Abstract
Measurement of the antioxidant potential using in vitro assays is paramount in the assessment of various food products and nutraceuticals. Researchers always attempt to develop more accurate assays which can be performed in unsophisticated conditions. This novel method, Ferric-Bipyridine reducing capacity of total antioxidants (FBRC) is a very simple, accurate assay performed based on the reduction of Fe (III) to Fe (II) by antioxidants with the formation of a colored complex with bipyridine (Bp) i.e, Fe(II)-Bp. The FBRC method thus developed was assessed under carefully adjusted parameters of oxidant concentration, pH, temperature, solvent, light and time in order to fix the optimum conditions for the assay. The spectrophotometric monitoring of Fe(II)-Bp complex was noted by the formation of an intense pink color at room temperature with absorption maxima at 535 nm, pH 4. The analytical performance of this method was fully validated, and the obtained results were satisfactory. It was successfully applied to measure the total antioxidant capacity of standard compounds such as gallic acid, ascorbic acid and butylated hydroxy toluene (BHT), in addition to some plant extracts and oils. The FBRC method is inexpensive, reproducible and simple to perform. In addition, the antioxidant activity of the tested compounds compared to common reference methods showed that the novel FBRC method is superior to the Ferric reducing antioxidant power (FRAP) with regard to its use of realistic pH and faster kinetics. Thus, the FBRC method is convenient for the estimation of total antioxidant in plants extracts, natural products, essential oils and food stuff.
Keywords: Analytical chemistry, Food science, Food analysis, Food chemistry, Biochemistry, Toxicology, Analytical biochemistry, Ferric-bipyridine assay, Ferric reducing power, Novel spectrophotometric assay, Total antioxidant activity
Analytical chemistry; Food science; Food analysis; Food chemistry; Biochemistry; Toxicology; Analytical biochemistry, Ferric-bipyridine assay, Ferric reducing power, Novel spectrophotometric assay, Total antioxidant activity.
1. Introduction
Antioxidants are compounds capable of inhibiting or delaying the oxidation processes that occurs under the influence of atmospheric oxygen or reactive oxygen species (ROS) [1]. They play an important role in preserving good health and are known to reduce the risk for chronic diseases such as cancer and heart disease [2]. The primary sources of antioxidant-rich food are whole grains, fruits and vegetables. Even antioxidants sourced from plants such as vitamin C, vitamin E, carotenes, phenolic acids and phytoestrogens have the potential to reduce disease risk [3, 4]. A typical plant-based diet provides most of the antioxidant compounds possessing various biochemical and physical properties. For example, gallic acid is known to possess strong antioxidant activity, while others, such as the mono-phenols are weak antioxidants [5]. Biological systems possess a number of free radicals and reactive oxygen species derived from a wide variety of sources [6]. These oxidize biomolecules such as nucleic acids, proteins, lipids or DNA and can initiate degenerative diseases [4, 7]. The distinguishing characteristic of an antioxidant is its ability to scavenge free radicals thereby inhibiting oxidative mechanisms that lead to degenerative diseases [8, 9]. This disease reducing capacity of antioxidants in vivo has strengthened the need to evaluate the antioxidant activity of food and nutraceuticals by simple, accurate and rapid assays that can be easily carried out in the nutrition laboratory. A number of such methods are available [10, 11] which differ from each other in terms of the reagents, substrates, experimental condition, reaction medium, and standard analytical evaluation methods [12]. There is however no easy methodology to compare and select the best assay method due to varied experimental conditions and difference in the physical and chemical properties of oxidizable substrates [13, 14]. Nevertheless, the assay may be divided in two systems (i) Antioxidant assays in aqueous system (DPPH, ABTS, DNA protection etc.) [9] and (ii) Antioxidant assays in lipid system thiobarbituric acid reactive substances (TBARS). In addition, based on the chemical reaction, they can be further divided into two categories- (i) Hydrogen atom transfer reaction (HAT) [15] and (ii) Single electron transfer (ET) reaction based system [16, 17].
A variety of ligands have been used in iron (III)-based assays for the determination of total antioxidant capacity (TAC). These are 1,10-phenanthroline, 4,7-diphenyl-1,10-phenanthroline (batho-phenanthroline) [18, 19], 2,4,6-tris(2-pyridyl)-1,3,5-triazine (TPTZ) [3, 20], ferrozine [21] and ferricyanide [22, 23]. The most widely used ferric reducing antioxidant assay (FRAP), which uses TPTZ as a ligand, was extensively criticized for the use of an unrealistic pH of 3.6 at which dissociation of phenolics is reduced thereby reducing susceptibility to oxidative attack by the reagent. Low pH also slows the kinetics of the reaction, hindering the completion of oxidation of specific compounds like hydroxycinnamic acids and thiols. Another disadvantage of the FRAP method is the higher affinity of the reagent towards hydrophilic antioxidants than hydrophobic ones. The redox reaction that may occur with antioxidants leads to the production of unbound Fe(II) which is suspected to cause redox cycling of antioxidants during the assay as a result of Fe(II)- mediated Fenton-type reactions [21].
