did not respond to medical treatment and proceeded to decompression. One patient developed sequential optic neuropathy in the other eye 2 months after contralateral orbital decompression. The average length of follow-up was 12 months (range, 6–40 months).
Three patients required septoplasty for deviated nasal septum and middle turbinectomy was performed for concha bullosa in three patients. Endonasal fat decompression was performed only in one patient in order to attain symmetry with the contralateral orbit. The average surgical time was 120 min (SD, 54 min), and ranged from 48 to 210 min. Cases operated by fellows under supervision took double the surgical time when compared to consultant alone (average 2.8 h versus 1.4 h). The median duration of hospital admission was one overnight stay; 5 of 17 patients stayed longer (3 days) due to associated comorbidities or social reasons.
Baseline characteristics are listed in Table 1. Preoperative assessment revealed mean BCVA of 1.0 ± 0.44 LogMAR (range, 0.2–1.8) in 17 eyes (Table 2). At the final review, the mean BCVA improved to 0.0 ± 0.15 (range, 0.2–1.2), with an average improvement of 0.41 ± 0.30 LogMAR (p 0.05, paired t-test). All cases except one (one line improvement) showed an improvement in BCVA of at least two lines (LogMAR). Dyschromatopsia was noted in 94.1% (16/17) eyes and 76.5% (13/17) of eyes had a relative afferent pupillary defect preoperatively. Color vision improved in all 16 eyes and all patients had resolution of the afferent pupillary defect at the final review. Twelve patients (71%) had preoperative visual fields testing, with ten (59%) demonstrating visual field defects. Five of these cases (50%) showed resolution of the visual field defect following orbital decompression.
Table 1.
Patient demographic and clinical data
| Variable | Values |
|---|---|
| Mean age (range) | 69 years (43–80 years) |
| Male:Female | 6:11 |
| Follow-up period (months) | 12 (6–40) |
| Dyschromatopsia | 94.1% |
| Relative afferent pupillary defect | 76.5% |
| Visual acuity (logMAR) | |
| Preoperative | 1.0 ± 0.44 |
| Postoperative | 0.0 ± 0.15 |
Table 2.
Results after augmented decompression of the orbital apex
| Operated eye | Pre-op VA | Post-op VA | Pre-op color vision | Post-op color vision |
|---|---|---|---|---|
| 80/R | 0.50 | 0.2 | 4/15 | 15/15 |
| 77/L | 1.80 | 0.6 | 1/17 | 7/17 |
| 65/R | 0.30 | 0.2 | 1/17 | 16/17 |
| 62/R | 0.20 | 0 | 11/17 | 15/15 |
| 62/L | 0.20 | 0 | 16/17 | 15/15 |
| 169/L | 0.30 | 0.2 | 4/15 | 8/15 |
| 60/R | 0.60 | 0 | 15/15 | 15/15 |
| 61/L | 1 | 0.2 | 0/15 | 15/15 |
| 68/R | 0.8 | 0.2 | 11/15 | 14/15 |
| 71/R | 0.50 | 0.1 | 6/15 | 15/15 |
| 71/L | 0.2 | 0 | 4/15 | 14/15 |
| 54/L | 0.5 | 0 | 3/17 | 16/17 |
| 58/R | 0.2 | 0.1 | 7/15 | 11/15 |
| 43/L | 0.3 | 0 | 9/15 | 15/15 |
| 50/R | 0.5 | 0.1 | 9/15 | 15/15 |
| 60/R | 0.6 | 0 | 11/15 | 15/15 |
| 60/L | 0.6 | 0.1 | 12/15 | 15/15 |
R right; L left; VA visual acuity in LogMAR
A crowded apex on CT was noted in all 17 cases. Preoperative exophthalmometry was 21 ± 10.9 mm (left-skewed values) and ranged from 16 to 26 mm with only four patients having preoperative exophthalmometry of less than 20 mm. At the final review, the mean globe position as measured by exophthalmometry was 18 ± 8.4 mm (14–21 mm). The mean proptosis reduction achieved was 2.7 ± 1.3 mm (median, 3 mm; range, 0–5 mm). Only one patient with 16 mm exophthalmometry had no change in Hertel’s reading postoperatively but had an improvement of 1.2 LogMAR in VA.
