Abstract
Curcuma karnatakensis, a member of Zingiberaceae, is endemic to the state of Karnataka, India. The structure and physicochemical properties of starch isolated from rhizomatous rootstocks of two samples - A and B were analyzed for the first time. Sample A contains 76.4 ± 0.3% of starch, of which 86.6 ± 0.4% is amylose, while sample B has 75.0 ± 0.4% of starch containing 84.6 ± 0.4% of amylose according to UV-Vis spectrophotometric analysis. The shape of the starch granules in both the samples is polygonal and cuboidal with a smooth surface, as revealed by SEM studies. The X-ray diffractogram indicated A type of polymorphs in contrast to other Curcuma species, where B types are reported. Since its high amylose content leads to an increased tendency to retrogradation and the formation of resistant starch, this taxon could become one of the major dietary sources of starch in the future. In addition, a source rich in amylose specifies its prospective application in the pharmaceutical and biodegradable film industry.
Keywords: Food science, Amylose, Curcuma karnatakensis, Endemic, Physicochemical properties, SEM studies, X-ray diffraction
Food science, Amylose; Curcuma karnatakensis; Endemic; Physicochemical properties; SEM studies; X-ray diffraction.
1. Introduction
The Curcuma genus includes more than 80 species of rhizomatous herbs known for their medicinal properties. The pharmacological properties of Curcuma species are mainly attributed to the presence of curcuminoids, whose therapeutic values are well established (Jayaprakasha et al., 2002, 2005; Lakshmi, Padmaja and Remani, 2011). In addition to their beneficial properties, many species are used as coloring agents and as dietary spices (Hansdah et al., 2015; Jyothi et al., 2003; Leonel et al., 2003; Policegoudra and Aradhya, 2008; Rani and Chawhaan, 2012; Ranjini and Vijayan, 2006). In recent decades, Curcuma species have emerged as an alternative source of starch (Braga et al., 2006; Hung and Vo, 2017; Rajeev Kumar, Rajeev, & Anilkumar, 2010). Starch is the most widely available carbohydrate and a major component of various rhizomatous perennial tubers/herbs. It is one of the major components of human nutrition and provides about 70–80% of the calories consumed by humans worldwide (Thomas and Atwell, 1999). Since, most of the conventional sources are being overexploited, exploring the new botanical sources of starch are required to ease the pressure on the traditional sources. Starch has been classified into rapidly digestible starch (RDS), slowly digestible starch (SDS) and resistance starch (RS) for nutritional purpose to specify its digestion properties in food products (Englyst et al., 1992; Zhang and Hamaker, 2009) Starch is not only known for its nutritional value, but also for its industrial applications. Starch is composed mainly of two polymers - amylose and amylopectin. The proportion of these two polymers in native starch varies from 20 to 30% for amylose and from 70 to 80% of amylopectin. These two components are major determinants of various physicochemical and functional properties of starch. Hence, knowledge about the composition, structure and physicochemical properties is prerequisite for processing and applications of starch for different uses (Zhu and Xie, 2018). High amylose starch attracts both nutritionists and technologists because of its wide application in both sectors. Various reports have favored the consumption of a diet rich in amylose resulting in a poor metabolic response by forming a highly resistant starch (Behall et al., 1989; Granfeldt et al., 1995: Regina et al., 2006), thereby reducing the risk of various chronic diseases such as heart related diseases, some cancers and especially diabetes (Frost et al., 1999; Salmeron et al., 1997a, Salmeron et al., 1997b). High amylose content in starch is known to form solid and flexible films that are widely used in the food and pharmaceutical industries (Myllarinen et al., 2002a; Palviainen et al., 2001; van Soest et al., 1996). Hence, the composition of starch in terms of amylose and amylopectin ratio plays the significant role in determining the functional properties. However, native starches have limited direct functionalities and they commonly modified for desired applications (Alcazar-Alay and Meireles, 2015).
Curcuma karnatakensis Amalraj, Velayudhan and Muralidharan, is an endemic perennial herb. It is reported in two localities of different geographical characteristics in Karnataka, India (Amalraj et al., 1991; Kotersha et al., 2008) (Figure 1). It hibernates in the soil until the season in the form of rhizomatous rootstock. The first leaf appears in April/May. After 3 to 4 leaves are formed, spike inflorescence initiates either lateral or terminal with flowers having three rose to rosy white coloured lobes enclosing pale yellow corolla tube. The flower has staminoids, which are multicolored, and anther is white in color. The fully developed plant has a short rhizomatous rootstock with stipitate tubers that are fusiform or conical and rich in starch. They form future rhizomatous rootstocks at the origin of new plants. The dried rhizomatous rootstocks give a creamy white powder when it is reduced to powder as seen in C. zedoaria, commonly known as white turmeric; while all other Curcuma species produce a characteristic yellowish orange powder. C. karnatakensis is a scientifically underutilized taxon, with the exception of two reports on in vitro regeneration from rhizomatous rootstocks and the estimation of curcuminoids (Tejavathi and Sujatha, 2016; Tejavathi et al., 2017).
Figure 1.
Curcuma karnatakensis: 1A-Plant with Inflorescence-in wild; 1B-Single flower; 1C-Rhizomatous root stock.
The present study deals with the isolation and analysis of the physicochemical properties of starch from rhizomatous rootstocks of two samples of Curcuma karnatakensis.
2. Material and methods
2.1. Material-plant source
Rhizomatous rootstocks of two samples were collected in their natural habitats; sample A was from Dharwad, Karnataka - a plain region, and sample B was from Hirehalli-Western ghat range of Karnataka during the flowering season. Authenticated herbaria of the two samples examined in this study are deposited in the Department of Botany, Bangalore University, Bangalore with designated voucher numbers: BUB 202 and BUB 203.
