Abstract
In the area of microbial production of valuable chemicals, plasmids have been widely applied for overexpressing rate-limiting enzymes with high yields. However, the effect of antibiotic concentrations on the transcription of target genes in E. coli is less involved in previous reports. In this study, we constructed E. coli strains expressing the reporter gene and the kanamycin resistant gene in an operon, and analyzed the transcription levels of the reporter gene and the fluorescent intensity of the recombinant E. coli under different kanamycin concentrations. We found that the growth and gene transcription of the recombinant strain were affected obviously by the concentration of kanamycin, indicating the importance of fine-tuning of antibiotic concentrations in microbial fermentation.
Keywords: E. coli, Plasmids, Kanamycin, Transcription level
Introduction
As possessing the advantages of fast growth, cheap culture medium, and relatively convenient metabolic engineering tools, various microorganisms have been used for bioproduction of valuable chemicals, such as amino acids (Li et al. 2017a; Oldiges et al. 2014; Trondle et al. 2018), succinate (Li et al. 2016; Zhu and Tang 2017), polyhydroxyalkanoates (Chen et al. 2018; Li et al. 2017b), and so on. To overexpress the rate-limiting enzymes with high yields, recombinant DNA technology is widely employed in Escherichia coli, a model microorganism which can easily achieve high cell density from less expensive substrates in a short cultivation time.
With the development of molecular biology, plasmids become an important tool for genetic engineering in microorganisms and plant cells. To a certain extent, notable development would not have occurred without plasmids. In addition, industrial production of high value proteins in microorganisms is also dependent on plasmids.
Although plasmids are easy to manipulate, their utilization suffers from genetic instability resulting from segregational instability, structural instability, and allele segregation (Friehs 2004). Since engineered plasmids are often lost during cultivation, it is essential to provide a selective pressure to ensure plasmids’ stability, which is often realized by antibiotics. However, metabolic burden will be also generated due to plasmid replication and consumption of precursor metabolites and energy (Bentley et al. 1990; Glick 1995).
Traditionally, antibiotics are supplemented into the medium at a recommended concentration, which depended on whether the plasmids are stringent or relaxed. However, the effect of antibiotic concentration on the transcription of target genes in E. coli has not been explored. Kanamycin, an aminoglycoside antibiotic, can inhibit the peptide synthesis of E. coli by blocking the translocation process (Semenkov et al. 1982). In this work, green fluorescent protein was selected as a reporter, and kanamycin was selected as a representative for antibiotics. By varying the concentration of kanamycin, transcription level and fluorescent intensity of recombinant E. coli were investigated.
Materials and methods
Bacterial strains
All strains, plasmids and oligonucleotides used in this study are listed in Tables 1 and 2. E. coli W3110 was selected as a host strain. E. coli strain DH5α was used as the host of recombinant DNA manipulation.
Table 1.
Strains and plasmids used in this study
| Strains | Genotype | References |
|---|---|---|
|
W3110 DH5a JZ-1 JZ-2 |
F-, l-, rph-1, IN (rrnD, rrnE) F−, endA1, hsdR17 (rK−, mK+), supE44, thi-l, l−, recA1, gyrA96, ΔlacU169 (F80lacZ ΔM15) W3110/ pJZ-1 W3110/ pJZ-2 |
Lab stock Lab stock This study This study |
| Plasmids | ||
| pTrc99a | oripBR322, bla(ApR) | Lab stock |
| pCL1920 | oripSC101, spc/str | Lerner and Inouye (1990) |
| pKD4 | oriR6Kγ, bla(ApR), kan, rgnB(Ter) | Datsenko and Wanner (2000) |
| pJZ-1 | pCL1920-kan-gfp | This study |
| pJZ-2 | pTrc99a-kan-gfp | This study |
Table 2.
