Abstract
Studies of host-parasite relationships largely benefit from adopting a multifactorial approach, including the complexity of multi-host systems and habitat features in their analyses. Some host species concentrate most infection and contribute disproportionately to parasite and vector population maintenance, and habitat feature variation creates important heterogeneity in host composition, influencing infection risk and the fate of disease dynamics. Here, we examine how the availability of specific groups of hosts and habitat features relate to vector abundance and infection risk in 18 vector populations along the Mediterranean-type ecosystem of South America, where the kissing bug Mepraia spinolai is the main wild vector of the parasite Trypanosoma cruzi, the etiological agent of Chagas disease. For each population, data on vectors, vertebrate host availability, vegetation, precipitation, and temperature were collected and analyzed. Vector abundance was positively related to temperature, total vegetation, and European rabbit availability. Infection risk was positively related to temperature, bromeliad cover, and reptile availability; and negatively to the total domestic mammal availability. The invasive rabbit is suggested as a key species involved in the vector population maintenance. Interestingly, lizard species –a group completely neglected as a potential reservoir–, temperature, and bromeliads were relevant factors accounting for infection risk variation across populations.
Subject terms: Community ecology, Ecological epidemiology
Introduction
Studies of host-parasite relationships largely benefit from adopting a multifactorial approach, including the complexity of multi-host systems and habitat features in their analyses1,2. For instance, because of their different susceptibility to acquire and propagate infection, not all host species have similar roles in infectious disease dynamics. Specifically, host reservoir species are expected to play a critical role as they often concentrate most of infection and have a disproportionate impact on parasite and vector populations3,4. While the impact of reservoir species on disease dynamics is a focus of important research at present5, most studies conducted to date have been carried out at the local spatial scale of analysis6,7 and few examine the extent to which the geographical turnover in host species composition influence infection risk8. This lack of emphasis is unfortunate as variation in species assemblages is the norm rather than the exception in ecological studies and variation in climatic factors is a major determinant of infectious disease dynamics9. In this way, habitat feature variation across localities is expected to create important heterogeneity in the type of host species, influencing the prevalence, infection risk and ultimately the fate of disease dynamics.
In this study, we assess how specific host assemblages and habitat features relate to vector abundance and infection risk (measured as infected vector abundance) in a range of abiotic conditions across populations. We focus on a system composed of the flagellated protozoan parasite Trypanosoma cruzi (the causative agent of Chagas disease), its hematophagous triatomine vector (kissing bugs), and vertebrate assemblages that include reptiles, birds, and native and non-native mammals from the Mediterranean-type ecosystem of South America (Chile). The climate of this ecosystem is of a semiarid Mediterranean type with most rainfall occurring in the winter season, with a mean annual rainfall between 25 and 700 mm depending on the latitude10. Vegetation is dominated by succulents, shrubs, and sclerophyllous species. Only mammal species have been identified as T. cruzi reservoirs and, in principle, they could contribute differently to infection maintenance since native mammals may be competent reservoirs as the result of their evolutionary history with T. cruzi and kissing bugs, while domestic mammals have a shorter history of interaction with kissing bug species and T. cruzi5,8.
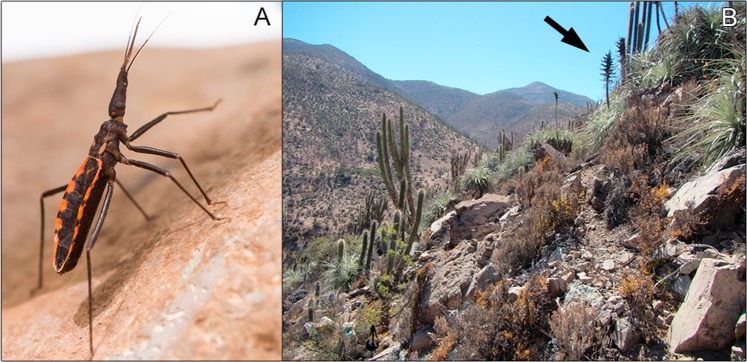
In this ecosystem, the most important kissing bug species is the wild triatomine Mepraia spinolai11 (Fig. 1A). This diurnal kissing bug species occurs from 26°S to 33°S12 and shows a conspicuous spatial aggregation, often using rocky outcrops and bromeliads as refuge (Fig. 1B), where it exhibits a sit-and-wait strategy to parasitize vertebrate hosts7,13–16. Mepraia spinolai feeds on the blood of vertebrates such as mammals, birds and reptiles17,18, albeit its preferred hosts consist on several small native mammals, reaching T. cruzi infection prevalence up to 70% in some rodent species16,19. Secondarily, the European rabbit Oryctolagus cuniculus, an invasive mammal present in this ecosystem since 1884, experiences T. cruzi infection prevalence up to 38%20. Overall, only 22 native mammal species overlap with M. spinolai geographic distribution; therefore, it is epidemiologically relevant to examine the role of non-native mammal species (free-ranging and domestic) in this mammal-depauperate ecosystem.
Figure 1.
(A) Micropterous adult male of Mepraia spinolai showing its extended proboscis. (B) Arborescent scrub habitat with predominance of bromeliads (Puya sp. highlighted by a black arrow).
The first goal of our study was to identify the vertebrate assemblages that influence vector abundance and the maintenance of the protozoan T. cruzi at regional scale. Because habitat features such as temperature, precipitation and vegetation cover, often affect host and insect vector demographic parameters such as survival and reproductive rates21–23, the infection risk found in natural populations may be also determined by variation in these factors24,25. Our second goal was to examine the importance of habitat features on vector abundance and infection risk at the regional scale. More specifically, in this study we address the following questions: (1) Do reptiles, birds, and mammals influence vector abundance and infection risk at the regional scale? (2) To what extent are these effects - if any - modulated by the habitat features? (3) What is the importance of non-native mammal species on vector abundance and infection risk across localities?
