Introduction
Real-time image-guided surgery is spreading in different surgical fields for its potential to improve surgical outcome and numerous types of imaging devices are available today [1–5]. The purpose of this study is to evaluate the role of intraoperative ultrasound biomicroscopy (iUBM) and optical coherence tomography (iOCT) for monitoring morphological changes of anterior chamber (AC) structures during ab externo canaloplasty for treating open angle glaucoma (OAG).
Material and methods
Consecutive patients affected by OAG refractory to topical therapy were enrolled and underwent ab externo canaloplasty (iTrackTM, Ellex iScience, Inc., Fremont, CA, USA) between January and June 2017. The study was approved by hospital ethics committee and informed consent from all patients was obtained. Surgery was performed by one surgeon (R.F.). Demographic and preoperative parameters were as follows: age, gender, eye, glaucoma type, intraocular pressure (IOP), best-corrected visual acuity (BCVA), lens status, and axial length. IOP and BCVA were monitored at 1, 3 and 6 postoperative months. iOCT integrated on to a microscope (OPMI Lumera 700, RESCAN 700 spectral domain-OCT wavelength 840 nm, Carl Zeiss Meditec, Jena, Germany) and iUBM (iUltrasound iscience interventionalTM, Menlo Park CA, 80 megaHertz transducer frequency) were used for evaluating the morphological changes of AC structures and for measuring the diameters of Schlemm canal (SC), trabecular meshwork (TM) thickness and AC angle during the surgery.
Results
Twenty eyes (20 patients) were recruited (Table 1). IOP decreased significantly during follow-up (from 27.3 ± 3.9 to 14.5 ± 2.5 mmHg). BCVA improved only in patients who underwent cataract surgery (6 out of 20).
Table 1.
Demographic and clincal preoperative data of patients
| No. | Age (years) | Gender male (m)/female (f) | IOP, mmHg | BCVA logMAR | lens status phakic (p)/pseudophakic (pp) | AL, mm | Glaucoma type |
|---|---|---|---|---|---|---|---|
| 1 | 67 | f | 34 | 0.6 | P | 24.7 | POAG |
| 2 | 72 | m | 36 | 0.5 | P | 23.5 | POAG |
| 3 | 68 | m | 26 | 0.8 | P | 22.8 | POAG |
| 4 | 50 | f | 22 | 0 | P | 25.8 | POAG |
| 5 | 75 | m | 31 | 0.1 | PP | 24.9 | POAG |
| 6 | 61 | m | 29 | 0.1 | P | 24 | POAG |
| 7 | 49 | f | 29 | 0 | P | 23.7 | POAG |
| 8 | 77 | m | 27 | 0 | PP | 22.9 | POAG |
| 9 | 69 | f | 30 | 0.6 | P | 23.4 | POAG |
| 10 | 45 | m | 24 | 0 | P | 24.2 | POAG |
| 11 | 71 | f | 26 | 0 | PP | 25.4 | POAG |
| 12 | 72 | m | 28 | 0.4 | P | 23.8 | POAG |
| 13 | 69 | f | 26 | 0.2 | PP | 22.5 | POAG |
| 14 | 49 | f | 24 | 0 | P | 22.9 | POAG |
| 15 | 42 | m | 31 | 0 | P | 24.2 | POAG |
| 16 | 51 | f | 28 | 0 | P | 25.1 | PEX |
| 17 | 47 | m | 26 | 0 | P | 25.7 | PEX |
| 18 | 58 | f | 24 | 0 | P | 22.8 | PG |
| 19 | 46 | m | 23 | 0 | P | 23.1 | PG |
| 20 | 33 | f | 21 | 0 | P | 24.6 | UG |
| Total mean ± (range) *% ** | 58.5 ± 12.96 (33–77) | 50%/50% | 27.3 ± 3.9 (21–36) | 0.14 ± 0.2 (0.8–0) | 80%/20% | 24 ± 1.02 (22.5–25.8) | 75%/10%/10%/5% |
AL axial lenght, BCVA best-corrected visual acuity, IOP intraoucular pressure, PEX pseudoespholiative glaucoma, PG pigmentary, POAG primary open angle glaucomaglaucoma, UG uveitic glaucoma
*Mean ± (range) for quantitative parameters
*Percentage for qualitative parameters
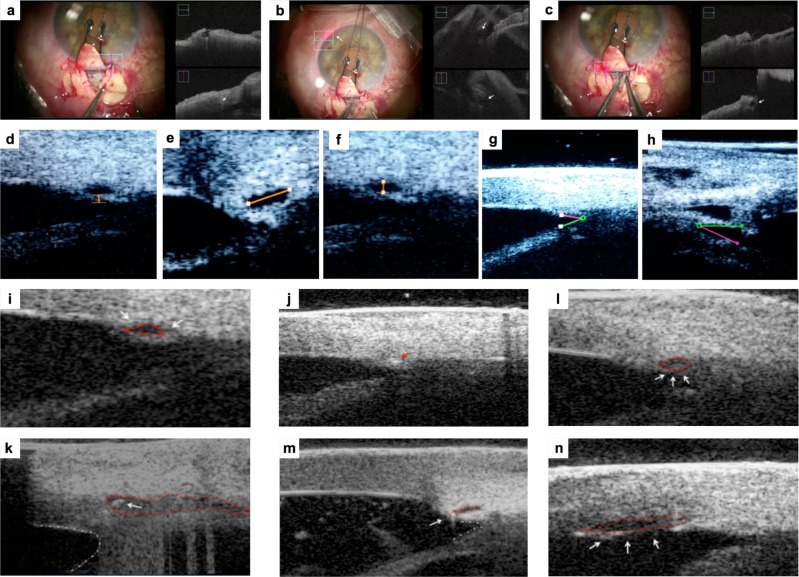
iOCT allowed the surgeon to monitor SC and AC angle changes during microcatheter passage and viscodilation. During viscodilation SC changed its profile from oval to round-shape with hyporeflective content (Fig. 1a–c). The poor quality of iOCT imaging did not allow the observer to obtain accurate measurements.
