Abstract
Oil mill wastewater (OMW) is the main liquid discharge from oil mills, it is considered as a dangerous pollutant due to its toxic chemical compounds which are unloaded directly in the environment without any treatment. The aims of this study were to evaluate the effectiveness of OMW adsorption on clay as a good method for the elimination of toxic chemical compounds and to study the application of treated OMW as an irrigation source in agricultural field. For this, Clay was collected from the city of Agourai (Meknes region, Morocco) and characterized by X-ray diffraction, X-ray fluorescence spectrometry, BET and FTIR analysis. Moreover, the treated OMW was analyzed using UHPLC-ESI-MS and the determination of total phenolic content (TPC) was also performed. However, the application of the treated OMW in agricultural field was performed by the determination of its effect on the germination of Lepidium sativum seeds (in vitro) and as a source of irrigation of Vicia faba plants (in situ). The results of this study showed that OMW had the following physicochemical characteristics: average pH of 4.88, TPC of 4.75 g/l, COD of 80 g/l, BOD5 of 18.72 g/l, conductivity of 16.05 cm-1, dry matter of 135.7 g/l and volatile matter of 58.7 g/l. The adsorption on clay had increased the pH from 4.88 to 6.14 and reduced significantly the organic matter (42% of COD and 57.4% of phenolic compounds). UHPLC-ESI-MS analysis showed the presence of a wide variety of organic compounds in OMW, with the appearance of new compounds after adsorption. Moreover, the use of treated OMW as a source of irrigation showed a significant effect on the germination of Lepidium sativum seeds and the growth of Vicia faba plants. From this study, we can conclude that the adsorption on clay is a good method for the treatment of OMW, which became non-toxic for environment and can be used as a source of irrigation in agricultural field.
Keywords: Agricultural science, Environmental science, Materials science, Oil mill wastewater, Adsorption, Moroccan clay, Chemical characterization, Biological application
Agricultural science; Environmental science; Materials science, Oil mill wastewater; Adsorption; Moroccan clay; Chemical characterization; Biological application.
1. Introduction
The Industrial production of olive oil is the main source of two byproducts: olive waste and OMW. The OMW is pollutant due to their wealth organic matter with the presence of a high percentage of phenolic compounds and is considered as a major problem of environment contamination [1]. OMW had a negative impact on the environment and public health by clogging the soil, polluting the water and releasing the disgusting smells [2].
The toxicity of OMW is related to the presence of long chain free fatty acids and phenolic compounds, which are very difficult for degradation at a high concentration exceeding the standards values (between 4 to 15 g/l) [3]. Phenols are organic volatiles compounds with a great economic and environmental impact, their study was increased during the over years due to their toxicity on environment and public health. In fact, phenolic compounds can become from various manufacturing processes like pharmaceutical industries, oil refineries, resins phenolic units and OMW [4, 5].
Treatment of OMW is very complex because of the quality and the quantity of their chemical constituents. In order, to eliminate the phenolic pollutants, several methods were described which can be divided into three categories: physical, chemical and biological methods [6]. Among them, adsorption was considered as an effective method used for the elimination of phenols and polyphenol existing in OMW. Therefore, the use of inorganic materials as adsorbents had attracted attention in recent years. However, The choice of adsorbents depends on the variation of the adsorption conditions, type of pollutants and the properties of the adsorbent such as specific surface area, pore size and homogeneity, structural properties, selective adsorption capacity and ease regeneration [7].
At present, activated carbon is considered to be one of the most versatile adsorbents and many studies show its effectiveness, but its use remains limited because of the difficulties of its regeneration and its high cost [8]. An alternative solution would be to use other efficient and more economical adsorbent materials [9]. Our choice focused on a material that is abundant in Morocco, it is clay [3]. Its potential for use in the natural state according to the varieties present in the various regions are well below the possibilities offered by their various properties [10], colloidal stability [11, 12], the structure [13] and the texture of these materials [14]. Management of water resources in arid and semi-arid regions had a major impact on the local population; indeed, the reduction of water resources in these regions was accompanied by a high demand for their uses in the agriculture field and other activities [15]. However, it is necessary to optimize the use of water resources by the treatment and recycling of water previously used in industrial or household activities. In this context, this study aims to characterize OMW by using physicochemical methods and to evaluate the effectiveness of its treatment by adsorption on Moroccan clay in order to be used as a source of irrigation in agriculture field.
2. Materials and methods
2.1. Collect and treatment of samples
OMW was collected from Meknes region during January 2019. Then, a centrifugation was performed to separate the liquid phase from the solid and oily phases. The liquid phase was split into two lots; the first one is the pure liquid phase (OMW) and the second one was diluted with distilled (50% of liquid phase +50% of distilled water) (D-OMW). Then all the samples were treated by adsorption on clay to get treated OMW (T-OMW) and treated diluted OMW (TD-OMW).
2.2. Collect and characterization of clay
The clay samples were collected in the city of Agourai (Meknes region) and characterized by using the following test:
2.2.1. X-ray diffraction
X-ray diffraction (XRD) patterns were recorded using an X’PERT MPD-PRO wide angle X-ray powder diffractometer provided with a diffracted beam monochromator and Ni filtered CuKɑ radiation (λ = 1.5406 Å). The 2θ angle was scanned between 4° and 30°range with a counting time of 2.0 s at steps of 0.02° [16].
2.2.2. BET analysis
N2 adsorption measurements at T = -196 °C were performed using a Micromeritics ASAP 2010. The specific surface area and the average pore diameter were determined according to the standard BET and BJH (Barrett, Joyner and Halenda) methods, respectively [16].