Materials and Methods
Field procedures
The study was carried out in 18 study sites encompassing a range of 500 km, between 28°58′ and 33°27′S (see Table 1; Fig. 2). During the austral summer, specimens of all stages of M. spinolai were collected at each study site (7 sites in 2015; 11 sites in 2016) by 3–4 trained researchers between 1100 and 1600 h the time of day with maximum M. spinolai activity14. Each site was geo-referenced in UTM coordinates (precision: ± 3 m) using a handheld GPS device. The collected kissing bugs were classified according their stage of development and individually stored to avoid potential cross-contamination with T. cruzi - infected feces. In the laboratory, kissing bugs were euthanized and subjected to abdominal extrusion to obtain a sample of its intestine and feces for subsequent DNA analyses. Each sample was diluted with 200 μL of double-distilled water. Whole genomic DNA was isolated from fecal samples (MOBIO, UltraClean Tissue & Cell DNA DNA Isolation Kit) and stored at −20 °C until molecular analyses. The Ethical Committee of the Faculty of Science of the University of Chile, and the National Forest Corporation (CONAF) reviewed and approved the animal-handling protocol for this study.
Table 1.
Descriptive information of the 18 populations of Mepraia spinolai under study.
| Site | Location | Latitude | Longitude | MS/h | IMS/h | TAP (mm) | TWT (°C) | VC | BC | NMA | DMA | OCA | BA | RA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Los Tambos | 28°58.6S | 70°11.2W | 7.05 | 3.04 | 40.4 | 16.6 | 0.21 | 0 | 19.3 | 0 | 0 | 2.3 | 0.7 |
| 2 | Pichasca | 30°25.3S | 70°51.0W | 4.2 | 4 | 53 | 18 | 0.3 | 0 | 0.7 | 0 | 0 | 0.7 | 1.3 |
| 3 | El Maitén | 30°47.8S | 70°35.4W | 8.22 | 0.23 | 95.4 | 18.2 | 0.4 | 0 | 4.3 | 1.7 | 0 | 4.7 | 2.3 |
| 4 | La Rinconada | 30°51.7S | 71°20.7W | 3.87 | 0.06 | 127.5 | 18.4 | 0.21 | 0 | 4.7 | 0 | 0 | 0 | 0 |
| 5 | Valle Hermoso | 31°17.0S | 70°59.9W | 9.59 | 0.12 | 82.1 | 17.3 | 0.37 | 0 | 4 | 0 | 0 | 1.3 | 0 |
| 6 | Los Pozos | 31°20.5S | 71°14.0W | 19.78 | 17.94 | 202.7 | 18.3 | 0.56 | 0.43 | 3.3 | 3.3 | 1 | 2 | 1 |
| 7 | Farellón Sánchez | 31°26.8S | 71°1.1W | 3.31 | 1.4 | 272.2 | 18.4 | 0.19 | 0.01 | 27.3 | 0 | 0 | 0.7 | 1 |
| 8 | Caña de Michío | 31°38.2S | 71°3.8W | 13.25 | 12.52 | 102.1 | 18.2 | 0.42 | 0.13 | 60.3 | 0 | 0 | 4.7 | 3.3 |
| 9 | San Agustín | 31°44.2S | 70°52.9W | 20.5 | 19.4 | 263 | 18.8 | 0.79 | 0.06 | 0.7 | 0.3 | 0 | 1.7 | 6 |
| 10 | La Higuerilla | 31°49.2S | 70°55.9W | 20.9 | 18.2 | 270 | 20 | 0.72 | 0.07 | 10 | 0 | 0 | 1.5 | 1.5 |
| 11 | La Patagua | 32°32.7S | 71°7.9W | 13.3 | 12.1 | 344 | 19.4 | 0.51 | 0.01 | 12.7 | 0.3 | 1.7 | 22 | 1 |
| 12 | Sahondé | 32°38.1S | 70°41.1W | 11 | 3.53 | 204 | 18.9 | 0.51 | 0 | 19 | 3.7 | 3.3 | 36 | 0.7 |
| 13 | Las Blancas | 32°53.2S | 70°47.4W | 9.06 | 6.09 | 255 | 18.1 | 0.55 | 0.06 | 4.7 | 0 | 0.3 | 11.7 | 0.7 |
| 14 | Pedrero | 32°54.1S | 70°37.1W | 22 | 20.77 | 200.9 | 19.6 | 0.64 | 0 | 2.3 | 2 | 1.3 | 8 | 4.7 |
| 15 | Chacabuco | 32°55.8S | 70°42.1W | 16.14 | 15.98 | 260.7 | 17.8 | 0.55 | 0.27 | 196.7 | 0 | 8.3 | 10.3 | 11.3 |
| 16 | La Campana | 32°57.7S | 71°7.8W | 8.21 | 5.62 | 422 | 15.9 | 0.75 | 0.39 | 28 | 0.3 | 0 | 6 | 3 |
| 17 | Til Til | 33°8.6S | 70°54.7W | 23.05 | 7.62 | 81.8 | 19.9 | 0.79 | 0.16 | 7.7 | 6.7 | 1.3 | 19.3 | 5 |
| 18 | Ciudad de los Valles | 33°27.2S | 70°50.4W | 15.21 | 7.24 | 161.6 | 19.9 | 0.39 | 0 | 0.7 | 0.7 | 4.7 | 20 | 0 |
MS/h: number of M. spinolai per hour; IMS/h: number of infected M. spinolai per hour; TAP: total annual precipitation; TWT: mean temperature of the warmest trimester; VC: vegetation cover; BC: bromeliad cover; NMA: mean number of native mammal recordings by month, DMA: mean number of domestic mammal recordings by month; OCA: mean number of Oryctolagus cuniculus recordings by month; BA: mean number of bird recordings by month; RA: mean number of reptile recordings by month.
