Abstract
The supplementation of 50 ppm dosed silver nanoparticles (AgNPs) as a disinfectant in broilers drinking water was investigated to examine their growth performance, blood serum biochemistry, and organ histology in the case group, compared to the control. The growth performance parameters, such as water intake, feed intake, and body weight were recorded 6 times, each in an interval of 7 days, over a period of 42 days. At the end of each 42 days, the blood and major organs of the 1 case boiler out of 75 and 1 control broiler out of 75 were collected in random. The procedure was repeated 3 sets one after another, each consisting 42 day intervening period. The liver enzyme, lipid profile, glucose level, organ histology, and concentration of AgNPs in liver, spleen, heart, and small intestine were determined. The obtained results show that the growth performance of the case broilers was significantly higher than the control section (p < 0.05). However, in all the three sets the changes in lipid profile, liver enzyme, and glucose level of the case broilers were not statistically significantly different compared to the control (p > 0.05). The histology of liver, kidney, heart, spleen, and small intestine of broilers has not shown any damages to the cells as compared to the control samples. In the case samples, the highest concentration of AgNPs was observed in the small intestine (5.44 µg/g) followed by liver (4.32 µg/g), kidney (3.94 µg/g), heart (3.82 µg/g), and spleen (3.49 µg/g). The present study concludes that the administering 50 ppm AgNPs of average 15 nm size in the poultry drinking water was found safe for consumption as well as for growth enhancing, due to better bird comfort.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2101-1) contains supplementary material, which is available to authorized users.
Keywords: AgNPs, Growth performance, Organ histopathology, Serum biochemistry
Introduction
In the poultry farming, good quality water is an essential factor to maintain the poultry metabolism; the metabolism depends on body temperature control, digestion, absorption of food, transport of nutrients, and urination from the body (Bobinienė et al. 2014). Jafari et al. (2006) reported in a study that the accumulation of microorganisms in the drinking water had increased the level of intestinal bacteria, which promoted the thickening of the intestinal mucosa and reduced its absorptive capacity-leading to ill health and early mortality. The demand of AgNPs as water disinfectant has been increasing due to the release of the elemental and ionic forms of silver with their large surface to volume ratio, a property of the nanoparticles that destroys the DNA and cell membrane of the bacteria. Several studies have reported about the properties of AgNPs having higher penetration capacity than the bulk materials like AgNO3, AgSO4, etc. AgNPs damage the bacterial cell wall via interaction with the thiol-group (–S–H), and blocks the electron transport-resulting in the death of the bacteria (Sawosz et al. 2007; Dasgupta et al. 2019). AgNPs could interact with mitochondrial respiratory chain and produce reactive oxygen species (ROS)-leading to DNA oxidation, and affect the adenosine triphosphate (ATP) synthesis causing cell death (Jiang et al. 2020; Samuel et al. 2020). As a water disinfectant, the AgNPs have a better antibacterial property at a small dosage compared to their bulk counterparts. Mishra et al. (2014) reported that the nanoparticles might reach the cells of the layer chicks intestine through the gastrointestinal tract (GIT) due to the smaller diameter of nanoparticles (≤ 5 nm). However, Park et al. (2011) found that the AgNPs were not absorbed in the GIT of rats but excreted in feces when administered orally. A similar study conducted in rats by Van der Zande et al. (2012) have revealed that 99% of AgNPs intake through oral route was found in feces while only a little fraction of AgNPs was absorbed in GIT after 28-days. Like many other drugs, the AgNPs might affect liver and kidney more than other tissues due to metabolization and excretion process in the liver and kidney, respectively (Loghman et al. 2012).
From the metabolic disorder perspective, the serum blood chemistry is an essential examination for the health of the bird species, which guides the deviation of blood parameters from their normal range and diagnosis of a disease condition (Arzour-Lakehal et al. 2015). A study conducted by Sirkorska et al. (2010) could demonstrate that the different doses of AgNPs in rat studies did not affect the serum blood chemistry, hematological parameter, organ weight, and bone marrow cytotoxicity. However, yet another study showed the retention of silver in the kidney, liver, intestine, spleen, heart, and stomach was increased when provided with a higher dose of silver (Kim et al. 2008). Furthermore, in the case of mice, the WHO provided a short term exposure limit of 4.5 mg of silver per kg of body weight per day for 125 days without risk to health (WHO 1993). From an immunological perspective, Pineda et al. (2012) reported that the AgNPs did not affect the immunoglobulin M (IgM) and immunoglobulin G (IgG) levels. Histological biomarkers examination can identify the physiological function of organs like tissue structure, cell damage, and toxicity (Louiz et al. 2018). The liver and kidney are the primary organs for the detoxification of chemicals; thus, any changes in its structure and anatomy can be linked to chemical contaminants (Ribeiro et al. 2013; Louiz et al. 2018). Kulak et al. (2018) reported that the accumulation of AgNPs in the cytoplasm might damage the function of mitochondria either by mechanical damage or by blocking electron transport in the respiratory chain. The effects of the addition of 50 ppm dosage of AgNPs in drinking water as a disinfectant improved the growth performance such as body weight, feed intake capacity, and reduced the mortality rate of the poultry that have been shown in our previous study (Kumar and Bhattacharya 2019). However, the effect of AgNPs in serum blood chemistry and histopathology of the different organs of broiler chickens has not been assessed so far. The objective of this reported study was to investigate the effect of AgNPs on organ histopathology (such as liver, kidney, intestine, spleen, and heart) and serum biochemistry of the broilers.
Materials and methods
Experimental AgNPs solution
The AgNPs were synthesized and characterized by the hydrothermal route using an autoclave instrument (Kumar and Bhattacharya 2019). Briefly, the size and shape of the AgNPs were 15 nm and spherical, respectively. The particles were stable, and no agglomeration could be seen for 3 months. The minimum inhibitory concentration (MIC) of AgNPs against Escherichia coli (E. coli O157:H7) considered was 50 ppm (mg/L). The stock solution of AgNPs was prepared by dispersion of nanoparticles in de-ionized (DI) water using an ultrasonic bath sonicator.
