Abstract
Neurotrophin-3 (NT-3), a neurotrophic factor that mainly binds to the tyrosine kinase C (trkC) receptor, has been shown to play a crucial role in proliferation, differentiation, and survival. However, the role of NT-3 in the hypoxia-induced retinopathy has not been investigated extensively. Here, we created a model of hypoxia (1% O2) in vitro and found that hypoxia promoted the apoptosis of mouse cone photoreceptor-derived 661W cells, increased the expression of TrkC and cleaved caspase-3. In contrast, the hypoxia-mediated 661W cell apoptosis was markedly alleviated by co-culturing with primary mouse Müller cells. Further mechanism studies revealed that hypoxia increased the synthesis and secretion of NT-3 by Müller cells, and exogenous NT-3 stimulation increased the phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 by binding to TrkC in 661W cells. Besides, both siRNA knockdown of TrkC expression and incubation with an ERK-specific inhibitor PD98059 triggered apoptosis in hypoxic 661W cells. Altogether, these data suggest that NT-3 originating from Müller cells protects photoreceptors from hypoxia-induced apoptosis through a TrkC/ERK-dependent pathway. Our findings may facilitate future studies on the therapeutic implications of NT-3 in the treatment of hypoxia-relevant retinal diseases.
Keywords: Neurotrophin-3, Photoreceptor apoptosis, Tyrosine kinase C, Müller cells, Hypoxia
Introduction
Retinal hypoxia has been proved to associate with the pathology of several blinding diseases such as diabetic retinopathy (DR), retinal detachment (RD) and age-related macular degeneration (AMD) (Arden and Sivaprasad 2011; Li et al. 2012; Blasiak et al. 2014). Insufficient supplies of oxygen in the retina may impair the structure and function of photoreceptor cells, and result in photoreceptor degeneration (Grimm and Willmann 2012). Otherwise, hypoxia-mediated photoreceptor apoptosis was greatly attenuated with oxygen supplementation or hypoxia-inducible factor 1 (HIF-1) inactivation (Mervin et al. 1999; Barben et al. 2018), suggesting that investigation of the detailed mechanisms triggering photoreceptor apoptosis under hypoxic environment may thus reveal factors, which may be targeted by therapeutic approaches to save and preserve vision in patients.
There is growing evidence that the neurotrophins (NTs), including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3) and neurotrophin 4 (NT-4), are involved in the neuronal retinal degeneration (Pardue and Allen 2018). The NT-3, which preferentially binds to receptor tropomyosin-related kinase C (TrkC), has been shown to be crucial for the development of the central nervous system (CNS) (Dawbarn and Allen 2003). After NT-3 binding, TrkC undergoes kinase activation, leading to the recruitment and phosphorylation of signaling proteins that regulate neuronal proliferation, differentiation, and survival (Das et al. 2000). Previous studies showed that intraocular injection of exogenous NT-3 can rescue photoreceptor apoptosis in several experimental models of retinal degeneration, such as selective Müller cell ablation (Shen et al. 2013) and high-intensity exposure to visible light (Harada et al. 2000).
Currently, there is no evidence that NT-3 participates in the process of hypoxia-mediated photoreceptor injury, and the corresponding molecular mechanism is unclear. In the present study, we investigated: (1) whether Müller cells were the potential sources of endogenous NT-3 and (2) the molecular mechanisms of NT-3 in the regulation of hypoxia-induced photoreceptor apoptosis. These findings will lead to a novel therapeutic approach for hypoxia-related retinal diseases.
Materials and methods
Cell cultures and hypoxic treatment
All of the procedures were performed in accordance with the Association for Research in Vision and Ophthalmology Statement (ARVO) for the use of animals in vision research and the guidelines of the Animal Care and Use Committee at Shanghai Jiaotong University Medical School, Shanghai, China. Primary Müller cells were isolated from retinas of C57BL/6J mice and cultured as described previously (Hicks and Courtois 1990). A mouse cone photoreceptor cell line (661W cone cells) was kindly provided by Dr. Muayyad Al-Ubaidi (Department of Cell Biology, University of Oklahoma Health Sciences Center, Oklahoma City, OK). The 661W cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/mL Penicillin and 100 µg/mL Streptomycin at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Hypoxic conditions were induced by placing dishes containing Müller cells or 661W cells in an anaerobic chamber with 1% O2, 95% N2 and 5% CO2 for 0–24 h.