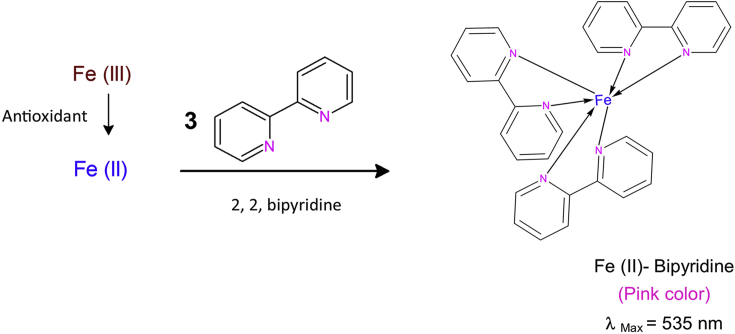
No assay has been reported in literature capable of measuring total antioxidant activity through Fe(III) reduction in the presence of bipyridine. Bipyridine is a partially selective and very sensitive reagent for Fe(II) emerging as a result of reduction [24].
The proposed assay depends on the reduction of Fe(III)-bipyridine reagent to the stable pink colored Fe(II)-bipyridine chelate by antioxidants in a buffered medium (Figure 1). The apparent molar absorptivity, linear concentration range and equivalent antioxidant capacity values of the studied antioxidants were found in the proposed assay.
Figure 1.
Stoichiometry outline of FBRC method.
2. Materials and methods
2.1. Chemicals
All chemicals used were of analytical grade. The TPTZ (tripyridyl tetra diazine), Bipyridine (Bp), Ferric chloride and 2,2- diphenylpicrylhydrazyl (DPPH) were obtained from Aldrich. Ascorbic acid, CH3COONa, CH3COOH, HCl and all metal ions were purchased from BDH. Gallic acid and butylated hydroxytoluene (BHT) were from Merck.
2.2. Instruments
All spectrophotometric measurements were made using UV-Vis Spectrophotometer (Specord200, Analytikjena, Germany) attached to HP computer. All assays were performed with a 1cm path length using a pair of matched quartz cuvettes. The pH measurements were made with the aid of a pH meter (Metrohm, Switzerland).
2.3. Parameters examined for the novel FBRC assay
A number of parameters were examined in order to determine the optimum conditions for total antioxidant assay using the FBRC method. The parameters evaluated were: effect of pH, time, temperature, solvent and light. The effect of pH was studied using 0.3 M sodium acetate buffer (pH 4, 5, 6) to evaluate the optimum pH for complexation between the metal ion reduced by antioxidants and bipyridine. The optimum temperature was determined by performing the reaction at 10, 15, 20, 30, 40 and 50 °C. The optimum time for completion of the reaction was gauged at intervals of 10 min. The effect of solvent was studied by using different percentages of ethanol and water (10%, 20%, 30%, 40%, 50%, 60%, 70% and 80%).
2.4. Ferric-bipyridine reducing capacity of total antioxidants (FBRC)
2.4.1. Calibration curve of standard antioxidant
To a fixed aliquot of the metal ion solution (1 ml, 10−2 M FeCl3.6H2O) taken in a 10ml volumetric flask, different volumes of the standard antioxidants (10−3 M) were added (0.01 ml, 0.02 ml, 0.04 ml, 0.06 ml, 0.08 ml, 0.1 ml, 0.2 ml, 0.4 ml, 0.6 ml, 0.8 ml, 1.0 ml and 2.0 ml). This was followed by 2.0 ml 0.3M acetate buffer (pH 4) and 1.0 ml bipyridine (6.4×10−3 M). The volume was made up to the mark with deionized water, incubated for 10 min at room temperature and measured calorimetrically at 535 nm against a blank composed of 1.0 ml FeCl3 solution, 2.0 ml 0.3M acetate buffer (pH 4) made up to 10 ml with deionized water. The absorbance values were plotted against the concentration of the various antioxidant solutions.
2.4.2. Antioxidant capacity of plant extract and essential oils
Similarly, 0.04 ml of 10 % of aqueous extract or essential oil was reacted with 1.0 ml 0.01M FeCl3 solution, 1.0 ml bipyridine and 2.0 ml 0.3M acetate buffer (pH 4). This mixture was diluted to 10 ml with deionized water for the antioxidant assay.