Figure 2.
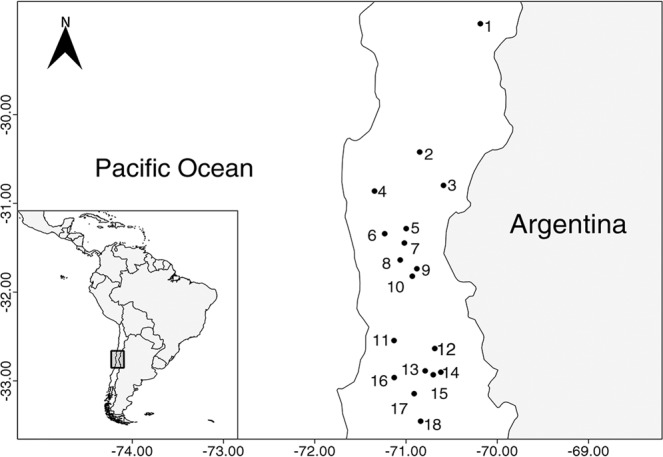
Map of north-central Chile with the geographic distribution of the 18 sampled populations of Mepraia spinolai.
For each vector population, mammal, bird and reptile species composition and availability were sampled via camera trapping (Bushnell Nature View HD Max) during peak kissing bug abundance from December to March (i.e., austral summer). Camera traps were placed to include the action area of the vector population. All photographic records were checked and classified using specific field guides for each vertebrate taxonomic group (mammals, birds, and reptiles). On this basis, the availability of each vertebrate group at each vector population was calculated as the mean number of records per month. In addition, we subdivided the mammals based on native (including all rodent, carnivore, and didelphimorph species), non-native feral (including the European rabbit), and non-native domestic (including all livestock, dogs and cats). Because we were mostly interested in reflecting blood meal availability to M. spinolai, instead of enumerating the individuals within each vertebrate species, repeated records of the same individual of vertebrate species were meaningful measures from the kissing bug’s perspective. To avoid the overestimation of availability, we considered photo records of the same species separated by one-hour interval.
In the surrounding of each vector population, the vegetation was sampled by means of three transects of 50 m each. Vegetation cover was calculated as the proportion of total vegetation on the three transects altogether, including herbs, succulents, shrubs and trees. Additionally, the specific cover of bromeliads (Puya sp.; Fig. 1B) was calculated because of the reported role of these plant species as kissing bug shelter15.
Regarding climatic variables, the mean temperature of the warmest trimester was obtained using the bioclimatic models developed by Pliscoff10 in the QGIS 2.18.1426 software using the “Point sampling tool” complement. Likewise, the total annual precipitation of the previous year of kissing bug collection - recorded by the nearest climatic station to each population (8.4 km distant on the average) - was downloaded from the Center of Climate and Resilience Research CR2 database (http://www.cr2.cl/recursos-y-publicaciones/bases-de-datos/). In this way, under any association, data on kissing bug abundance and infection would respond to rainfall at the appropriate temporal scale.
Molecular procedure for Trypanosoma cruzi detection
The amplification reaction for fecal samples was performed in triplicate with oligonucleotides 121 (5′-AAA TAA TGT ACG GK GAG ATG CAT GA 3′) and 122 (5′ GGG TTC GAT TGG GGT TGG TGT-3′) which anneal to the four conserved regions of T. cruzi minicircles as described27. A sample of 5 μL of the elution of the extract of kissing bug intestine and feces was used as DNA template in 50 μL of final volume. Each experiment included a blank that contained water instead of DNA and a positive control that contained purified kinetoplast DNA of T. cruzi. The minicircle hypervariable region PCR product of 330 bp was analyzed by electrophoresis in a 2% agarose gel and visualized by GelRed staining (For more information about methodology see ESM1-Fig. S1). A sample was considered positive when at least one of the three assays showed amplification. DNA concentration was measured on all T. cruzi negative samples to verify extraction success (See detailed information in ESM2-Excel file).
Statistical analyses
Generalized linear models (GLM hereafter) with identity link function were employed to assess the effect of variables on vector abundance and infection risk (measured as infected vector abundance). We constructed separated models to account for vector abundance and infection risk using the mean temperature of the warmest trimester, total annual precipitation, total vegetation cover, bromeliad cover, and the mean availability of each vertebrate group (i.e., mean records of: total native mammal species, O. cuniculus, total domestic mammal species, total reptile species and total bird species) as predictor variables. The model structure providing the lowest Akaike Information Criterion (AIC)28 was chosen in each case using the “stepAIC” function, included in the “MASS” library of the R software29. Models were validated in the R software: residual normality was checked using graphic methods and the Shapiro-Wilk test, and homocedasticity assumption was validated with graphic methods30.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Results
A total of 3044 M. spinolai were collected from the 18 populations. Localities were highly variables in their habitat features, vertebrate composition and availability, vector abundance and infection risk. Kissing bug abundance ranged from 3.3 to 23.1 individuals/hour, and T. cruzi-infection risk ranged from 0 to 20.8 infected M. spinolai/hour (See electrophoresis results of a subset of the samples in EMS1-Fig. S1; Fig. 3). The mean temperature of the warmest trimester (TWT) ranged from 15.9 to 20.0 °C and total annual precipitation (TAP) from 40.4 to 421.6 mm. Vegetation cover (VC) ranged from 19.1 to 78.9% and Puya sp. (BC) were detected in 10 out of 18 populations, covering from 1.0 to 42.9% (See detailed information per population in Table 1). Reptile availability (RA) ranged from 0 to 11.3 records/month and bird availability (BA) from 0 to 36.0 records/month. The total availability of native mammals (NMA) ranged from 0.7 to 196.7 records/month, and domestic mammals (DMA) from 0 to 6.7 records/month. The rabbit O. cuniculus (OCA) was present in eight populations, with a range of 0 to 8.3 records/month. See Tables 2–4 for complete lists of recorded mammal, bird and reptile species, respectively.