Experimental design
This study was conducted in a commercial poultry farm, which was located at Gokulpur village 25 km away from Kharagpur, West Bengal, India. The farm was in a controlled condition with cooling, heating, and ventilation system. A total of 150 seven-day-old Cobb strain chicks (average body weight = 40 ± 0.5 g) of each sex were obtained from a commercial hatchery (Suguna poultry farm limited, Kolkata, India) and raised until the 42-days of age, before selling them. The experiment was designed into two experimental cases and control groups with 75 chicks in each group and the experiments were replicated three times one after another. The case group received 50 ppm dosed AgNPs solution through drinking water during the entire period of the experiment while the control group was not provided with such water. In both cases, the broilers were inspected once in a day and at the end of every week, their growth, feed intake, and water consumption were recorded. Moreover, during the entire experiment, any clinical signs and abnormal behavior were also examined for both case and control. All broilers were fed with commercial starter, grower, and finisher diets from Suguna Foods Pvt. Ltd. Kolkata, India, as standard practice. The dietary mixtures were composed of corn, soybean, mineral, and vitamin.
Serum blood chemistry studies
For blood sampling, at the end of each experiment, the blood samples were collected in serum separator tubes which contain a gel that separates blood cells from serum as suggested by Odetola et al. (2019) from two broilers from each case and control group. The serum was collected from the blood samples. The serum was obtained by centrifugation of the blood at 3500g for 10 min. at 4 °C and stored at − 20 °C for biochemical studies. The concentration of blood serum was analyzed for liver enzymes level test [total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein, total albumin, globulin, and alkaline phosphate], lipid profile test [cholesterol, triglycerides, HDL (high-density lipoprotein), VDL (low-density lipoprotein) and VLDL (very low-density lipoprotein)], and blood glucose at a standard pathological laboratory having quality certification. The liver and lipid profile tests were analyzed to check the health condition of both case and control groups in each experiment.
Organ histopathology examination
After the blood collection, the sample broilers were slaughtered and the liver, heart, spleen, kidney, and intestine were collected. These organ samples were washed thrice with phosphate buffer saline (PBS) solution and then fixed in 4% formalin within 24 h at room temperature as outlined by Sawosz et al. (2007). For histopathological examination, the tissues were rinsed in water, dehydrated alcohol, xylene, and embedded in paraffin blocks in a tissue processor as described by Ognik et al. (2016a). Now, the tissues were sectioned in size range of 5–8 µm, placed on glass slides, and stained with hematoxylin and eosin (H&E staining) at Ashok Laboratory Clinical Testing Pvt. Ltd. Kolkata India, following a method reported by Kristiansen et al. (2008). After that, the pathological changes in stained tissues were examined under a light microscope (ZEISS, Scope.A1) assisted with a digital camera (Canon, EOS 1100D).
Determination of silver concentration in tissues
The total silver content in tissues was determined by atomic absorption spectroscopy (AAS) (ICE 3300 AAS Thermo Fisher Scientific) as described by Gallocchio et al. (2017). Before the analysis, the tissue samples (liver, kidney, heart, spleen, and intestine) of 1 g each were digested in 8 mL HNO3 and 3 mL H2O2 (30%) using microwave digester (Microwave Digestion System Start-D, Milestone, USA). The digested samples were filtered with cellulose membrane filter paper with a 0.2 µm pore size. Then, 1 mL of filtrate of each sample was diluted to 10 mL of DI water and analyzed with AAS. The control organ digested samples were not diluted because in the AAS instrument, the diluted sample concentration showed the blank. It was considered that the silver concentration was zero in the control organ samples. The calibration curve was prepared by plotting the absorbance versus known concentration of silver within the range of 0–2 mg/L, as shown in supplementary Fig. S1. From the AAS, we have got a sample concentration value in mg/L (w/v). The concentration mg/L (w/v) was converted into µg/g (w/w) by the formula as given below:
where C = concentration of the sample solution (mg/L) as obtained by AAS, V = volume of undiluted sample solution (mL), W = weight of the organ sample (g), df = dilution factor = volume of diluted sample solution (mL)/volume of aliquot taken for dilution (mL).
Statistical analysis
To find the statistical significance of the samples, paired sample test, and one way ANOVA statistics were done of the data by using SPSS software of version 20. The significant difference value was considered as p value < 0.05, whereas the value p > 0.05 would show no significant difference.