Cell transfection
RNA interference was used to silence the expression of TrkC in 661W cells. TrkC-siRNA (5′-GGACGATGGGAACCTCTTC-3′) and scramble siRNA (si-NC) were synthesized by GenePharma Biotechnology (Shanghai, China). siRNA transfection was conducted using Lipofectamine 2000 transfection reagent (Invitrogen) following the manufacturer’s instruction. Briefly, 1 × 105 cells were seeded into 6-well plates containing an antibiotic-free medium and incubated overnight. For each well, 5 µL siRNA were mixed with 250 µL OPTI-MEM I (Invitrogen). The mixture was then combined with a solution of 5 µL lipofectamine in 250 µL OPTI-MEM I. After a 20-min incubation period at room temperature (RT), the mixture was applied to the cells in an appropriate volume of Opti-MEM I so as to achieve a final concentration of 50 nmol/L. After incubation for 6 h at 37 °C, DMEM supplemented with serum was added to the wells. Cells were cultured for an additional 24 h at 37 °C before analysis.
Co-culture of 661W cells with Müller cells
The primary Müller cells (1 × 105 cells/well) were plated in upper chambers of 24-well transwell plates (collagen coated porous membrane, 0.4-µm pore size, Corning, NY, US). After 24 h of adherence period, these inserts were placed on top of the 24-well plate previously seeded with 661W cells (1 × 105 cells/well) in the lower chambers. The porous membrane of the inserts allowed free diffusion of soluble molecules but not of whole cells.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
Total RNAs were isolated from cells using a Trizol reagent (Invitrogen) and cDNA was prepared using PrimeScript® RT reagent Kit (Takara, RR036A, Dalian, China) following standard protocols. qPCR was performed using SYBR® Premix Ex TaqTM II (Takara, RR420A) on an Applied Biosystems 7500 Real-time PCR Systems (Applied Biosystems, USA) with the following two-stage program parameters: pre-incubated for 60 s at 95 °C and then 40 cycles of 5 s 95 °C and 34 s at 60 °C. The β-actin was selected as internal standard for gene expression and relative gene expression was calculated using the 2−ΔΔCT formula. The sequences of the primers used are as follows: NT-3 (forward, 5′-GAAACGCGATGTAAGGAAGC-3′; reverse, 5′-CCAGCCCACGAGTTTATTGT-3′), β-actin (forward, 5′-ACCACAGTCCATGCCATCAC-3′; reverse, 5′-TCCACCACCCTGTTGCTGTA-3′)
Western blot
Cells were lysed in RIPA buffer containing 1% proteinase inhibitor and phosphatase inhibitor cocktails (Beyotime Institute of Biotechnology, Jiangsu, China). Lysates were centrifuged at 12,000×g at 4 °C for 15 min and the protein concentration in the supernatant was determined using a BCA Protein Assay Kit (Beyotime Institute of Biotechnology). The protein sample (40 µg) was separated on 10% SDS-PAGE and then transferred onto PVDF membranes (Millipore, Bedford, MA) after electrophoresis. Following blocking with 0.05 M Tris-buffered saline (TBS), containing 5% non-fat skim milk and 0.1% Tween-20, for 2 h at room temperature, the membranes were incubated with primary antibodies overnight at 4 °C. Then, the membranes were incubated with HRP-conjugated secondary antibody (cat. no. 14708 or 14709; 1:5000; Cell Signaling Technology, Inc., Danvers, MA, USA) at RT for 1 h, and detected with ECL detection reagents (Thermo Fisher Scientific, Waltham, MA). The following primary antibodies were used: TrkC (cat. no. ab227289, 1:500; Abcam, Cambridge, MA, USA), Bax (cat. no. 199677; 1:2000; Abcam); cleaved caspase-3 (cat. no. ab32042; 1:500; Abcam), NT-3 (cat. no. ab53685; 1:1000; Abcam), p-ERK (cat. no. 4370; 1:2000; Cell Signaling Technology), ERK (cat. no. 4695; 1:2000; Cell Signaling Technology), p-P38 (cat. no. 4511; 1:2000; Cell Signaling Technology), P38 (cat. no. 8690; 1:2000; Cell Signaling Technology), p-JNK (cat. no. 9255; 1:2000; Cell Signaling Technology), JNK (cat. no. 9252; 1:2000; Cell Signaling Technology) and β-actin (cat. no. 4970; 1:1000; Cell Signaling Technology).