Primers used in this study
| Primers | Sequences |
|---|---|
|
kan-CF kan-CR gfp-CF gfp-CR kan-AF kan-AR |
5′-CCCAAGCTTAAGGAGATATACATATGATTGAACAAGATGGATTGC-3′ 5′-ACGCGTCGACTCAGAAGAACTCGTCAAGAAGGCGATAGA-3′ 5′-ACGCGTCGACAAGGAGATATACATATGCGTAAAGGCGAAGAGCTG-3′ 5′-CGCGGATCCTCATTTGTACAGTTCATCCATACCAT-3′ 5′-CCGGAATTCAAGGAGCATCTATGATTGAACAAGATGGATTGCACGCAGGT-3′ 5′-CGCGGATCCTCAGAAGAACTCGTCAAGAAGGCGATAGAA-3′ |
| gfp-AF | 5′-CGCGGATCCAAGGAGCATCTATGCGTAAAGGAGAAGAACT-3′ |
| gfp-AR | 5′-CGCGGATCCTCATTTGTACAGTTCATCCATACCAT-3′ |
| Primers for qRT-PCR | |
| rrsG-QF | GGTGTAGCGGTGAAATGCGTAG |
| rrsG-QR | TCAAGGGCACAACCTCCAAGTC |
| kanr-F | CTGCTATTGGGCGAAGTG |
| kanr-R | GATGTTTCGCTTGGTGGTC |
| gfp-QF | TCACTACTTTCGGTTATGG |
| gfp-QF | AAACTTGACTTCAGCACG |
Growth conditions
Strains for cloning and inoculation were grown in Luria–Bertani (LB) medium (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl) at 37 °C for 8–12 h. Ampicillin (100 mg/L), kanamycin (25 mg/L), and spectinomycin (50 mg/L) were incorporated into the medium when necessary. IPTG was added at a final concentration of 0.2 mM.
Construction of recombinant plasmids
The pTrc99a and pCL1920 plasmids were selected to overexpress kan and gfp genes encoding the kanamycin resistant protein and green fluorescent protein, respectively. The kan and gfp genes were amplified by PCR using pKD4 and pG-1 as templates, and kan-AF/kan-AR and gfp-AF/gfp-AR were used as primers. The ribosome binding site of the plasmid pET-28a was designed in primers kan-AF and gfp-AF, respectively. Then, these two fragments were ligated into pTrc99a by restriction endonucleases EcoRI, SalI and HindIII, and T4 DNA ligase. Similarly, to ligate the kan and gfp fragments into pCL1920, pKD4 and pG-1 were selected as templates, and kan-CF/kan-CR and gfp-CF/gfp-CR were used as primers. The ribosome binding site of the plasmid pET-28a was also designed in primers kan-CF and gfp-CF, respectively. And then, these two fragments were ligated into pCL1920 by restriction endonucleases HindIII, SalI and BamHI, and T4 DNA ligase. As a result, recombinant plasmids, pJZ-1 and pJZ-2 were obtained, respectively. At last, these two plasmids were transformed into wild type W3110 separately to generate strains, JZ-1 and JZ-2.
qRT-PCR
Samples for mRNA preparation were cultivated 6 h after the addition of inducer. Total mRNA of samples was extracted by an RNA Simple Total RNA kit (Tiangen, Beijing, China). Reverse transcription was performed using random 6-mers and oligo dT primers with a PrimeScript RT reagent kit (Takara, Dalian, China). RT-PCR was conducted by SYBR Premix Ex Taq II (Takara) following the LightCycler 480 RT-PCR System protocol (Roche, Basel, Switzerland). The qRT-PCR primers are listed in Table 2.
Analytical methods
Cell growth was monitored by measuring the optical density at 600 nm (OD600) with a UV5100H spectrophotometer (METASH, Shanghai China). The fluorescence of recombinant strains was determined as described previously (Li et al. 2012).
Results and discussion
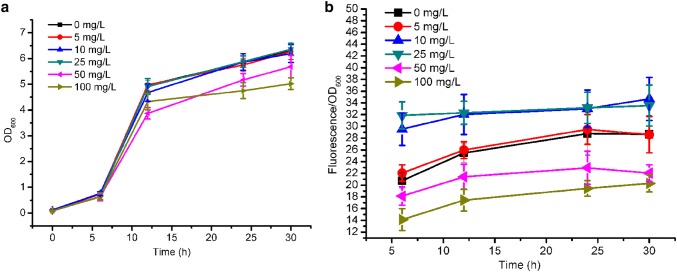
To explore the relationship between the concentration of kanamycin and the expression level of report genes, the kan and gfp genes were assembled into an operon and ligated into pCL1920 and pTrc99a to generate pJZ-1 and pJZ-2, respectively (Fig. 1). And then, batch fermentation with different concentrations of kanamycin was first performed for the recombinant strain JZ-1, and the results are shown in Fig. 2. When 0, 5, 10 or 25 mg/L kanamycin was used, the recombinant strain exhibited similar growth curve. However, 50 or 100 mg/L of kanamycin interfered with the normal growth of JZ-1 seriously, indicated by the maximum OD600 decrease to 4.68 or 4.02 separately. This result was consistent with our expectations, as the normal concentration of kanamycin for E. coli was 25 mg/L. In a previous study, Marini et al. explored the effects of kanamycin concentrations on cell growth (Marini et al. 2014). Different from our results, they found that varying the concentration of kanamycin at 10, 30 or 50 mg/L did not interfere with the growth of E. coli. Similar results were also obtained from another report (Einsfeldt et al. 2011). The maximum applied concentration of kanamycin in these two reports was only 50 mg/L, while 100 mg/L of kanamycin was used in this study. On the other hand, different plasmids with different genetic backgrounds were employed in this work compared to other reports. Maybe these discrepancies led to different effects of kanamycin on cell growth of E. coli.