2.5. FRAP assay for total antioxidant activity
The FRAP assay developed by Benzie and Strain [25] measures the reduction of ferric 2,4,6-tripyridyl-s-triazine (TPTZ) to a colored product. FRAP reagent was prepared fresh just before use by mixing CH3COOH/CH3COONa buffer (30 mM; pH 3.6), TPTZ solution (10 mM) and ferric chloride solution (20 mM) in the ratio 10:1:1. 40μL of the 10% plant extract (v/v) was added to 2.0 ml FRAP reagent and incubated for 15 min in the dark. The absorbance was measured at 610 nm with a spectrophotometer. Ascorbic acid was employed as a standard in this assay. The result of antioxidant power was expressed as ascorbic acid equivalents (AAE) μmol/ml extract, mean ± SD of five determinations.
2.6. DPPH radical scavenging assay of total antioxidant activity
A solution of the DPPH radical was prepared in methanol to a final concentration of 3×10−3 M. Ascorbic acid as DPPH scavenging compound was used as the positive control. To 1.0 ml of the methanolic solution of DPPH (3 ×10−3 M), 10 μl of essential oil or extract were added and the mixture was incubated at room temperature for 30 min [21]. The absorbance was measured at 517 nm against a suitable blank. % radical scavenging activity was calculated using the equation (% of scavenging activity = Ac -At/Ac × 100).where A is the absorbance at a wavelength of 517 nm IC50 was calculated from the linear regression algorithm of the graph plotted for % inhibition against the extract concentration. IC50 values denote the concentration of the sample required to scavenge 50% of DPPH free radicals [26]. All experiments were carried out in five replicates.
2.7. Plant material and extraction
The plant material collected from nature included Thyme, Eugenol, and Cisuss. The plant samples were dried, powdered and extracted by two different methods namely, steam distillation and Soxhlet extraction. The extracts were filtered and evaporated using rotary evaporator [27]. Almond oil and olive oil were collected from the market.
2.8. Calculation of total antioxidant capacity of herbal material
The molar absorptivity of ascorbic acid, gallic acid and BHT in the above reference methods and the FBRC methods were calculated. The antioxidant capacity calculated from the calibration curve was expressed both in μM equivalents of gallic acid and ascorbic acid, reported in 1 ml plant extract.
2.9. Statistical analysis
All presented data are expressed as mean ± SD of five measurements. The statistical significance between the two methods was analyzed using F-test and t-test with Prism 6 software (GraphPad, San Diego, CA, USA). A value of p < 0.05 was considered significant.
3. Results and discussion
3.1. Parameters examined for FBRC assay
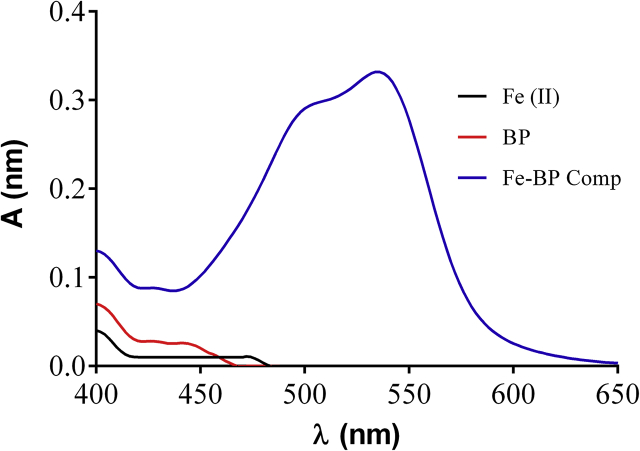
Fe(II)-bipyridine complex produced by the reaction of Fe(III) with antioxidants followed by bipyridine exhibited maximum absorption at 535 nm (Figure 2). Based on the appearance of this peak, a method was proposed to detect the antioxidant activity.
Figure 2.
Absorption spectra of Fe2+ -Bp complex (10−3M Fe3+ +10−3 M Bp + 10−2M ascorbic acid).