Table 3.
Bird species detected by camera traps in the 18 populations of Mepraia spinolai.
| Taxon | Population | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Caprimulgiformes | ||||||||||||||||||
| Systellura longirostris | x | x | ||||||||||||||||
| Columbiformes | ||||||||||||||||||
| Zenaida auriculata | x | x | x | x | ||||||||||||||
| Galliformes | ||||||||||||||||||
| Callipepla californica | x | x | x | x | x | x | x | x | ||||||||||
| Passeriformes | ||||||||||||||||||
| Asthenes humicola | x | x | x | x | x | x | x | x | ||||||||||
| Curaeus curaeus | x | |||||||||||||||||
| Diuca diuca | x | x | ||||||||||||||||
| Mimus thenca | x | x | x | x | x | |||||||||||||
| Ochetorhynchus melanurus | x | x | x | x | x | x | ||||||||||||
| Phrygilus fruticeti | x | x | x | x | ||||||||||||||
| Phrygilus gayi | x | x | ||||||||||||||||
| Pteroptochos megapodius | x | x | x | x | x | x | x | x | x | x | x | x | ||||||
| Scelorchilus albicollis | x | x | x | x | x | |||||||||||||
| Sturnella loyca | x | |||||||||||||||||
| Troglodytes aedon | x | x | x | x | x | |||||||||||||
| Turdus falcklandii | x | |||||||||||||||||
| Xolmis pyrope | x | |||||||||||||||||
| Zonotrichia capensis | x | x | x | x | x | |||||||||||||
| Tinamiformes | ||||||||||||||||||
| Nothoprocta perdicaria | x | x | x | x | x | |||||||||||||
Figure 3.
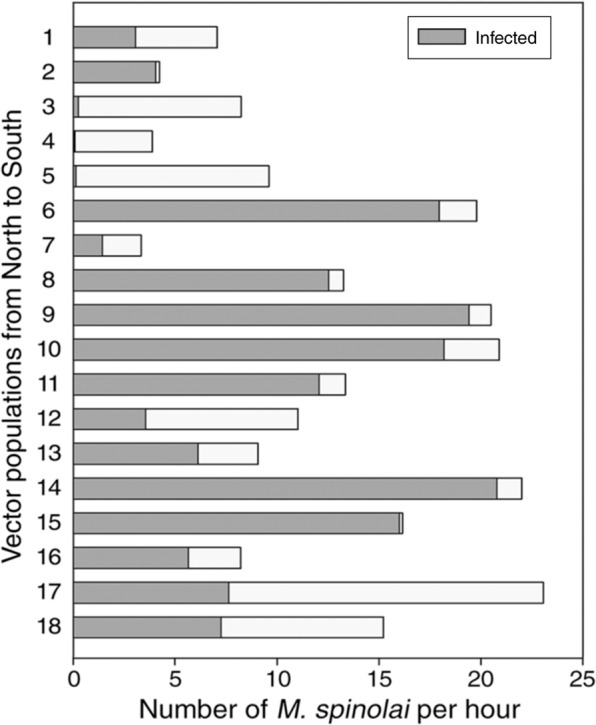
Total number of Mepraia spinolai (whole bar) in the 18 sampled populations. The number of infected individuals per hour are depicted in dark grey.
Table 2.
Mammal species detected by camera traps in the 18 populations of Mepraia spinolai. *Introduced species.
| Taxon | Population | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Didelphimorphia | ||||||||||||||||||
| Thylamys elegans | x | x | x | x | x | x | x | x | x | x | x | x | ||||||
| Artriodactyla | ||||||||||||||||||
| Bos Taurus* | x | |||||||||||||||||
| Capra hircus* | x | x | x | x | ||||||||||||||
| Ovis aries* | x | x | ||||||||||||||||
| Carnivora | ||||||||||||||||||
| Canis familiaris* | x | x | x | x | ||||||||||||||
| Felis silvestris* | x | x | x | |||||||||||||||
| Galictis cuja | x | |||||||||||||||||
| Leopardus colocolo | x | |||||||||||||||||
| Lycalopex culpaeus | x | x | x | x | x | x | x | x | ||||||||||
| Puma concolor | x | |||||||||||||||||
| Lagomorpha | ||||||||||||||||||
| Oryctolagus cuniculus* | x | x | x | x | x | x | x | x | x | |||||||||
| Rodentia | ||||||||||||||||||
| Abrocoma benetti | x | x | x | |||||||||||||||
| Abrothrix olivaceus | x | x | x | x | ||||||||||||||
| Octodon degus | x | x | x | x | ||||||||||||||
| Octodon lunatus | x | x | x | x | ||||||||||||||
| Phyllotis darwini | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||
Table 4.