Clinical activity score was less than four in six patients, with a mean value of 4 (range, 1–7). Diplopia was preexisting in 12 patients (70.5%; mean Gorman score of 2), of whom five had diplopia resolution after subsequent muscle surgery. One patient with gaze-evoked diplopia had complete diplopia resolution after decompression alone. No patients developed new-onset diplopia after surgery. Complications such as postoperative hemorrhage, visual loss, cerebrospinal fluid leak, and sinusitis were not encountered in any of the patients.
Discussion
Techniques for orbital decompression in thyroid orbitopathy have evolved over the past two decades since the original description by Kennedy et al. in 1990. Approaches used for apical decompression include transantral, transcutaneous, transconjunctival, transcaruncular, and endoscopic transethmoidal approaches for medial wall with inferomedial floor removal [15–22]. In the present study, bone removed during ETMOWD included the medial wall of the orbit and the posteromedial portion of the orbital floor including the OPPB up to the medial edge of the inferior orbital fissure.
The rationale for this technique is to achieve the maximum possible degree of apical decompression for patients with DON who have crowding at the orbital apex. Removal of the MWS plus additional removal of the OPPB and the bone inferior to the apex provides an estimated 140–160° of apical decompression. In the standard endoscopic technique, the posterior limit of decompression lies at the MWS within 2 mm of sphenoid face medially, and the posterior wall of the maxillary sinus inferiorly [7]. The bone below the apex representing the posterosuperior edge of the posterior maxillary wall is often left intact. The annulus of Zinn lies approximately within 2 mm of face of the sphenoid sinus [23]. Enlarged EOMs exert pressure on the optic nerve in the anterior part of the orbital apex within the intraconal space. We believe that decompression in this area provides an added anatomical space for the expansion of enlarged medial and inferior recti, thereby relieving pressure being exerted on the optic nerve. The OPPB forms the posterior most part of the orbital floor along with the maxillary bone. Morphometric analysis of the OPPB by Mueller et al. [24] demonstrated an increase in dissection volume of 0.36 ± 0.42 cm3 after the OPPB removal. Access to the OPPB can be attained via both transconjunctival and transnasal routes. The OPPB can be removed with the bone rongeurs while decompressing via transcaruncular or transconjunctival swinging eyelid approach. Due to its thickness, we found removal of the OPPB required powered instrumentation in all cases. One can encounter a pneumatised OPPB during its removal, although its exact prevalence is not known. The optic canal was not decompressed in any of the cases. A few authors in the past have decompressed the optic canal and incised the nerve sheath as well, however the added risks and benefits are inconclusive [22].
Michel et al. [6] reported the initial outcomes of transnasal decompression in a large series of 145 orbits. Out of 145 orbits, 78 eyes had DON and subsequent decompression resulted in a mean reduction in proptosis of 3.94 mm, but ocular motility imbalance increased from 53.7% to 81.2%. Thereafter, some surgeons modified their technique to preserve the inferomedial, but the rate of new-onset diplopia remained 5–33% [7, 10, 15, 18, 20]. Selective removal of the medial orbital wall alone in a series of five patients with DON resulted in mean reduction in proptosis of 3.1 mm and visual recovery in all but one patient [20]. Our technique differs in terms of sphenoidotomy and removal of the MWS, which was not performed in any of their cases. Schafer et al. [8] performed a sphenoidotomy and removal of the medial wall of the sphenoid sinus anterior to the internal carotid artery in addition to the routine inferomedial orbital decompression. They reported an improvement in VA of more than two lines in 89% patients (25/28 eyes) with DON. The mean proptosis reduction was 3.6 mm, and five patients had new-onset diplopia. However, our technique differs in terms of decompression of the inferior portion of the orbital apex as well. The OPPB and the posterosuperior aspect of the maxillary sinus was left intact in their series. We did not encounter any new-onset diplopia in our patients. Sowerby and associates recently reported substantial improvement in Snellen VA following urgent endoscopic orbital decompression in seven patients (three bilateral) with DON unresponsive to corticosteroid therapy [25]. No cases of new-onset diplopia were encountered in their series. The posterior extent of decompression in their series was described as up to the annulus of Zinn. Our technique differs in terms of the additional removal of the OPPB.
