Abstract
Purpose
To describe the foveal characteristics of children with a history of retinopathy of prematurity (ROP) using optical coherence tomography angiography (OCTA).
Methods
All eyes were examined by OCTA (RTVue AVANTI, Optovue Inc, Fremont, CA) with a scan of 3 × 3 mm cantered on the fovea. The size of the foveal avascular zone (FAZ), central retinal thickness (CRT), and foveal bulge were measured.
Results
Forty-eight eyes of 26 children with a history of ROP and a mean age of 8.8 years with a range of 4–16 years (ROP group) were studied. Sixty-six eyes of 36 children without any fundus abnormalities and with an average age of 10.5 years and a range of 3–17 years (control group) were studied as controls. The mean FAZ area in the ROP group was 0.18 mm2 which was significantly smaller than the 0.32 mm2 in the control group (p < 0.01). The mean CRT was significantly thicker in the ROP group (228 µm) compared to the control group (189 µm; p < 0.01). The size of FAZ was not measurable in 5 eyes (10.4%) of 3 children in the ROP group. The correlation between the FAZ area and CRT was significant in both the ROP and control groups (r = −0.53 in ROP; r = −0.57 in control; both p < 0.01). There was no significant difference in the height of the foveal bulge between two groups (p = 0.64).
Conclusions
The FAZ is smaller in ex-preterm children with a history of ROP (including laser treatment for ROP) than in children who were not premature.
Subject terms: Retinal diseases, Epidemiology
Introduction
Retinopathy of prematurity (ROP) is a disorder of early childhood that is associated with reduced visual acuity and possible blindness in severe cases of ROP [1, 2]. ROP is a retinal disorder that is associated with a failure of blood vessels to develop in the peripheral fundus and is associated with an increased expression of vascular endothelial growth factor (VEGF). Recent optical coherence tomographic (OCT) studies have shown that the fovea was abnormal in eyes with a history of ROP [3, 4].
OCT angiography (OCTA) is a non-invasive technique for imaging the microvasculature of the retina and choroid [5, 6]. Cross-sectional OCT images can also be obtained at the same time by OCTA. OCTA is able to obtain high resolution en face images of the vascular pattern around the fovea which can then be used to quantify the dimensions of the foveal avascular zone (FAZ) with greater detail than fluorescein angiography [7, 8]. The clinical significance of the FAZ has not been definitively determined [9]. For example, the mechanism(s) causing the larger FAZ in diabetic patients even before the onset of diabetic retinopathy has not been determined [10, 11]. Although there have been some reports on the FAZ in children determined by OCTA, there have been few studies on the FAZ in children with ROP which is known to be associated with vascular abnormalities during the early postpartum period [12–14].
Thus, the purpose of this study was to determine the FAZ area and the foveal structures in children with a history of ROP using OCTA and to compare these to children of normal gestation period and normal birth weight.
Methods
This was a single-center, retrospective, observational, comparative study of patients examined in the ROP clinic of the Tokyo Women’s Medical University Hospital. Patients examined between 1 October 2016 and 30 September 2017 were studied. All cases had a best-corrected visual acuity (BCVA) of ≥20/20 and had good fixation. Cases with Zone I ROP that affected the foveal vasculature and fixation were excluded. Children with a history of ROP, the ROP group, were recruited from the ROP clinic, and full-term birth children with normal ocular examination were recruited from the Strabismus and Amblyopia Clinic of our hospital for the control group. All examinations were performed after informed consent was obtained from the parents.
All eyes were examined by OCTA (RTVue AVANTI, Optovue Inc, Fremont, CA) with a scan of 3 × 3 mm cantered on the fovea. Cross-sectional OCT images were also obtained with this device. We examined both eyes but excluded the eyes with poor image quality.
