Abstract
Background
Syrian Sumac, scientifically defined as Rhus coriaria, is a commonly used spice powder in the Middle East. Rhus coriaria has been shown to contain numerous compounds that have a substantial role in the food industry and in homeopathic therapy. From the retardation of oxidative processes to the treatment of fungal and bacterial infections and many more, these compounds are of great importance in improving human health and economy.
Scope and approach
Several studies have been done to explore the benefits and potential uses of Rhus coriaria. In the following review, the relevant phytochemical and biological research available on Rhus coriaria have been explored. A comprehensive account of its healing activity is shown. Also presented are its phytochemical components which have medicinal, nutritional and industrial significance.
Key findings and conclusions
Sumac has been studied for its use as an antibacterial, antioxidant, colorant, food and animal feed supplement, steel inhibitor in sea water and much more. Its antibacterial, antifungal and antioxidant properties make it a great and versatile tool to be used in the food industry, where it can be used as an efficient food preservative and natural, harmless food additive.
Keywords: Food science, Rhus coriaria, Sumac properties, Antibacterial, Antifungal, Antioxidant, Phytochemical characteristics
Food science; Rhus coriaria, Sumac properties, Antibacterial, Antifungal, Antioxidant, Phytochemical characteristics
1. Introduction
Sumac is a commonly used spice in the Arab world. Although used as a powder, its natural state is a fruit. From the species Anacardiaceae and genus Rhus, Sumac is the generic name used to indicate the spice product of the plant Rhus coriaria. The etymology of the word Sumac is believed to come from “summāq” which means “dark red” in Arabic (AbouReidah et al., 2014). Others believe it is derived from the Syriac word “Sumaga”, which also translates to “red” (Raodah et al., 2014).
Rhus coriaria has an attractive economic importance due to its increasing use in food, cosmetic and pharmaceutical industries, coloring or preservation of foods and veterinary practices (Kizil & Turk, 2010).
Several studies have been performed on identifying the major components of Rhus coriaria (Shabir, 2012) and its bioactive compounds (Asgarpanah and Saati, 2013) as well as fatty acid composition (Dogan and Celic, 2015). Moreover, its potential therapeutic effect has been evaluated by several studies which identified its antibacterial power (AlJaber, 2008; Raodah et al., 2014), antifungal (Ertürk, 2010, Ferk et al., 2007), antioxidant (Ertürk, 2010, Ferk et al., 2007; Raodah et al., 2014) and anaelgesic effects (Mohammadi et al., 2016). Moreover, its antilipidemic (Boroujeni et al., 2016) and hypoglycemic effects (Shidfara and Hosseinic, 2014; Anweri et al., 2013) has been proved effective. In addition, researchers have been analyzing its potential as preservatives (Abdelmalek, 2013; Obais et al., 2013), antioxidant (Bursal, 2010; Almouwaly et al., 2013), meat tendirizer (Sakhr and El Khatib, 2019; El Khatib and Salame, 2019), meat preservative (El El Khatib and Salame, 2019) and colorant (Dabas, 2016) for its use in the food industry. Not only restricted to that, it also has been shown to have a potential for inhibiting steel in sea water (Anaee et al., 2016) and in dentistry, where it efficiently suppressed Streptococcus mutans, the main bacteria causative agent of dental caries and plaque (Dastjerdi et al., 2014).
In order to explore all the promising potential of this plant, many studies can still be done for its appealing source of functional food and nutraceuticals. Moreover, further mechanisms of action of Rhus coriaria components should be established in order to have a better understanding of the plant extracts and components’ bioactivity and thus, a wider use of the plant extracts in food science.
2. Physiochemical analysis of Rhus coriaria
Rhus coriaria is the scientific name of Sumac, which is a flowering shrub that usually grows wildly or in orchards in Lebanese rural areas. It is part of the Anacardiaceae family in the order of Sapindales (Asgarpanah and Saati, 2013). It can be found in subtropical and temperate regions. Africa, Mediterranean area and Western Asia are the places where it is mostly popular (AbouReidah et al., 2014). The genus “Rhus” is composed of almost 250 species spread throughout the planet. In the Middle Eastern area that includes Lebanon, Syria, Palestine and Jordan, it is referred to as Syrian sumac (Raodah et al., 2014). Anatomically, it is a small tree that can grow up to ten meters in height. Sumac propagates both by seed and by new roots that propagate underground called rhizomes (AbouReidah et al., 2014).