2.2.3. FTIR analysis
The characterization and the structural changes, which took place during the thermal degradation of the nanocomposite, were collected using JASCO 4100 FTIR spectrometer with a resolution of 4 cm−1 and accumulation of at least 64 scans. The nanocomposite and the residues resulting from each stage of thermal degradation processes of nanocomposite were prepared using KBr pellets [16].
2.2.4. X-ray fluorescence spectrometry
The X-ray fluorescence was carried out using an "Axion" X-ray fluorescence spectrometer, with 1 kW wavelength dispersion according to the protocol described previously by Ouallal and his group [17].
2.3. Adsorption of OMW on clay
2.3.1. Adsorption experience and characterization of OMW
In a beaker of 1L of OMW puts in contact with a 10g of clay during 36h, with the duration of reaction of the samples are made to analyze it and to destroy the physicochemical parameters of OMW, the acidity measured by a pH- meter (Thermo Scientific Orion 2 STAR), the Suspended matter (SM) are determined by centrifugation of a volume of 20 ml of OMW at 8000rpm for 20 min. The pellet is placed in a porcelain dish weighed and then dried in an oven at 105 °C for 24 h. The difference between the weight of the dried sample and that of the cup determines the rate of SM. It is expressed in g/l. The volatile matter is determined by differentiating between the dry matter obtained by evaporation at 105 °C and ash residues from calcination at 550 °C for 2 h. It is expressed in g/l. The COD corresponds to the oxygen consumption necessary for the complete oxidation of the organic matter of OMW. It is expressed in grams of oxygen per liter of sample. The determination of the COD is carried out by the potassium dichromate method, the BOD5 (AFNOR T90-103) was determined by a BOD-meter.
2.3.2. UHPLC-ESI-MS
UHPLC-ESI-MS analysis was performed with a UHPLC (Nexera X2 system consisting of LC-30AD, SIL-30AC, CTO-20AC, Shimadzu, Tokyo) coupled to a triple quadrupole mass spectrometer (TQ8040, Shimadzu) with the Jet Stream electrospray ionization source. Two different core-shell C18 columns with different dimensions were tested. One is a longer core-shell column (Kinetex, 2.1 mm × 100 mm, 2.6 μm particle size, Phenomenex, Torrance, Calif). The other column was a shorter core-shell column (Kinetex, 2.1 mm × 50 mm, 2.6 μm particle, Phenomenex). The mobile phase system for the positive ionization UHPLC-ESI-MS was composed of water (A) and ACN (B). The analyzes are carried out under the following conditions: Analysis time of 15 min, Scan interval of 50–400 m/z, collision energy of -35V, Nebulizing gas of 3 l/min, DL temperature of 250 °C, Heat block temperature of 400 °C, Drying gas flow of 0.2 ml/min, Pump A flow of 0.2 ml/min, and Pump B flow of 0.2 ml/min.
2.3.3. Determination of total phenolic content (TPC)
The determination of total phenolic content in OMW was performed using the Folin Ciocalteu method as described by Singleton et al. [18]. Briefly, 0.3 ml of the crude OMW was added to 1.5 ml of Folin-Ciocalteu reagent (10/100). The mixture was incubated for 6 min and mixed with 1.2 ml of Na2CO4 (7.5%). The samples prepared were incubated in dark for 2 h and the absorbance was measured at 760 nm. The total phenolic compounds were expressed as gallic acid equivalents (GAE) in mg/g of OMW. The total phenolic content was calculated using a calibration curve which was made according to the protocol described by Barbouchi and his collaborators [19].
2.4. Biological application of treated OMW
2.4.1. Effect of OMW on the germination de Lepidium sativum seeds
In order to evaluate the toxicity of different samples of OMW on the germination of seeds, the garden cress Lepidium sativum L. (Brassicaceae) was used as a matrix in this study [20]. The seeds of Lepidium sativum were carefully selected by removing any damaged and small seed to obtain the uniform size. Then, they were washed with distilled water to remove the impurities. However, 10 ml of pure OMW, T-OMW, DT-OMW and distilled water (as control) were pipetted onto three layers of filter paper fitted into a 90 mm Petri dish for each one. Then, 10 healthy locking L. sativum seeds were distributed evenly on filter paper. The Petri dishes were kept in the dark at 24 ± 1 °C for two days. Afterward, seed germination and the root length of seedlings were measured. The experimental set of each testing scheme involved three control dishes and three replicates for each sample.
2.4.2. Effect OMW on the growth of Vicia faba plants
In this study, the seeds of Vicia faba were cultivated and divided into 4 batches (containing 4 seeds for each one), and each batch was irrigated periodically by the same volume of OMW, T-OMW, DT-OMW and normal water as a control. Then, all the samples were deposited in the same environmental conditions. The growth of Vicia faba was evaluated periodically, and after the end of growth (fruit production), we measured the length of the stems, the dry mass of the stems, the dry mass of the roots, and the number of nitrogen knot.
2.5. Statistical analysis
Measurements were carried out in triplicate. The data obtained were presented as means ± standard error and the significance of the difference between test and control groups was statistically analyzed using the Student test. A probability level of P < 0.05 was used in testing the statistical significance of all experimental data. The statistical analyses were achieved using Microsoft Excel software.