The FAZ area (mm2) was determined from the en face OCTA images and the central retinal thickness (CRT, µm) from the cross-sectional OCT images with the embedded software. If a foveal bulge, a lengthening of the outer segments of the photoreceptors, was detected at the central fovea, the images showing the foveal bulge were used for the measurements of CRT. The length of foveal bulge was also measured using ImageJ (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). The area of the FAZ was automatically calculated by the non-flow mode in each image of the auto-segmented superficial capillary plexus. (Supplementary Fig. 1A, B) We calculated the correlation between the FAZ area and the CRT. In the eyes with unmeasurable FAZ, the FAZ area was set to zero for the statistical analyses.
The density of the vessels (%) of the auto-segmented superficial capillary plexus was also measured by the vessel density mode automatically (Supplementary Fig. 1C). The measurements were made in the following areas; a 3 × 3 mm circle as the whole image, a 1 × 1 mm circle as the fovea, and a 3 × 3 mm annulus other than the fovea as the parafovea.
The procedures used in this retrospective study conformed to the tenets of the Declaration of Helsinki. The Institutional Review Board of the Tokyo Women’s Medical University approved this study.
Statistical analyses
All tests to determine the significance of the differences were two-tailed, and p-values < 0.05 were considered statistically significant. Chi-square tests were used for categorical analysis. The means were compared by Mann-Whitney U tests. Spearman’s tests were used to determine the significance of the correlations. Multiple regression analysis was also performed to evaluate the contribution of each initial factor. All statistical analyses were performed with EZR free software (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphic user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [15].
Results
One-hundred and fourteen eyes of sixty-two children (28 boys, 34 girls) with a mean age of 9.8 ± 3.1 years and a range of 3 to 17-years were studied. Forty-eight eyes of 26 children with a history of ROP (8 boys, 18 girls, mean age 8.8 ± 2.6 years, range 4–16 years) were in the ROP group, and 66 eyes of 36 children (20 boys, 16 girls; mean age 10.5 ± 3.2 years with a range 3 to 17 years) without any fundus abnormalities were in the control group. The mean gestational age was 27.8 [23.3 to 34.0] weeks and the mean birth weight was 954 (523 to 2035) grams in the ROP group. The gestational age in the control group was ≥37 weeks that was obtained from the mothers’ medical records, however there was no birth weight recorded in the control group. The children in the ROP group were significantly younger than in control group (p = 0.02). There were fewer boys in the ROP group than in the control group, but the difference was not significant (p = 0.07). All inborn preterm infants in the ROP group underwent ROP examinations at the neonatal intensive care unit of the Tokyo Women’s Medical University and were diagnosed according to the International Classification of Retinopathy of Prematurity. Thirty-eight eyes (81.3%) of 21 children in the ROP group had a history of laser therapy for severe stage 3 ROP (35 eyes in Zone II, 13 eyes in Zone III), however there was no child with stage 4 or 5 of ROP. There were 3 children with a history of unilateral laser therapy and only 5 children without laser therapy in both eyes. The 5 children without any laser therapy had relatively longer gestational period and were heavier at birth than the other cases (n = 21) with laser therapy for both eyes. These differences were statistically significant (gestational age 31.3 vs 27.0 weeks, p = 0.02; and birth weight 1411 vs 845 grams, p = 0.03). Representative cases are shown in Fig. 1.
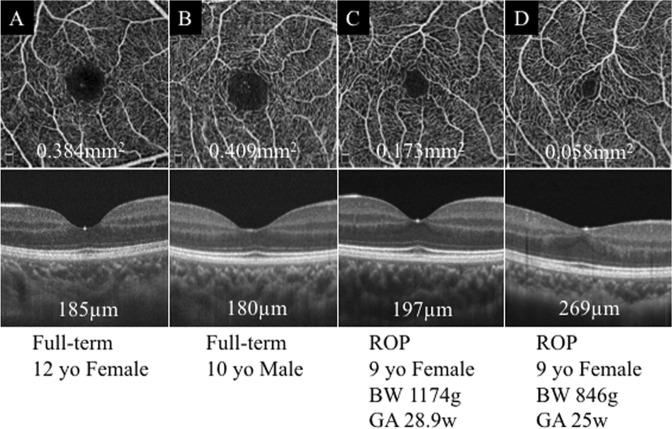
Fig. 1.