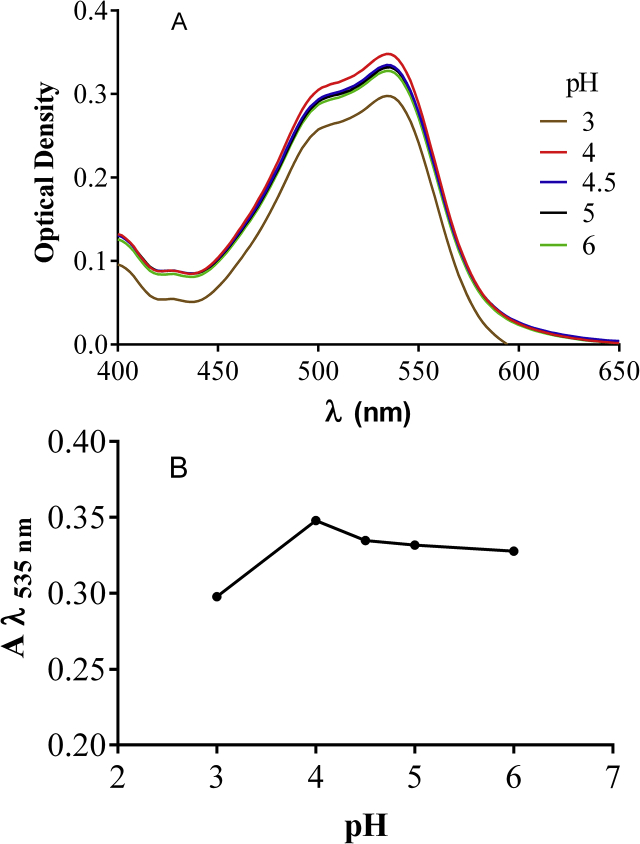
A series of experiments were then conducted to establish the optimum analytical conditions for the detection of total antioxidant activity. From the reaction of bipyridyl with Fe(III) it was evident that pH 4 favors complex formation (Figure 3 A and B). This value was selected as the working value.
Figure 3.
Effect of pH on Fe2+-Bp complex (10−3MFe3++10−3M Bp + 10−3M ascorbic acid) (A) on λmax (B) Absorption at λmax = 535 nm.
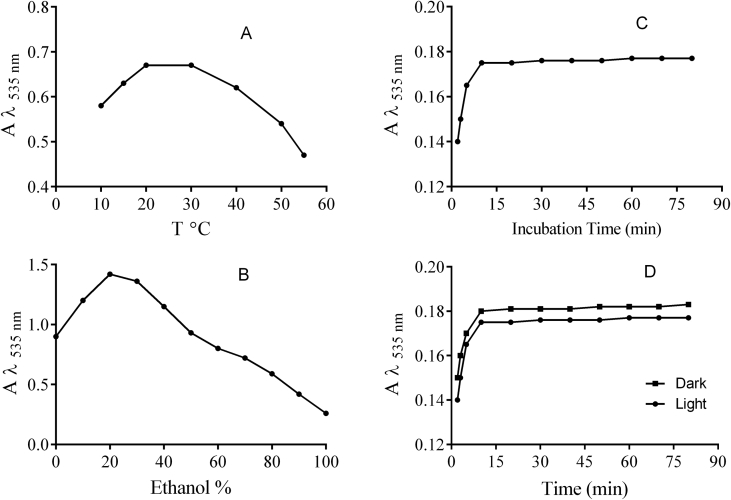
Temperature is known to affect complexation reactions. Hence, a range of temperature between 10-50 °C was examined to determine the optimum temperature for the assay. It was found that the FBRC method works at all temperatures under 55 °C (Figure 4A).
Figure 4.
Effect of some factors on the absorption of Fe2+-Bp complex (10−3M Fe3++10−3 M Bp + 10−3 M ascorbic acid) at pH 4 with λmax = 535 nm. Temperature (A), solvent (B), time (C) and light (D).
The next parameter evaluated was the stability of the complex formed at different ratios of ethanol and water. It was found that stability was maximum at 20% ethanol concentration and hence this was chosen as the best ratio for completing the reaction (Figure 4B).
While the exact redox potential of the Fe2+-bipyridine complex is not known in literature, it can be estimated that this potential would be higher than the standard Fe3+-Fe2+ potential of 0.77 V due to preferential stabilization of the Fe2+ state by binding to bipyridine. Since the standard potentials of most antioxidants lie in the range of 0.2–0.8 V, it can be estimated that Fe2+-bipyridine should be able to oxidize the antioxidants under study. As a general rule, it may be envisaged that as the redox potentials of the oxidant and reductant approach each other, complete oxidation of the antioxidant by the oxidizing reagent would take more time. Figure 4C exhibits changes in the absorbance at 535 nm with time during the first few minutes from the initiation of the reaction. As can be seen in Figure 4C, the absorbance reaches a maximum at 10 min and remains constant afterwards. In general, the reaction kinetics for the proposed FBRC assay is faster than that of the FRAP assay. Therefore, absorbance measurements were performed after 10 min from initiation of the reaction.