The mean improvement in VA in this study was 0.41 LogMAR units, which is comparable to that reported in other studies (0.25–0.55) [6–8, 12, 15, 17, 19]. Sixteen out of 17 eyes (94.1%) showed an improvement of more than or equal to two lines of VA on LogMAR testing. In terms of decompression effect, the mean reduction in proptosis was 2.7 mm with a maximum of 5.1 mm. Most cases with DON do not have a large amount of proptosis, hence the extent of the orbital floor or fat decompression can be tailored in each case. Fat decompression was performed only in one case in our cohort to achieve symmetry with the contralateral side. Visual outcomes reported in this study serve the aim of decompression surgery in DON. Out of 17 patients, 13 had a preoperative RAPD, which resolved after decompression. Being a teaching institute, some of the cases had fellows as an operating surgeon as well, which may be responsible for the large variation in the duration of surgery.
The extent of apical decompression performed in the current technique might not be necessary in every case of DON. Considering the different techniques being reported in literature with good outcomes in terms of VA, decompression in DON cases can be graded according to the degree of apical crowding and/or vision loss [2]. We add the possibility of decompressing the additional areas (OPPB, MWS) with good outcomes and minimal complications. Future clinical and radiological studies employing this technique in a larger number of cases will give us better insight regarding its efficacy. Enhanced visualization of the orbital apex and the increased extent of bony removal in the area of orbital apex are the advantages of this technique. Potential risks include injury to the optic canal, or inadvertent entry into the pterygopalatine fossa, hence we advocate careful drilling with continuous irrigation in the vicinity of these areas.
In conclusion, we describe an augmented decompression of the orbital apex, by removal of the medial orbital wall, the MWS, the OPPB, and the floor under the apex to the inferior orbital fissure, as an alternative technique in the surgical management of DON.
Summary
What was known before
Endoscopic technique of orbital decompression in DON involves removal of the medial wall of the orbit.
The extent of apical decompression can be increased by performing sphenoidotomy in severe cases.
What this study adds
Augmented apical decompression can be achieved by removing MWS and OPPB endoscopically in DON patients.
No cases of new-onset diplopia were observed with this technique.
Acknowledgements
We wish to acknowledge help of Luke Halladay in archiving skull model images.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neigel JM, Rootman J, Belkin RI, et al. Dysthyroid optic neuropathy: the crowded orbital apex syndrome. Ophthalmology. 1988;95:1515–21. doi: 10.1016/S0161-6420(88)32978-7. [DOI] [PubMed] [Google Scholar]
- 2.Saeed P, Tavakoli Rad S, Bisschop PHLT. Dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg. 2018;34:S60–S67. doi: 10.1097/IOP.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 3.Kazim M, Trokel SL, Acaroglu G, et al. Reversal of dysthyroid optic neuropathy following orbital fat decompression. Br J Ophthalmol. 2000;84:600–5. doi: 10.1136/bjo.84.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korkmaz S, Konuk O. Surgical treatment of dysthyroid optic neuropathy: long-term visual outcomes with comparison of 2-wall versus 3-wall orbital decompression. Curr Eye Res. 2016;41:159–64. doi: 10.3109/02713683.2015.1008641. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy DW, Goldstein ML, Miller NL, et al. Endoscopic transnasal orbital decompression. Arch Otolaryngol Head Neck Surg. 1990;116:275–82. doi: 10.1001/archotol.1990.01870030039006. [DOI] [PubMed] [Google Scholar]
- 6.Michel O, Oberlander N, Neugebauer P, et al. Follow-up of transnasal orbital decompression in severe Graves’ ophthalmopathy. Ophthalmology. 2001;108:400–4. doi: 10.1016/S0161-6420(00)00533-9. [DOI] [PubMed] [Google Scholar]