3. Results and discussion
3.1. Characterization of clay
3.1.1. XRD
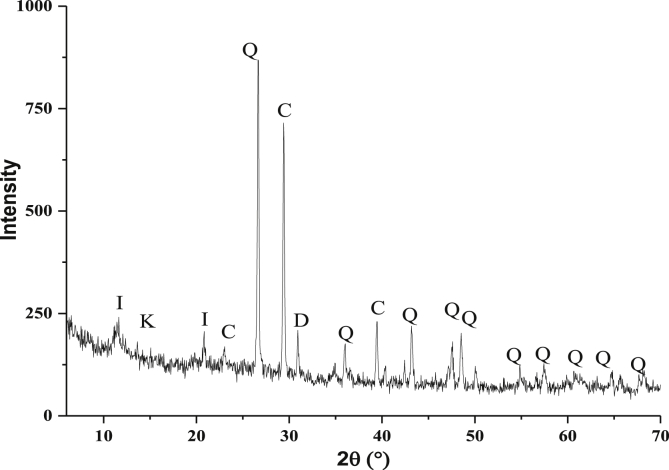
The XRD diagram of the raw clay is illustrated in Figure 1. The spectral analysis indicates that the studied clay was composed of Quartz (SiO2), Calcite (CaCO3), Kaolinite (Al2Si2O5 (OH)4), Illite [(K, H3O) Al2Si3AlO10 (OH)2] and vermiculite [(Mg, Al)3 (Si, Al)4O10 (OH)2, 4H2O]. It mainly reveals the presence of two intense peaks; one corresponds to Calcite and the other to a mixture of Quartz, Illite, Kaolinite, and Vermiculite, which implies that our clay is heterogeneous [21]. The diffractogram of the raw clay is illustrated in Figure 1. The spectral analysis indicates that the studied clay was composed of Quartz (SiO2), Calcite (CaCO3), Kaolinite (Al2Si2O5 (OH)4), Illite [(K, H3O) Al2Si3AlO10(OH)2] and vermiculite [(Mg, Al)3 (Si, Al)4O10 (OH)2, 4H2O]. It mainly reveals the presence of two intense peaks; one corresponds to Calcite and the other to a mixture of Quartz, Illite, Kaolinite and Vermiculite, which implies that our clay is heterogeneous, the diagram shows the presence of quartz characterized by the following lines: (2θ = 36, 43, 47.5, 48.5, 50, 55, 57.5, 62, 65, 78, 26.5°) and carbonates in the form of calcite characterized by lines located at (2θ = 39, 26, 29°). Other low-intensity reflections occur in the area of low diffraction angles and can be attributed to clay minerals: kaolinite (2θ = 14°) Illite (2θ = 12°) and Dolimite (2θ = 32°) [22].
Figure 1.
XRD pattern of clay.
3.1.2. BET
The adsorption of nitrogen at -196 °C on the surface of a material is made by physisorption of nitrogen molecules. The process is reversible depending on the pressure. As a result, isothermal adsorption-desorption will be represented by the volume of adsorbed gas relative pressure function (p/p0) between 0 and 1. The determination of a isothermal adsorption-desorption is therefore to measure the volume of gas that adsorbs (or desorbs) on the surface of the solid at a given temperature. The desorption isotherm is rarely superimposable to that of adsorption thus leading to a phenomenonhysteresis. The software of acquisition and data processing allow the calculation of the textural parameters of analyzed powders. The specific surface area of the solid was calculated using the following BET equation:
with:
C: constant that defines the shape of the isotherm
P: equilibrium pressure
P °: saturation vapor pressure of the adsorbate
Vm: Volume of gas necessary to form a monolayer of gas on the surface of the solid
Vads: Adsorbed volume at pressure P.
The slope and the ordinate at the origin of the line of BET make it possible to calculate Vm and C.
Thus, the specific surface of the solid is given by the following equation:
with:
σ = 16.2 Å2: area occupied by a molecule of nitrogen gas
Nm = : Number of moles of gas corresponds to the formation of a monolayer (V: Volume occupied by one mole of steam is worth 22414 cm3.mol-1)
NAV: Avogadro number
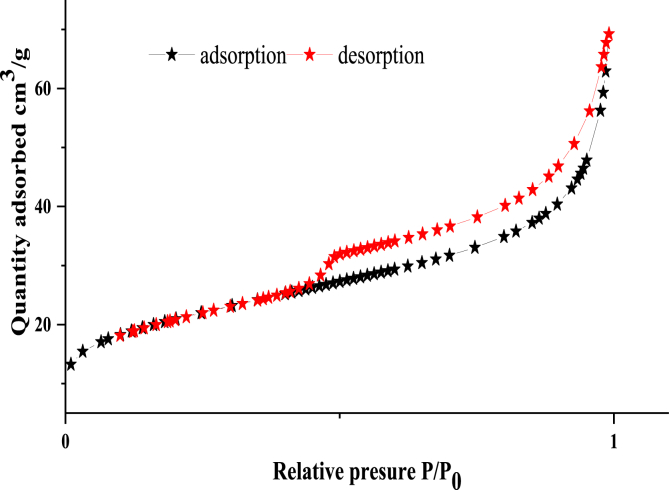
BET method (Brunuer Emmet and Teller) was used to characterize the clay texture, according to the standard classification of physical adsorption isotherms [23] proposed by the International Union of Pure and Applied Chemistry (IUPAC) [24]. The results showed that the clay isotherms are of type IV (Figure 2), with a Hestryse loop of type H3, the calculations show that the specific surface is of 51.41 m2 g−1, a diameter of 92Ǻ and a pore volume of 0.13 cm3 g−1. The textural data of our solid can open the possibility of their uses in the adsorption of polyphenols of OMW [25].
Figure 2.
N2 adsorption/desorption isotherms of clay.