Foveal avascular zone (FAZ) and central retinal thickness (CRT) in representative optical coherence tomography angiographic (OCTA) images. FAZ is smaller and CRT is thicker in the eyes with a history of retinopathy of prematurity than in the eye of a full-term child. a and b Eyes of a full-term child. c and d Eyes with a history of retinopathy of prematurity. BW birth weight, GA gestational age
The mean area of the FAZ was 0.18 ± 0.15 mm2 in the ROP group which was significantly smaller than the 0.32 ± 0.09 mm2 in the control group (p < 0.01). The FAZ area was too small to measure in 5 eyes (10.4%) of 3 children in the ROP group (Fig. 2), and the FAZ was measurable in all of the control eyes. The mean FAZ area in the eyes with a history of laser therapy was 0.182 ± 0.15 mm2 which was not significantly different from the ROP eyes without prior laser therapy (0.174 ± 0.12 mm2, p = 0.79).
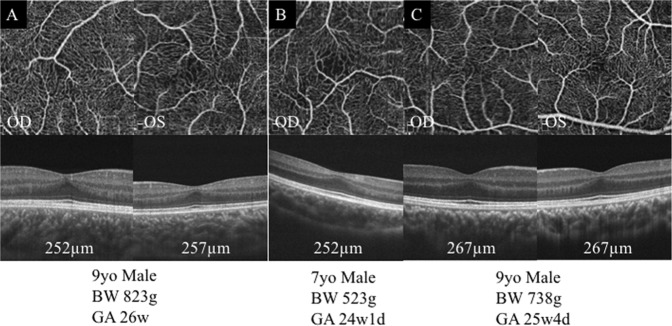
Fig. 2.
OCTA images of eyes with unmeasurable size of foveal avascular zone in the retinopathy of prematurity group. All eyes have a residual inner retinal layer in the foveal region. a Both eyes of a 9-year-old boy. Gestational age was 26 weeks and birth weight was 823 g. b Right eye of a 7-year-old boy. Gestational age was 24 weeks and birth weight was 523 grams. c Both eyes of a 9-year-old boy. Gestational age was 25 weeks and birth weight was 738 g. BW birth weight, GA gestational age
The foveal bulge was present in the cross-sectional OCT images in all eyes. The mean length of the foveal bulge was 56.0 ± 12.0 µm in the ROP group which was not significantly longer than the 54.8 ± 11.9 µm in the control group (p = 0.64). The mean CRT was 228 ± 30 µm in the ROP group which was significantly thicker than the 189 ± 13 µm in the control group (p < 0.01; Fig. 3). The mean CRT in the eyes with a history of laser therapy was 237 ± 28 mm2 which was significantly thicker than that in the ROP eyes without prior laser therapy (206 ± 25 mm2, p < 0.01).
Fig. 3.
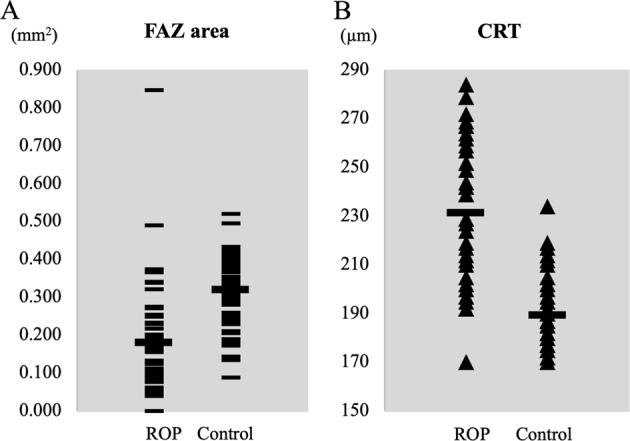
Comparisons of the area of the foveal avascular zone (FAZ) and the central retinal thickness (CRT) between eyes of the retinopathy of prematurity (ROP) group and control group. a The mean FAZ area is 0.18 ± 0.15 mm2 in the ROP group and 0.32 ± 0.09 mm2 in the control group (P < 0.01). b The mean CRT is 228 ± 30 µm in the ROP group which is significantly thicker than the 189 ± 13 µm in the control group (P < 0.01). Long horizontal bars indicate the mean values in each group
There was a residual inner retinal layer in the cross-sectional images through the fovea in 20 eyes (76.9%) of 12 children with a history of ROP including the eyes in which the FAZ size could not be measured. There was no residual inner retinal layer in any of the control eyes.