Reptile species detected by camera traps in the 18 populations of Mepraia spinolai.
| Taxon | Population | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Squamata | ||||||||||||||||||
| Callopistes maculatus | x | x | x | x | ||||||||||||||
| Liolaemus fuscus | x | x | x | x | ||||||||||||||
| Liolaemus monticola | x | x | ||||||||||||||||
| Liolaemus nitidus | x | x | x | x | x | |||||||||||||
| Liolaemus platei | x | x | x | x | x | |||||||||||||
| Liolaemus pseudolemniscatus | x | |||||||||||||||||
| Philodryas chamissonis | x | |||||||||||||||||
The best model for kissing bug abundance, included mean temperature of the warmest trimester, total annual precipitation, vegetation cover, O. cuniculus availability and bird availability as predictor variables (Table 5). Significant positive estimates were detected for vegetation cover, mean temperature of the warmest trimester, and availability of the free-ranging European rabbit O. cuniculus. The best model for infection risk included mean temperature of the warmest trimester, bromeliad cover, total domestic mammal availability, and reptile availability as predictor variables (Table 6). Significant positive effects were detected for mean temperature of the warmest trimester, bromeliad cover and reptile availability, and negative for total domestic mammals.
Table 5.
Generalized linear model testing the relationship between Mepraia spinolai abundance and the predictor variables.
| Model | AIC | |||||
|---|---|---|---|---|---|---|
| MS/h = Intercept + TAP + TWT + VC + BC + RA + BA + NMA + DMA + OCA | 100.55 | |||||
| MS/h = Intercept + TAP + TWT + VC + OCA + BA | 94.5 | |||||
| Response | Variables | Estimates | SE | p | r² | p |
| MS/h | 0.82 | <0.001 | ||||
| Intercept | −40.259 | 12.295 | 0.007 | |||
| TAP | −0.016 | 0.008 | 0.055 | |||
| TWT | 2.331 | 0.686 | 0.005 | |||
| VC | 27.409 | 4.144 | <0.001 | |||
| OCA | 0.822 | 0.358 | 0.041 | |||
| BA | −0.162 | 0.086 | 0.083 | |||
MS/h: total number of M. spinolai per hour; TAP: total annual precipitation; TWT: mean temperature of the warmest trimester; VC: vegetation cover; BC: cover of bromeliads; RA: mean records of reptiles per month; BA: mean records of birds per month; NMA: mean records of native mammals per month; DMA: mean records of domestic mammals per month; OCA: mean records of Oryctolagus cuniculus per month. The complete model is shown in the top. The selected model with the lowest AIC value is shown in the bottom. Statistically significant p-values in bold.
Table 6.
Generalized linear model testing the relationship between Trypanosoma cruzi infection risk (i.e., infected vector abundance) and the predictor variables.
| Model | AIC | |||||
|---|---|---|---|---|---|---|
| IMS/h = Intercept + TAP + TWT + VC + BC + RA + BA + NMA + DMA +OCA | 117.34 | |||||
| IMS/h = Intercept + TWT + BC + NMA + DMA + RA | 110.77 | |||||
| Response | Variables | Estimates | SE | p | r² | p |
| IMS/h | 0.63 | 0.003 | ||||
| Intercept | −79.133 | 22.023 | 0.004 | |||
| TWT | 4.581 | 1.199 | 0.002 | |||
| BC | 33.024 | 9.886 | 0.006 | |||
| NMA | −0.072 | 0.040 | 0.096 | |||
| DMA | −1.991 | 0.742 | 0.020 | |||
| RA | 1.723 | 0.621 | 0.017 | |||
IMS/h: number of infected M. spinolai per hour; TAP: total annual precipitation; TWT: mean temperature of the warmest trimester; VC: vegetation cover; BC: cover of bromeliads; RA: mean records of reptiles per month; BA: mean records of birds per month; NMA: mean records of native mammals per month; DMA: mean records of domestic mammals per month; OCA: mean records of Oryctolagus cuniculus per month. The complete model is shown in the top. The selected model with the lowest AIC value is shown in the bottom. Statistically significant p-values in bold.
Discussion
In this study, we examined the vertebrate hosts and habitat features associated with kissing bug abundance and infection risk at regional scale. The results showed that temperature, vegetation cover and the European rabbit availability affected positively vector abundance. On the other hand, the presence of T. cruzi in vector populations was associated positively to temperature, bromeliad cover and reptile availability, but negatively to the total domestic mammal availability.
Rabbit availability accounted for considerable variation in kissing bug abundance, and this association may relate to the fact this mammal is a highly accessible blood meal to M. spinolai. This suggestion is supported by two facts: (i) rabbit burrows frequently occur next to M. spinolai colonies18, and (ii) rabbits have mostly nocturnal habits while M. spinolai is a predominantly diurnal species, therefore, facilitating feeding activity on resting rabbits20. Furthermore, when fed separately on different mammal species under laboratory conditions, kissing bugs reach the highest fecundity when fed on rabbits31. Our result stresses the importance of the alien European rabbit, described as a plague in the Mediterranean-type ecosystem of South American20, as a critical feeding resource for kissing bug populations32.
Total vegetation cover influenced positively vector abundance. It is likely that, as reported in other kissing bugs33 and ticks (e.g., Ixodes rictus, the vector of Lyme borreliosis)34, vegetation provides shelter and stable abiotic conditions allowing high population growth rates. Some rodent species such as Octodon degus and Phyllotis darwini use thorny shrubs as permanent refuges, which increases their susceptibility to parasitism by kissing bugs. Indeed, these rodents constitute an important proportion of M. spinolai diet18, suggesting that these microsites represent critical patches for vector establishment and population growth. However, it has also been described that when M. spinolai is associated with rural housing, they feed on domestic animals such as dogs, cats, goats, among others35.