Enzyme-linked immunosorbent assay (ELISA)
Müller cells were seeded in 12-well plates and grown to 80% confluence. Then, cells were exposed to hypoxic condition for 6, 12 and 24 h, and the culture medium were collected and subsequently centrifuged at 1500 rmp for 5 min to pellet cell debris. Supernatants were analyzed using mouse ELISA development kits for NT-3 (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
TUNEL staining
661W Cell apoptosis was detected by using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit (Roche Applied Science, Penzberg, Germany), following the manufacturer’s instructions. In brief, 661w cells were fixed with 4% paraformaldehyde for 30 min. After washing with PBS, cells were permeabilized using 0.5% Triton X-100. Then they were incubated with 50 µL TUNEL reaction fluid in a humid environment at 37 °C for 1 h. And after washing twice with PBS, 661W cells were incubated with 4′,6-diamidino-2-phenylindole (DAPI, 1:1000, 5 min; Beyotime) to stain nuclei. Finally, 661W cells were observed under fluorescence microscope (Olympus, Tokyo, Japan).
Immunofluorescence
661W cells were fixed in 4% paraformaldehyde for 30 min and permeabilized with 0.2% Triton X-100 in PBS for 15 min, followed by blocking with 1% BSA in PBS with 10% goat serum at RT for 1 h. The cells were then incubated with primary antibodies against TrkC (1:100; Abcam) overnight at 4 °C. The primary antibody binding was detected with an Alexa Fluor 594-conjugated donkey anti-mouse IgG (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h at 37 °C, followed by DAPI staining (1:1000, 5 min; Beyotime), and visualized using a fluorescence microscope (Olympus, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5 software (GraphPad Software, Inc., USA). All experiments were performed in triplicate and data are presented as mean ± SD. The differences between two groups were analyzed using Student's t test, while a one-way ANOVA was used for three or more groups. A P < 0.05 was considered to be a significantly different.
Results
Hypoxia induced cell apoptosis and TrkC expression in 661W cells
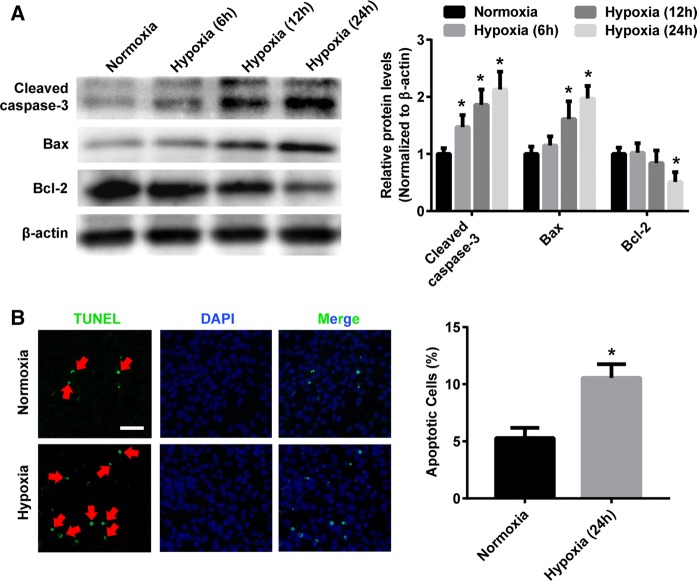
We first examined the effect of hypoxia on photoreceptor apoptosis. Western blot analyses showed the expressions of cleaved-caspase3 and Bax were significantly elevated, while Bcl-2 were declined in 661W cells exposed to hypoxic conditions for 24 h, compared with the normoxic control (Fig. 1a). In addition, Fig. 1b showed that the hypoxic group had a significantly more number of cell apoptosis (TUNEL-positive stain) than that in the normoxic group.
Fig. 1.