Results
Characterization of AgNPs
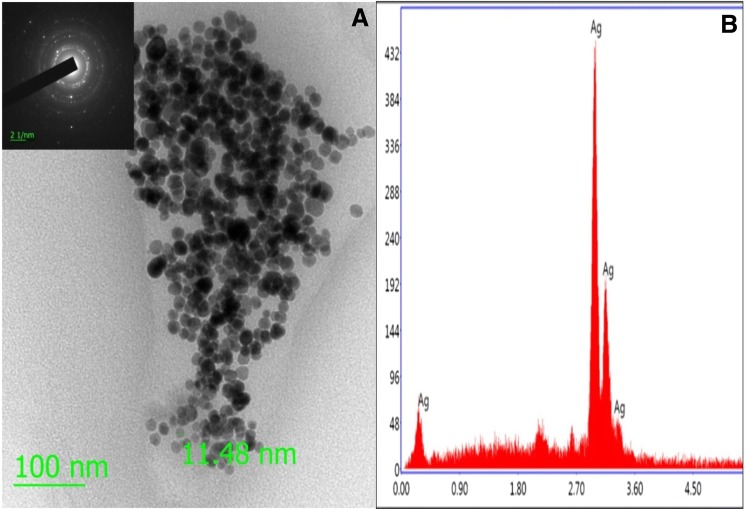
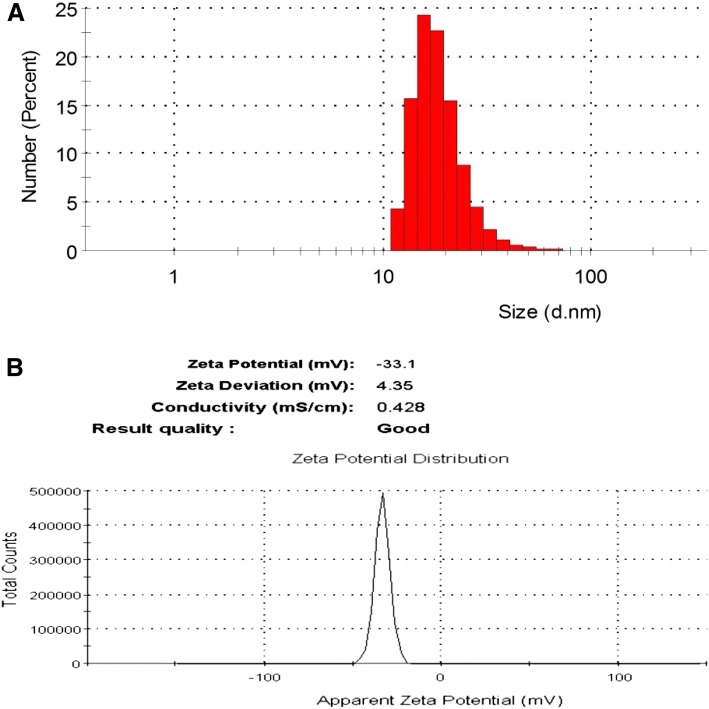
The high resolution transmission electron microscopy (HRTEM) and energy dispersive X-ray spectroscopy (EDX) analysis were used to characterize morphology, size, shape, dispersion, and elemental properties. The HRTEM image of AgNPs revealed that the size of AgNPs was less than 20 nm as using scale bar 100 nm, spherical in shape, and well disperse, as shown in Fig. 1a. The EDX analysis of synthesized nanomaterial revealed that the elements present in the nanomaterial was silver (Fig. 1b). Furthermore, the average size and zeta potential charge of AgNPs were determined by dynamic light scattering (DLS) and found to be 15 nm and − 33.1 mV, respectively, as presented in Fig. 2a, b. Therefore, the obtained average size of AgNPs by DLS confirmed the similar result of HRTEM.
Fig. 1.
a HRTEM (inset SEAD pattern), and b EDX image of AgNPs
Fig. 2.
a Size distribution, and b zeta potential charge of AgNPs by DLS analysis
The influence of AgNPs on water intake and growth performance
During the experiment, the difference in drinking water, eating, and physical activity of the broilers were observed in both groups and at the end of the experiment, the growth performance of the broilers was measured as shown in supplementary Table 1. The descriptive statistics data showed that the supplementation of AgNPs in drinking water significantly increased (p < 0.05) the water and food intake capacity in the case group and as a result of this, the body weight of broiler was significantly increased (p < 0.05) as presented in Table 1. Till day 42, the average water intake (WI) and feed intake (FI) in the case group were 0.645 L/bird/day and 0.531 kg/bird/day, respectively while in control group was 0.564 L/bird/day and 0.487 kg/bird/day, respectively which was statistically significant (p < 0.05). The significant body weight (BW) gain (p < 0.05) of the broilers in the case group was 2.392 kg/bird while in the control group was 1.933 kg/bird. Furthermore, based on the data of FI and BW, the food conversion ratio (FCR) value in the case and control group was 0.221 and 0.251, respectively.
Table 1.
Descriptive statistics of the growth performance of per broiler chicken (n = 12)
| Parameters | Farm | Descriptive | Statistic | Std. error | T test |
|---|---|---|---|---|---|
| P value | |||||
| Water intake (L) | Case | Mean | 0.645 | 0.0144 | 0.001 |
| Std. deviation | 0.035 | ||||
| Minimum | 0.597 | ||||
| Maximum | 0.687 | ||||
| Control | Mean | 0.564 | 0.0090 | ||
| Std. deviation | 0.022 | ||||
| Minimum | 0.541 | ||||
| Maximum | 0.590 | ||||
| Feed intake (kg) | Case | Mean | 0.531 | 0.0051 | 0.000 |
| Std. deviation | 0.012 | ||||
| Minimum | 0.510 | ||||
| Maximum | 0.543 | ||||
| Control | Mean | 0.487 | 0.0021 | ||
| Std. deviation | 0.005 | ||||
| Minimum | 0.477 | ||||
| Maximum | 0.492 | ||||
| Body weight (kg) | Case | Mean | 2.392 | 0.0230 | 0.000 |
| Std. deviation | 0.056 | ||||
| Minimum | 2.310 | ||||
| Maximum | 2.454 | ||||
| Control | Mean | 1.933 | 0.0117 | ||
| Std. deviation | 0.028 | ||||
| Minimum | 1.907 | ||||
| Maximum | 1.977 | ||||
| FCR | Case | Mean | 0.221 | 0.0006 | 0.000 |
| Std. deviation | 0.001 | ||||
| Minimum | 0.220 | ||||
| Maximum | 0.224 | ||||
| Control | Mean | 0.251 | 0.0019 | ||
| Std. deviation | 0.004 | ||||
| Minimum | 0.245 | ||||
| Maximum | 0.257 |
Serum biochemical profile
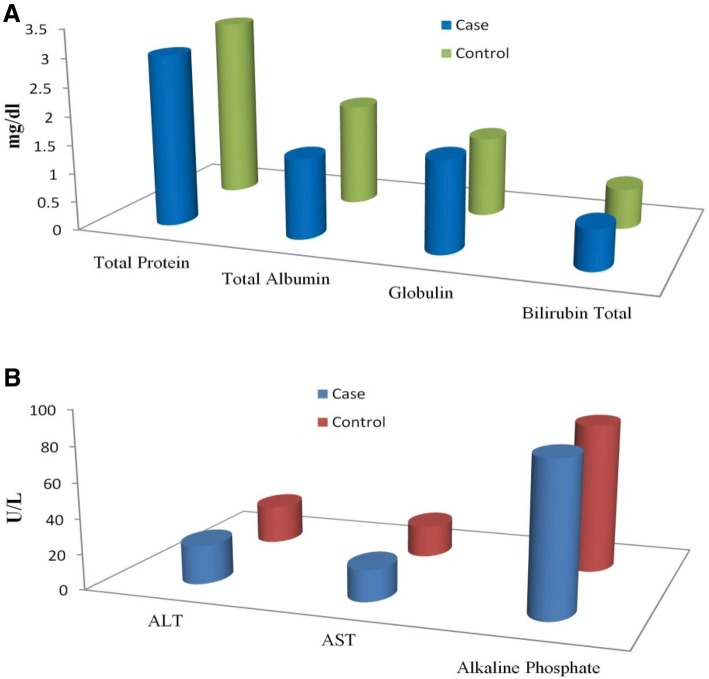
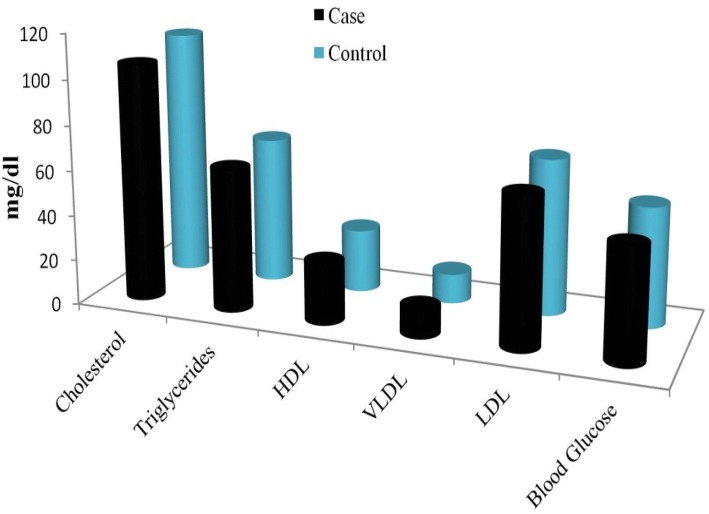