The warmest temperature showed a positive association with vector abundance, which is consistent with reports describing that insect mortality increases at low temperatures as most insects need an optimal temperature to complete their development, above which survival can decline24,36,37. Temperature has been described as playing an important role on kissing bug’s behavior, since it regulates essential processes in their biological cycles, such as feeding, dispersal, molting time and reproduction, all of them ultimately affecting population abundances21,23. We suggest that the critical thermal maximum is not approached at our sites and this might be relevant to predict changes in kissing bug abundance with a warming climate.
The total availability of domestic mammals was negatively related with infected vector abundance (i.e., infection risk). One potential explanation for this pattern is based on a simple host numerical effect assuming an opportunistic vector feeding behavior. If infected and/or more competent mammals are less abundant, reducing their contact rates with vectors, the net result will be a lower density of infected vectors. For instance, some mammal species might be less competent than others in acquiring and transmitting T. cruzi to vectors because of a lack common evolutionary history with the protozoan (e.g., domestic mammals). Some host species-specific features that prevent parasite inoculation or settlement are skin thickness, fur density and length, grooming behavior, repulsion behavior to kissing bug bites38. We suggest that Chagas disease risk should be assessed considering all the complexity associated to this multi-host system and evaluated in a wide spatial scale.
Surprisingly, reptile availability was a good predictor of T. cruzi-infected vector abundance (i.e., infection risk). The reptile species, mostly lizards, cohabitating with M. spinolai are mainly insectivorous (e.g., Liolaemus platei, L. fuscus, L. monticola, L. nitidus)39, which suggests they probably include kissing bugs in their diet40. The higher infection risk observed at increasing lizard availability can be tentatively explained if lizards become infected and amplify T. cruzi, which is consistent with a previous report in the North American lizard Gerrhonotus multicarinatus webbii41. There is evidence that Mepraia species feed on reptiles17 and probably lizards prey on kissing bugs40; therefore, lizards could become infected by vectorial transmission during thermoregulation activities, when reptiles are largely inactive, or by infected kissing bug consumption (i.e., oral transmission). Further research is needed to test the mechanistic role of lizards in the wild transmission cycle of T. cruzi.
Temperature and bromeliad cover were relevant habitat features positively associated with the protozoan-infection risk. Laboratory evidence indicates that T. cruzi replication in triatomines tends to increase with temperature up to an optimum ca. 27–30 °C42,43, which is in line with our regional findings. Regarding the positive contribution of bromeliad cover to T. cruzi infection, it is likely that this association is mediated through changes in blood meal sources such as rodent availability. Vegetation (and thus shelter) is scarce and often constitutes a limiting resource in semiarid Mediterranean-type environments. In this situation, bromeliads may provide shelter and appropriate thermal conditions to small mammals, creating kissing bug aggregations and overcrowding. As kissing bug feces contain aggregation pheromones, this may increase disproportionally disease transmission rate within colonies. Horizontal transmission through blood stealing and coprophagy has been reported in dense triatomine colonies44,45, and suggested in M. spinolai7, which may help to understand the mechanisms underlying the positive association between bromeliad cover and infection risk across populations.
In summary, the free-ranging European rabbit was identified as an important species accounting for variation in vector abundance across populations, suggesting this mammal species plays a key role in regulating vector abundance and, therefore, parasite transmission37. Infection risk, in turn, increased with lizards but decreased with the availability of domestic mammals. While the mechanisms involved in the geographical patterns here described remain to be tested, our results showing the relevance of bromeliads and temperature, and more importantly the role of lizards and the invasive European rabbit in the epidemiology of Chagas disease, contribute to a more complete understanding of the factors involved in the transmission of one of the major neglected infectious diseases46.
Supplementary information
Acknowledgements
We thank R. Cares, M. Ehrenfeld, D. Estay, R. Montero and N. Peña for contribution in field and laboratory work, P. Ferrer for statistical advice, and R. Medel, R.S. Ostfeld and A. Solari for invaluable comments on our manuscript. We thank the Regional Ministerial Secretary of Chile for logistic support. A.S.R., R.A.D. and E.S.J. were supported by CONICYT-Beca de Doctorado 21181241, CONICYT-Beca de Magister 22172284, and CONICYT-Beca de Magister 22190109, respectively. C.B.M. specially thanks the Cary Institute at Millbrook, NY, USA, for a sabbatical visit. This study was funded by CONICYT-FONDECYT No. 1170367 & 1140521 (C.B.M.).
Author contributions
C.B.M. and E.S.J. conceived the idea and designed the study. C.B.M., E.S.J., A.S.R., A.Y.M. and R.A.D. performed fieldwork. A.S.R., N.Q. and A.Y.M. performed the experiments. E.S.J. and R.A.D. analyzed the data. C.B.M., E.S.J. and R.A.D. wrote the manuscript; other authors provided editorial advice.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Esteban San Juan and Raúl Araya-Donoso.
Supplementary information
is available for this paper at 10.1038/s41598-020-59054-8.