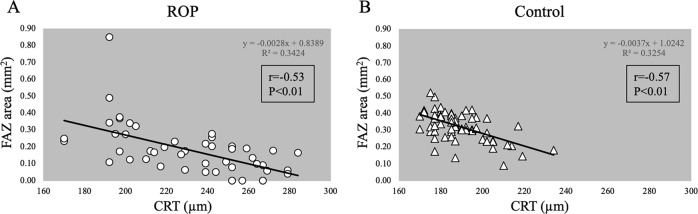
There were significantly correlations between the FAZ area and the CRT in both the ROP and control groups (r = −0.53 in ROP; r = −0.57 in control; both p < 0.01; Fig. 4).
Fig. 4.
The size of the foveal avascular zone (FAZ) is significantly correlated with the central retinal thickness (CRT) in both the retinopathy of prematurity group (ROP) and the control group (r = −0.53 in ROP, r = −0.57 in control, both p < 0.01)
The FAZ area in the ROP group was significantly correlated with the gestational age (r = 0.54, p < 0.01), however it was not significantly correlated with the birth weight (r = 0.14, p = 0.36; Fig. 5a, b). On the other hand, the CRT in the ROP group was significantly correlated with both the gestational age (r = −0.67, p < 0.01) and birth weight (r = −0.59, p < 0.01; Fig. 5c, d). Multiple regression analysis showed the correlation of the FAZ area to a history of laser therapy was not significant (p = 0.23) but not significantly correlated with the gestational age, the birth weight, and the CRT (all p < 0.01).
Fig. 5.
Correlations between area of FAZ and central retinal thickness and gestational age and birth weight. a and b Foveal avascular zone (FAZ) area of an eye in the retinopathy of prematurity (ROP) group is significantly correlated with the gestational age (r = 0.50, p < 0.01). However, it is not significantly correlated with the birth weight (r = 0.07, p = 0.65). c and d Central retinal thickness (CRT) in ROP group is significantly correlated with both the gestational age (r = −0.67, p < 0.01) and birth weight (r = −0.59, p < 0.01). GA gestational age, BW birth weight
The vessel density of the superficial capillary plexus of the whole image in the ROP group was significantly lower than in the control group (52.6% vs 53.9%, p < 0.01; Supplementary Fig. 2A). Five eyes in the ROP group and eight eyes in the control group were excluded from the measurements of the vessel density due to poor image quality of the retinal capillaries. Although the vessel density at the fovea in the ROP group was significantly higher than in the control group (35.7 vs 30.1%, p < 0.01; Supplementary Fig. 2B), the vessel density at the parafovea was significantly lower in the ROP group than in the control group (54.1 vs 56.4%, p < 0.01; Supplementary Fig. 2C). The vessel density in the ROP group was not significantly correlated with the gestational age (r = 0.15, p = 0.35; Supplementary Fig. 3A), however it was significantly correlated with the birth weight (r = 0.47, p < 0.01; Supplementary Fig. 3B).