The majority of studies made on Rhus coriaria have shown many potential compounds that have a substantial role in the food industry as well as in homeopathic therapy. From the retardation of oxidative processes to the treatment of fungal and bacterial infections and many more, these compounds are of great importance in improving human health and economy.
2.1. Physical characteristics of Rhus coriaria
Sumac trees have its leaves arranged in spirals as well as its fruit inside each cluster. The red fruits are tightly grouped together into an inverted cone-shaped spike of 5–30 cm. The fruits are tiny little spheres tightly packed together forming dense clusters of reddish drupes called sumac bobs (Figure 1). These drupes are sun-dried and ground producing the reddish commonly known sumac powder spice (AbouReidah et al., 2014).
Figure 1.
Sumac fruit and leaves (Photo by Khaula Sakhr, 2019)
A study of the Faculty of agriculture in Selcuk University in Turkey has studied the proximate physical characteristics of sumac fruits on a moisture content of 4.79%, measured directly upon arrival, and the dimensional properties of these fruits were measured (Table 1). The knowledge of the physical properties of sumac is necessary for the initial design of equipment for the handling, collecting, transport, processing and storage of the fruit crops (Ozcan and Haciseferogullari, 2004). In addition, an evaluation of the physical properties of the fruits was undergone (Table 1).
Table 1.
Dimensional and physical properties of Rhus coriaria (Ozcan and Haciseferogullari, 2004).
| Length (mm) | 4.72 ± 0.030 |
|---|---|
| Width (mm) | 3.90 ± 0.028 |
| Thickness (mm) | 2.64 ± 0.025 |
| Weight (g) | 0.018 ± 0.001 |
| Mean diameter (mm) | 3.64 ± 0.023 |
| Volume (mm3) | 19.49 ± 0.442 |
| Sphericity (-) | 0.773 ± 0.003 |
| Projected area (cm2) | 0.164 ± 0.005 |
| Bulk density (kg/m3) | 304.25 ± 1.364 |
| Porosity (%) | 68.52 ± 0.578 |
| Terminal velocity (m/s) | 3.52 ± 0.128 |
| Coefficient of static friction | |
| Iron sheet | 0.532 ± 0.036 |
| Galvanized iron sheet | 0.482 ± 0.022 |
| Plywood | 0.607 ± 0.068 |
| Rubber | 0.675 ± 0.334 |
2.2. Chemical characteristics of Rhus coriaria
A study conducted in Iran on the characterization and identification of chemical compounds of Sumac using HPLC-MS method identified 191 compounds in Rhus coriaria and classified them as being:
-
•
78 hydrolysable tannins
-
•
59 flavonoids
-
•
9 anthocyanins
-
•
2 isoflavonoids
-
•
2 terpenoids
-
•
1 diterpene
-
•
38 other unidentified compounds (Ardalani et al., 2016)
In order to isolate, determine and identify the compounds from the Rhus coriaria fruits, different extracts were taken from the fruit and leaves of the Sumac plant. Some were isolated from aqueous extracts, others from alcoholic extracts and some from lipid extracts. Hydrolysable tannins compose the highest percentage in the Sumac fruits, followed by flavonoids (Ardalani et al., 2016). This emphasizes the antioxidant potential of the fruit. Following hydrolysable tannins, comprising almost 20% of the fruit's mass, are other still unidentified compounds. Further studies should be done on Rhus coriaria in order to isolate and identify these substances. Subsequently there are anthocyanins, isoflavonoids, terpenoids and diterpenes (Ardalani et al., 2016).
A study on the chemical properties of sumac fruit was conducted on ripe fruits and have found a 2.6% protein content, 7.4% fat content, 14.6% fiber content, 1.8% ash (Table 2). Also, a calorimetric calculation showed that 100g of sumac fruit contains 147.8 kcal (Ozcan and Haciseferogullari, 2004).
Table 2.