References
- 1.Swei A, Meentemeyer R, Briggs CJ. Influence of abiotic and environmental factors on the density and infection prevalence of Ixodes pacificus (Acari: Ixodidae) with Borrelia burgdorferi. J. Med. Entomol. 2011;48:20–28. doi: 10.1603/ME10131. [DOI] [PubMed] [Google Scholar]
- 2.Gottdenker NL, Chaves LF, Calzada JE, Saldaña A, Carroll CR. Host life history strategy, species diversity, and habitat influence Trypanosoma cruzi vector infection in changing landscapes. PLoS Negl. Trop. Dis. 2012;6:e1884. doi: 10.1371/journal.pntd.0001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans. R. Soc. Trop. Med. Hyg. 2009;103:109–121. doi: 10.1016/j.trstmh.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pongsiri MJ, et al. Biodiversity loss affects global disease ecology. Bioscience. 2009;59:945–954. doi: 10.1525/bio.2009.59.11.6. [DOI] [Google Scholar]
- 5.Gürtler RE, Cardinal MV. Reservoir host competence and the role of domestic and commensal hosts in the transmission of Trypanosoma cruzi. Acta Trop. 2015;151:32–50. doi: 10.1016/j.actatropica.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Brownstein JS, Skelly DK, Holford TR, Fish D. Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia. 2005;146:469–475. doi: 10.1007/s00442-005-0251-9. [DOI] [PubMed] [Google Scholar]
- 7.Oda E, Solari A, Botto-Mahan C. Effect of mammal host diversity and density on the infection level of Trypanosoma cruzi in sylvatic kissing bugs. Med. Vet. Entomol. 2014;28:384–390. doi: 10.1111/mve.12064. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PT, Preston DL, Hoverman JT, Richgels KL. Biodiversity decreases disease through predictable changes in host community competence. Nature. 2013;494:230–233. doi: 10.1038/nature11883. [DOI] [PubMed] [Google Scholar]
- 9.Poulin R. Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology. 2006;132:143–151. doi: 10.1017/S0031182005008693. [DOI] [PubMed] [Google Scholar]
- 10.Pliscoff P, Luebert F, Hilger HH, Guisan A. Effects of alternative sets of climatic predictors on species distribution models and associated estimates of extinction risk: A test with plants in an arid environment. Ecol. Modell. 2014;288:166–177. doi: 10.1016/j.ecolmodel.2014.06.003. [DOI] [Google Scholar]
- 11.Botto-Mahan C, Ortiz S, Rozas M, Cattan PE, Solari A. DNA evidence of Trypanosoma cruzi in the Chilean wild vector Mepraia spinolai (Hemiptera: Reduviidae) Mem. Inst. Oswaldo Cruz. 2005;100:237–239. doi: 10.1590/S0074-02762005000300003. [DOI] [PubMed] [Google Scholar]
- 12.Ihle-Soto C, et al. Spatio-temporal characterization of Trypanosoma cruzi infection and discrete typing units infecting hosts and vectors from non-domestic foci of Central Chile. PLoS Negl. Trop. Dis. 2019;13:e7170. doi: 10.1371/journal.pntd.0007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cattan PE, Pinochet A, Botto-Mahan C, Acuña M, Canals M. Abundance of Mepraia spinolai in a periurban zone of Chile. Mem. Inst. Oswaldo Cruz. 2002;97:285–287. doi: 10.1590/S0074-02762002000300001. [DOI] [PubMed] [Google Scholar]
- 14.Botto-Mahan C, Cattan PE, Canals M, Acuña M. Seasonal variation in the home range and host availability of the blood-sucking insect Mepraia spinolai in wild environment. Acta Trop. 2005;95:160–163. doi: 10.1016/j.actatropica.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Bacigalupo A, et al. Primer hallazgo de vectores de la enfermedad de Chagas asociados a matorrales silvestres en la Región Metropolitana, Chile. Rev. Med. Chil. 2006;134:1230–1236. doi: 10.4067/S0034-98872006001000003. [DOI] [PubMed] [Google Scholar]
- 16.Correa JP, et al. Spatial distribution of an infectious disease in a small mammal community. Sci. Nat. 2015;102:51. doi: 10.1007/s00114-015-1304-5. [DOI] [PubMed] [Google Scholar]
- 17.Sagua H, Araya J, González J, Neira I. Mepraia spinolai in the Southeastern Pacific Ocean Coast (Chile)-First insular record and feeding pattern on the Pan de Azucar Island. Mem. Inst. Oswaldo Cruz. 2000;95:167–170. doi: 10.1590/S0074-02762000000200006. [DOI] [PubMed] [Google Scholar]
- 18.Canals M, Cruzat L, Molina MC, Ferreira A, Cattan PE. Blood host sources of Mepraia spinolai (Heteroptera: Reduviidae), wild vector of Chagas disease in Chile. J. Med. Entomol. 2001;38:303–307. doi: 10.1603/0022-2585-38.2.303. [DOI] [PubMed] [Google Scholar]
- 19.Botto-Mahan C, et al. Temporal variation of Trypanosoma cruzi infection in native mammals in Chile. Vector Borne Zoonotic. Dis. 2010;10:317–319. doi: 10.1089/vbz.2009.0006. [DOI] [PubMed] [Google Scholar]
- 20.Botto-Mahan C, Acuña-Retamar M, Campos R, Cattan PE, Solari A. European rabbits (Oryctolagus cuniculus) are naturally infected with different Trypanosoma cruzi genotypes. Am. J. Trop. Med. Hyg. 2009;80:944–946. doi: 10.4269/ajtmh.2009.80.944. [DOI] [PubMed] [Google Scholar]
- 21.Ehrenfeld MJ, Canals M, Cattan PE. Population parameters of Triatoma spinolai (Heteroptera: Reduviidae) under different environmental conditions and densities. J. Med. Entomol. 1998;35:740–744. doi: 10.1093/jmedent/35.5.740. [DOI] [PubMed] [Google Scholar]
- 22.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect. Dis. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fresquet N, Lazzari CR. Response to heat in Rhodnius prolixus: the role of the thermal background. J. Insect Physiol. 2011;57:1446–1449. doi: 10.1016/j.jinsphys.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Lehane, M.J. The Biology of Blood-Sucking inInsects (Cambridge University Press, 2005).