The proposed FBRC method was examined in dark and light to comprehend the influence of light on the reaction. Most assays determine total antioxidant activity performed in the dark under special conditions. As seen, there is no significant difference between the reaction in dark and light (Figure 4D). The experiment can hence be done both in light and dark, whereas FRAP and DPPH methods require complete darkness for accuracy.
3.2. Spectrophotometric method development
The indirect FBRC spectrophotometric method developed for determining total antioxidant activity is based on the redox reaction between phenolic compounds and Fe(III) at room temperature. The initial antioxidant concentration is indicated by the concentration of the oxidizing Fe(III). Therefore, selection of the maximum absorption wavelength for Fe(III) is important. Also, solvent acidity should be properly adjusted such that this wavelength does not shift with pH.
3.3. Calibration lines
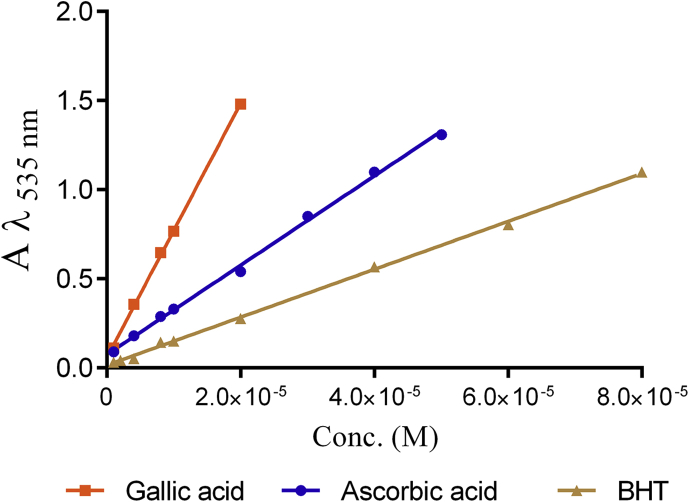
The calibration lines of the selected antioxidants (i.e., ascorbic acid, gallic acid and BHT) with respect to the proposed method were drawn as shown in Figure 5 and Table 1.
Figure 5.
Calibration curves of three standard antioxidants with Fe-Bp complex.
Table 1.
The FBRC method performed well for ascorbic acid, gallic acid and BHT.
| Antioxidant compound | Concentration range for ideal linearity (mol/L) | Molar absorptivity L.mol−1.cm−1 | r2 |
|---|---|---|---|
| Ascorbic acid | 1.0×10−6 – 5.0×10−5 | 13445.1 | 0.9979 |
| Gallic acid | 1.0×10−6 – 2.0×10−5 | 76189.4 | 0.9992 |
| BHT | 1.0×10−6 – 8.0×10−5 | 25068.5 | 0.9989 |
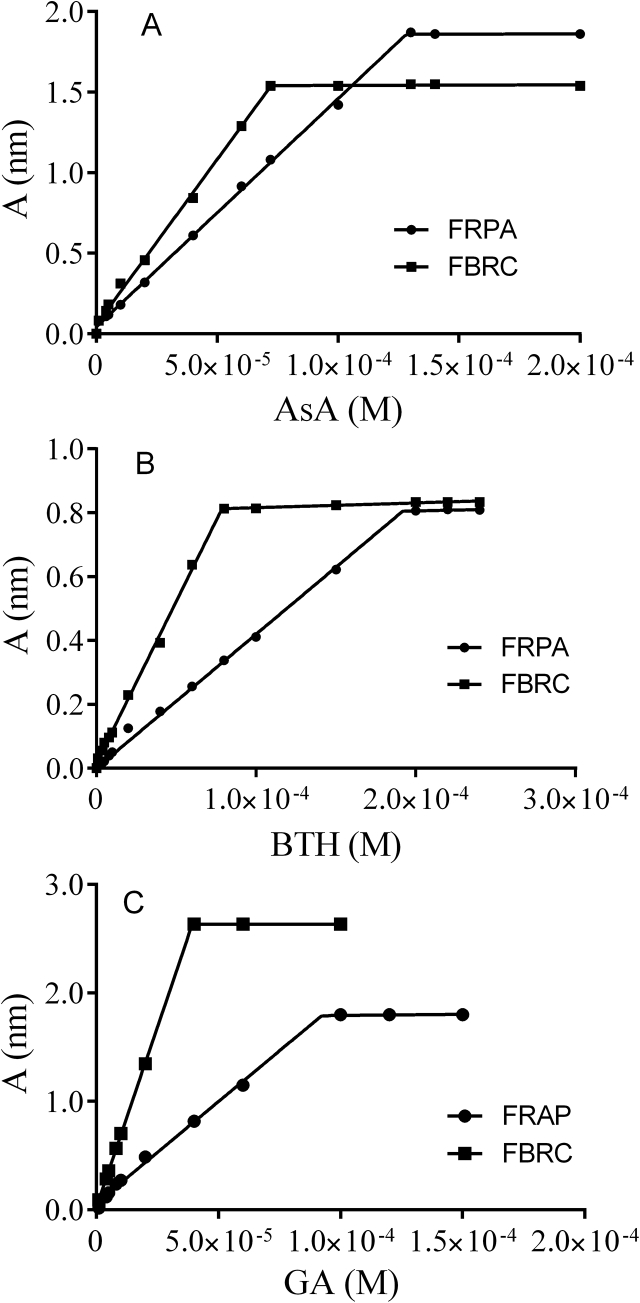
The statistical details of the new developed method in comparison with reference method are presented in Table 2 and Figure 6.