Chemical composition of Sumac (Ozcan and Haciseferogullari, 2004; Raodah et al., 2014).
| Properties | References |
|
|---|---|---|
| Ozcan and Haciseferogullari (2004) | Raodah et al. (2014) | |
| Moisture (%) | 10.6 (fresh) | 2.43 (dried) |
| Crude oil (%) | 7.4 | 18.74 |
| Crude protein (%) | 2.6 | 4.69 |
| Crude fiber (%) | 14.6 | ND |
| Carbohydrate (%) | ND | 71.21 |
| Crude energy (kcal/100g) | 147.8 | ND |
| Ash (%) | 1.8 | 2.93 |
| Water soluble extract (%) | 63.8 | ND |
| Acidity (%) | 4.6 | ND |
| pH | 3.7 | 3.02 |
2.2.2. Chemical compounds extracted from Rhus coriaria
2.2.2.1. Phenolic compounds
The phenolic compounds in Sumac are the compounds that constitute its phytochemical activity along with anthocyanins. The most abundant phenolic compound in sumac fruits was found to be Gallic acid (Bozan et al., 2002).
A study to optimize the polyphenols extraction in sumac fruit was made, where the authors managed and varied the conditions in which the extraction took place. It was determined that the optimal conditions are as follows: Ethanol concentration is 80%; extraction time is 1 h; extraction temperature is 40 °C; particle size 1.0mm; and solvent to sumac ratios 15:1 ml/g (Kossah et al., 2010).
On the other hand, a study conducted by Zalacain et al. on the optimization of extraction of Gallotannins from the leaves of sumac found that the best extraction conditions are macerating the leaves in water at 45 °C for 60 min (Zalacain et al., 2003). Gallotannins are the most abundant tannin compounds found in the Rhus genus (Romeo et al., 2015).
In addition to that, a study made in Turkey on sumac fruit showed the extraction yield percentage and total phenolic content of fractions obtained from aqueous methanol extracts of sumac. It showed that the highest phenolic content was present in the ethyl acetate fraction of the extract (Bozan et al., 2002). A similar evaluation was made by Raodah et al. wherein the methanolic extract showed the highest Total Phenolics content, followed by ethyl acetate and aqueous extracts (Raodah et al., 2014).
Moreover, in order to determine the total phenolic content of Syrian Sumac, Raodah et al. applied the Folin-Ciocalteau procedure followed by absorbance measurement using a spectrophotometer and found that the highest phenolic compounds extraction was obtained from the methanolic sumac extract which contained 151.71 mg/g of phenols, followed by 65.31 mg/g from the ethyl acetate extract and 6.10 mg/g from the aqueous extract of sumac (Raodah et al., 2014).
Also, Romeo et al. identified the phenolic compounds in sumac using an HPLC. The first component identified was gallic acid, followed by flavonoid identified as Quercetin, Myrecetin 3-rhamnoside and Quercetin 3-glucoside (Romeo et al., 2015).
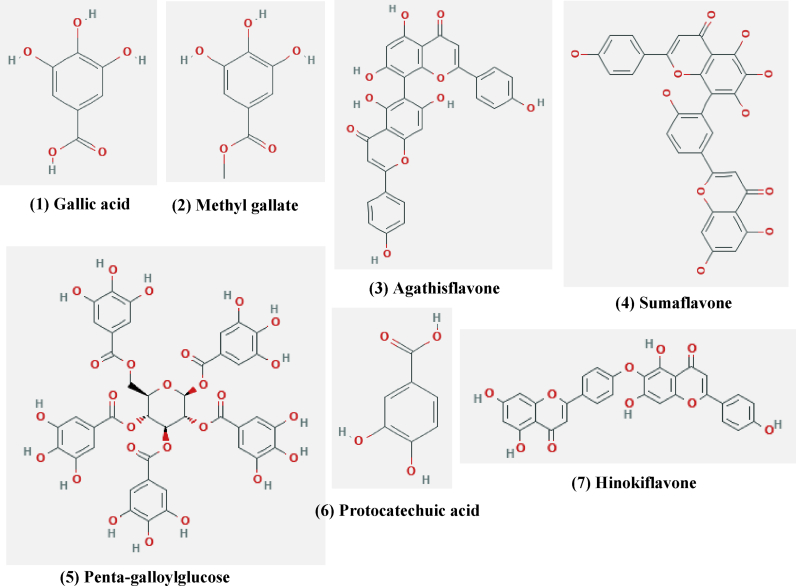
Moreover, 6 gallotannins were identified. These were penta, hexa, hepta, octa, nona and decagalloyl-glucoside (Romeo et al., 2015). Tannins are the compounds responsible for the astringent taste of sumac (AbouReidah et al., 2014). The chemical structures of some phenolic compounds present in Sumac fruit are represented in Figure 2 (AbouReidah et al., 2014).