2.2. Methods
2.2.1. Extraction of starch
Starch extraction from both samples was performed following the method of Moorthy (1991) with slight modifications in ammonia solution (0.03 M). The freshly harvested rhizomatous rootstocks were thoroughly washed under running water. After taking off the outer layer, they were cut into small pieces. 2.5g of each sample were homogenized separately using a laboratory blender in 0.03M ammonia solution. The pulp was allowed to stand for 30 min before being filtered through a fine muslin cloth. The residue retained on the muslin was again homogenized in a 0.03M ammonia solution. The process was repeated 4 to 5 times. Finally, the supernatant layer was discarded and the sedimented starch was collected. Purification of the sedimented starch was performed by treating with the saline suspension at room temperature (Badenhuizen, 1964). The saline suspension was then mixed with toluene (0.1v) to denature and remove residual cellular proteins. The process was repeated 3 to 4 times. Finally, the toluene protein layer was discarded. The sedimented starch was defatted by treating with methanol (80%), followed by washing with acetone and ether. After washing, it was dried under reduced pressure. The starch yield was calculated using the following formula
2.2.2. Amylose content
The amylose content of the samples was determined by a slight modification of Hoover and Ratnayake (2002) method. 20mg of starch from both samples were dissolved in 8ml of 90% DMSO (dimethyl-sulfoxide). After stirring well by shaking vigorously, the reaction mixture was diluted by adding water to 25ml. 0.2–1ml was taken from this solution individually and mixed with 40ml of water and 5ml of potassium iodide. A final volume of 50ml was prepared for each batch by adding the required amount of water. The OD values were recorded for all concentrations at 600nm using a UV-Vis spectrophotometer (ELICO, India). The total amylose content was calculated by plotting a standard curve using standard amylose (99.99%) (Sigma, USA).
2.2.3. Moisture, ash and fiber content
The starches isolated from the samples were analyzed for moisture, fiber and ash content in accordance with the official AOAC (1990) procedures with slight modifications.
2.2.3.1. Total moisture content
Thoroughly washed crucible along with the lid was dried in oven at 105°Cfor 3 h and transferred to desiccator to cool. Empty crucible and lid was weighed and recorded. 3 g of both the samples was transferred to the weighed dish (W1). The samples were spread evenly on the surface of the crucible and placed in the oven at 150 °C for 3h. After drying, the dish was transferred to desiccator with partially closed by the lid. Finally, the dishes along with dried samples were weighed and reading was recorded (W2). Percent moisture content was calculated using the following formula.
2.2.3.2. Ash content
Crucible along with the lid was placed in the furnace overnight at 550 °C to ensure that impurities on the surface of crucible are burned off. Crucible was kept in desiccator for 30 min for cooling after removed from the furnace. Crucible along with the lid was weighed to three decimal places (W1). 5g of each sample were then transferred to crucible and heated on low Bunsen flames with half covered lid. When fumes are no longer produced, the crucible along with the contents was removed and kept in furnace for overnight at 550°c without the lid. The crucible was removed from furnace, closed with the lid to prevent the loss of fluffy ash, and cooled in desiccator. Finally weighed the ash in the crucible along with the lid (W2). Percent of ash was calculated as follows.
2.2.3.3. Fiber content
2.5g of both the samples were weighed and transferred to extraction apparatus (soxhlet extractor) (W) and extracted with petroleum ether. The extracted samples were air dried and transferred to conical flask of 1L capacity. The dried extracts were heated with mixture of acetone and petroleum benzene for 30 min. The flask was then connected to water-cooled reflux condenser and heated so that the contents of the flask started boiling within 1min. The flask was rotated frequently while boiling for 30 min. Then the contents were filtered through fine linen (about 18 threads to a cm) held in a funnel and the residue was washed with 200 ml of boiling sodium hydroxide solution. Immediately the filtrate was transferred to a flask and connected to reflux condenser, boiled for 30 min. Then the flask was removed and the content was filtered through the filtering cloth. Thus, obtained residue was washed with hot water and followed by ethyl alcohol (15ml) before transferring it to a Gooch crucible. It was dried at 105 °C in an oven until constant weight is achieved (W1). After cooling, incinerate the contents in a muffle furnace until the carbonaceous matter is burnt. Finally, the contents were cooled and weighed (W2). Percent of fiber was calculated following the formula as given below.
2.2.4. Solubility, swelling power and water holding capacity
The solubility in water and water holding capacity of starch from two samples were recorded at 50, 60, 70, 80, 90 and 100 °C according to the method of Ju and Mittal (1995) with slight modifications. The swelling power of the starch was determined according to the method of Leach et al. (1959).
2.2.4.1. Solubility
One gram of each sample was added to 10ml of water and stirred manually with a help of glass rod for through mixing. The samples were then centrifuged at a speed of 4000 rpm for 15 min at room temperature. Total mass of the supernatant liquid was recorded. From that, 0.5g of each sample was transferred onto an aluminum foil placed on a watch glass and dried in a vacuum oven at various temperatures ranging from 50 to100 °C for 4 h. The mass of the solid left on the aluminum foil was weighed and percent of solubility was calculated using the following formula.
2.2.4.2. Swelling power
0.1 g of each sample was heated in 10ml of distilled water in a water bath at 60˚c for 30 min with constant mixing. Then they were centrifuged for 15 min at 1600 rpm. The precipitated part was weighed and the swelling power was determined using the following formula.
2.2.4.3. Water holding capacity
1.2 g of each sample was added to 15 ml of distilled water in a centrifugation tube and kept in oven at various temperatures ranging from 50 to 100 °C Then, centrifuged at a speed of 6000 rpm for 15 min at room temperature. The supernatant water was removed and weighed. Percent of water holding capacity was calculated using the following equation
2.2.5. Scanning electron microscopy (SEM)
The morphology of the starch grains was determined using TESCAN SEM (Czech Republic). A circular aluminum section is glued to one side of the double-sided carbon ribbon. The samples were spread on the other surface of the carbon ribbon using an air blower so that the material was evenly distributed over the surface and adhered firmly. Additional material was cleared. An acceleration potential of 25Kv with a resolution of 3nm was used during the micrography.
2.2.6. X-ray diffraction pattern
A PAN analytical powder X-ray diffractometer (Netherlands) was used to obtain the diffraction patterns of the samples. The diffractometer is equipped with a Cu kα radiation operating at 45KV and 30mA. The diffracted intensity was measured from 0° to 140° as a function of 2θ. The scanning speed is 1°/second from the diffraction angle (2θ).