3.1.3. FTIR
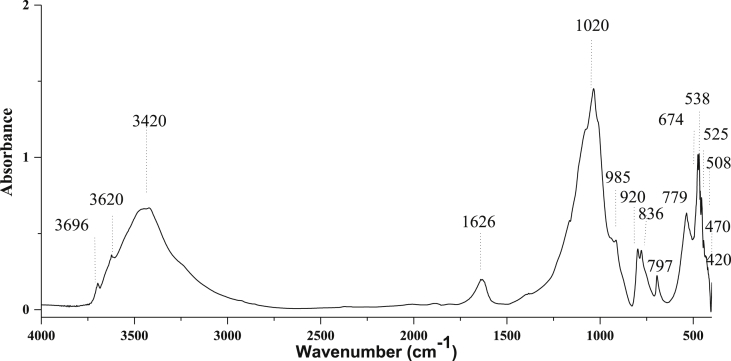
The FTIR technique was used for the determination of the functional groups present in the Agouraï natural clay (Figure 3). Each link has a defined vibration frequency characteristic The valence vibration of OH hydroxyl groups leads to the presence of three absorption bands refracted by the frequency of 3620 cm−1 (external OH) and 3420 and 3696 cm−1 (internal OH). The hydroxyl groups can be distinguished by a doublet in 3620, 3420 and 3696 cm−1 that has been indexed in the work Hanae Ouaddari and these collaborators [26]. In addition, bands at 420 cm−1, 470 cm−1, and 525 cm−1 correspond to the Si–O–Fe, Si–O–Mg and Si–O–Al deformations. Thus, a band observed at 1020 cm−1 corresponds to the stretching of Si–O–Si. The assignment of the bands centered at 985, 836, 797, 674 and 508 cm−1 are assigned to the vibratory deformation of the Al–OH–Al, Si–O–Al/Al–Mg–OH, cristobalite, Si–O bonds, - and Mg–OH–Mg, respectively [26]. The band centered at 920 cm−1 is not attributed solely to the connections of the Al–OH–Al deformation vibrations but is also attributed to the presence of kaolinite. The intensity of the absorption bands at 797 cm−1 and 779 cm−1, corresponding to quartz [27, 28].
Figure 3.
FTIR spectra of clay.
3.1.4. X fluorescence
The percentage of Silica and Aluminum is very important; which indicates the presence of Kaolinite (Al2Si2O5 (OH)4). As well as for iron oxide which is relatively high, so this material is rich in iron. The ratio Alumina/Silica, information on the permeability of the material towards moisture, plus this ratio is large plus permeability is important [21]. In our case, this ratio is small Al2O3/SiO2 = 0.27, This low value is in agreement with the low percentage of moisture (1.41%) estimated by the loss on fire (Table 1) [21]. The SiO2/Al2O3 molar ratio = 3.73 maximum substitution of Si4+ by Al3+ is greater than the standard value of bentonites which is 2.7. This difference indicates the presence of free Quartz in the clay fraction in a large proportion [21]. The overall composition of the other oxides (P2O5 MgO, K2O and CaO) reaches a percentage of 10%. The X-ray fluorescence data confirm the results of the X-ray diffraction heterogeneity of the raw clay [29].
Table 1.
Chemical composition of clay.
| element | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | S | BaO | P2O5 | ND |
|---|---|---|---|---|---|---|---|---|---|
| (% mass) | 38.74 | 10.6 | 16.8 | 0.927 | 6 | 0.19 | 0.026 | 0.1046 | 16.61 |
ND: Not determined.
3.2. Adsorption of OMW on clay
It is difficult to propose a general method of separation of phenolic products contained OMW because of the diversity of physicochemical properties such as water content, acidity, composition, and viscosity, which vary not only with time but also with the temperature, the place and the period of culture. For this, the adsorption of OMW on clay presents a simple, efficient and reducible process for the elimination of phenolic compactions. Chromatographic characterization was used for the determination of the organic compounds existing in the OMW before and after adsorption.
3.2.1. Characterization of OMW before and after adsorption
According to the results shown in Table 2, the OMW has a pH of 4.88, which can be explained by the existence of organic acids. The quantity of dry matter of the OMW and its volatile matter is 135.7 and 18.72 g/l, respectively. The volatile matter presents 80% of the material dries which shows the organic nature of this pollutant. The Chemical Oxygen Demand (COD) and the Biochemical Oxygen Demand (BOD5) are very high compared to other pollutants which are also characterized by a predominance of toxic substances and the presence of the phenolic compound (6,75 g/l) [3, 30, 31]. The measurement of Physico-chemical parameters of OMW after adsorption on the clay showed that the pH remains stable and the conductivity increases because of the presence of mineral salts in the medium of treatment. However, three parameters suffered reductions, which are phenolic compounds, COD and BOD. Their allowances were 50%, 43% and 54%, respectively. These reductions can be explained by the adsorption of a part of phenolic compounds and organic matter on the clay [32].
Table 2.