Figure 2.
Structure of some selected phenolic compounds from Rhus coriaria Fruit (PubChem).
In addition, a comparative study made in Baghdad on different extracts (aqueaous, methanolic and ethanolic) found that methanolic extracts contain a higher amount of phenolics and flavonoids than ethanolic and aqueous extracts thus making it the best solvent for the extraction of phenolics from sumac (AlMouwaly et al., 2013), confirming previous statements (Raodah et al., 2014).
2.2.2.2. Anthocyanins
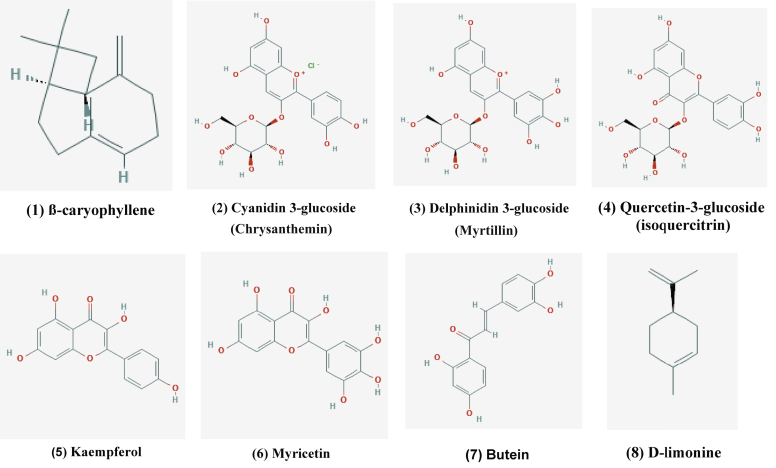
Anthocyanins are abundant in sumac fruits (AbouReidah et al., 2014). In increasing order of content in the sumac fruit, these anthocyanins were identified as being: Delphidin-3-glucoside, Cyanidin 3-(2″-galloyl)galactoside, Cyanidin-3-glucoside, 7-methyl-cyanidin-3-(2″galloyl)galactoside, 7-methyl-cyanidin-3-galactoside (Romeo et al., 2015). Figure 3 shows some anthocyanins' chemical structures identified in Rhus coriaria (AbouReidah et al., 2014).
Figure 3.
Structure of some anthocyanins from Rhus coriaria (structures 1–8) (Pubchem).
2.2.2.3. Volatile compounds
The main volatiles found in Rhus coriaria are Terpene hydrocarbons (AbouReidah et al., 2014). According to a study published by the Journal of Chemical Biodiversity which studied the different volatile substances in Rhus coriaria during different stages of growth, monoterpene and sesquiterpene hydrocarbons, specifically β-caryophyllene and α-pinene, were the most abundant class in the Rhus coriaria extracts analyzed, followed by diterpenes (Reidel, 2017).
Moreover, a study conducted in New Delhi, India, identified three new compounds from the Rhus coriaria plant. These were defined as Coririanaphthyl ether, Coriarioic acid and Coriariacthracenyl ester (Onkar et al., 2011).
2.2.2.4. Fatty acids
A study conducted in Turkey studied the content of fatty acids in sumac by means of Gas chromatography and mass spectrometry and reported that sumac fruits contain 37.7% oleic acid, 34.8% linoleic acid, 27.4% palmitic acid and 17.3% stearic acid (Kizil and Turk, 2010).
This implies that sumac fruit could be a good source of beneficial unsaturated fatty acids that could support a healthy diet (Kizil and Turk, 2010).
A study conducted on harvests of sumac from Iran identified a total of 21 oil compounds representing 86.6 % of the fatty acids in the fruit. Iranian Sumac essential oils are in its majority β-caryophillene (30.7%) and cembrene (21.4%) (Dogan and Akgul, 2005).
Additionally, different types of Sumac fruits in Turkey were studied and the proximate composition of oil and protein was found to be 12.5% and 3.5%, respectively. The fatty acid composition was determined to be around 0.25% Myristic acid, 23.1% Palmitic acid, 3.1% Stearic acid. The highest amounts of fatty acids are also mostly of oleic acid comprising 37.5%. The polyunsaturated fatty acids known as linoleic (omega 6) and α-linolenic acid (omega 3) contents were found to be between 34.84 and 1.88% respectively (Dogan and Akgul, 2005).