Hypoxia induces the apoptosis of 661W cells. a Western blotting representative and densitometry quantification of cleaved caspase-3, Bax and Bcl-2 expression in 661W cells exposed to normoxic or hypoxic conditions for 6, 12 and 24 h. b TUNEL staining of cell apoptosis in 661W cells exposed to normoxic or hypoxic conditions for 24 h. Red arrows indicate the apoptotic cell. Scale bars, 100 µm. Data are shown as mean ± SD (n = 3 independent tests, *P < 0.05 versus 0 normoxia group). (Color figure online)
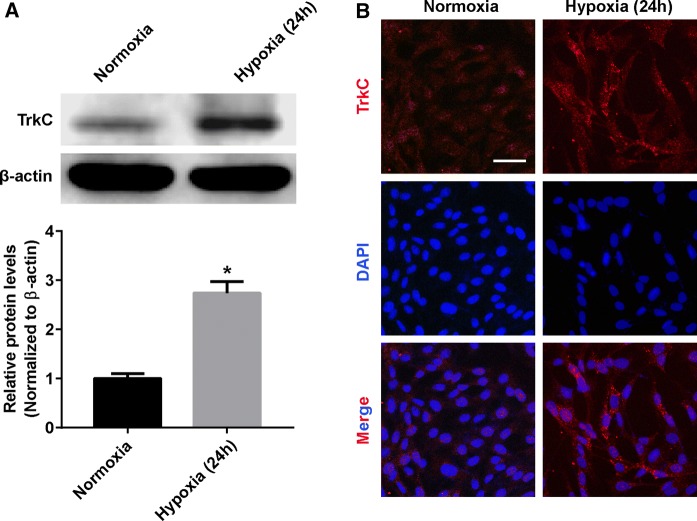
Previous studies have reported that TrkC was mainly expressed on photoreceptors (Nag and Wadhwa 1999). Thus, we examined the expression of TrkC in 661W cells under normoxia and hypoxia. RT-PCR (Fig. 2a) and Immunofluorescence (Fig. 2b) showed that the expression of TrkC was significantly upregulated in the hypoxia-exposed 661W cells compared with the normoxic control.
Fig. 2.
Hypoxia induces the expression of TrkC in 661W cells. a Western blotting representative and densitometry quantification of TrkC expression in 661W cells exposed to normoxic or hypoxic conditions for 24 h. b Representative images of immunofluorescence stain for TrkC in 661W cells exposed to normoxic or hypoxic conditions for 24 h. Data are shown as mean ± SD (n = 3 independent tests, *P < 0.05 versus normoxia group)
Müller-cell derived NT-3 inhibited 661W cells apoptosis under hypoxia
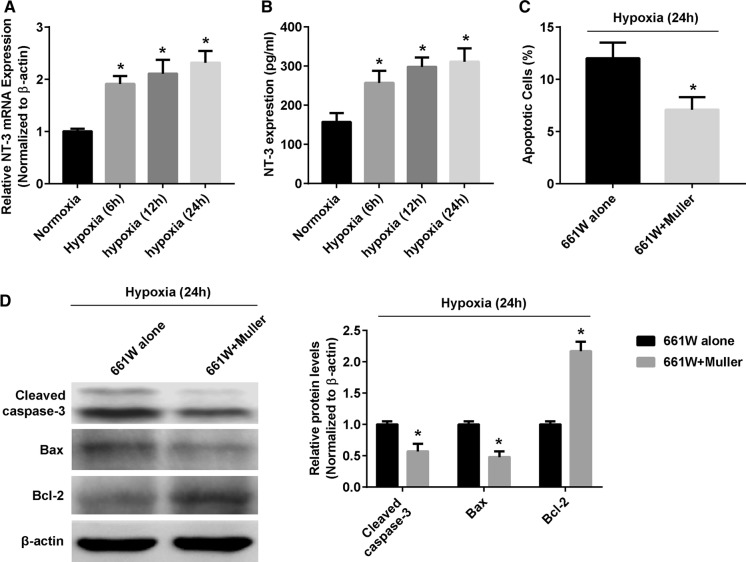
There is increasing evidence that Müller cells are important for photoreceptor health because they are an important source of various neuroprotectants (Bringmann et al. 2006). Therefore, we speculated that the Müller cells are one major source of NT-3. RT-PCR analysis showed that the mRNA expression of NT-3 in Müller cells exposed to hypoxia was significantly upregulated in a time-dependant manner (Fig. 3a). Moreover, ELISA analysis revealed a higher level of NT-3 in Müller cell supernatant under hypoxia than normoxia (Fig. 3b).
Fig. 3.
Hypoxia triggeres the NT-3 release by primary mouse Müller cells. a Relative expression of NT-3 mRNA in Müller cells exposed to normoxic or hypoxic conditions for 24 h. b ELISA analyses of NT-3 concentrations in Müller cell culture supernatants under normoxia or hypoxia for 24 h. c TUNEL staining of cell apoptosis in 661W cells which cultured alone or co-cultured with Müller cells under hypoxia for 24 h. d Western blotting representative and densitometry quantification of Bax, Bcl-2 and cleaved caspase-3 expression in 661W cells which cultured alone or co-cultured with Müller cells under hypoxia for 24 h. Data are shown as mean ± SD (n = 3 independent tests, *P < 0.05 versus 0 normoxia or 661W alone groups)
To investigate the possibility that Müller-cell derived NT-3 acts as a neuroprotectant for 661W cells, we performed co-cultures of Müller cells and 661W cells under hypoxia for 24 h, and determined 661W cell apoptosis. As shown in Fig. 3c, the apoptosis rate of 661W was significantly lower when cultured together with Müller cells than alone. Compared with the 661W cells that not co-cultured with Müller cells, the 661W cells co-cultured with Müller cells showed increased Bcl-2 expression, and decreased Bax and cleaved caspase-3 expression, after exposing to hypoxia for 24 h (Fig. 3d).