- 7.Metson R, Dallow RL, Shore JW. Endoscopic orbital decompression. Laryngoscope. 1994;104:950–7. doi: 10.1288/00005537-199408000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer SD, Soliemanzadeh P, Della Rocca DA, Yoo GP, Maher EA, Milite JP, et al. Endoscopic and transconjunctival orbital decompression for thyroid-related orbital apex compression. Laryngoscope. 2003;113:508–13. doi: 10.1097/00005537-200303000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Lund VJ, Larkin G, Fells P, et al. Orbital decompression for thyroid eye disease: a comparison of external and endoscopic techniques. J Laryngol Otol. 1997;111:1051–5. doi: 10.1017/S0022215100139313. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RA, Shorr N, Cohen MS. The medial orbital strut in the prevention of postdecompression dystopia in dysthyroid ophthalmopathy. Ophthalmic Plast Reconstr Surg. 1992;8:32–34. doi: 10.1097/00002341-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Metson R, Samaha M. Reduction of diplopia following endoscopic orbital decompression: the orbital sling technique. Laryngoscope. 2002;112:1753–7. doi: 10.1097/00005537-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Lv Z, Selva D, Yan W, Daniel P, Tu Y, Wu W. Endoscopical orbital fat decompression with medial orbital wall decompression for dysthyroid optic neuropathy. Curr Eye Res. 2016;41:150–8. doi: 10.3109/02713683.2015.1008640. [DOI] [PubMed] [Google Scholar]
- 13.Martins C, Costa E, Silva IE, Campero A, Yasuda A, Aguiar LR, Tatagiba M, Rhoton A., Jr. Microsurgical anatomy of the orbit: the rule of seven. Anat Res Int. 2011;2011:468727. doi: 10.1155/2011/468727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu W, Selva D, Bian Y, Wang X, Sun MT, Kong Q, Yan W. Endoscopic medial orbital fat decompression for proptosis in type 1 graves orbitopathy. Am J Ophthalmol. 2015;159:277–84. doi: 10.1016/j.ajo.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Tyler MA, Zhang CC, Saini AT, Yao WC. Cutting-edge endonasal surgical approaches to thyroid ophthalmopathy. Laryngoscope Investig Otolaryngol. 2018;3:100–4. doi: 10.1002/lio2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu EA, Miller NR, Grant MP, Merbs S, Tufano RP, Lane AP. Surgical treatment of dysthyroid orbitopathy. Otolaryngol Head Neck Surg. 2009;141:39–45. doi: 10.1016/j.otohns.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Garrity JA, Fatourechi V, Bergstralh EJ, et al. Results of transantral orbital decompression in 428 patients with severe Graves’ ophthalmopathy. Am J Ophthalmol. 1993;116:533–47. doi: 10.1016/S0002-9394(14)73194-0. [DOI] [PubMed] [Google Scholar]
- 18.Perry JD, Kadakia A, Foster JA. Transcaruncular orbital decompression for dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg. 2003;19:353–8. doi: 10.1097/01.IOP.0000083645.19368.99. [DOI] [PubMed] [Google Scholar]
- 19.Liao SL, Chang TC, Lin LL. Transcaruncular orbital decompression: an alternate procedure for Graves ophthalmopathy with compressive optic neuropathy. Am J Ophthalmol. 2006;141:810–8. doi: 10.1016/j.ajo.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Chu EA, Miller NR, Lane AP. Selective endoscopic decompression of the orbital apex for dysthyroid optic neuropathy. Laryngoscope. 2009;119:1236–40. doi: 10.1002/lary.20240. [DOI] [PubMed] [Google Scholar]
- 21.Kingdom TT, Davies BW, Durairaj VD. Orbital decompression for the management of thyroid eye disease: an analysis of outcomes and complications. Laryngoscope. 2015;125:2034–40. doi: 10.1002/lary.25320. [DOI] [PubMed] [Google Scholar]
- 22.Luxenberger W, Stammberger H, Jebeles JA, Walch C. Endoscopic optic nerve decompression: the Graz experience. Laryngoscope. 1998;108:873–82. doi: 10.1097/00005537-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Dallan I, Castelnuovo P, de Notaris M, Sellari-Franceschini S, Lenzi R, Turri-Zanoni M, Battaglia P, Prats-Galino A. Endoscopic endonasal anatomy of superior orbital fissure and orbital apex regions: critical considerations for clinical applications. Eur Arch Otorhinolaryngol. 2013;270:1643–9. doi: 10.1007/s00405-012-2281-3. [DOI] [PubMed] [Google Scholar]
- 24.Mueller SK, Freitag SK, Bleier BS. Morphometric analysis of the orbital process of the palatine bone and its relationship to endoscopic orbital apex surgery. Ophthalmic Plast Reconstr Surg. 2018;34:254–7. doi: 10.1097/IOP.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 25.Sowerby LJ, Rajakumar C, Allen L, Rotenberg BW. Urgent endoscopic orbital decompression for vision deterioration in dysthyroid optic neuropathy. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;S1879-7296(18)30125-X. [DOI] [PubMed]