Fig. 1.
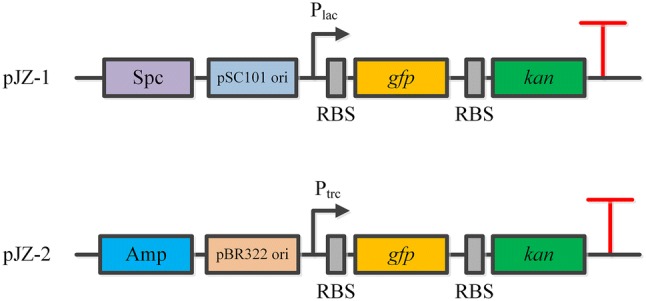
The structure of recombinant plasmids pJZ-1 and pJZ-2
Fig. 2.
The OD600 (a) and fluorescence (b) in JZ-1 with different concentrations of kanamycin. The error bars represent standard deviations from three replicate fermentations. The fluorescence intensity of gfp was normalized by OD600
And then, the fluorescence of JZ-1 was also examined. When no kanamycin or only 5 mg/L of kanamycin was added into the media, the expression level of GFP was relatively low, probably due to partial loss of plasmids. In contrast, 10 or 25 mg/L of kanamycin caused the fluorescence of GFP increase to 34.68 ± 3.67 and 33.55 ± 3.50, respectively. This indicated that a lower concentration of kanamycin (10 mg/L) could also achieve similar expression level of proteins compared to that of the recommended concentration. Surprisingly, JZ-1 with 50 or 100 mg/L kanamycin exhibited even weaker fluorescence than the control with no kanamycin added. Maybe the transcription level of kan increased with the increased concentration of kanamycin, while the expression of gfp was severely harmed. To test our suspect, qRT-PCR was performed for JZ-1, with different kanamycin concentrations added to the medium. As shown in Fig. 3, when 50 or 100 mg/L kanamycin was supplemented into the medium, the transcription level of kan increased over 2.5-fold compared to the same strain with no kanamycin supplement. In contrast, the transcription level of gfp decreased by over 50% when the transcription of kan increased. In a previous study, different genes in the same operon could exhibit different transcription levels, and the gene expression in the operon has linear relationship with the transcription distance (Lim et al. 2011). In this study, we also found different transcription levels of the genes in an operon, but this difference was induced by improving the concentration of kanamycin. In 2011, the relationship between the expression of protease ClpP and kanamycin concentration was investigated by Karen Einsfeldt and co-researchers (Einsfeldt et al. 2011). Compared to recombinant E. coli with 0 or 50 mg/L kanamycin, supplement of 25 mg/L of kanamycin into the medium led to higher expression of ClpP, which also indicates the effect of kanamycin concentration on gene expression (Fig. 4).
Fig. 3.
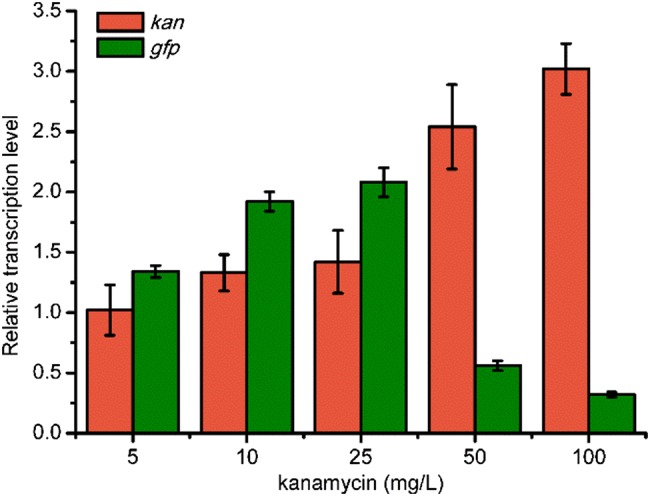
Relative transcription level of gfp and kan in E. coli JZ-1 with different concentrations of kanamycin. E. coli JZ-1 with no kanamycin added was selected as the control. The rrsG transcripts were selected as the standard and each measurement was repeated three times. The error bars indicate standard deviations from three replicate samples
Fig. 4.