3. Results and discussion
3.1. Starch and amylose content
Aqueous extraction of starch from various botanical sources is the most commonly used method (Badenhuizen, 1964; Moorthy, 1991). However depending on the requirement, several modifications are being incorporated in traditional method of extraction from time to time. Supercritical fluid extraction is another method which is frequently employed for extraction of starch from turmeric and ginger (Moreschi et al., 2006; Santana et al., 2017). In the present study, starch was extracted using ammonia solution (0.03M) as per the method of Moorthy (1991). The use of ammonia enables faster settling of starch, thereby prevents the microbial damage (Moorthy, 1991; Rani and Chawaan, 2012). Extraction of starch in ammonia solution has improved the starch yield in several tuber crops than with water (Moorthy, 1991). Purification and defatification of the isolated starch was carried out by following the method of Badenhuizen (1964) by treating with toluene and methanol followed by washing with acetone and ether. Finally, it was dried under reduced pressure to remove all the traces of solvents. Purification of starch is required to remove the protein fraction of the starch, which may affect starch quality and make it not suitable for some food applications; thereby reducing its commercial value (Chen, Schols and Voragen, 2003; Tester et al., 2004). Defatification of starch is needed for further characterization, since lipid-amylose comlex thus formed results in low clarity, slow swelling and reduces starch's iodine-binding capacity (Chen et al., 2003; Tester et al., 2004).
The defatted starch thus isolated from the rhizomatous rootstocks of two samples is creamy white in color and the yield varies according to the sample. Sample A growing in the plain region contained 76.4 ± 0.3% starch, while the sample B collected from western ghats had 75.0 ± 0.4% according to UV-Vis spectrophotometric analysis (Table 1). The highest percent yield (98.12 ± 0.03%) was reported by Hung and Vo (2017) in C. longa and C. caesia. However, Braga et al. (2006) and Leonel et al. (2003) have recorded 77 ± 2% and 86.62 ± 1.15% of starch yield in C. longa respectively, while Sajitha & Sasikumar (2015) have reported 45.24% starch yield in C. caesia. The variation in starch yield of the same species depends on various factors such as its habitat, time of harvest, extraction method and genetic make-up (Asare et al., 2011; Ping et al., 2013; Rahman, Wheatley and Rakshit, 2003; Sharma and Tejinder, 2014; Zhu, 2017). Sajitha & Sasikumar (2015) have studied the qualitative and quantitative variations of starch from four Curcuma species and concluded that Curcuma could become an alternative source of commercial starch.
Table 1.
Physicochemical characteristics of starch obtained from Curcuma karnatakensis.
| Samples | Starch yield (%) | Amylose (%) | Solubility (%) at 600c | Swelling power (g/g) at 600c | Water holding capacity (%) at 600c | Moisture content (%) | Fiber (%) | Ash (%) | pH |
|---|---|---|---|---|---|---|---|---|---|
| A | 76.4 ± 0.3 | 86.6 ± 0.4 | 0.9 ± 0.9 | 5.06 ± 0.07 | 9.0 ± 0.01 | 16.0 ± 0.6 | 0.9 ± 0.03 | 0.38 ± 0.03 | 7.6 |
| B | 75.0 ± 0.4 | 84.6 ± 0.4 | 0.6 ± 0.4 | 5.03 ± 0.04 | 8.04 ± 0.03 | 15.0 ± 0.4 | 0.98 ± 0.02 | 0.36 ± 0.01 | 7.5 |
As the starch content, the amylose content also varies within and between Curcuma species. Sample A contains 86.6 ± 0.4%, while Sample B contains 84.6 ± 0.4% according to UV-Vis spectroscopic analysis (Table 1). However, the colorimetric analysis recorded 90% of amylose in both samples. While the starch yield was about 98% in C. longa and C. caesia, the amylose content was only 26.3 ± 0.7 and 24.9 ± 0.7 respectively (Hung and Vo, 2017). Similarly, Leonel et al. (2003) reported a high starch content (approximately 86%) in C. longa and C. zedoaria, with an amylose content of 22.32 ± 0.2 and 20.53 ± 0.63 respectively. Different methods of measurement may give different results for the same starch (Regina et al., 2006; Zhu, 2017). As Zhu (2017) has pointed out, quantification methods should be mentioned for any comparison of amylose content reported by different studies. In-depth review of the literature has confirmed that C. karnatakensis (present study) has the highest percentage of amylose in starch among Curcuma species (Braga et al., 2006; Hung and Vo, 2017; Jyothi et al., 2003. Leonel et al., 2003; Policegoudra and Aradhya, 2008; Rani and Chawhaan, 2012).
The ratio of amylose to amylopectin is important for the nutritional and technological properties of starch (Frost et al., 1999; Granfeldt et al., 1995; Salomonsson and Sundberg, 1994; Zhang et al., 2006). In starches obtained from conventional sources, the ratio of amylose to amylopectin is about 20–30:80–70%. Increasing the consumption of resistant starch containing mainly amylose is one of the ways to reduce the risk of various chronic diseases such as cardiovascular diseases, certain cancers and diabetes (Salmeron et al., 1997a, Salmeron et al., 1997b; Frost et al., 1999). Behall et al. (1989) suggest that long-term consumption of a high amylose corn diet improves fasting triglyceride and cholesterol levels in healthy men compared to amylopectin-rich diets. Studies of postprandial glycemic and insulinemic responses to cornstarch have shown that high amylose products favor low metabolic responses and the formation of high resistant starch contents (Granfeldt et al., 1995). An in vivo method of extended glycemic index was proposed by Zhang and Hamaker (2009) to evaluate metabolic effect and related health consequences of slowly digestible starch (SDS). Regina et al. (2006) concluded from their rat experiments that a diet rich in amylose has considerable potential for improving health and reducing the risk of serious non-infectious diseases. In addition, high amylose starches have been associated with a greater tendency to retrogradation (Whistler and Be Miller, 1999). As retrogradation decreases starch digestibility, its potential in the food industry can be exploited further (Kong and Singh, 2011). In addition to its nutritional value, it is known that a high amylose content in starch forms strong and flexible films (Myllarinen et al., 2002a, Myllarinen et al., 2002b; Palviainen et al., 2001). Starch-based films have better applicability in food and pharmaceutical products than protein-based films because of their cost-effectiveness and non-allergenicity (Guilbert, 2000; Han, 2002). Strong gelation properties and a linear helical polymer structure of amylose make a high amylose starch as a useful film forming material (Eliasson and Tatham, 2001). A high amylose cornstarch is known to produce strong, flexible films probably due to the crystallization of amylose (Myllarinen et al., 2002a, Myllarinen et al., 2002b; van Soest et al., 1996). Other important properties of the high amylose starch are excellent oxygen barrier properties, lower water solubility, and more stable mechanical properties at high RHs compared to those made of native starches (Rindlav-Westling et al., 1998). Therefore, the starch of C. karnatakensis containing a high percent of amylose has the potential for producing biodegradable films and is a promising alternative source.