2.3. Mineral composition of Rhus coriaria
Sumac fruit is rich in potassium, calcium, magnesium and phosphorus predominantly, followed by aluminum, iron, sodium, boron and zinc (Ozcan and Haciseferogullari, 2004). Sumac can be a good addition to increase the dietary intake of some minerals like potassium and calcium (Ozcan and Haciseferogullari, 2004). The mineral of highest concentration was found to be potassium with a value of 7963 ppm, followed by calcium and phosphorus with 3661 ppm and 1238 ppm, respectively. On the other hand, cadmium content was shown to be almost negligible with 0.03 ppm followed by selenium with 0.47 ppm.
A comparative study was made on the selenium content of sumac and other herbal plants. It showed that sumac had the lowest content of selenium amongst the plants studied, compared for example to Basil which had the highest content (Ozkutlu et al., 2011).
2.4. Vitamins composition of Rhus coriaria
Studies made on the vitamins composition of Rhus coriaria are still lacking. A comparative study done in China on the difference between Syrian sumac and Chinese sumac have listed some of the vitamins present in Rhus coriaria. The main vitamins found in sumac were stated to be thiamin B1, riboflavin B2, pyridoxine B6, cyanocobalamin B12, nicotinamide, biotin and ascorbic acid. From these vitamins, pyridoxine was the most abundant, followed by ascorbic acid, thiamine and riboflavin. According to the study, the amount of vitamins found in Syrian sumac was relatively higher than that found in Chinese sumac (Kossah et al., 2010).
3. Therapeutic uses of Rhus coriaria
The use of herbs is very common in the treatment of several health problems from headaches, arthritis, urinary and digestive problems to many others. Similarly, Sumac is one of these super-powered herbs. Rhus coriaria's bioactive compound has been shown to have antibacterial, antifungal, anti-inflammatory, antioxidant, antiemetic, antidiarrheal, antiviral, hypoglycemic, leukopenic and antifibrinogenic potential (AbouReidah et al., 2014; Shabir, 2012). The use of Rhus coriaria in homeopathic therapy and the potential of its extracts in the pharmaceutical industry is what makes Sumac fruits such a versatile food ingredient.
3.1. Antimicrobial power of Rhus coriaria
The antimicrobial aspect of sumac's active compounds are not only useful in the therapeutic field but are also used to overcome several problems in the food industry.
Therapeutically, the use of Rhus coriaria extracts against different bacterial infections has been highlighted. A study of Al Mustansiriya University in Baghdad evaluated the effect of two Rhus coriaria extracts, water based and ethanol based extracts, on three strains of Pseudomonas aeruginosa and two strains of Escherichia coli bacteria on epithelial cells isolated from humans. They compared the inhibition of bacterial adhesion zones of the bacteria treated with the two extracts to one treated with Tetracycline. The results showed a higher effect of the hot ethanol extracts than those of hot water extracts. This was theorized to be due to the lethal effect of ethanol on some types of bacteria by damaging its cell membrane. Both extracts showed a higher effectiveness than the control and distilled water (Alwan et al., 2009a, Alwan et al., 2009b).
Furthermore, they evaluated the degree of effectiveness of the ethanol 40mg/ml extract (which had the highest efficiency) on the adhesion diameter compared to the control (Tetracycline). The results showed a greater efficiency of the ethanol extracts emphasizing its potential in the safe treatment of urinary and other infections as an alternative for other antibiotics which may have many side effects (Alwan et al., 2009a, Alwan et al., 2009b).
An additional study of the Basra University in Iraq evaluated the effect of two phenolic extracts A and B of Rhus coriaria on four bacterial species: E. coli, Staphylococcus aureus, Brucella sp. and Klebsiella pneumoniae. They used three different extracts: methanol, chloroform and lipid crude extracts. They also evaluated two other extracts A and B, which were tested for their phenolic content (positive blue color using FeCl3) and aldehyde, ketone content (positive orange color using Brady's reagent) and analyzed using Thin Layer Chromatography and, Infra Red and Ultra Violet light. The crude extracts were evaluated separately from extracts A and B. The methanolic extract showed a high effectiveness against all four bacteria by having an average adhesion diameter of 26, 23, 22 and 19 cm for E. coli, S. aureus, Brucella sp. and Klebsiella pneumoniae, respectively. Meanwhile, the chloroform and lipid crude extracts showed no antibacterial effect whatsoever (AlJaber, 2008). On the other hand, extracts A and B showed no antibacterial effect when used separately. However, when used together, it showed a synergetic antibacterial effect (AlJaber, 2008).