Table 2.
Statistical result comparison of FBRC and FRAP for determination of total antioxidant activity using three standard antioxidants.
| Parameters | Ascorbic acid |
Gallic acid |
BHT |
|||
|---|---|---|---|---|---|---|
| FBRC | FRAP | FBRC | FRAP | FBRC | FRAP | |
| n | 5 | 5 | 5 | 5 | 5 | 5 |
| Mean of blank | 1.80E-04 | 2.60E-04 | 1.60E-04 | 4.00E-04 | 1.20E-04 | 1.60E-04 |
| SD | 7.48E-05 | 4.90E-05 | 4.90E-05 | 5.77E-05 | 6.83E-05 | 4.47E-05 |
| Sm | 3.35E-05 | 2.19E-05 | 2.19E-05 | 2.58E-05 | 3.06E-05 | 2.00E-05 |
| RSD | 7.483E-05 | 4.9E-05 | 4.9E-05 | 6.32E-05 | 7.48E-05 | 4.9E-05 |
| RSD% | 7.48E-03 | 4.90E-03 | 4.90E-03 | 1.22E-01 | 7.48E-03 | 4.90E-03 |
| variance | 5.6E-09 | 2.4E-09 | 2.4E-09 | 3.33E-09 | 4.67E-09 | 2E-09 |
| LOD mol/L | 2.47E-04 | 1.62E-04 | 1.62E-04 | 1.91E-04 | 2.25E-04 | 1.48E-04 |
| LOQ mol/L | 7.48E-04 | 4.90E-04 | 4.90E-04 | 5.77E-04 | 6.83E-04 | 4.47E-04 |
| F. test (6.39)a | 1.53 | 0.04 | 1.53 | |||
| T. test (2.26)a | 1.69 | 1.33 | 0.95 | |||
n: number of replicates; SD: Standard deviation of the blank; Sm: Standard division of the mean; LOQ: limits of quantitation; LOD: limit of detection; RSD: Relative standard division. BTH: Butylated hydroxy toluene.
Values between parenthesis are the tabulated t and F values respectively, at p = 0.05 [28].
Figure 6.
Comparison of calibration curves for FBRC and FRAP (A)ascorbic acid (AsA), (B) BHT, and (C) gallic acid.
3.4. Divalent ions interferences
Since the method developed was based on measuring the complex of Bp with Fe (II) oxidized by antioxidants, transition and heavy metal cations like Cu2+, Cd2+, Co2+, Hg2+, Pb2+ and Zn2+ did not interfere with Fe(II)-Bp of the proposed method. This reflects a factual result even in presence of the above ions in the antioxidant extract.
3.5. Analysis of herbal samples and comparison of results
Spectrophotometric determination of some plant extracts in ethanol using the developed FBRC method was reported (Table 3). The ascorbic-equivalent antioxidant capacities of the herbal samples (thyme, eugenol, cisuss, almond and olive oils) and measured on five different runs with the proposed FBRC method were comparable to those found by DPPH and FRAP reference methods (Table 3). It is accepted that the results obtained with a variety of electron-transfer based antioxidant assays would give comparable but not identical results for real samples due to the reagents used, stability issues during storage and application. This is the first documentation of a ferric-bipyridine complex formation based total antioxidant assay. For potential researchers who wish to practice and improve the developed method, we propose the name ferric-bipyridine reducing capacity of antioxidants ‘FBRC’ to be further used for the assay of the antioxidants capacity of plants extracts, natural products, essential oils and food stuff.
Table 3.