Discussion
Our results showed that the area of the FAZ was significantly smaller and the CRT was significantly thicker in the OCTA images of eyes with a history of ROP than in eyes of full-term children. In addition, there were 5 eyes (10.4%) of 3 children in the ROP group whose FAZ was not measurable. Interestingly, the FAZ area in the ROP group was not significantly correlated with the birth weight but was with the gestational age. This suggests that the gestational age was more important for the normal development of the foveal vasculature. Moreover, there was no difference in foveal bulge between two groups. This suggests that the development of the outer retinal layer is not associated with a history of prematurity. However, the majority of the cases in the current study had had laser therapy for the ROP, and we cannot state that our results were not affected by the ROP alone.
The development of the fovea begins with all retinal layers being present. Then, the cells of the inner retinal layers including the retinal ganglion cells migrate away from the fovea and undergo apoptosis at around 25 weeks of gestation as the second step. The foveal depression finally forms with an absence of the inner retinal layers [16, 17]. During these processes, the retinal vessels do not invade the foveal region which remains avascular. There have been several OCT studies that reported abnormalities in the development of the fovea in preterm birth infants and ROP eyes [3, 4]. Villegas et al. [4] reported that patients with a history of ROP had a higher incidence of macular morphological abnormalities including retention of the inner retinal layers and an absence of a foveal depression in the spectral domain OCT images. Our results also showed a residual inner retinal layer in 76.9% of the eyes. A small or absent FAZ has also been found in fluorescein angiograms [18–21]. Mintz-Hittner et al. [18] measured the area of the FAZ in the eyes with a history of ROP using digital fluorescein angiography. Their mean FAZ area was 0.044 mm2 in preterm infants (average gestational age 27.8 weeks and birth weight 993.5 g), which is much smaller than the FAZ area in our cohort. Interestingly, they had excluded eyes with severe ROP to avoid the influences of treatment such as laser therapy. In contrast, Lepore et al. [19, 20] reported the macular abnormalities including absence of FAZ in the fluorescein angiograms improved 9 months after the laser treatment (64.6%) compared to intravitreal injection of bevacizumab (25%). They also reported that there were fewer macular abnormalities even at 4-years-of-age in the same laser treatment groups [20]. These reports suggest that a local suppression of the VEGF level by the laser treatment had a better effect on the foveal development than complete suppression of VEGF by a bevacizumab injection.
An absence of a FAZ and a foveal depression are often observed in the eyes with foveal hypoplasia [22, 23]. Some of these eyes with low grade foveal hypoplasia and without a FAZ have been reported to have good visual acuity [24, 25]. We have also reported an unmeasurable small size of FAZ without visual impairment in 1.5% of the 267 normal adult eyes [26]. We also evaluated the foveal bulge which might suggest that the integrity of the retinal outer layer including the cone density in the eyes with a history of ROP. The foveal bulge was seen in all ROP eyes, and the mean length of the outer segments in the foveal bulge was not significantly different from that in the eyes of control group. These results suggest that the development of the retinal outer layer was not associated with the inner retinal layer even in those with a history of ROP. Cone packing at the fovea is thought to gradually form after birth [22]. Thus, a history of ROP might have little influence on retinal outer layer development unless there were genetic factors. However, further studies are needed in the eyes with poor vision and/or the history of the severe ROP because the current study only included eyes with good vision and fixation for the OCTA examinations.
Earlier studies have reported on the area of the FAZ in the eyes with a history of ROP using OCTA [12–14]. For example, Falavarjani et al. [13] reported that the mean FAZ area was 0.06 mm2 in 28 eyes of preterm infants which was much smaller than our findings. They also reported that an absence of FAZ was observed in 12 eyes (42.8%) which is much higher than that in our cohort. Their cohort consisted of patients whose mean gestational age was 27.1 weeks and a mean birth weight was 990 grams. In addition, 10 eyes (35.7%) had been treated with laser for ROP. In their study, the FAZ area in the eyes with a history of laser therapy was significantly smaller than the eyes without laser therapy. However, the difference might not be due to the laser therapy itself because of the significant lower birth weight in the patients with laser therapy. The mean gestational age and birth weight were comparable to that of our cohort, and the differences in the mean FAZ area and the rates of absence FAZ might be due to other more complex factors, e.g., there was no difference of FAZ area between the eyes with and without a history of laser therapy in the current study. Although this is a small number, it is also supported from the results of multiple regression analysis. The higher proportion of previous laser treatments of similar gestational ages and birth weights might lead to the relative larger FAZ areas. Nonobe et al. [14] reported that the FAZ area was 0.103 mm2 in ten eyes with a history of laser or cryopexy for stage 3 ROP. Although it is not possible to simply compare their results with that of others because of the small number of cases, their FAZ area was relatively smaller than our FAZ area but larger than the FAZ area of Falavarjani et al. [13] Their results did not provide evidence for adverse effects of laser on FAZ formation.