Moreover, a study conducted in the Jordan University of Science and Technology studied the antibacterial activity of ethanol extract of 15 plant species used in the traditional medicine in Jordan and other Middle Eastern countries. It was shown that Rhus coriaria, along with Punica granatum L. and Quercus infectoria Olive, exhibited a broad spectrum antibacterial activity. The MIC for Rhus coriaria was 1.95–31.25 mg/ml. According to their results, tannins may be the main antimicrobial agent of the plant, since it was present in all the plants evaluated (Nimri et al., 1999).
A similar study evaluated the antimicrobial effect of Syrian sumac and found that the methanolic extract was the most effective and had the highest inhibitory activity percentage. Bacillus subtilis was found to be the most sensitive to sumac extracts and salmonella sp. was found to be the most resistant. The inhibitory effect was found to increase with increased concentration in all sumac extracts (Raodah et al., 2014).
3.2. Antifungal power of Rhus coriaria
Many studies have shown the antimicrobial efficacy of Sumac to a degree that it overcomes the effectiveness of antibiotics (Digrak et al., 2001). Its antifungal effect is also a point for healthy homeopathic treatments.
A study investigated extracts of Rhus coriaria and identified new xanthones that were all active against fungal infections. They extracted different samples using methanol (1, 5, 10, 20 mg/ml concentrations) and corianaphtyl ether, coriarioic acid and coriariacthracenyl ester (all with 25, 50, 100, 200 mcg/ml concentrations). The tests were performed on petri dishes incubated with Alpergillus flavus, Candida albicans and Penicillium citrinium. The results were based on the mean zone of inhibition caused by the extract compared to that of the control (Fluconazole). The methanolic extract showed the highest average mean zone inhibition followed by corirariacthracenyl ester extract (Singh, 2011a, Singh, 2011b).
Another study, by the University of Jiangnan in China, evaluated the antimicrobial and antifungal effect of Rhus coriaria fruits on different bacteria strands and yeast. The fungi studied were Candida albicans and Aspergillus niger. Their suspensions were adjusted to 107 cells/ml and the inhibition zone was recorded after an incubation of 48 h at 27 °C. According to their results, Rhus coriaria showed a potent effect on both bacteria and fungi with an average MIC <0.25–0.5 mcg/ml. The antifungal activity of Rhus coriaria's extract was more effective than that of the standard antifungal Nystatin (Ertürk, 2010).
An additional antifungal investigation of Rhus coriaria extracts was performed on Pichia pastoris, Kluyveromyces lactis and Saccharomyces cerevisiae (Kossah et al., 2013). The MIC observed for fungal strains were found to be between 5200 and 7000 μg/ml (Ertürk, 2010).
These yeasts are also main causative agents of food spoilage, which emphasizes its importance in food preservation as well (Obais et al., 2013; Abdelmalek, 2013).
3.3. Hypoglycemic power of Rhus coriaria
Diabetes Mellitus is a condition that not only affects blood sugar levels causing metabolic dysfunctions but it also mediates cardiac and hepatic problems. In order to manage it, not only blood glucose levels should be controlled but also the lipids profile. Rhus coriaria has been showing great effect in managing hyperglycemia and hyperlipidemia, being a potential treatment for the prevention and management of Diabetes.
Several studies have been done in vivo to evaluate the effect of different sumac extracts on blood sugar levels by analyzing its activity on insulin resistance (Anweri et al., 2013), serum biomarkers (Dogan and Celic, 2015) and on gene expression and the enzymes responsible for the metabolism of glucose (Mohammadi et al., 2010). Few studies have been done in humans.
In a study by Dogan et al., 54 rats were induced into diabetes by injecting them with streptozotocin, a compound toxic to the beta cells of the pancreas. Some rats were treated with Metformin, a drug used to treat hyperglycemia and some were treated with different levels of sumac extract. A significant weight loss was observed in the diabetic rats after treatment with sumac. Also, a significant decrease in blood glucose and lipid profile were observed (Dogan and Celic, 2015).