3.2. Morphological characteristics - SEM and X-ray diffraction analysis
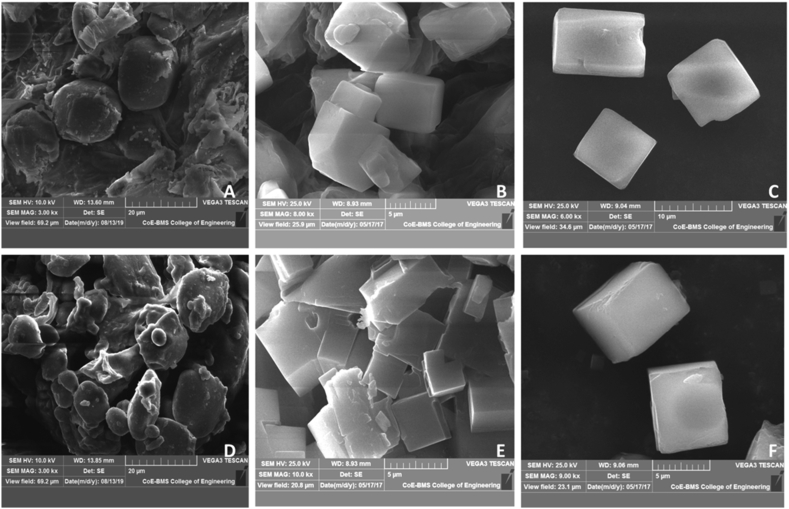
The analysis of scanning electron micrographs of the starch granules of the two samples obtained before and after the extraction has revealed the similarity in both shape and structure (Figure 2). The shape and structure of the granules are retained even after the extraction indicating the efficacy of the extraction method. The starch granules are cuboid to polygonal in shape. The diameter of the granules is between 1 and 10μm. The surface of the granules is smooth unlike C. longa and C. leucorrhiza, where the starch granules are elliptic with fissures (Braga et al., 2006; Hansdah et al., 2015). While starch granules of C. aromatica are distinguished from others by their concentric rings (Sajitha & Sasikumar, 2015).
Figure 2.
Curcuma karnatakensis: Fig. 2A-C Sample A. 2A: SEM Micrograph of starch granules before extraction, 2B&2C: SEM Micrographs of starch granules after extraction. Fig. 2D-2F Sample B. 2D: SEM Micrograph of starch granules before extraction, 2E&2F: SEM Micrographs of starch granules after extraction.
The size and shape of the starch granules vary according to the species. Various types and forms such as round, oval, elliptical, triangular, cuboidal and polygonal have been reported in various Curcuma species (Braga et al., 2006; Hansdah et al., 2015; Hung and Vo, 2017; Jyothi et al., 2003; Leonel et al., 2003; Policegoudra and Aradhya, 2008; Rani and Chawhaan, 2012; Sajitha & Sasikumar, 2015). The size of starch granules in Curcuma species ranges from 3 to 50μm (Hansdah et al., 2015; Policegoudra and Aradhya, 2008). The morphological variation of starch granules has been attributed to plant physiology, which is itself dependent on the habitat and other environmental conditions (Singh et al., 2003; Zhou et al., 2013).
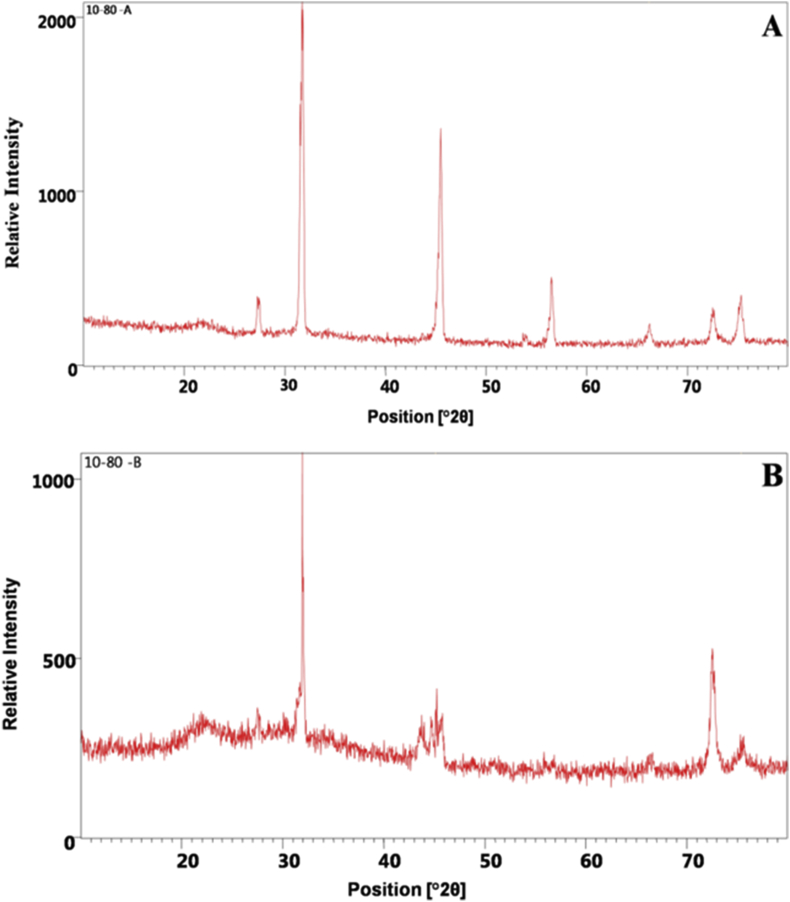
X-ray diffraction patterns have been widely used to determine the degree of crystallinity of starch granules and are considered as a fingerprint of the crystal structure (Zeng et al., 2011). Katz and Van Itallie (1930) categorized the crystal structures of the starch granules into three types - A, B, C based on the XRD pattern. Type A is characteristic of cereal starches; Type B is tubers and Type C is present in legumes (Singh et al., 2003). The A type granules have a sharper XRD pattern than other types of wheat varieties (Zeng et al., 2011). The starch isolated from the two samples of the present study shows A type of crystalline structure with 8 diffraction peaks in samples A and 5 in sample B at 2θ (Figure 3). The d-spacing values observed on different peaks range from 3.25 to 1.26A° in sample A and 2.79 to 1.25A° in sample B. Sharp peaks observed in the XRD pattern of the sample A indicate the fine crystalline structure of the material. Further, the distribution of the patterns is very sharp and smooth revealing the presence of synergistic effect of particles in the materials. More number of counts in the pattern presents the good structural stability. The presence of fine particles in the material improves the structural stability. Whereas the XRD pattern of sample B shows, single sharp band and scattered patterns. Presence of sharp peaks was very less and scattered pattern was observed. This indicates the presence of particles with coarse grains, which cannot improve the structural stability of the material. Further, the number of counts are less compared to the pattern of sample A. Based on these observations on XRD pattern of two samples, it can be concluded that sample A is better than sample B in structural stability. Hence, sample A is more suitable for relevant applications where structural stability is concerned. Previous studies in other Curcuma species had reported polymorphs of type B; characteristic of other tuber starches (Braga et al., 2006; Hansdah et al., 2015; Hung and Vo, 2017; Jyothi et al., 2003; Policegoudra and Aradhya, 2008; Ranjini and Vijayan, 2006). However, wheat and potato starches comprise both starch A and B granules with type A polymorphs containing more amylose than type B (Dundar et al., 2009; Zeng et al., 2011). On the other hand, high amylose starch in C. aeruginosa has type B granules (Ranjini and Vijayan, 2006). Starch types determine the functional properties of starch, which specify their use in food products and their industrial applications.