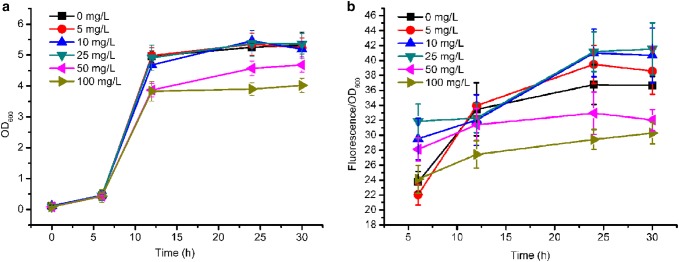
The OD600 (a) and fluorescence (b) in JZ-2 with different concentrations of kanamycin. The error bars represent standard deviations from three replicate fermentations. The fluorescence intensity of gfp was normalized by OD600
To examine whether different plasmid copy-number leads to similar results, pTrc99a with medium copies in a cell was selected to construct pJZ-2, and the kan and gfp genes are in the same operon as well. In batch fermentation, strain JZ-2 containing pJZ-2 with different concentrations of kanamycin exhibited similar growth curve with JZ-1. When 10 or 25 mg/L kanamycin was supplemented, the expression level of GFP was almost the same. In addition, the fluorescence of JZ-2 was higher than JZ-1, probably because of the higher copy-number of pTrc99a than pCL1920. However, with 50 or 100 mg/L of kanamycin added into the medium, the maximum fluorescence of gfp decreased to 32.95 ± 2.83 and 30.29 ± 1.45. This result suggested that the impact of kanamycin on gene transcription in the same operon was not interfered by the copy-number of plasmids. To further verify this speculation, qRT-PCR was also performed for JZ-2. As shown in Fig. 5, the transcription of kan increased obviously when the concentration of kanamycin was 50 or 100 mg/L. In contrast, the transcription of gfp decreased to only 0.46- and 0.24-fold, respectively, compared to the control. In 2014, Lin et al. found that the expression of LamB and Odp1 complex were crucial for antibiotic resistance in E. coli. Lower expression of a LamB–Odp1 complex was a general response of resistance to different classes of antibiotics (Lin et al. 2014). These results indicated that expression of several different genes can be affected by the pressure of antibiotics.
Fig. 5.
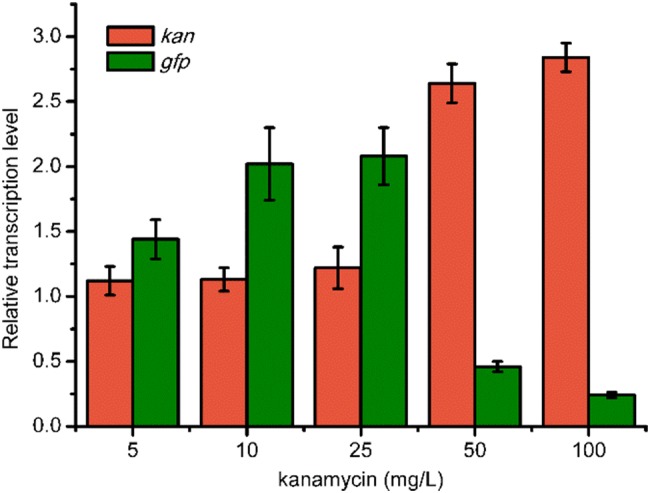
Relative transcription level of gfp and kan in E. coli JZ-2 with different concentrations of kanamycin. E. coli JZ-2 with no kanamycin added was selected as the control. The rrsG transcripts were selected as the standard and each measurement was repeated three times. The error bars indicate standard deviations from three replicate samples
In conclusion, recombinant E. coli strains overexpressing GFP and kanamycin resistant genes in an operon with two different plasmids were constructed. We found that the growth and gene transcription of recombinant strains were affected by the concentration of kanamycin. This work provides a useful supplement for the effect of antibiotics on gene expression levels.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31600066, 31870105, 31741007).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
References
- Bentley WE, Mirjalili N, Andersen DC, Davis RH, Kompala DS. Plasmid-encoded protein: the principal factor in the "metabolic burden" associated with recombinant bacteria. Biotechnol Bioeng. 1990;35:668–681. doi: 10.1002/bit.260350704. [DOI] [PubMed] [Google Scholar]
- Chen J, Li W, Zhang ZZ, Tan TW, Li ZJ. Metabolic engineering of Escherichia coli for the synthesis of polyhydroxyalkanoates using acetate as a main carbon source. Microb Cell Fact. 2018;17:102. doi: 10.1186/s12934-018-0949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsfeldt K, Severo Junior JB, Correa Argondizzo AP, Medeiros MA, Alves TL, Almeida RV, Larentis AL. Cloning and expression of protease ClpP from Streptococcus pneumoniae in Escherichia coli: study of the influence of kanamycin and IPTG concentration on cell growth, recombinant protein production and plasmid stability. Vaccine. 2011;29:7136–7143. doi: 10.1016/j.vaccine.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Friehs K. Plasmid copy number and plasmid stability. Adv Biochem Eng Biotechnol. 2004;86:47–82. doi: 10.1007/b12440. [DOI] [PubMed] [Google Scholar]