Physicochemical properties of OMW.
| Parameters | Before treatment | After treatment |
|---|---|---|
| pH | 4,88 ± 0,01 | 6.14 ± 0,01 |
| Conductivity | 16,05 ± 0,4 mSC-1 | 12.56 ± 0,4 mSC-1 |
| SM | 135,7±2 g/l | - |
| VM | 58,7±2 g/l | - |
| BOD5 | 18,72 ± 0,07 O2g/l | 9,22O ±0,07 2 g/l |
| COD | 80 ± 1 g d’O2/l | 45 ± 1 g d’O2/l |
| Phenolic compounds | 4,75 ± 0,40 g/l | 2,45 ± 0,40 g/l |
3.2.2. UHPLC-ESI-MS analysis
Chromatography and mass spectrometry are techniques that can help to identify the chemical compounds of OMW [33]. Ultra-High Performance Liquid Chromatography (UPLC) is a valuable tool for the detection of trace analytes. This technique, which has higher sensitivity, higher resolution and shorter runtime than HPLC, has recently been used to study flavonoids in plant material [34, 35]. The works performed by Obeid et al. [36] and Dermeche et al. [37] showed the presence of more than 50 different phenolic compounds in OMW, which they are very diverse and their structure is highly variable. They are generally derived from the enzymatic hydrolysis of carbohydrates and esters of olive pulp during the crushing process and are water-soluble, which explains their high concentration in OMW. Several aromatic monomers have been identified in OMW by chromatographic techniques, essentially represented by alcohol and phenolic acids. The latter is the most abundant, which explains the acidity of the OMW. Tannins also are high molecular weight phenolic compounds mainly identified in OMW as one of the most visible effects of coloring pollution in natural waters. The UPLC chromatogram has shown the presence of a wide variety of identified compounds and highlights a significant heterogeneity in the organic composition of OMW. It shows by way of example the presence of the following polar compounds: Esters, aromatic fractions (2-benzylbiphenyl), alcohols and phenol fractions (catechol, tyrosol), acidic fractions (benzoic acid, 3-cyclohexene-1-carboxylic acid, 3- (4-hydroxyphenyl) propanoic acid and ketones, all the results for the identification of organic compounds of OMW and T-OMW by adsorption on clay are grouped together in Table 3. In fact, these results showed a significant variation in organic compounds that are present in OMW. It should be noted also that only benzoic acid, catechol, tyrosol and para-coumaric acid have been identified as compounds already identified in OMW. In addition, after absorption, other new compounds were identified in our sample, which justifies the variation in toxicity and adverse effects of OMW on the environment [38, 39]. The elimination of the phenolic compounds of this pollutant interjected a variation of its effects on the environment and become useful as a source of irrigation in the agricultural field.
Table 3.
The main compounds identified by UHPLC-ESI-MS from OMW, T-OMW, and DT-OMW.
| Organic compounds | m/z |
||
|---|---|---|---|
| OMW | T-OMW | DT-OMW | |
| Nd | 79 | - | 79 |
| Benzoic acid | 120 | 120 | 120 |
| 2,2,5-Triméthyl-3,4-hexanedione | 157 | - | 158 |
| 1,3-Diméthyl-2(1H)-quinoxalinone | 179 | - | - |
| Nd | 215 | - | - |
| 1,2,3-propanetriyl triacetate | 217 | 217 | - |
| 1,2-benzenediol | 254 | - | - |
| 4-Hydroxy-3-methoxyphenethyl alcohol | 319 | 319 | 319 |
| 3,4-dihydroxybenzoic acid | 407 | - | - |
| 4-hydroxy-3-methoxyphenethyl glycol | 445 | - | - |
Nd: not identified.
3.2.3. Determination of total phenolic content (TPC)
OMW are considered highly polluting because they are heavily loaded with organic matter, and they particularly affect the quality of the waters in which they are discharged. The recovery or elimination of polyphenols by adsorption on clays is an important process for the valorization of these two products.
In Figure 4, a variation in the polyphenol content in the OMW and D-OMW samples as a function of time was observed. A removal rate of 50% for both OMW samples demonstrated the adsorption efficiency of these phenolic compounds on the clay with an average dilution factor. These results can be useful for studying the effects of these pollutants in soils and plants. This is in agreement with the previous result of the reduction of the polyphenol content that can explain and confirm the elimination of the organic compounds by the adsorption process. The decrease in the polyphenol content reflects the abrupt variation of the physicochemical properties listed in Table 3. This variation in polyphenol content can change the effect of this pollutant on the soil and the environment.
Figure 4.
Variation in total phenolic content (TPC) of OMW and D-OMW treated by adsorption on clay.
3.2.4. Characterization of clay by infrared after adsorption
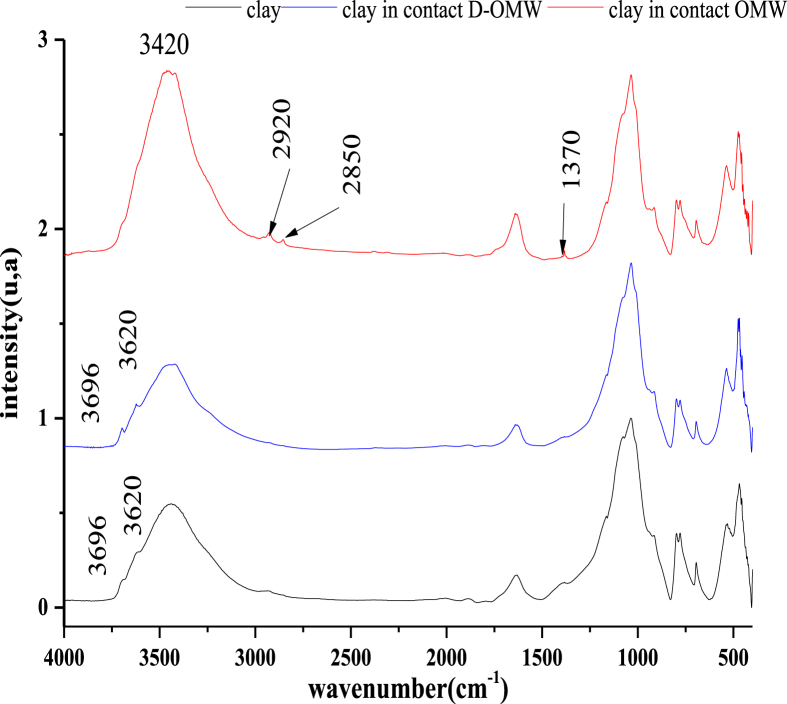
From Figure 5, we notice that we have a fixation of organic matter on the clay. In the range of 1788–3050 cm−1, the appearance of bands assigned to the organic matter with a decrease in the band intensity of 1020 cm−1 corresponds to the Si–O vibration. These results confirm the variation of the physicochemical properties of OMW and the reduction of phenolic compounds. This structural variation of material confirms all the previous results of the characterization of OMW. the biological results are justified by this structural variation on the clay, a decrease in the weight of organic matter in the OMW influences a variation on the physical and chemical properties of OMW, which has an effect on the growth of plants, so it is possible to use these waters after treated in the righteousness which offers a door of valorization of these real pollutants.