Figure 3.
X-ray diffractogram of starch of Curcuma karnatakensis 3A-Sample A. 3B-Sample B.
3.3. Solubility, swelling power and water holding capacity
Solubility, swelling capacity, and water holding capacity are directly correlated with temperature (Ronden-Sanabria and Finardi-Filho, 2009). Factors that can affect the solubility of starch are the source, the swelling capacity; inter-association forces in the amorphous and crystalline domain and the presence of other compounds (Kumoro et al., 2012). Solubility is one of the important physicochemical characteristics that determine the functional properties of starch. Both samples of the present study showed very low solubility in water at 60 °C. Samples A and B showed a solubility of 0.9 ± 0.9% and 0.6 ± 0.4% respectively (Table 1). However, it increases at 100 °C to 15 ± 0.8% in sample A and 8.04 ± 0.2% in sample B. There is a linear correlation between the solubility of starch and the increase in temperature. Solubility and swelling power are positively correlated. The swelling capacity of the starch granules is closely related to the proportion of amylose and amylopectin and their characteristics tend to vary during the heating process. The hydroxyl groups of amylose and amylopectin thus exposed are then bound to the water molecules by hydrogen bonding, resulting in increased swelling and solubility of the granules (Hoover, 2001). The available binding sites determine the water retention capacity of the starch grains. The water holding capacity of the starch in sample A and sample B was found to be 9.00 ± 0.01% and 8.04 ± 0.03% at 60 °C (Table 1). However, WHC of starch of both the samples was increased linearly with increase in temperature to 16.0 ± 0.3% in sample A and 14.25 ± 0.1% in sample B at 100 °C. Swelling powers of 5.06 ± 0.07 and 5.03 ± 0.04 g/g were recorded for samples A and B, respectively (Table 1). The starch of C. amada (mango ginger) has a solubility of 1.5% in water at 25 °C and 1.21 ± 0.02 at 85 °C (Policegoudra and Aradhya, 2008; Sajitha & Sasikumar, 2015). The lowest solubility and swelling capacity of 0.47 ± 0.01% at 85 °C and 3.74 ± 0.04 g/g was reported in C. caesia (Sajitha & Sasikumar 2015). Higher solubility among tuber crops has been reported for cassava starch and lower solubility for aroid starches (Moorthy, 2002). The low solubility of starch in the present study is correlated with the high amylose content. Amylose molecules have a strong tendency to form lipid complexes that prevent their leaching and hence their swelling capacity (Leach et al., 1959; Singh et al., 2003). The swelling capacity indicates the water holding capacity of the starch, which has generally been used to highlight the differences between the various types of starches (Crosbie, 1991).
3.4. Moisture, fiber, ash content, and pH
The moisture content of the two samples studied did not vary significantly with 16.00 ± 0.6% in sample A and 15.00 ± 0.4% in sample B (Table 1). Higher moisture levels may result in deterioration of quality due to microbial growth. A maximum of 1.7–18.6% moisture was recorded in the starch of Dioscorea rotundata (Rasper, 1967; Moorthy, 2002). However, the maximum recommended moisture content for safe storage is 13% (ISI, 1970; Radley, 1976). Moisture content has been found to strongly influence the density of potato starch (Statsiak et al., 2014). Climatic factors play an important role in regulation of moisture content.
The fiber content of the starch of the two samples was found to be 0.9 ± 0.03% in sample A and 0.98 ± 0.02% in sample B (Table 1). A significant variation in the fiber content of various tuber crops has been documented (Moorthy, 2002). In sweet potatoes, the fiber content ranges from 0.7 to 1.3% (Gracia and Walter, 1998). In C. leucorrhiza, C. longa and C. zedoaria, the fiber content was 0.83%, 0.05% and 0.37%, respectively (Hansdah et al., 2015; Leonel et al., 2003). In general, fiber reduces the incidence of cardiovascular diseases and obesity and is effective for insulin sensitivity (Chambers et al., 2011; Slavin, 2013).