In the present study, we have analyzed liver enzyme, lipid profile, and blood glucose test of the broilers at 42nd day, the average rearing period. The liver enzyme concentration in the blood serum of the broilers is presented in Fig. 3. The concentration of total protein and total albumin was lower in the case group than control, was not statistically significant (p > 0.05). The globulin concentration, though lower in the control group, was significantly different (p < 0.05). Other parameters like total bilirubin, ALT, AST, and alkaline phosphate were found similar in both the groups. The lipid profile and blood glucose level of the serum of both case and control have been presented in Fig. 4. In the case group, cholesterol, triglycerides, HDL, VDL, VLDL, and blood glucose in the serum were not statistically significant (p > 0.05) as compared to the control group. The descriptive statistics of the serum biochemical parameter have been presented in Table 2. The obtained results of the biochemical profile suggest that the application of AgNPs in drinking water of the broilers did not have any significant toxicological effect on the case group as compared to the control group.
Fig. 3.
The liver enzyme concentration in blood serum of the case and control broilers
Fig. 4.
The lipid profile and blood glucose level of the case and control broilers
Table 2.
Descriptive statistics deviation of serum biochemical parameters of broiler chicken (n = 6)
| Blood parameters | Farm | Descriptive | Statistic | Std. error | T test |
|---|---|---|---|---|---|
| P value | |||||
| Total protein (mg/dL) | Case | Mean | 3.05 | 0.0500 | 0.095 |
| Std. deviation | 0.0707 | ||||
| Variance | 0.005 | ||||
| Control | Mean | 3.15 | 0.0500 | ||
| Std. deviation | 0.0707 | ||||
| Variance | 0.005 | ||||
| Total albumin (mg/dL) | Case | Mean | 1.575 | 0.1750 | 0.375 |
| Std. deviation | 0.2474 | ||||
| Variance | 0.061 | ||||
| Control | Mean | 1.775 | 0.0250 | ||
| Std. deviation | 0.0353 | ||||
| Variance | 0.001 | ||||
| Globulin (mg/dL) | Case | Mean | 1.595 | 0.0050 | 0.001 |
| Std. deviation | 0.0070 | ||||
| Variance | 0.000 | ||||
| Control | Mean | 1.445 | 0.0450 | ||
| Std. deviation | 0.0636 | ||||
| Variance | 0.004 | ||||
| Cholesterol (mg/dL) | Case | Mean | 106.65 | 1.3500 | 0.186 |
| Std. deviation | 1.9091 | ||||
| Variance | 3.645 | ||||
| Control | Mean | 109.50 | 0.5000 | ||
| Std. deviation | 0.7071 | ||||
| Variance | 0.500 | ||||
| Triglycerides (mg/dL) | Case | Mean | 64.00 | 0.8000 | 0.306 |
| Std. deviation | 1.1313 | ||||
| Variance | 1.280 | ||||
| Control | Mean | 65.10 | 0.1000 | ||
| Std. deviation | 0.1414 | ||||
| Variance | 0.020 | ||||
| HDL (mg/dL) | Case | Mean | 26.90 | 0.1000 | 0.070 |
| Std. deviation | 0.1414 | ||||
| Variance | 0.020 | ||||
| Control | Mean | 27.70 | 0.2000 | ||
| Std. deviation | 0.2828 | ||||
| Variance | 0.080 | ||||
| VLDL (mg/dL) | Case | Mean | 12.75 | 0.1500 | 0.238 |
| Std. deviation | 0.2121 | ||||
| Variance | 0.045 | ||||
| Control | Mean | 12.95 | 0.0500 | ||
| Std. deviation | 0.0707 | ||||
| Variance | 0.005 | ||||
| LDL (mg/dL) | Case | Mean | 66.95 | 1.0500 | 0.191 |
| Std. deviation | 1.4849 | ||||
| Variance | 2.205 | ||||
| Control | Mean | 69.00 | 0.1000 | ||
| Std. deviation | 0.1414 | ||||
| Variance | 0.020 | ||||
| Blood glucose (mg/dL) | Case | Mean | 51.50 | 1.5000 | 0.808 |
| Std. deviation | 2.1213 | ||||
| Variance | 4.50 | ||||
| Control | Mean | 52.00 | 1.0000 | ||
| Std. deviation | 1.4142 | ||||
| Variance | 2.00 |
Histopathology examination
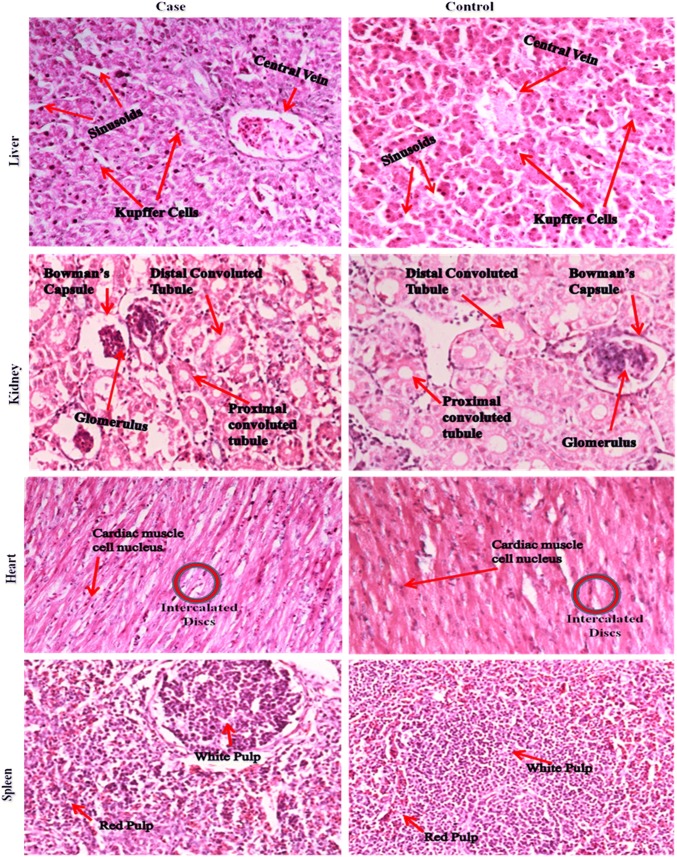