NT-3 inhibited the apoptosis of 661W cells via ERK1/2 pathway
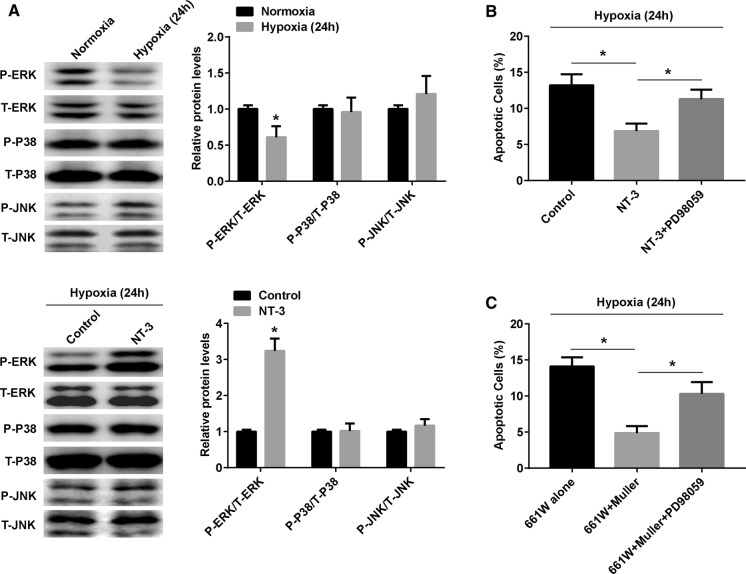
Several studies have shown that NT-3 binds to TrkC, triggers the receptor and signals cascades (Ivanov et al. 2013; Han et al. 2016; Li et al. 2016). To identify the signaling pathway through which NT-3 on 661W cells, cells were treated with exogenous NT-3 (20 nM) for 24 h and the phosphorylation state of the mitogen-activated protein kinase (MAPK) signaling pathway was examined, including ERK1/2, p38 and JNK. Western blot analyses showed that the phosphorylation level of ERK1/2 was significantly decreased in 661W cells under hypoxia (Fig. 4a, upper panel), and this reduction was reversed by exogenous NT-3 stimulation (Fig. 4a, lower panel).
Fig. 4.
NT-3 protected hypoxia-induced 661W cell apoptosis via ERK signaling. a Western blotting representative and densitometry quantification of phosphorylated and total ERK1/2, P38 and JNK in 661W cells which cultured under normoxic and hypoxic conditions (upper panel), and treated with or without recombinant NT-3 (20 nM) under hypoxia for 24 h (lower panel). b TUNEL staining of cell apoptosis in 661W cells treated with recombinant NT-3 (20 nM) or NT-3 combining with PD98059 (5 µM) under hypoxia for 24 h. c TUNEL staining of cell apoptosis in 661W cells which cultured alone, co-cultured with Müller cells or Müller cells combining with PD98059. Data are shown as mean ± SD (n = 3 independent tests, *P < 0.05 versus untreated or 661W alone groups)
To verify that NT-3 inhibits the apoptosis of 661W via the ERK1/2 pathway, ERK-specific inhibitor PD98059 was used to inhibit ERK1/2 activity. Figure 4b showed that PD98059 pre-treatment attenuated the anti-apoptosis effect of exogenous NT-3 stimulation in 661W cells. In addition, PD98059 pre-treatment also reversed the anti-apoptosis effect of Muller cells (Fig. 4c).