Nonobe et al. [14] also reported that there was no significant difference between the median vessel densities at the fovea (1 mm circle) in the eyes with ROP and the normal control group, and the vessel density at the parafovea (1–3 mm annulus) in the ROP group was significantly lower than that in the normal control group. Their results were comparable to our vessel density data. In our study, the vessel density at the fovea in the ROP group was significantly higher than in the control group. However, this might be due to the vascular invasion into the foveal area in the eyes with a history of ROP, and it might not indicate the maturity of foveal capillaries.
On the other hand, the mean CRT in the eyes with a history of laser therapy was significantly thicker than in the ROP eyes without prior laser therapy despite there being no difference in the FAZ area. Falavarjani et al. [13] also reported on the CRT and their results showed that the eyes with a history of laser therapy for ROP had thicker CRT than without laser therapy. These results might also be associated with not only the laser therapy but also the gestational ages and birth weights.
There are several limitations in this study including the low numbers of cases, the older age of the control group than the ROP group, and the absence of eyes with poorer vision because of the difficulty in maintaining fixation for the OCTA examinations. In addition, there were fewer boys in the ROP group than in the control group although the difference was not significant. This difference might suggest some bias in our selection of our cohort because severe ROP had been associated more frequently with boys in earlier studies [27, 28]. Because this was a retrospective study, we did not have enough cases without laser therapy, and we could not include the preterm and premature cases without develop ROP. In addition, we used the data of both eyes of an individual which might have affected the results. Also, the birth weight of the full-term children in the control group was not recorded. Thus, further studies of a larger number of ROP cases that includes some with poor visual acuity are needed. In addition, more data are needed to determine whether the laser treatment affected the development of the FAZ and/or vessel density around the fovea.
In conclusion, the area of the FAZ and vessel density around the fovea might be associated with the abnormal formation of the fovea excluding the foveal bulge during development in eyes with a history of ROP. OCTA is useful method to evaluate the foveal retinal vasculature and structure noninvasively even in children.
Summary
What was known before
A smaller foveal avascular zone was observed in eyes with a history of retinopathy of prematurity than in normal eyes using optical coherence tomography angiography.
What this study adds
In eyes with a history of retinopathy of prematurity the foveal avascular zone was smaller than in normal control group and could not be measured in 5 eyes (10.4%) because the it was too small or indistinguishable. There was no difference in the size of the foveal bulge due to cone packing between retinopathy of prematurity and normal control groups.
Supplementary information
SF1 - Method of measuring foveal avascular zone (FAZ) and vessel density
SF2 - Differences in vessel density between the retinopathy of prematurity (ROP) group and control group
SF3 - Correlation between the vessel density of the superficial capillary plexus in the whole image and gestational age and birth weight in the eyes of the retinopathy of prematurity group
Acknowledgements
The authors thank Professor Emeritus Duco Hamasaki of the Bascom Palmer Eye Institute, University of Miami, for his critical discussion and editing of the final manuscript. The authors thank the orthoptists at the Tokyo Women’s Medical University Hospital, Wakana Sagara, Tomoko Shikama, and Yukari Sakurai for data collection. The authors also thank Dr. Noriyuki Azuma of Department of Ophthalmology and Laboratory for Visual Science, National Center for Child Health and Development, for his important advice and suggestions.