In histopathology examination, a parallel to the serum test, the toxicological effect of AgNPs and deposition within the cells are investigated to check whether any sort of tissue inflammations, damages, changes in normal and regular morphology caused towards the major organs. The histological section images of the major organs of the case and control broilers have been shown in Fig. 5. As from the figures, no signs inflammations or damages could be seen in the major organs such as liver, kidney, heart, spleen, and intestine in the case study. We have found normal and similar morphology with respect to the control study. The liver histological section showed a normal hepatic architecture, sinusoids, and no aggregation of hepatocyte and Kupffer cells by the application of AgNPs in water as compared to control. The normal sinusoids morphology in the liver section characterized the uniform distribution of blood to the liver. The renal glomeruli and tubules in the kidney histological section have shown normal structure in the case group with respect to the control group. There were no signs of fibrosis observed in the cortex and medulla of the kidney section. In the homogenous cytoplasm and round nuclei of the heart and spleen tissues, no lesions or abnormalities were found. In the small intestine, we have taken the jejunum section (middle segment) for histology. The histological section showed no evidence of intestinal lesions on villi, muscularis externa, sub mucosa, and crypt for both the groups, as shown in Fig. 6.
Fig. 5.
Hematoxylin and eosin (H&E) stained section images of major organs of the case and control broiler
Fig. 6.
The histopathological H&E stained images of different section of small intestine of broiler
Silver concentration in the tissues
The retention of silver content in the major organs was determined at the end of the experiment by AAS and presented in Table 3. The silver content was not detected in the control organ samples. However, in the case group, the silver concentration was higher in the small intestine (5.44 µg/g), followed by liver, kidney, spleen, and heart. The silver content in the muscles of chicken was found below the detection limit, therefore not shown. The current findings suggest that the small intestine was the residence of a level of AgNPs, which indicates that the excretion of silver from the intestine to the feces. A similar result was found in the rat study by Park et al. (2011).
Table 3.
Retention of silver in major organs of case group broiler chicken
| Parameter | Organ | ||||
|---|---|---|---|---|---|
| Liver | Kidney | Heart | Spleen | Small intestine | |
| Silver concentration (µg/g) | 4.32 | 3.94 | 3.82 | 3.49 | 5.44 |
Discussion
In animal production, the use of AgNPs as an antimicrobial agent has been experimented with to reduce the population of harmful bacteria like Salmonella, E. coli, and Streptococcus spp. in food and water; however, no effect was observed on the beneficial Lactobacillus spp. in the microflora that are present inside the intestine (Elkloub et al. 2015; Duffy et al. 2018; Anwar et al. 2019). In the previous studies, the supplementation of AgNPs in drinking water as a disinfectant was not focused to check their effect on the serum biochemical and histological changes of the major organs, rather than focusing only the feed additives of AgNPs as growth promoter (Ahmadi 2009; Sawosz et al. 2009; Ahmadi and Kurdestany 2010; Ognik et al. 2017). The present study found that the supplementation of 50 ppm dosed AgNPs as a disinfectant in broilers drinking water had no negative effects on the growth performance of the broilers. It was observed that the FI, WI, and BW gain significantly increased (p < 0.05) in the case group as compared to control (Table 1). Simultaneously, during the entire experiment, no significant clinical and histological signs were observed among the broilers due to the application of AgNPs. In context to this result, Ahmadi (2009) reported that the supplementation of AgNPs (300–900 ppm) in solid feed improved the BW, FI, and FCR of the broilers chicken.