The total ash content in the sample is almost identical, with 0.38 ± 0.03 in sample A and 0.36 ± 0.01 in sample B (Table 1). The fiber/ash content in tubers, particularly in cassava and sweet potato, increases with maturity (Moorthy, 2002). The maximum amount of ash among Curcuma species was recorded in C. aromatica (11.45%) by Sajitha and Sasikumar (2015); minimum in C. longa (0.32 ± 0.02) by Leonel et al. (2003). The low ash content is an indication of the good quality of the starch because a high content of minerals retards the growth of certain microorganisms (Nielson, 1998).
The pH of the starch in both samples was found to be acidic and closer to the pH range of 3–9 required for use in the pharmaceutical, cosmetic and food industries (Coursey and Rasper, 1967). The pH of the starch of sample A was 7.6, while that of sample B was 7.5 (Table 1). The pH variation can alter the physicochemical properties of native starches and can impart new functionality (Builders et al., 2014).
4. Conclusion
The present study indicates that Curcuma karnatakensis is a good source of amylose and found highest among Curcuma species. The variations in the physicochemical characteristics of the two samples studied may be due to their geographically different habitats. The role of high amylose starch in the formation of resistant starch and in the production of biodegradable films is well established. C. karnatakensis, therefore, has a potential to emerge as an alternative source in the food, pharmaceutical and film industries. The possibility of modifying the native starch to meet the requirements could be exploited.
Declarations
Author contribution statement
D.H. Tejavathi: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
B. S. Sujatha: Performed the experiments.
C. S. Karigar: Contributed reagents, materials, analysis tools or data.
Funding statement
D.H. Tejavathi was supported by the University Grants Commission, New Delhi, through a BSR Fellowship.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Alcazar-Alay S.C., Meireles M.A.A. Physiochemical properties, modifications and applications of starch from different botanical sources. Food Sci. Technol. 2015;35:215–236. Campinas. [Google Scholar]
- Amalraj V.A., Velayudhan K.C., Muralidharan V.K. Curcuma karnatakensis sp.nov. (Zingiberaceae) - a new species from Uttar Kannada district of Karnataka state. J. Econ. Bot. Taxon. 1991;15:490–492. [Google Scholar]
- AOAC . In: Helrich S., editor. Vol. 1. 1990. (Official Methods of Analysis of the Association of Official Analytical Chemists). Arlington, Virginia. [Google Scholar]
- Asare E.K., Jaiswal S., Maley J., Bage M., Summynaiken R., Rossnagel B.G., Chibbar R.N. Barley grain constituents, starch composition, and structure affect starch in vitro enzymatic hydrolysis. J. Agric. Food Chem. 2011;59:4743–4754. doi: 10.1021/jf200054e. [DOI] [PubMed] [Google Scholar]
- Badenhuizen N.P. General methods for starch isolation. In: Whistler R.L., editor. Vol. 4. Academic Press; New York: 1964. pp. 14–15. (Methods in Carbohydrate Chemistry). [Google Scholar]
- Behall K.M., Scholfield D.J., Yuhaniak I., Canary J. Diets containing high amylose vs amylopectin starch: effects on metabolic variables in human subjects. Am. J. Clin. Nutr. 1989;49:337–344. doi: 10.1093/ajcn/49.2.337. [DOI] [PubMed] [Google Scholar]
- Braga M.E.M., Moreschi S.R.M., Meireles M.A.A. Effects of supercritical fluid extraction on Curcuma longa L. and Zingiber Officinale R. starches. Carbohydr. Polym. 2006;63:340–346. [Google Scholar]
- Builders P.F., Mbah C.C., Adama K.K., Andu M.M. Effect of PH on the physicochemical and binder properties of tigermint starch. Starch. 2014;66:281–293. [Google Scholar]
- Chambers E., Guess N., Viardot A., Frost G. Dietary starch and Fibre: potential benefit to body weight and glucose metabolism. Diabetes Manag. 2011;1:521–528. [Google Scholar]
- Chen Z., Schols H.A., Voragen A.G.J. Physicochemical properties of starches obtained from three varieties of Chinese sweet potatoes. J. Food Sci. 2003;68:431–437. [Google Scholar]
- Coursey D.G., Rasper V. Properties of starches of some west African Yams. J. Sci. Food Agric. 1967;18:240–248. [Google Scholar]
- Crosbie G.B. The relationship between starch swelling properties, past viscosity and boiled noodle quality in wheat flours. J. Cereal Sci. 1991;13:145–150. [Google Scholar]
- Dundar E., Turan Y., Blaurock A.E. The large scale structure of wheat, rice, and potato starch revealed by ultra-small angle X-ray diffraction. Int. J. Biol. Macromol. 2009;45:206–212. doi: 10.1016/j.ijbiomac.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Eliasson A., Tatham A. Cereal starch and proteins. In: Dendy D.A.V., Dobraszczyk B.J., editors. Cereals and Cereal Products: Chemistry and Technology. Aspen Publishers; Gaithersburg, Md: 2001. pp. 68–89. [Google Scholar]
- Englyst H.N., Kingman S.M., Cummings J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992;46:S33–50. [PubMed] [Google Scholar]
- Frost G., Leeds A.A., Dore D.J., Madeiros S., Brading S., Dornhorst A. Glycaemic index as a determinant of serum HDL- cholesterol concentration. Lancet. 1999;535:1045–1048. doi: 10.1016/s0140-6736(98)07164-5. [DOI] [PubMed] [Google Scholar]
- Gracia A.M., Walter W.M., Jr. Water: physico – Chemical Characterization of starch from Peruvian sweet potato selections. Starch. 1998;50:334–337. [Google Scholar]
- Granfeldt Y., Drews A., Bjorck I. Arepas made from high amylose corn flour produce favorably low glucose and insulin responses in healthy humans. J. Nutr. 1995;125:459–465. doi: 10.1093/jn/125.3.459. [DOI] [PubMed] [Google Scholar]