The values of FBRC method and reference methods for some common natural extracts.
| sample | DPPH μmL/mL* |
FRAP μmol/mL |
FBRC μmol/mL |
|---|---|---|---|
| Thyme oil | 11.5 | 25.42 ± 2.94 | 9.38 ± 0.33 |
| Cisuss extract | 14 | 45.28 ± 2.94 | 27.66 ± 0.32 |
| Eugenol oil | 11.4 | 29.54 ± 2.94 | 24.54 ± 0.29 |
| Menthol | 13 | 19.84 ± 4.85 | 5.96 ± 0.32 |
| Almond Oil | 13.2 | 18.14 ± 2.94 | 4.6 ± 0.29 |
| Olive Oil | 13.6 | 12.6 ± 2.94 | 3.44 ± 0.28 |
n = 5, * DPPH values are IC50.
3.6. Advantages of the new developed FBRC assay
The new proposed FBRC method is superior to the widely used FRAP method [21] with regard to determination of ascorbic acid equivalent (AAE) for plant extracts. This is because in the present study, the AAE values for Thyme, Eugenol, Cisuss, Almonds oil and Olive oil were better than that of FRAP (Table 3). In addition, the reagent Bipyridine used for the FBRC assay is in the linear range of the calibration curve possessing larger linearity compared to that of FRAP (Figures 5 and 6). The near neutral pH used in this assay (pH 4–6) is significantly higher than that of the widely used FRAP assay. The acidic medium of FRAP is rather unrealistic (pH 3.6) in regard to simulation of antioxidant action under physiological conditions. The reducing capacity of antioxidants may be suppressed due to protonation at significantly more acidic conditions. The proposed method is also easy, flexible, and of low-cost.
4. Conclusions
The novel FBRC method has been developed for the inexpensive, simple and versatile assay of antioxidants from food. Fe(III) easily oxidizes antioxidants in the presence of the bipyridine ligand while itself undergoes reduction to the Fe(II)-Bp complex thereby yielding a high molar absorptivity ensuring a highly sensitive assay. The assay involves the non-laborious use of a spectrophotometer common to most laboratories. Bipyridine is purchased by many food laboratories for use in iron binding capacity assays and may also be advantageously used for total antioxidant capacity measurements. The Fe(II)-Bp chelate is stable over a wide range of pH i.e., 4–6. The FRAP method commences with the light blue complex of Fe(III)-TPTZ which is then converted to a more intense blue complex of Fe(II)-TPTZ. In the developed FBRC method, no complex is formed with Fe(III) and hence no interferences with colors or light when compared to DPPH and FRAP methods. The FBRC assay is expected to be used by researchers for the assay of antioxidant capacities of plants extracts, natural products, essential oils and food stuff. In addition, it will be useful for conventional food laboratories not necessitating sophisticated equipment and highly skilled operators.
Declarations
Author contribution statement
Khalid Mohammed Naji: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Faten Hameed Thamer: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Abdulqawi Ahmed Numan: Performed the experiments; Analyzed and interpreted the data.
Eqbal Mohammed A. Dauqan, Yahya Mohammed Alshaibi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Myrene Roslen D'souza: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to acknowledge the Faculty of Science and The Central Research Lab of the Faculty of Science, Sana'a University for providing all general research facilities.
References
- 1.Badarinath M.R., Chetty M.S. A review on In-vitro antioxidant methods: comparisions, correlations and considerations. Int. J. PharmTech Res. 2010;2(2):1276–1285. [Google Scholar]
- 2.Formagio A., Volobuff C., Santiago M., Cardoso C., Vieira M., Valdevina Z.P. Evaluation of antioxidant activity, total flavonoids, tannins and phenolic compounds in psychotria leaf extracts. Antioxidants. 2014;3(4):745–757. doi: 10.3390/antiox3040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad S., Arshad M.A., Ijaz S., Khurshid U., Rashid F., Azam R. Review on methods used to determine Antioxidant activity. Int. J. Multidiscip. Res. Dev. 2014;1(1):35–40. [Google Scholar]
- 4.Benzie I.F.F., Choi S.W. first ed. Vol. 71. Elsevier Inc.; 2014. (Antioxidants in Food: Content, Measurement, Significance, Action, Cautions, Caveats, and Research Needs). [DOI] [PubMed] [Google Scholar]
- 5.Katalinic V., Milos M., Kulisic T., Jukic M. Food Chemistry Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–557. [Google Scholar]