Moreover, from our study of the blood serum of the broilers, it was seen there were no significant (p > 0.05) changes in the liver enzyme, lipid profile, and blood glucose as compared to the control. The serum blood parameters of the present study were found within the normal range for healthy broilers, as reported by Scott et al. (2018). In a mice study, Arora et al. (2009) reported that the higher concentration of 20 nm-sized AgNPs (> 200 ppm) could cause oxidative stress, apoptosis, and decreased cell viability in fibroblasts. Ognik et al. (2016a, b) hypothesized that the liver damage or cardiac infarction caused by toxins and viruses and usually increases the activity of the liver enzyme, releasing hepatocytes to the blood. In the present study, within the MIC applied, we did not find any such damages or lesions in the liver, kidney, and heart by the histology images of liver, kidney, and heart, as shown in Fig. 5. According to Aragon and Younossi (2010), kidney and bone growth disorder usually occur due to the reduced levels of AST and ALT or ALP. Our current findings of similar values of AST, ALT, and ALP in both the case and control groups were not enough to bring any visible changes in the cell and tissue histology to prompt any kidney dysfunction and bone growth disorder due to the supplementation of AgNPs. The present results were in agreement with Sawosz et al. (2009), Andi et al. (2011), and Ognik et al. (2016a, b) who found no effect of the administration of AgNPs to the broilers on the activity of AST, ALT or ALP. Furthermore, the lipid profile of the case group broilers has been lower than the control group (Fig. 4), which revealed that the addition of AgNPs in the water had no negative impact on the cholesterol, LDL, triglycerides, and blood glucose level. In contrast, Ahmadi and Branch (2012) have found that the feed additive of AgNPs (20–40 ppm/kg feed) to the broilers had a negative impact on the blood lipid profile due to increased level of cholesterol, LDL, and triglycerides. Moreover, another study of Ahmadi et al. (2013) have found that the lower concentration of AgNPs (4–12 ppm/kg) feed additive on feed had no negative effect on the lipid profile of the blood. Ognik et al. (2016a, b) reported that the modification in the cholesterol and LDL was usually occurred due to degradation of polyunsaturated fatty acid and as a result of this lipid peroxidation initiated, followed by an increased level of the formation of lipid, triglycerides, and phospholipid peroxides from cholesterol esters.
According to the 42 days supplementation of AgNPs in drinking water, the higher retention of AgNPs was shown in the small intestine, followed by liver and kidney, and this seemed to be excreted via feces, bile, and urine, respectively. In present findings, the presence of silver concentration in organs is agreed with published studies. Espinosa-Cristobal et al. (2013) worked with Wistar rats during 55 days and showed that higher retention of AgNPs in the small intestine was 41.7 µg/g, followed by liver (18.7 µg/g) and kidney (15.4 µg/g). Our results are in agreement with Park et al. (2011) who reported that the accumulation of AgNPs in different organs was not significant in orally treated rats rather than intravenous injection, and concluded that the orally administered AgNPs was passed through the GIT tract and excreted via feces without translocation in the bloodstream. In another research, Nabinejad et al. (2016) have shown that the high concentration of AgNPs (185 µg/g) in the liver was found to be detoxifying like other toxins and excreted through bile, while silver residue in the kidney was filtered by kidneys and excreted via urine. The finding of the present study with respect to silver distribution in muscles concur with Kulak et al. (2018), who have demonstrated that the varying dose of AgNPs (2.87–63.74 mg/bird) was not accumulated in the breast muscles. As depicted in Figs. 5 and 6, the organ histological response showed that the 15 nm-sized AgNPs did not show any indications of damage in the liver, kidney, heart, spleen, and intestine as 50 ppm dosed AgNPs supplemented in drinking water of broilers. In contrast with Ibrahim et al. (2018) who reported that the exposure of small-sized gold nanoparticles (≤ 5 nm) induced more pathological changes in the liver, kidney, or spleen rather than the medium to larger sized (10–50 nm) nanoparticles. In a previous study, Samani et al. (2018) have reported that the application of AgNPs (40 nm) with the concentration of 10–100 mg/L reduced the oxidative stress and had negative effects on the liver and kidneys where an inflammatory reaction occurs. In addition, they reported that the early stages of vacuolar degeneration of hepatocytes could occur due to a high dose of AgNPs (100 ppm). In brief, the results of the experiment show that relatively larger AgNPs of 15 nm and a certain concentration would be able to reduce the pathogenic mortality of chickens, but without any health effects on humans.
Conclusion
The reported study is about finding the probable effects of administering AgNPs as a disinfectant in the drinking water of the broilers by the growth, serum biochemical, and histopathological responses. The study reports the major findings:
The supplementation of 50 ppm dosed AgNPs (15 nm) in broilers drinking water showed no negative effects on blood serum biochemical properties, and histopathological parameters.
The obtained results also showed that the average WI, FI, and BW in the case group per broiler was 0.645 L, 0.531 kg, and 2.392 kg, respectively whereas in the control group was 0.564 L, 0.487 kg, and 1.933 kg, respectively which was statistically significant (p < 0.05).
In the testing of case broilers there were observed no changes due to AgNPs dosed drinking water, in the tissues regarding histological morphology, that were different from the control group, to suggest any visible effects.