TrkC silencing in 661W cells attenuated the Müller-cell mediated anti-apoptosis
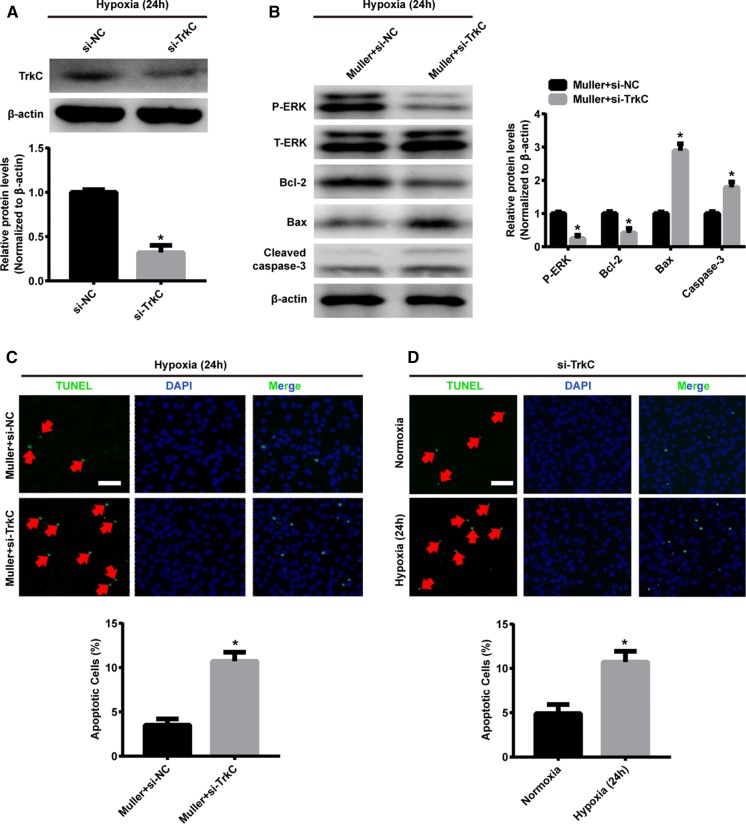
To further confirm the role of NT-3/TrkC pathway in the Müller-cell mediated anti-apoptosis, TrkC in 661W cells was silenced by RNA interference. After confirmation of TrkC inhibition by Western blot (Fig. 5a), 661W cells were then co-cultured with Müller cells under hypoxia for 24 h. The TUNEL staining in Fig. 5c showed the number of apoptotic cell was significantly increased in the 661W cells transfected with si-TrkC when compared to those transfected with si-NC. Moreover, the protein levels of Bax and cleaved caspase-3 were upregulated, whereas Bcl-2 and phosphorylation of ERK1/2 was downregulated in si-TrkC group (Fig. 5b). Furthermore, the apoptotic rate of si-TrkC transfected 661W cells was significantly higher under hypoxia than those under normoxia (Fig. 5d), which was similar to the tendency observed in wild-type 661W cells under normoxic and hypoxic conditions (Fig. 1b).
Fig. 5.
TrkC silencing in 661W cells attenuated the Müller-cell mediated anti-apoptosis. a Western blotting representative and densitometry quantification of TrkC expression in 661W cells transfected with si-NC or si-TrkC. b Western blotting representative and densitometry quantification of Bax, Bcl-2, cleaved caspase-3 expression and phosphorylated ERK1/2 in 661W cells transfected with si-NC or si-TrkC, and both of which co-cultured with Müller cells under hypoxia for 24 h. c TUNEL staining of cell apoptosis in si-TrkC transfected 661W cells cultured under normoxia and hypoxia for 24 h. Red arrows indicate the apoptotic cell. Scale bars, 100 µm. d TUNEL staining of cell apoptosis in 661W cells transfected with si-TrkC, and both of which co-cultured with Müller cells under hypoxia for 24 h. Red arrows indicate the apoptotic cell. Scale bars, 100 µm. Data are shown as mean ± SD (n = 3 independent tests, *P < 0.05 versus si-NC or si-NC + Müller cell groups). (Color figure online)
Discussions
Photoreceptors are the specialized cells that have the highest oxygen requirements of any cell in the body (Steinberg 1987). Tissue oxygen levels are important determinants of photoreceptor death and survival in both developing and adult retina (Wellard et al. 2005). Hypoxia-induced photoreceptor injury is obvious in several hypoxic diseases of the retina such as DR, RD and AMD (Wangsa-Wirawan and Linsenmeier 2003; Li et al. 2012; Kurihara et al. 2016). In the current study, we created a model of hypoxia to assess photoreceptor cells in vitro and found that hypoxia led to photoreceptor apoptosis, along with TrkC upregulation. We also showed that NT-3 derived from hypoxic Müller cells could alleviate hypoxia-induced photoreceptor apoptosis. Further mechanism studies by NT-3 receptor TrkC silencing and PD98059 pretreatment indicated that the ERK pathway was involved in the NT-3/TrkC mediated anti-apoptosis in photoreceptor cells (Fig. 6).
Fig. 6.