Nevertheless, the activities of serum plasma liver damage enzymes significantly increased in the DM group compared to the NC group. Interestingly, oral administration of the plant extract in diabetic groups significantly restored the enzyme levels close to NC group. Also, diabetic rats administrated Rhus coriaria-extract. The extract caused a significant decrease in the levels of HbA1c and α-glucosidase activity ( Dogan, 2016).
The results of the above studies are also proven true by Anwer et al. with similar results. They also studied the effect of sumac extract on serum sensitivity with an oral administration of Rhus coriaria extracts on rats by calculating HOMA-IR, which is the Homeostatic Model Assessment of Insulin Resistance. This method quantifies insulin resistance as well as beta-cell function. They also calculated the KITT (insulin sensitivity index by Insulin Tolerance Test ITT), which is used to assess peripheral insulin resistance. The results obtained showed that insulin sensitivity of the rats was significantly improved after administering sumac. Additionally, sumac treatment significantly prevented the rise in insulin resistance (Anweri et al., 2013). This showed the effectiveness of sumac use pharmacologically in the treatment and management of insulin resistance.
On the same scope, Shidfar et al. have studied the effectiveness of Rhus coriaria powder on serum glycemic status, ApoB, ApoA-I and Total Antioxidant Capacity (TAC) in humans suffering from Type 2 DM. The authors conducted a clinical trial on 41 diabetic volunteer patients divided into 2 groups: 22 sumac treated patients and 19 patients treated with placebo. During the intervention, each participant consumed 3.0 g sumac powder daily over three months while the participants of the placebo group received the same amount of lactose and some biochemical parameters were analyzed before and after the intake. There was shown to be a significant difference between the sumac and placebo groups with a notably decreased serum glucose and HbA1c levels (Shidfara and Hosseinic, 2014). Apo B markers are analyzed to evaluate the risks of CHD (Coronary Heart Diseases). Apo B has shown a significant decrease in the sumac group and no effect in the placebo group. ApoA-I is removed excess cholesterol from tissues and incorporates it into HDL cholesterol which transports it to the liver. So, the ratio of apoB/apoA-I reflects the balance of cholesterol transport. Therefore, the higher the value, the higher the propensity for cholesterol deposition, and consequently the higher the risk for atherogenesis. A significant decrease in the ratio and the average differences before and after intervention were seen in sumac group, but there weren't significant in placebo group which emphasizes the ability of sumac to manage metabolic syndrome as a whole (Shidfara and Hosseinic, 2014).
On the other hand, a study has shown that sumac has negative effects on blood sugar by elevating serum glucose (Mirhadi et al., 2011).
3.4. Hypolipidemic power of Rhus coriaria
Sumac has been found to be effective in patients with hyperlipidemia as it was shown to increase HDL levels (Hajmohammadi et al., 2016) as well as decrease LDL (Low-density lipoprotein) levels (Boroujeni et al., 2016). Additionally, it has shown a great potential in the treatment and prevention of atherosclerosis (Zargham, 2008).
In an attempt to try to reduce the side effects of hyperlipidemic drugs, Sumac was evaluated as an aid to anti-lipidemic drugs in decreasing blood cholesterol in hyperlipidemic patients. In this study, there were two groups of patients: one group received lovastatin and the other received a combination of lovastatin and sumac.The serum LDL level was measured after three months. The results showed that the LDL levels of the combination treatment group was 105.75 mg/dL while in the single Lovastatin therapy treatment was 117.04, which emphasizes the great help Sumac had in decreasing LDL levels when combined with anti-lipidemic drugs (Boroujeni et al., 2016).
Moreover, Sumac was tested on patients with hyperlipidemia to evaluate its effect on serum TG, total LDL, and HDL cholesterol. A notable increase in HDL cholesterol level was observed in the sumac group compared to the placebo receiving patients after 2 months of intervention (Hajmohammadi et al., 2016).
Furthermore, the tannins present in sumac fruits have shown an effect in inhibiting vascular smooth muscle cell (VSMC) migration and thus being a great alternative for drugs used in the treatment and prevention of atherosclerosis. The accumulation in VSMC is critical in the pathogenesis of vascular diseases and tannins were shown to have the ability to inhibit VSMC migration. The results showed that sumac decreased VSMC migration by 62%, making it a potent aid in the treatment of atherosclerosis (Zargham, 2008).