On the basis of the above, it can be said 15 nm AgNPs of 50 ppm dose while being effective as a disinfectant, did not have any effect on the examined tissues of the broilers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to the ministry of human resource and development (MHRD), Government of India for financial assistantship for the first author. The authors would like to acknowledge Mr. Bhuvaneshwaran Subramanian, Research Scholar of School of Medical Science and Technology, IIT Kharagpur, for his consistent support in the laboratory work. Zelence Industries Private Limited is also thanked for their help in providing access to the poultry farms and making the farmers agree to the tests.
Compliance with ethical standards
Conflict of interest
The authors do not have conflict of interest in this present work.
References
- Ahmadi J. Application of different levels of silver nanoparticles in food on the performance and some blood parameters of broiler chickens. World Appl Sci J. 2009;7:24–27. [Google Scholar]
- Ahmadi F, Branch S. Impact of different levels of silver nanoparticles (Ag–NPs) on performance, oxidative enzymes and blood parameters in broiler chicks. Pak Vet J. 2012;32:325–328. [Google Scholar]
- Ahmadi F, Kurdestany AH. The impact of silver nano particles on growth performance, lymphoid organs and oxidative stress indicators in broiler chicks. Glob Vet. 2010;5:366–370. [Google Scholar]
- Ahmadi F, Khah MM, Javid S, Zarneshan A, Akradi L, Salehifar P. The effect of dietary silver nanoparticles on performance, immune organs, and lipid serum of broiler chickens during starter period. Int J Biosci. 2013;3:95–100. [Google Scholar]
- Andi MA, Hashemi M, Ahmadi F. Effects of feed type with/without nanosil on cumulative performance, relative organ weight and some blood parameters of broilers. Glob Vet. 2011;7:605–609. [Google Scholar]
- Anwar M, Awais M, Akhtar M, Navid M, Muhammad F. Nutritional and immunological effects of nano-particles in commercial poultry birds. World's Poult Sci J. 2019;75:261–272. [Google Scholar]
- Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Clevel Clin J Med. 2010;77:195–204. doi: 10.3949/ccjm.77a.09064. [DOI] [PubMed] [Google Scholar]
- Arora S, Jain J, Rajwade JM, Paknikar KM. Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells. Toxicol Appl Pharmacol. 2009;236:310–318. doi: 10.1016/j.taap.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Arzour-Lakehal N, Benlatreche C, Boukerrou A, Siliart B, Ldh O. Effect of age on selected plasma indices of biochemical and mineral metabolism in two strains of broiler chickens. Int J Poult Sci. 2015;14:20. [Google Scholar]
- Bobinienė R, Miškinienė M, Gudavičiūtė D, Mackiewicz Z. Health indicators of the poultry drinking water treated with electromagnetic vibrations. Vet Zootech. 2014;67:20. [Google Scholar]
- Dasgupta N, Ranjan S, Ramalingam C, Gandhi M. Silver nanoparticles engineered by thermal co-reduction approach induces liver damage in Wistar rats: acute and sub-chronic toxicity analysis. 3 Biotech. 2019;9:125. doi: 10.1007/s13205-019-1651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy LL, Osmond-McLeod MJ, Judy J, King T. Investigation into the antibacterial activity of silver, zinc oxide and copper oxide nanoparticles against poultry-relevant isolates of Salmonella and Campylobacter. Food Control. 2018;92:293–300. [Google Scholar]
- El-katcha MI, Soltan MA, Sharaf MM, Hasen A. Growth performance, immune response, blood serum parameters, nutrient digestibility and carcass traits of broiler chicken as affected by dietary supplementation of garlic extract (Allicin) Alex J Vet Sci. 2016;49:20. [Google Scholar]
- Elkloub K, Moustafa ME, Ghazalah AA, Rehan AAA. Effect of dietary nanosilver on broiler performance. Int J Poult Sci. 2015;14:177–182. [Google Scholar]
- Espinosa-Cristobal LF, Martinez-Castanon GA, Loyola-Rodriguez JP, Patino-Marin N, Reyes-Macias JF, Vargas-Morales JM, Ruiz F. Toxicity, distribution, and accumulation of silver nanoparticles in Wistar rats. J Nanopart Res. 2013;15:1702. [Google Scholar]
- Gallocchio F, Biancotto G, Cibin V, Losasso C, Belluco S, Peters R, van Bemmel G, Cascio C, Weigel S, Tromp P, Gobbo F. Transfer study of silver nanoparticles in poultry production. J Agric Food Chem. 2017;65:3767–3774. doi: 10.1021/acs.jafc.7b00670. [DOI] [PubMed] [Google Scholar]
- Ibrahim K, Al-Mutary M, Bakhiet A, Khan H. Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules. 2018;23:1848. doi: 10.3390/molecules23081848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari RA, Fazlara A, Govahi M. An investigation into Salmonella and fecal coliform contamination of drinking water in broiler farms in Iran. Int J Poult Sci. 2006;5:491–493. [Google Scholar]
- Jiang S, Liu X, Liu Y, Liu J, He W, Dong Y. Synthesis of silver@hydroxyapatite nanoparticles based biocomposite and their assessment for viability of osseointegration for rabbit knee joint anterior cruciate ligament rehabilitation. J Photochem Photobiol B. 2020;202:111677. doi: 10.1016/j.jphotobiol.2019.111677. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH, Kwon IH. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague–Dawley rats. Inhal Toxicol. 2008;20:575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- Kristiansen S, Ifversen P, Danscher G. Ultrastructural localization and chemical binding of silver ions in human organotypic skin cultures. Histochem Cell Biol. 2008;130:177–184. doi: 10.1007/s00418-008-0415-x. [DOI] [PubMed] [Google Scholar]