Author contributions
Designing and conducting of study: MT, IM, AY. Data collection: MT, IM, AY, MK. Analysis and interpretation of data: MT, IM, AY, MK. Manuscript preparation and review: MT, IM, AY, MK, TH, TI. Approval of the manuscript: MT, IM, AY, MK, TH, TI.
Compliance with ethical standards
Conflict of interest
MT has nothing to disclose. IM reports grants from JSPS KAKENHI (Grant Number JP16K11274), grants and personal fees from Novartis Pharma K.K., personal fees from Bayer Yakuhin, Ltd., personal fees from Santen Pharmaceutical Inc., personal fees from Alcon Japan, Ltd., personal fees from Topcon Co., Ltd., personal fees from Senju Pharmaceutical Co., Ltd., personal fees from NIDEK Co., Ltd., outside the submitted work. Dr. Yamaguchi has nothing to disclose. MK has nothing to disclose. TH has nothing to disclose. TI reports grants and personal fees from Novartis Pharma K.K. (Japan), personal fees from Bayer Yakuhin, Ltd. (Japan), grants and personal fees from Santen Pharmaceutical Co., Ltd. (Japan), grants from Nidek, grants from Kowa, grants and personal fees from Canon, grants and personal fees from Topcon, grants and personal fees from Senju Seiyaku, grants from AMO, grants and personal fees from JFC, outside the submitted work. The remaining author declares that she has no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41433-019-0500-5) contains supplementary material, which is available to authorized users.
References
- 1.Smith BT, Tasman WS. Retinopathy of prematurity: late complications in the baby boomer generation. Trans Am Ophthalmol Soc. 2005;1946>–1964:225–34. [PMC free article] [PubMed] [Google Scholar]
- 2.Salvin JH, Lehman SS, Jin J, Hendricks DH. Update on retinopathy of prematurity: treatment options and outcomes. Curr Opin Ophthalmol. 2010;21:329–34. doi: 10.1097/ICU.0b013e32833cd40b. [DOI] [PubMed] [Google Scholar]
- 3.Wu WC, Lin RI, Shih CP, Wang NK, Chen YP, Chao AN, et al. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology. 2012;119:1907–16. [DOI] [PubMed]
- 4.Villegas VM, Capó H, Cavuoto K, McKeown CA, Berrocal AM. Foveal structure function correlation in children with history of retinopathy of prematurity. Am J Ophthalmol. 2014;158:508–12. [DOI] [PubMed]
- 5.Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710–25. [DOI] [PMC free article] [PubMed]
- 6.Spaide RF, Klancnik JM, Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 7.Samara WA, Say EA, Khoo CT, Higgins TP, Magrath G, Ferenczy S, et al. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina. 2015;35:2188–95. doi: 10.1097/IAE.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 8.Carpineto P, Mastropasqua R, Marchini G, Toto L, Di Nicola M, Di Antonio L. Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol. 2016;100:671–6. doi: 10.1136/bjophthalmol-2015-307330. [DOI] [PubMed] [Google Scholar]
- 9.Chui TY, VanNasdale DA, Elsner AE, Burns SA. The association between the foveal avascular zone and retinal thickness. Invest Ophthalmol Vis Sci. 2014;55:6870–7. doi: 10.1167/iovs.14-15446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58:190–6. doi: 10.1167/iovs.16-20531. [DOI] [PubMed] [Google Scholar]
- 11.Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35:2377–83. doi: 10.1097/IAE.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 12.Gołębiewska J, Olechowski A, Wysocka-Mincewicz M, Odrobina D, Baszyńska-Wilk M, Groszek A, et al. Optical coherence tomography angiography vessel density in children with type 1 diabetes. PLoS ONE. 2017;12:e0186479. [DOI] [PMC free article] [PubMed]