Figure 5.
FTIR spectra of clay before and after adsorption of OMW.
3.3. Biological application of treated OMW
3.3.1. Effect of OMW on the germination de Lepidium sativum seeds
The effect of OMW on the germination of Lepidium sativum seeds was calculated by measuring the size of roots and stems of each sample. The results showed that no grain treated with pure OMW was sprouted (Table 4). However, the grains treated by DT-OMW and T-OMW present interesting results, respectively. The results showed that OMW had high toxicity on the germination of seeds and the growth of different plant species. These results were supported by those performed by Mekki and his collaborators, which confirm the toxicity of OMW on soil [40]. Moreover, a study carried out by Danellakis and his group confirmed also the toxicity of OMW in the marine environment [41].
Table 4.
The size of roots and stems of L. sativum seeds treated with different samples of oil mill wastewater.
| Samples | Roots∗(cm) | Stems∗(cm) |
|---|---|---|
| T | 1.966 ± 0.25a | 1.735 ± 0.5a |
| DT-OMW | 1.65 ± 0.484a | 0.983 ± 0.411b |
| T-OMW | 0.666 ± 0.12b | 0.416 ± 0.213bc |
| D-OMW | 0.333 ± 0.265c | 0.3 ± 0.236c |
| OMW | 0±0d | 0±0d |
The same letter was assigned to the values of the same column that doesn't have a significant difference (P < 0.05).
3.3.2. Effect of OMW on the growth of Vicia faba plants
The In vivo effect of treated and non-treated OMW on the growth of plant species showed that its direct release into the environment has adverse effects on agriculture fields and the environment (Table 5). However, our results showed that the treatment of OMW by the adsorption process may eliminate the toxic compounds and improve their uses in irrigation. These results were confirmed by worldwide studies, which confirm the benefits of the elimination of phenolic compounds from OMW on the agriculture field [42]. The use of T-OMW in irrigation has major benefits; it helps to solve the problem of environmental pollution and to obtain additional amounts of water that can be used in irrigation especially in arid and semi-arid regions [43, 44].
Table 5.
Effect of OMW samples on the growth of Vicia faba.
| Length of the stem (cm) | The dry mass of the stems (%) | The dry mass of the roots (%) | Number of nitrogen knot | |
|---|---|---|---|---|
| T | 43.28 ± 0.92a | 64.17 ± 1.25a | 14.83 ± 0.28a | 30.33 ± 0.57a |
| OMW | 14.74 ± 0.49b | 28.9 ± 0.79b | 0±0b | 0±0b |
| D-OMW | 15.12 ± 0.81b | 18.77 ± 0.75c | 0±0b | 0±0b |
| T-OMW | 45.30 ± 0.55a | 56.33 ± 0.57d | 7.47 ± 0.5c | 27.33 ± 1.15c |
| DT-OMW | 46.44 ± 0.5a | 72.83 ± 0.76e | 19.33 ± 0.57d | 29.66 ± 0.57ac |
* The same letter was assigned to the values of the same column that doesn't have a significant difference (P < 0.05).
4. Conclusion
From this work, we can conclude that the studied OMW is an acid pollutant (pH = 4.88), with a high COD (80 g/l), high content of polyphenols, conductivity, dry matter and volatile matter. Moreover, the characterization of clay showed that it has a specific surface area of 51.41 m2 g−1, and is rich in Iron (15.44) with the presence of two intense peaks; one corresponds to calcite and the other to a mixture of quartz, illite, kaolinite, and vermiculite. However, the treatment of OMW by adsorption on clay allows the elimination of toxic chemical compounds, which makes it useful for irrigation in the agricultural field.