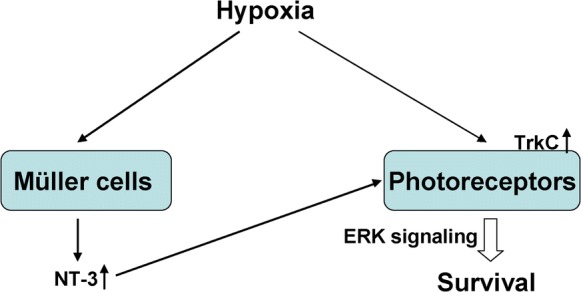
Schematic representation of NT-3/TrkC/ERK signaling in photoreceptor survival under hypoxia
NT-3 is a member of the neurotrophin family and regulates the survival of neurons that express the receptor TrkC. It has been previously shown that TrkC was uniquely present in photoreceptors in the infant and adult human retina, but was apparently absent on the murine photoreceptors (Nag and Wadhwa 1999). However, Harada et al. (2000) reported that the expression of the trkC gene was induced in light-degenerated rat photoreceptors, suggesting that photoreceptor survival in rat retina may be partly the result of direct effect of NT-3. Similar to these studies, our results showed that the expression of TrkC was extremely low in 661W cells, while upregulated in the homotypic cells exposing to hypoxia for 24 h. In this study, therefore, we are underlining the contribution of NT-3/TrkC signaling in the neuroprotection of 661W cells.
Müller cells are a major cellular source of regulating survival signals for retinal neurons under hypoxic conditions (Bringmann et al. 2006). One important function of Müller cells is to release neurotrophic factors such as NT3, BDNF and NGF to maintain the health of photoreceptors and other neurons (Oku et al. 2002). Moreover, it has been documented that Müller cells are able to rapidly increase their level of NT-3 release in response to glutamate-induced toxicity (Taylor et al. 2003) or inflammatory stimulus (Boss et al. 2017). Here, we investigated, for the first time, the effects of hypoxia on the production of NT3 in primary mouse Müller cells and found that hypoxia increased NT3 synthesized and secreted by Müller cells. Notably, co-culture with Müller cells under hypoxic condition could protect 661W cells from apoptosis. Therefore, NT-3 might act more effectively on photoreceptors when produced by their glial neighbour cells, and intraretinal NT-3 levels governed by Müller cells may represent the very first line of defense against photoreceptor aggressions.
To gain further insight into the mechanism by which NT-3 promotes photoreceptor survival, we evaluated the activity of signaling pathways downstream of TrkC. A key pro-growth signaling downstream of TrkC activation is the MAP kinase pathway that includes ERK, JNK and p38. We therefore measured phospho-ERK1/2, P38 and JNK levels in response to exogenous NT-3 stimulation in 661W cells. The results of our present study suggest that NT-3 promotes 661W cell survival via the ERK signaling pathway, and not through the activation of JNK or p38. Furthermore, inhibition of ERK1/2 pathway suppressed the NT-3-mediated anti-apoptotic effect. Our results were partly consistent with a previous study which reported that NT-3 promotes the growth of human neurons cells primarily through the TrkC/ERK pathway (Li et al. 2016).
In summary, the major finding of our study is that the NT-3 originating from Müller cells protects photoreceptors from hypoxia-induced apoptosis through a TrkC/ERK-dependent pathway. Our results may facilitate future studies on the therapeutic implications of NT-3 in the treatment of hypoxia-relevant retinal diseases.
Acknowledgements
This study was supported by Grants from National Natural Science Foundation of China (Nos. 81670861, 81800826).