- Guilbert S. Edible films and coating and biodegradable packaging. Bull. Int. Dairy Fed. 2000;346:10–16. [Google Scholar]
- Han J.H. Protein-based edible films and coatings carrying antimicrobial agents. In: Gennadios A., editor. Protein-Based Films and Coatings. CRC Press; Boca Raton, Florida, USA: 2002. pp. 485–489. [Google Scholar]
- Hansdah R., Prabhakar P.K., Srivastav P.P., Mishra H.N. Physico-chemical characterization of lesser-known Palo (Curcuma leucorrhiza) starch. Int. J. Food Res. 2015;22:1368–1373. [Google Scholar]
- Hoover R. Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr. Polym. 2001;45:253–267. [Google Scholar]
- Hoover R., Ratnayake W.S. Starch characteristics of black bean, chickpea, lentil, navy bean and pinto bean cultivars grown in Canada. Food Chem. 2002;78:489–498. [Google Scholar]
- Hung P.V., Vo T.N.D. Structure, physicochemical characteristics, and functional properties of starches isolated from yellow (Curcuma longa) and black (Curcuma caesia) turmeric rhizomes. Starch. 2017;69:1–8. [Google Scholar]
- ISI . Indian Standard Institution; New Delhi: 1970. Specifications for Tapioca Starch for Use in Cotton Textile Industries, IS 1605-1906. [Google Scholar]
- Jayaprakasha G.K., Rao L.J.M., Sakariah K.K. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J. Agric. Food Chem. 2002;50 doi: 10.1021/jf025506a. 3668-3072. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha G.K., Rao L.J.M., Sakariah K.K. Chemistry and biological activities of C. longa. Trends Food Sci. Technol. 2005;16:533–548. [Google Scholar]
- Ju J., Mittal G.S. Physical properties of various starch-based fat-substitutes. J. Food Process. Preserv. 1995;19:361–383. [Google Scholar]
- Jyothi A.N., Moorthy S.N., Vimala B. Physicochemical and functional properties of starch from two species of Curcuma. Int. J. Food Prop. 2003;6:135–145. [Google Scholar]
- Katz J.R., Van Itallie T.B. Uber die Anderungen in Rontgenspektrum der starke beim Backen und beim Altbackenwerden des Brotes. Z. Phys. Chem. 1930;150:37–59. [Google Scholar]
- Kong F., Singh R.P. Chemical deterioration and physical instability of food and beverages. In: Kilcast D., Subramaniam P., editors. Food and Beverage Stability and Shelf Life, Woodhead Publishing Series in Food Science, Technology and Nutrition. Woodhead publishing limited; UK: 2011. pp. 29–62. [Google Scholar]
- Kotersha K., Kambhar S.V., Harihar N.S. Proceedings of XVIII. The Annual Conference of Indian Association of Angiosperm Taxonomy and an International Seminar on Multidisciplinary Approaches in Angiosperm Systematics. University of Kalyani; Kalyani, India: 2008. Curcuma karnatakensis Amalaraj, Velayudhan, and Muralidharan. A new record from Dharward, Karnataka state. [Google Scholar]
- Kumoro A., Retnowati D.S., Budiyati C.S., Manurung T., Siswanto Water solubility, swelling and gelatinization properties of raw and ginger oil modified Gadung (Dioscorea hispida Dennst) flour. Res. J. Appl. Sci. Eng. Technol. 2012;4:2854–2860. [Google Scholar]
- Lakshmi S., Padmaja G., Remani P. Antitumor effects of Isocurcumenol isolated from Curcuma zedoaria rhizomes on human and Murine Cancer cells. Int. J. Med. Chem. 2011;2011:1–13. doi: 10.1155/2011/253962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach H.W., McCowen L.D., Schoch T.J. Structure of the starch granule. I. Swelling and solubility patterns of various starches. Cereal Chem. 1959;36:534–544. [Google Scholar]
- Leonel M., Sarmento S.B.S., Cereda M.P. New starches for the food industry: Curcuma longa and Curcuma zedoaria. Carbohydr. Polym. 2003;54:385–388. [Google Scholar]
- Moorthy S.N. Extraction of starches from tuber crops using Ammonia. Carbohydr. Polym. 1991;16:391–398. [Google Scholar]
- Moorthy S.N. Physicochemical and functional properties of tropical tuber starches: a Review. Starch. 2002;54:559–592. [Google Scholar]
- Moreschi S.R.M., Leal J.C., Braga M.E.M., Meireles M.A.A. Ginger and turmeric starches hydrolysis using subcritical water+ co2: the effect of the SFE pre-treatment. Braz. J. Chem. Eng. 2006;23:235–242. [Google Scholar]
- Myllarinen P., Buleon A., Lehtinen J., Forssell P. The crystallinity of amylose and amylopectin films. Carbohydr. Polym. 2002;48:41–48. [Google Scholar]
- Myllarinen P., Partanen R., Seppala J., Forssell P. Effect of glycerol on the behavior of amylose and amylopectin films. Carbohydr. Polym. 2002;50:355–361. [Google Scholar]
- Nielson S.S. second ed. Aspen Publication; Gaithersburg, Maryland: 1998. Food Analysis; pp. 40–250. [Google Scholar]
- Palviainen P., Heinamaki J., Myllarinen P., Lahtinen R., Yliruusi J., Forssell P. Corn starches as film formers in aqueous–based film coating. Pharm. Dev. Technol. 2001;6:353–361. doi: 10.1081/pdt-100002617. [DOI] [PubMed] [Google Scholar]
- Ping H., Wang J., Ren G. Prediction of the total starch and amylose content in barley using near-infrared reflectance spectroscopy. Intell. Autom. Soft Comput. 2013;19:231–237. [Google Scholar]
- Policegoudra R.S., Aradhya S.M. Structure and biochemical properties of starch from an unconventional source – mango ginger (Curcuma amada Roxb.) rezone. Food Hydrocolloids. 2008;22:513–519. [Google Scholar]
- Radley J.A. Applied Science Publishers Ltd; London: 1976. Examination and Analysis of Starch and its Derivatives. [Google Scholar]
- Rahman S.M.M., Wheatley C., Rakshit S.K. Selection of sweet potato variety for high starch extraction. Int. J. Food Prop. 2003;6:419–430. [Google Scholar]