- 6.De Araújo K., de Lima A., Silva J.N., Rodrigues L.L., Amorim G.N.A., Quelemes P.V. Identification of phenolic compounds and evaluation of antioxidant and antimicrobial properties of Euphorbia tirucalli L. Antioxidants. 2014;3:159–175. doi: 10.3390/antiox3010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcão S.I., Freire C., Vilas-Boas M. A proposal for physicochemical standards and antioxidant activity of Portuguese propolis. JAOCS, J. Am. Oil Chem. Soc. 2013;90(11):1729–1741. [Google Scholar]
- 8.Panda S.K. Intech; 2012. Assay Guided Comparison for Enzymatic and Non-enzymatic Antioxidant Activities with Special Reference to Medicinal Plants; pp. 381–400. [Google Scholar]
- 9.Wink M., Setzer W.N. Radical scavenging and antioxidant activities of essential oil components – an experimental and computational investigation NPC. Nat. Prod. Commun. 2015;10(1):153–156. [PubMed] [Google Scholar]
- 10.Marinova G., Batchvarov V. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg. J. Agric. Sci. 2011;17(1):11–24. [Google Scholar]
- 11.Tirzitis G., Bartosz G. Determination of antiradical and antioxidant activity: basic principles and new insights. Acta Biochim. Pol. 2010;57(1):139–142. [PubMed] [Google Scholar]
- 12.Antolovich M., Prenzler P.D., Patsalides E., McDonald S., Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–198. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- 13.Alam M.N., Bristi N.J., Rafiquzzaman M., Alam N., Bristi N.J. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013;21(2):143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moniruzzaman S.H.G.M., Khalil M.L., Sulaiman S.A. Advances in the analytical methods for determining the antioxidant properties of honey: a review. Afr. J. Tradit., Complementary Altern. Med. 2011;9(1):36–42. doi: 10.4314/ajtcam.v9i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorinstein S., Böhm V., Schaich K.M., Özyürek M., Güçlü K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report) Pure Appl. Chem. 2013;85(5):957–998. [Google Scholar]
- 16.Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 17.Apak R., Güçlü K., Demirata B., Özyürek M., Çelik S.E., Bektaşoğlu B., Berker K.I., Özyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12(7):1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmagirbi A., Sulistyarti H. Study of ascorbic acid as iron (III) reducing agent for spectrophotometric iron speciation. J. Pure App. Chem. 2012;1(1):11–17. [Google Scholar]
- 19.Mutaftchiev K. Determination of manganese in some medicinal plants and their infusions by a catalytic spectrophotometric method. Ann. Chim. 2004;94(11):829–836. doi: 10.1002/adic.200490103. [DOI] [PubMed] [Google Scholar]
- 20.Kumar C.S.C., Then L.Y., Chia T.S., Chandraju S., Win Y.F., Sulaiman S.F., Hashim N.S., Ooi K.L., Quah C.K., Fun H.K. Benzofuranyl esters: synthesis, crystal structure determination, antimicrobial and antioxidant activities. Molecules. 2015;4(2):16566–16581. doi: 10.3390/molecules200916566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berker K.I., Demirata B. A novel antioxidant assay of ferric reducing capacity measurement using ferrozine as the colour forming complexation reagent. R. Soc. Chem. 2010;2:1770–1778. 2010. [Google Scholar]
- 22.Tupe R.S., Kemse N.G., Khaire A.A. Evaluation of antioxidant potentials and total phenolic contents of selected indian herbs powder extracts. Int. Food Res. J. 2013;20(3):1053–1063. [Google Scholar]
- 23.Aliyu A.B., Ibrahim M.A., Musa A.M., Musa A.O., Kiplimo J.J., Oyewale A.O. Free radical scavenging and total antioxidant capacity of root extracts of anchomanes difformis engl. (Araceae) Acta Pol. Pharm. 2013;70(1):115–121. [PubMed] [Google Scholar]
- 24.Syamala P., Rao P.V.S. Kinetics of dissociation oftris-2, 2’- bipyridyl iron(II) in the water pools of CT AB reverse micelles. Indian J. Chem. 2000;39:643–645. [Google Scholar]
- 25.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 26.Dutta P., Dey T., Manna P., Kalita J. Antioxidant potential of Vespa affinis L., a traditional edible insect species of North East India. PLoS One. 2016;11(5):1–19. doi: 10.1371/journal.pone.0156107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naji K.M., Al-Shaibani E.S., Alhadi F.A., Al-Soudi S.A., D’souza M.R. Hepatoprotective and antioxidant effects of single clove garlic against CCl4-induced hepatic damage in rabbits. BMC Complement Altern. Med. 2017;17:411. doi: 10.1186/s12906-017-1916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walash M.I., Saad S. A new spectrophotometric method for determination of phenylpropanolamine HCl in its pharmaceutical formulations via reaction with 2,3,5,6-tetrachloro-1,4-benzoquinone. Int. J. Biomed. Sci. 2010;6(2):150–157. [PMC free article] [PubMed] [Google Scholar]