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to report.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7:291–304. doi: 10.2174/157339911797415620. [DOI] [PubMed] [Google Scholar]
- Barben M, Ail D, Storti F, Klee K, Schori C, Samardzija M, Michalakis S, Biel M, Meneau I, Blaser F, Barthelmes D, Grimm C. Hif1a inactivation rescues photoreceptor degeneration induced by a chronic hypoxia-like stress. Cell Death Differ. 2018;25:2071–2085. doi: 10.1038/s41418-018-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak J, Petrovski G, Vereb Z, Facsko A, Kaarniranta K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. Biomed Res Int. 2014;2014:768026. doi: 10.1155/2014/768026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss JD, Singh PK, Pandya HK, Tosi J, Kim C, Tewari A, Juzych MS, Abrams GW, Kumar A. Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Investig Ophthalmol Vis Sci. 2017;58:5594–5603. doi: 10.1167/iovs.17-21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Das I, Sparrow JR, Lin MI, Shih E, Mikawa T, Hempstead BL. Trk C signaling is required for retinal progenitor cell proliferation. J Neurosci. 2000;20:2887–2895. doi: 10.1523/JNEUROSCI.20-08-02887.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawbarn D, Allen SJ. Neurotrophins and neurodegeneration. Neuropathol Appl Neurobiol. 2003;29:211–230. doi: 10.1046/j.1365-2990.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- Grimm C, Willmann G. Hypoxia in the eye: a two-sided coin. High Alt Med Biol. 2012;13:169–175. doi: 10.1089/ham.2012.1031. [DOI] [PubMed] [Google Scholar]
- Han KA, Woo D, Kim S, Choii G, Jeon S, Won SY, Kim HM, Heo WD, Um JW, Ko J. Neurotrophin-3 regulates synapse development by modulating TrkC-PTPsigma synaptic adhesion and intracellular signaling pathways. J Neurosci. 2016;36:4816–4831. doi: 10.1523/JNEUROSCI.4024-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Harada C, Nakayama N, Okuyama S, Yoshida K, Kohsaka S, Matsuda H, Wada K. Modification of glial–neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron. 2000;26:533–541. doi: 10.1016/S0896-6273(00)81185-X. [DOI] [PubMed] [Google Scholar]
- Hicks D, Courtois Y. The growth and behaviour of rat retinal Muller cells in vitro. 1. An improved method for isolation and culture. Exp Eye Res. 1990;51:119–129. doi: 10.1016/0014-4835(90)90063-Z. [DOI] [PubMed] [Google Scholar]
- Ivanov SV, Panaccione A, Brown B, Guo Y, Moskaluk CA, Wick MJ, Brown JL, Ivanova AV, Issaeva N, El-Naggar AK, Yarbrough WG. TrkC signaling is activated in adenoid cystic carcinoma and requires NT-3 to stimulate invasive behavior. Oncogene. 2013;32:3698–3710. doi: 10.1038/onc.2012.377. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Westenskow PD, Gantner ML, Usui Y, Schultz A, Bravo S, Aguilar E, Wittgrove C, Friedlander M, Paris LP, Chew E, Siuzdak G, Friedlander M. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. Elife. 2016;5:e14319. doi: 10.7554/eLife.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Fu ZJ, Lo AC. Hypoxia-induced oxidative stress in ischemic retinopathy. Oxid Med Cell Longev. 2012;2012:426769. doi: 10.1155/2012/426769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wu Y, Jiang D. NT-3 attenuates the growth of human neuron cells through the ERK pathway. Cytotechnology. 2016;68:659–664. doi: 10.1007/s10616-014-9813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervin K, Valter K, Maslim J, Lewis G, Fisher S, Stone J. Limiting photoreceptor death and deconstruction during experimental retinal detachment: the value of oxygen supplementation. Am J Ophthalmol. 1999;128:155–164. doi: 10.1016/S0002-9394(99)00104-X. [DOI] [PubMed] [Google Scholar]
- Nag TC, Wadhwa S. Neurotrophin receptors (Trk A, Trk B, and Trk C) in the developing and adult human retina. Brain Res Dev Brain Res. 1999;117:179–189. doi: 10.1016/S0165-3806(99)00121-2. [DOI] [PubMed] [Google Scholar]
- Oku H, Ikeda T, Honma Y, Sotozono C, Nishida K, Nakamura Y, Kida T, Kinoshita S. Gene expression of neurotrophins and their high-affinity Trk receptors in cultured human Muller cells. Ophthalmic Res. 2002;34:38–42. doi: 10.1159/000048323. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Allen RS. Neuroprotective strategies for retinal disease. Prog Retin Eye Res. 2018;65:50–76. doi: 10.1016/j.preteyeres.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Zhu L, Lee SR, Chung SH, Gillies MC. Involvement of NT3 and P75(NTR) in photoreceptor degeneration following selective Muller cell ablation. J Neuroinflamm. 2013;10:137. doi: 10.1186/1742-2094-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RH. Monitoring communications between photoreceptors and pigment epithelial cells: effects of "mild" systemic hypoxia. Friedenwald lecture. Investig Ophthalmol Vis Sci. 1987;28:1888–1904. [PubMed] [Google Scholar]
- Taylor S, Srinivasan B, Wordinger RJ, Roque RS. Glutamate stimulates neurotrophin expression in cultured Muller cells. Brain Res Mol Brain Res. 2003;111:189–197. doi: 10.1016/S0169-328X(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- Wellard J, Lee D, Valter K, Stone J. Photoreceptors in the rat retina are specifically vulnerable to both hypoxia and hyperoxia. Vis Neurosci. 2005;22:501–507. doi: 10.1017/S0952523805224112. [DOI] [PubMed] [Google Scholar]