Declarations
Author contribution statement
Younes Dehmani, Abdelaziz Ed-Dra, Omar Zennouhi, Aziz Bouymajane: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Fouzia Rhazi Filali, Laila Nassiri, Sadik Abouarnadasse: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ibrahimoglu B., Yilmazoglu M.Z. Disposal of olive mill wastewater with DC arc plasma method. J. Environ. Manag. 2018;217:727–734. doi: 10.1016/j.jenvman.2018.03.134. [DOI] [PubMed] [Google Scholar]
- 2.Chehab H., Tekaya M., Ouhibi M., Gouiaa M., Zakhama H., Mahjoub Z., Laamari S., Sfina H., Chihaoui B., Boujnah D., Mechri B. Effects of compost, olive mill wastewater and legume cover cropson soil characteristics, tree performance and oil quality of olive trees cv.Chemlali grown under organic farming system. Sci. Hortic. (Amst.) 2019;253:163–171. [Google Scholar]
- 3.Flores N., Brillas E., Centellas F., Rodríguez R.M., Cabot P.L., Garrido J.A., Sirés I. Treatment of olive oil mill wastewater by single electrocoagulation with different electrodes and sequential electrocoagulation/electrochemical Fenton-based processes. J. Hazard Mater. 2018;347:58–66. doi: 10.1016/j.jhazmat.2017.12.059. [DOI] [PubMed] [Google Scholar]
- 4.Yang S., Li W., Zhang H., Wen Y., Ni Y. Treatment of paper mill wastewater using a composite inorganic coagulant prepared from steel mill waste pickling liquor. Separ. Purif. Technol. 2019;209:238–245. [Google Scholar]
- 5.El-Desoky H., Farouk S., Heikal M., El-Mahallawy M., Wahid A. Geochemical and technical investigation of some clay materials in the Bahariya Oasis, Western Desert, Egypt: implication in the vitrified clay pipes industry. J. Afr. Earth Sci. 2019;160:103612. [Google Scholar]
- 6.Mushtaq F., Zahid M., Bhatti I.A., Nasir S., Hussain T. Possible applications of coal fly ash in wastewater treatment. J. Environ. Manag. 2019;240:27–46. doi: 10.1016/j.jenvman.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 7.Abdelkreem M. Adsorption of phenol from industrial wastewater using olive mill waste. APCBEE Procedia. 2013;5:349–357. [Google Scholar]
- 8.Ma L., He M., Fu P., Jiang X., Lv W., Huang Y., Liu Y., Wang H. Adsorption of volatile organic compounds on modified spherical activated carbon in a new cyclonic fluidized bed. Separ. Purif. Technol. 2020;235 [Google Scholar]
- 9.Azarkan S., Peña A., Draoui K., Sainz-Díaz C.I. Adsorption of two fungicides on natural clays of Morocco. Appl. Clay Sci. 2016;123:37–46. [Google Scholar]
- 10.Cavallaro G., Grillo I., Gradzielski M., Lazzara G. Structure of hybrid materials based on halloysite nanotubes filled with anionic surfactants. J. Phys. Chem. C. 2016;120:13492–13502. [Google Scholar]
- 11.Lazzara G., Cavallaro G., Panchal A., Fakhrullin R., Stavitskaya A., Vinokurov V., Lvov Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018;35:42–50. [Google Scholar]
- 12.Lisuzzo L., Cavallaro G., Parisi F., Milioto S., Lazzara G. Colloidal stability of halloysite clay nanotubes. Ceram. Int. 2019;45:2858–2865. [Google Scholar]
- 13.Cavallaro G., Chiappisi L., Pasbakhsh P., Gradzielski M., Lazzara G. A structural comparison of halloysite nanotubes of different origin by Small-Angle Neutron Scattering (SANS) and Electric Birefringence. Appl. Clay Sci. 2018;160:71–80. [Google Scholar]
- 14.Lisuzzo L., Cavallaro G., Lazzara G., Milioto S., Parisi F., Stetsyshyn Y. Stability of halloysite, imogolite, and boron nitride nanotubes in solvent media. Appl. Sci. 2018;8:1068. [Google Scholar]
- 15.Ragab R., Prudhomme C. Climate change and water resources management in arid and semi-arid regions: prospective and challenges for the 21st century. Biosyst. Eng. 2002;81:3–34. [Google Scholar]
- 16.Aazza M., Ahlafi H., Moussout H., Maghat H. Ortho-Nitro-Phenol adsorption onto alumina and surfactant modified alumina: kinetic, isotherm and mechanism. J. Environ. Chem. Eng. 2017;5:3418–3428. [Google Scholar]
- 17.Ouallal H., Dehmani Y., Moussout H., Messaoudi L., Azrour M. Kinetic, isotherm and mechanism investigations of the removal of phenols from water by raw and calcined clays. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 19.Barbouchi M., Elamrani K., El Idrissi M., Choukrad M. A comparative study on phytochemical screening, quantification of phenolic contents and antioxidant properties of different solvent extracts from various parts of Pistacia lentiscus L. J. King Saud Univ. Sci. 2018 [Google Scholar]
- 20.Studzińska S., Buszewski B. Study of toxicity of imidazolium ionic liquids to watercress (Lepidium sativum L.) Anal. Bioanal. Chem. 2009;393:983–990. doi: 10.1007/s00216-008-2523-9. [DOI] [PubMed] [Google Scholar]
- 21.da Silva Favero J., dos Santos V., Weiss-Angeli V., Gomes L.B., Veras D.G., Dani N., Mexias A.S., Bergmann C.P. Evaluation and characterization of Melo Bentonite clay for cosmetic applications. Appl. Clay Sci. 2019;175:40–46. [Google Scholar]
- 22.Bentahar Y., Draoui K., Hurel C., Ajouyed O., Khairoun S., Marmier N. Physico-chemical characterization and valorization of swelling and non-swelling Moroccan clays in basic dye removal from aqueous solutions. J. Afr. Earth Sci. 2019;154:80–88. [Google Scholar]