- 13.Falavarjani KG, Iafe NA, Velez FG, Schwartz SD, Sadda SR, Sarraf D, et al. Optical coherence tomography angiography of the fovea in children born preterm. Retina. 2017;37:2289–94. doi: 10.1097/IAE.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 14.Nonobe N, Kaneko H, Ito Y, Takayama K, Kataoka K, Tsunekawa T, et al. Optical coherence tomography angiography of the foveal avascular zone in children with a history of treatment-requiring retinopathy of prematurity. Retina. 2019;39:111–7. [DOI] [PubMed]
- 15.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013;48:452–8. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91:603–12. doi: 10.1016/S0161-6420(84)34247-6. [DOI] [PubMed] [Google Scholar]
- 17.Provis JM. Development of the primate retinal vasculature. Prog Retin Eye Res. 2001;20:799–821. doi: 10.1016/S1350-9462(01)00012-X. [DOI] [PubMed] [Google Scholar]
- 18.Mintz-Hittner HA, Knight-Nanan DM, Satriano DR, Kretzer FL. A small foveal avascular zone may be an historic mark of prematurity. Ophthalmology. 1999;106:1409–13. doi: 10.1016/S0161-6420(99)00732-0. [DOI] [PubMed] [Google Scholar]
- 19.Lepore D, Quinn GE, Molle F, Baldascino A, Orazi L, Sammartino M, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology. 2014;121:2212–9. [DOI] [PubMed]
- 20.Lepore D, Quinn GE, Molle F, Orazi L, Baldascino A, Ji MH, et al. Follow-up to age 4 years of treatment of type 1 retinopathy of prematurity intravitreal bevacizumab injection versus laser: fluorescein angiographic findings. Ophthalmology. 2018;125:218–26. [DOI] [PubMed]
- 21.Vajzovic L, Hendrickson AE, O'Connell RV, Clark LA, Tran-Viet D, Possin D, et al. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol. 2012;154:779–89. [DOI] [PMC free article] [PubMed]
- 22.Thomas MG, Kumar A, Mohammad S, Proudlock FA, Engle EC, Andrews C, et al. Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography a predictor of visual acuity? Ophthalmology. 2011;118:1653–60. [DOI] [PMC free article] [PubMed]
- 23.Wolfson Y, Fletcher E, Strauss RW, Scholl HP. Evidence of macular pigment in the central macula in albinism. Exp Eye Res. 2016;145:468–71. doi: 10.1016/j.exer.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Pakzad-Vaezi K, Keane PA, Cardoso JN, Egan C, Tufail A. Optical coherence tomography angiography of foveal hypoplasia. Br J Ophthalmol. 2017;101:985–8. doi: 10.1136/bjophthalmol-2016-309200. [DOI] [PubMed] [Google Scholar]
- 25.Bazvand F, Karkhaneh R, Roohipoor R, Rajabi MB, Ebrahimiadib N, Davoudi S, et al. Optical coherence tomography angiography in foveal hypoplasia. Ophthalmic Surg Lasers Imaging Retin. 2016;47:1127–31. doi: 10.3928/23258160-20161130-06. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama T, Maruko I, Koizumi H, Ishikawa Y, Iida T. Unmeasurable small size of foveal avascular zone without visual impairment in optical coherence tomography angiography. Eye. 2018;32:1062–6. doi: 10.1038/s41433-017-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darlow BA, Hutchinson JL, Henderson-Smart DJ, Donoghue DA, Simpson JM, Evans NJ. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics 2005;115:990–6. [DOI] [PubMed]
- 28.Matsumoto T, Itokawa T, Shiba T, Tomita M, Hine K, Mizukaki N, et al. Intravitreal bevacizumab treatment reduces ocular blood flow in retinopathy of prematurity: a four-case report. Graefes Arch Clin Exp Ophthalmol. 2018;256:2241–7. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SF1 - Method of measuring foveal avascular zone (FAZ) and vessel density
SF2 - Differences in vessel density between the retinopathy of prematurity (ROP) group and control group
SF3 - Correlation between the vessel density of the superficial capillary plexus in the whole image and gestational age and birth weight in the eyes of the retinopathy of prematurity group