- Kulak E, Ognik K, Stępniowska A, Drażbo A. Effect of nanoparticles of silver on redox status and the accumulation of Ag in chicken tissues. J Sci Food Agric. 2018;98:4085–4096. doi: 10.1002/jsfa.8925. [DOI] [PubMed] [Google Scholar]
- Kumar I, Bhattacharya J. Assessment of the role of silver nanoparticles in reducing poultry mortality, risk and economic benefits. Appl Nanosci. 2019;9:1293–1307. [Google Scholar]
- Loghman A, Iraj SH, Naghi DA, Pejman M. Histopathologic and apoptotic effect of nanosilver in liver of broiler chickens. Afr J Biotech. 2012;11:6207–6211. [Google Scholar]
- Louiz I, Palluel O, Ben-Attia M, Aït-Aïssa S, Hassine OKB. Liver histopathology and biochemical biomarkers in Gobius niger and Zosterisessor ophiocephalus from polluted and non-polluted Tunisian lagoons (Southern Mediterranean Sea) Mar Pollut Bull. 2018;128:248–258. doi: 10.1016/j.marpolbul.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Mishra A, Swain RK, Mishra SK, Panda N, Sethy K. Growth performance and serum biochemical parameters as affected by nano zinc supplementation in layer chicks. Indian J Anim Nutr. 2014;31:384–388. [Google Scholar]
- Nabinejad AR, Noaman V, Khayyam NM. Evaluation of silver residues accumulation in tissues of Broilers treated with nanosilver using MNSR (A Clinical Trial) Arch Razi Inst. 2016;71:51–55. [Google Scholar]
- Odetola OM, Adejinmi OO, Owosibo OA, Banjo OT, Awodola-Peters OO. Growth response, serum biochemistry and organ histopathology of broilers fed diets supplemented with graded levels of Petiveria alliacea root meal. Int J Poult Sci. 2019;18:45–50. [Google Scholar]
- Ognik K, Sembratowicz I, Cholewińska E, Wlazło Ł, Nowakowicz-Dębek B, Szlązak R, Tutaj K. The effect of chemically-synthesized silver nanoparticles on performance and the histology and microbiological profile of the jejunum in chickens. Ann Anim Sci. 2016;16:439–450. [Google Scholar]
- Ognik K, Cholewińska E, Czech A, Kozłowski K, Nowakowicz-Dębek B, Szlązak R, Tutaj K. Effect of silver nanoparticles on the immune, redox, and lipid status of chicken blood. Czech J Anim Sci. 2016;61:450–461. [Google Scholar]
- Ognik K, Stępniowska A, Kozłowski K. The effect of administration of silver nanoparticles to broiler chickens on estimated intestinal absorption of iron, calcium, and potassium. Livestock Sci. 2017;200:40–45. [Google Scholar]
- Park K, Park EJ, Chun IK, Choi K, Lee SH, Yoon J, Lee BC. Bioavailability and toxicokinetics of citrate-coated silver nanoparticles in rats. Arch Pharmacal Res. 2011;34:153–158. doi: 10.1007/s12272-011-0118-z. [DOI] [PubMed] [Google Scholar]
- Pineda L, Chwalibog A, Sawosz E, Lauridsen C, Engberg R, Elnif J, Hotowy A, Sawosz F, Gao Y, Ali A, Moghaddam HS. Effect of silver nanoparticles on growth performance, metabolism and microbial profile of broiler chickens. Arch Anim Nutr. 2012;66:416–429. doi: 10.1080/1745039X.2012.710081. [DOI] [PubMed] [Google Scholar]
- Ribeiro CADO, Katsumiti A, França P, Maschio J, Zandoná E, Cestari MM, Vicari T, Roche H, Assis HCSD, Filipak NF. Biomarkers responses in fish (Atherinella brasiliensis) of paranaguá bay, southern Brazil, for assessment of pollutant effects. Braz J Oceanogr. 2013;61:1–11. [Google Scholar]
- Samani PY, Samani PY, Arabi M, Shadkhast M, Samani PY, Piraei E. Repeated-dose toxicity in mouse liver and kidney after skin exposure to silver nanoparticles. J Clin Diagn Res. 2018;12:20. [Google Scholar]
- Samuel MS, Jose S, Selvarajan E, Mathimani T, Pugazhendhi A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J Photochem Photobiol B. 2020;202:111642. doi: 10.1016/j.jphotobiol.2019.111642. [DOI] [PubMed] [Google Scholar]
- Sawosz E, Binek M, Grodzik M, Zielińska M, Sysa P, Szmidt M, Niemiec T, Chwalibog A. Influence of hydrocolloidal silver nanoparticles on gastrointestinal microflora and morphology of enterocytes of quails. Arch Anim Nutr. 2007;61:444–451. doi: 10.1080/17450390701664314. [DOI] [PubMed] [Google Scholar]
- Sawosz E, Grodzik M, Zielińska M, Niemiec T, Olszańska B, Chwalibog A. Nanoparticles of silver do not affect growth, development and DNA oxidative damage in chicken embryos. Arch Geflügelkd. 2009;73:208–213. [Google Scholar]
- Scott A, Vadalasetty KP, Łukasiewicz M, Jaworski S, Wierzbicki M, Chwalibog A, Sawosz E. Effect of different levels of copper nanoparticles and copper sulphate on performance, metabolism and blood biochemical profiles in broiler chicken. J Anim Physiol Anim Nutr. 2018;102:364–373. doi: 10.1111/jpn.12754. [DOI] [PubMed] [Google Scholar]
- Sikorska J, Szmidt M, Sawosz E, Niemiec T, Grodzik M, Chwalibog A. Can silver nanoparticles affect the mineral content, structure and mechanical properties of chicken embryo bones? J Anim Feed Sci. 2010;663:127. [Google Scholar]
- Van der Zande M, Vandebriel RJ, Van Doren E, Kramer E, Herrera Rivera Z, Serrano-Rojero CS, Gremmer ER, Mast J, Peters RJ, Hollman PC, Hendriksen PJ. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS NanoNano. 2012;6:7427–7442. doi: 10.1021/nn302649p. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Guidelines for drinking-water quality. 2. Geneva: World Health Organization; 1993. pp. 281–283. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.