- Glick BR. Metabolic load and heterologous gene expression. Biotechnol Adv. 1995;13:247–261. doi: 10.1016/0734-9750(95)00004-A. [DOI] [PubMed] [Google Scholar]
- Lerner CG, Inouye M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 1990;18:4631. doi: 10.1093/nar/18.15.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gu P, Kang J, Wang Y, Wang Q, Qi Q. Extending homologous sequence based on the single gene mutants by one-step PCR for efficient multiple gene knockouts. Folia Microbiol (Praha) 2012;57:209–214. doi: 10.1007/s12223-012-0111-z. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang B, Wu H, Li Z, Ye Q, Zhang YP. Production of succinate from acetate by metabolically engineered Escherichia coli. ACS Synth Biol. 2016;5:1299–1307. doi: 10.1021/acssynbio.6b00052. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Current status on metabolic engineering for the production of L-aspartate family amino acids and derivatives. Bioresour Technol. 2017;245:1588–1602. doi: 10.1016/j.biortech.2017.05.145. [DOI] [PubMed] [Google Scholar]
- Li ZJ, Qiao K, Che XM, Stephanopoulos G. Metabolic engineering of Escherichia coli for the synthesis of the quadripolymer poly(glycolate-co-lactate-co-3-hydroxybutyrate-co-4-hydroxybutyrate) from glucose. Metab Eng. 2017;44:38–44. doi: 10.1016/j.ymben.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Lim HN, Lee Y, Hussein R. Fundamental relationship between operon organization and gene expression. Proc Natl Acad Sci USA. 2011;108:10626–10631. doi: 10.1073/pnas.1105692108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XM, Yang MJ, Li H, Wang C, Peng XX. Decreased expression of LamB and Odp1 complex is crucial for antibiotic resistance in Escherichia coli. J Proteom. 2014;98:244–253. doi: 10.1016/j.jprot.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Marini G, et al. Experimental design approach in recombinant protein expression: determining medium composition and induction conditions for expression of pneumolysin from Streptococcus pneumoniae in Escherichia coli and preliminary purification process. BMC Biotechnol. 2014;14:1. doi: 10.1186/1472-6750-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldiges M, Eikmanns BJ, Blombach B. Application of metabolic engineering for the biotechnological production of L-valine. Appl Microbiol Biotechnol. 2014;98:5859–5870. doi: 10.1007/s00253-014-5782-8. [DOI] [PubMed] [Google Scholar]
- Semenkov YuP, Katunin VI, Makarov EM, Kirillov SV. Quantitative study of kanamycin action on different functions of Escherichia coli ribosomes. FEBS Lett. 1982;144:121–124. doi: 10.1016/0014-5793(82)80583-8. [DOI] [PubMed] [Google Scholar]
- Trondle J, Trachtmann N, Sprenger GA, Weuster-Botz D. Fed-batch production of L-tryptophan from glycerol using recombinant Escherichia coli. Biotechnol Bioeng. 2018;115:2881–2892. doi: 10.1002/bit.26834. [DOI] [PubMed] [Google Scholar]
- Zhu LW, Tang YJ. Current advances of succinate biosynthesis in metabolically engineered Escherichia coli. Biotechnol Adv. 2017;35:1040–1048. doi: 10.1016/j.biotechadv.2017.09.007. [DOI] [PubMed] [Google Scholar]