Fig. 1.
Picture a Microscope image (left picture) shows trabeculo-descemetic window after dissection of the deep scleral flap. iOCT image (right picture) shows SC before dilating the ostium of SC using the iTrack microcatheter (white arrow). Picture b Microscope image (left picture) shows the progress of microcatheter into SC. Red light indicates the position of the microcatheter tip in the SC. iOCT image (right picture) shows the microcatheter in the SC (white arrows) Picture c Microscope imaging shows the final step of retraction of the microcatheter with simultaneous injection of Healon GV into the SC. iOCT image (right picture) shows the enlarged SC (white arrow) Picture d Intraoperative UBM image: TM thickness measurement after SC viscodilation. Picture e, f Intraoperative UBM image: horizontal and vertical diameter of SC after viscodilation. Picture g, h Intraoperative UBM image: AC angle before (g) and after surgery (h) Picture i Preoperative iUBM shows SC that appears as an anechogenic oval-shaped space (red dashed line), and TM (white arrow) Picture j iUBM shows the microcatheter in the SC that appears hyperechogenic in comparison to the surrouding tissue (red arrow) Picture l: iUBM shows enlarged SC after viscodilation (white arrows, red dashed line) Picture k: iUBM shows SC with suture before tensioning suture (white arrow), deep scleral dissection and filtering window (red dashed line). AC angle appears with a concave and rounded profile (white dashed line) Picture m iUBM shows SC and suture after tensioning suture (white arrow). SC appears elongated (red dashed line) and AC angle becomes acute (white dashed line) Picture n iUBM shows Descemet’s membrane detachment after viscodilation (white arrows and red dashed line)
TM thickness, SC diameters and AC angle were measured by iUBM. TM thickness mean was 92 ± 20.6 µm. Horizontal and vertical diameters mean of SC were, respectively, 410 ± 56 and 110 ± 23 µm during viscodilation. AC angle mean was reduced from 25.8° ± 11° to 24.5° ± 14° after tensioning suture and its profile changed from concave to acute. SC appeared as an anechogenic oval-shaped space. Microcatheter into SC appeared hyperechogenic. In one case endothelial detachment was detected during viscodilation (Fig. 1d–n).
Discussion
iOCT allowed the surgeon to monitor morphological changes of AC structures during surgery but scanning acquisition was not immediate requiring continual adjustment of the focus and scanning point due to the eye movements during surgery that changed the depth of the plan of visualization. The limit of iOCT was the short penetration depth, responsible for shadowing of scleral tissue and of overlapping and inverted images. Anyhow, iOCT was the best option to obtain a high resolution for imaging superficial areas. Instead iUBM was useful in detecting deeper structures, giving sharper images to measure morphological changes of AC structures. However, the image acquisition required interrupting surgery and the contact of the probe with the eye.
Intraoperative real-time image-guided surgery represents the future even if it still requires further efforts to make the instrumentation functional and simple to use.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li X, Wei L, Dong X, Huang P, Zhang C, He Y, et al. Microscope-integrated optical coherence tomography for image-aided positioning of glaucoma surgery. J Biomed. 2015;20:76001. doi: 10.1117/1.JBO.20.7.076001. [DOI] [PubMed] [Google Scholar]
- 2.Kumar RS, Jariwala MU, V SA, Venugopal JP, Puttaiah NK, Balu R, et al. A pilot study on feasibility and effectiveness of intraoperative spectral-domain optical coherence tomography in glaucoma procedures. Transl Vis Sci Technol. 2015;4:1–9. doi: 10.1167/tvst.4.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan X, Li M, Chen Z, Zhu Y, Song Y, Zhang H. Schlemm’s canal and trabecular meshwork in eyes with primary open angle glaucoma: a comparative study using high-frequency ultrasound biomicroscopy. PLoS One. 2016;4:11. doi: 10.1371/journal.pone.0145824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siebelmann S, Cursiefen C, Lappas A, Dietlein T. Intraoperative optical coherence tomography enables noncontact imaging during canaloplasty. J Glaucoma. 2016;25:236–8. doi: 10.1097/IJG.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 5.Xj Zhu, Zhang KK, He WW, Sun XH, Meng FR, Lu Y. Diagnosis of pupillary block glaucoma after removal of congenital cataract cataracts with intraoperative ultrasound biomicroscopy: a case report. BMC Ophthalol. 2016;16:58. doi: 10.1186/s12886-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]