- Rajeev Kumar P., Rajeev R., Anil Kumar N. Studies on Curcuma Angustifolia starch as a pharmaceutical excipient. Int. J. Pharm. Tech. Res. 2010;2:2456–2460. [Google Scholar]
- Rani A., Chawhaan H. Extraction and scanning electron microscopic studies of Curcuma angustifolia Roxb. Starch. Indian J. Nat. Prod. Resour. 2012;3:407–410. [Google Scholar]
- Ranjini C.E., Vijayan K.K. A high amylose starch isolated from the tubers of Curcuma aeroginosa. Indian J. Chem. 2006;453:2773–2775. [Google Scholar]
- Rasper V. Vol. 2. 1967. Investigations on some starches from some West African root crops; pp. 48–61. (Proceedings of International Symposium of Tropical Root Crops, St.Augustine, Trinidad). [Google Scholar]
- Regina A., Bird A., Topping D., Bowden S., Freeman J., Barsby T., Kosar-Hashemi B., Li Z., Rahman S., Morcll M. High amylose wheat generated by RNA interference improves indices of large bowel health of rats. Proc. Natl. Acad. Sci. 2006;103:3546–3551. doi: 10.1073/pnas.0510737103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindlav-Westling A., Standing M., Hermansson A.M., Gatenholm P. Structure, mechanical and barrier properties of amylose and amylopectin films. Carbohydr. Polym. 1998;36:217–224. [Google Scholar]
- Ronden-Sanabria G.G., Finardi-Filho F. Physical-chemical and functional properties of maca root starch (Lepidium meyenii Walpers) Food Chem. 2009;114:492–498. [Google Scholar]
- Sajitha P.K., Sasikumar B. Qualitative and quantitative variation in starch from four species of Curcuma. Cytologia. 2015;80:45–50. [Google Scholar]
- Salmeron J., Ascherio A., Rimm E.B., Colditz G.A., Spiegelman D.C., Jenkins D.J., Stampfer M.J., Wing A.L., Willett W.C. Dietary fiber, glycemic load and risk of NIDDM in men. Diabetes Care. 1997;20:545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- Salmeron J., Manso J.E., Stampfer M.J., Colditz G.A., Wing A.L., Willet W.C. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. J. Am. Med. Assoc. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- Salomonsson A., Sundberg B. Amylose content and chain profile of amylopectin from normal, high amylose and waxy Barleys. Starch. 1994;46:325–328. [Google Scholar]
- Santana A.L., Zabot G.L., Osorio-Tobon F., Johner J.C.F., Coelho A.S., Schmiele M., Steel C.J., Meireles M.A.A. Starch recovery from turmeric wastes using supercritical technology. J. Food Eng. 2017;214:266–276. [Google Scholar]
- Sharma P., Tejinder S. Extraction of starch from hulled and hull-less barley with paper in an aqueous sodium hydroxide. J. Food Sci. Technol. 2014;51:3870–3877. doi: 10.1007/s13197-013-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Singh J., Kaur L., Sodhi N.S., Gill B.S. Morphological, thermal, and rheological properties of starches from different botanical sources. Food Chem. 2003;81:219–231. [Google Scholar]
- Slavin J.L. Carbohydrates, Dietary fiber, and resistant starch in white vegetables: links to health outcomes. Adv. Nutr. 2013;4:351S–355S. doi: 10.3945/an.112.003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statsiak M., Molenda M., Horabik J., Mueller P., Opalinskin I. Mechanical properties of potato starch modified by moisture content and addition of lubricant. Acta Agrophysica. 2014;28:501–509. [Google Scholar]
- Tejavathi D.H., Sujatha B.S. In vitro propagation of Curcuma karnatakensis – an endemic taxon. IJTA (Int. J. Trop. Agric.) 2016;34:1755–1760. [Google Scholar]
- Tejavathi D.H., Sujatha B.S., Kannan R. Estimation of Curcuminoids in Curcuma karnatakensis (white turmeric) – an endemic taxon. Asian J. Pharmaceut. Clin. Res. 2017;10:360–363. [Google Scholar]
- Tester R.F., Karkalas J., Qi X. Starch-Composition, fine structure and architecture. J. Cereal Sci. 2004;39:151–165. [Google Scholar]
- Thomas D.J., Atwell W.A. Eagen Press; USA: 1999. Starches. Handbook Series; pp. 1–11. [Google Scholar]
- van Soest J.J.G., Hulleman S.H.D., De Wit D., Vliegenthart J.F.G. Change in the mechanical properties of thermoplastic potato starch in relation with changes in B-type crystallinity. Carbohydr. Polym. 1996;29:225–232. [Google Scholar]
- Whistler R.L., Be Miller J.N. American Association of Cereal Chemists, USA: Eagen Press; St. Paul, MN: 1999. Carbohydrate Chemistry for Food Scientists. [Google Scholar]
- Zeng J., Li G., Gao H., Ru Z. Comparison of A and B starch granules from three wheat varieties. Molecules. 2011;16:10570–10591. doi: 10.3390/molecules161210570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Hamaker B.R. Slowly Digestible Starch: concept, mechanism, and proposed extend Glycemic index. Crit. Rev. Food Sci. Nutr. 2009;49:852–867. doi: 10.1080/10408390903372466. [DOI] [PubMed] [Google Scholar]
- Zhang G., Ao Z., Hamaker B.R. Slow digestion property of native cereal starches. Biomacromolecules. 2006;7:3252–3258. doi: 10.1021/bm060342i. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Shi W., Meng X., Liu Y. Studies on the morphological, crystalline, thermal properties of an underutilized starch from Yam, Dioscoreae zingiberensis C. H. Wright. Starch. 2013;65:123–133. [Google Scholar]
- Zhu F. Barley starch: composition, structure, properties, and modifications. Compr. Rev. Food Sci. Food Saf. 2017;16:558–579. doi: 10.1111/1541-4337.12265. [DOI] [PubMed] [Google Scholar]
- Zhu F., Xie Q. Structure and Physicochemical properties of starch. In: Sui Z., Kong X., editors. Physical Modifications of Starch. Springer Nature; Singapore: 2018. pp. 1–4. [Google Scholar]