- 23.Michot L.J. Determination of surface areas and textural properties of clay minerals. Dev. Clay Sci. 2018;9:23–47. [Google Scholar]
- 24.Li Z., Shen X., Qi Z., Hu R. Study on the pore structure and fractal characteristics of marine and continental shale based on mercury porosimetry, N2 adsorption and NMR methods. J. Nat. Gas Sci. Eng. 2018;53:12–21. [Google Scholar]
- 25.Mkaouar S., Maherzi W., Pizette P., Zaitan H., Benzina M. A comparative study of natural Tunisian clay types in the formulation of compacted earth blocks. J. Afr. Earth Sci. 2019;160:103620. [Google Scholar]
- 26.Ouaddari H., Beqqour D., Bennazha J., El Amrani I.E., Albizane A., Solhy A., Varma R.S. Natural Moroccan clays: comparative study of their application as recyclable catalysts in Knoevenagel condensation. Sustain. Chem. Pharm. 2018;10:1–8. [Google Scholar]
- 27.Nabbou N., Belhachemi M., Boumelik M., Merzougui T., Lahcene D., Harek Y., Zorpas A.A., Jeguirim M. Removal of fluoride from groundwater using natural clay (kaolinite): optimization of adsorption conditions. Compt. Rendus Chem. 2019;22:105–112. [Google Scholar]
- 28.Bentahar S., Dbik A., El Khomri M., El Messaoudi N., Lacherai A. Adsorption of methylene blue, crystal violet and Congo red from binary and ternary systems with natural clay: kinetic, isotherm, and thermodynamic. J. Environ. Chem. Eng. 2017;5:5921–5932. [Google Scholar]
- 29.Ouaddari H., Karim A., Achiou B., Saja S., Aaddane A., Bennazha J., El Amrani El Hassani I., Ouammou M., Albizane A. New low-cost ultrafiltration membrane made from purified natural clays for direct Red 80 dye removal. J. Environ. Chem. Eng. 2019;7:103268. [Google Scholar]
- 30.García C.A., Hodaifa G. Real olive oil mill wastewater treatment by photo-Fenton system using artificial ultraviolet light lamps. J. Clean. Prod. 2017;162:743–753. [Google Scholar]
- 31.Vuppala S., Bavasso I., Stoller M., Di Palma L., Vilardi G. Olive mill wastewater integrated purification through pre-treatments using coagulants and biological methods: experimental, modelling and scale-up. J. Clean. Prod. 2019;236:117622. [Google Scholar]
- 32.Azzam M.O.J. Olive mills wastewater treatment using mixed adsorbents of volcanic tuff, natural clay and charcoal. J. Environ. Chem. Eng. 2018;6:2126–2136. [Google Scholar]
- 33.Atallah E., Kwapinski W., Ahmad M.N., Leahy J.J., Al-Muhtaseb A.H., Zeaiter J. Hydrothermal carbonization of olive mill wastewater: liquid phase product analysis. J. Environ. Chem. Eng. 2019;7:102833. [Google Scholar]
- 34.Papoušková B., Bednář P., Hron K., Stávek J., Balík J., Myjavcová R., Barták P., Tománková E., Lemr K. Advanced liquid chromatography/mass spectrometry profiling of anthocyanins in relation to set of red wine varieties certified in Czech Republic. J. Chromatogr., A. 2011;1218:7581–7591. doi: 10.1016/j.chroma.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Prokudina E.A., Havlíček L., Al-Maharik N., Lapčík O., Strnad M., Gruz J. Rapid UPLC-ESI-MS/MS method for the analysis of isoflavonoids and other phenylpropanoids. J. Food Compos. Anal. 2012;26:36–42. [Google Scholar]
- 36.Obied H.K., Allen M.S., Bedgood D.R., Prenzler P.D., Robards K., Stockmann R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005;53:823–837. doi: 10.1021/jf048569x. [DOI] [PubMed] [Google Scholar]
- 37.Dermeche S., Nadour M., Larroche C., Moulti-Mati F., Michaud P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013;48:1532–1552. [Google Scholar]
- 38.Atallah E., Kwapinski W., Ahmad M.N., Leahy J.J., Zeaiter J. Effect of water-sludge ratio and reaction time on the hydrothermal carbonization of olive oil mill wastewater treatment: hydrochar characterization. J. Water Process Eng. 2019;31:100813. [Google Scholar]
- 39.Dammak I., Khoufi S., Sayadi S. A performance comparison of olive oil mill wastewater enzymatic treatments. Food Bioprod. Process. 2016;100:61–71. [Google Scholar]
- 40.Mekki A., Dhouib A., Sayadi S. Polyphenols dynamics and phytotoxicity in a soil amended by olive mill wastewaters. J. Environ. Manag. 2007;84:134–140. doi: 10.1016/j.jenvman.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Danellakis D., Ntaikou I., Kornaros M., Dailianis S. Olive oil mill wastewater toxicity in the marine environment: alterations of stress indices in tissues of mussel Mytilus galloprovincialis. Aquat. Toxicol. 2011;101:358–366. doi: 10.1016/j.aquatox.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Mekki A., Dhouib A., Sayadi S. Review: effects of olive mill wastewater application on soil properties and plants growth. Int. J. Recycl. Org. Waste Agric. 2013;2:15. [Google Scholar]
- 43.Kokkora M., Vyrlas P., Papaioannou C., Petrotos K., Gkoutsidis P., Leontopoulos S., Makridis C. Agricultural use of microfiltered olive mill wastewater: effects on maize production and soil properties. Agric. Agric. Sci. Procedia. 2015;4:416–424. [Google Scholar]
- 44.Nethononda V.G., Elumalai V., Rajmohan N. Irrigation return flow induced mineral weathering and ion exchange reactions in the aquifer, Luvuvhu catchment, South Africa. J. Afr. Earth Sci. 2019;149:517–528. [Google Scholar]