- 25.Lafferty KD. Calling for an ecological approach to studying climate change and infectious diseases. Ecology. 2009;90:932–933. doi: 10.1890/08-1767.1. [DOI] [PubMed] [Google Scholar]
- 26.QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project, https://qgis.org (2018).
- 27.Wincker P, et al. Use of a simplified polymerase chain reaction procedure to detect Trypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. Am. J. Trop. Med. Hyg. 1994;51:771–777. doi: 10.4269/ajtmh.1994.51.771. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JB, Omland KS. Model selection in ecology and evolution. Trends Ecol. Evol. 2004;19:101–108. doi: 10.1016/j.tree.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 29.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. URL, https://www.R-project.org/ (2018).
- 30.Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A. & Smith, G. M. Mixed Effects Models and Extensions in Ecology with R (Spring Science and Business Media, 2009).
- 31.Acuña-Retamar M, Botto-Mahan C, Canals M, Correa JP, Cattan PE. Comparative population dynamics of the bug Mepraia spinolai, a sylvatic vector of Chagas’ disease, in different hosts. Med. Vet. Entomol. 2009;23:106–110. doi: 10.1111/j.1365-2915.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- 32.Hill WA, Brown JP. Zoonoses of rabbits and rodents. Vet. Clin. North Am. Exot. Anim. Pract. 2011;14:519–531. doi: 10.1016/j.cvex.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Abad-Franch F, Palomeque FS, Aguilar H, Miles MA. Field ecology of sylvatic Rhodnius populations (Heteroptera, Triatominae): risk factors for palm tree infestation in western Ecuador. Trop. Med. Int. Health. 2005;10:1258–1266. doi: 10.1111/j.1365-3156.2005.01511.x. [DOI] [PubMed] [Google Scholar]
- 34.Lindström A, Jaenson TG. Distribution of the common tick, Ixodes ricinus (Acari: Ixodidae), in different vegetation types in southern Sweden. J. Med. Entomol. 2003;40:375–378. doi: 10.1603/0022-2585-40.4.375. [DOI] [PubMed] [Google Scholar]
- 35.Chacón F, et al. Feeding profile of Mepraia spinolai, a sylvatic vector of Chagas disease in Chile. Acta Trop. 2016;162:171–173. doi: 10.1016/j.actatropica.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 36.Bardosh KL, Ryan S, Ebi K, Welburn S, Singer B. Addressing vulnerability, building resilience: community-based adaptation to vector-borne diseases in the context of global change. Infect. Dis. Poverty. 2017;6:166. doi: 10.1186/s40249-017-0375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gozlan, R. E. & Marine, C. Environmental change and pathogen transmission in Ecology and Evolution of Infectious Diseases: Pathogen Control and Public Health Management in Low-Income Countries (eds. Roche, B., Broutin, H. & Simard, F.) 59–76 (Oxford University Press, Oxford, 2018).
- 38.Dobson AD, Auld SK. Epidemiological implications of host biodiversity and vector biology: key insights from simple models. Am. Nat. 2016;187:405–422. doi: 10.1086/685445. [DOI] [PubMed] [Google Scholar]
- 39.Vidal, M.A. & Labra, A. Dieta de anfibios y reptiles in Herpetología de Chile (eds. Vidal, M. A. & Labra, A.) 453–482 (Science Verlag Ediciones, 2008).
- 40.Ramírez PA, González A, Botto-Mahan C. Masking behavior by Mepraia spinolai (Hemiptera: Reduviidae): Anti-predator defense and life history trade-offs. J. Insect. Behav. 2013;26:592–602. doi: 10.1007/s10905-012-9371-3. [DOI] [Google Scholar]
- 41.Ryckman R. Lizards: a laboratory host for Triatominae and Trypanosoma cruzi Chagas (Hemiptera: Reduviidae) (Protomonadida: Trypanosomidae) Trans. Am. Microsc. Soc. 1954;73:215–218. doi: 10.2307/3223760. [DOI] [Google Scholar]
- 42.Asin S, Catala S. Development of Trypanosoma cruzi in Triatoma infestans: influence of temperature and blood consumption. J. Parasitol. 1995;81:1–7. doi: 10.2307/3283997. [DOI] [PubMed] [Google Scholar]
- 43.Ferreira RC, Teixeira CF, de Sousa VFA, Guarneri AA. Effect of temperature and vector nutrition on the development and multiplication of Trypanosoma rangeli in Rhodnius prolixus. Parasitol. Res. 2018;117:1737–1744. doi: 10.1007/s00436-018-5854-2. [DOI] [PubMed] [Google Scholar]
- 44.Schaub GA. Direct transmission of Trypanosoma cruzi between vectors of Chagas’ disease. Acta Trop. 1988;45:11–19. [PubMed] [Google Scholar]
- 45.Falvo ML, Lorenzo Figueiras AN, Manrique G. Spatio-temporal analysis of the role of faecal depositions in aggregation behaviour of the triatomine Rhodnius prolixus. Physiol. Entomol. 2016;41:24–30. doi: 10.1111/phen.12120. [DOI] [Google Scholar]
- 46.Roche, B., Broutin, H. & Simard, F. Ecology and Evolution of Infectious Diseases: Pathogen Control and Public Health Management in Low-Income Countries (Oxford University Press, 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.