3.5. Analgesic power of Rhus coriaria
Also with the objective of finding an alternative to drugs having undesirable side effects, scientists have reached for herbal alternatives in search of natural bio actives that may substitute these drugs with an effective yet healthier result, with little or no side effects and more accessible prices (Mohammadi et al., 2016).
In order to evaluate the effectiveness of sumac extracts on pain, several tests were done. The writhing test is done by counting the abdominal contractions after injection. The Tail Flick test is assessed by using a tail flick analgesimeter apparatus and the chronic pain was assessed by the Formalin test in which they assessed and rated the animals' motor response to pain. The results showed that the extract was effective in inhibiting pain. The tail flick test is done by a thermal stimulation and injecting of doses of Sumac extract decreased the pain. Since this test is used to evaluate spinal reflexes, it was suggested that the anti-nociceptive effect of Sumac extract affects the central nervous system. Also, it was found that it’s even more efficient in decreasing chronic pain than acute pain (Mohammadi et al., 2016).
3.6. Antioxidant power of Rhus coriaria
Sumac has shown to have great antioxidant activity. In a study by Bozan et al., ethyl acetate fractions of sumac extract exhibited free radical scavenging activities higher than those of BHT and BHA (butylated hydroxytoluene and butylated hydroxyanisole which are synthetic antioxidant compounds) at all concentrations tested (Bozan et al., 2002).
A study was made on aqueous, methanolic and ethanolic extracts of sumac to evaluate which had the highest antioxidant activity. It was found that the methanolic extract showed the highest antioxidant power and a greater reducing power compared to ascorbic acid. Moreover, as the concentration of sumac extract increased, the scavenging activity against nitric oxide, the hydroxyl radical inhibition, metal chelating activities, peroxides decomposition and oxygen quenching was found to be increased (AlMouwaly et al., 2013).
4. The uses of Rhus coriaria in the food industry
4.1. Preservative effect
As discussed previously, Rhus coriaria has a potent antimicrobial and antifungal effect (Alwan et al., 2009a, Alwan et al., 2009b; AlJaber, 2008 Singh, 2011a, Singh, 2011b; Ertürk, 2010; Nimri et al., 1999; Digark et al., 2001). These qualities are not only important therapeutically but are a crucial weapon in the food industry, allowing sumac to be used as a natural preservative to increase products’ shelf life maintaining its quality (Gulmez at al., 2006; El Khatib and Salame, 2019).
A comparative study evaluated the effect of sumac extracts, distilled water and lactic acid on broiler meat with the objective of improving its microbiological quality and increasing its shelf life. To do so, they divided broiler wings into 3 groups each of them was decontaminated separately by the addition of distilled water, sumac extract and lactic acid treatments. Then, a microbiological analysis was carried out, as well as a sensory evaluation (Gulmez at al., 2006).
In all experiments, lactic acid showed a higher antimicrobial effect on psychrotrophs, mesophilic aerobes, enterobacteriaceae, coliforms, and presumptive fecal coliforms compared to both sumac-treated and distilled water treated wings. However, the effectiveness of sumac extract was comparable to that of lactic acid and higher than that of distilled water making it a good alternative to decontaminate foods other than using synthetic and chemical antimicrobials. It's also a cheaper, natural and safer option (Gulmez at al., 2006).
As a plus, sumac-treated wings showed a good color in the sensory evaluation. In contrast, both distilled water-treated wings and lactic acid- treated wings developed an unpleasant color. This gives a positive score for the use of sumac in poultry processing (Gulmez at al., 2006).
Moreover, the preservative effect of sumac was studied on poultry by analyzing its effect on Bacillus cereus and Salmonella typhimurium. The results showed that sumac extract was effective on preventive bacterial growth for 7 days and it was more effective on S. typhimurium than on B. cereus (Abdelmalek, 2013).
The antimicrobial effect of sumac cannot only be used as a food safety matter, but also to prevent the microbiological degradation of food and thus maintaining its quality for longer periods of time. A study was conducted to evaluate the efficiency of sumac extracts to control the causal agent of soft rot disease, the bacterium Erwinia carotovora, on potato tuber. Water and ethanol extracts were prepared and studied and the results showed that both extracts had a high efficiency in inhibiting the growth of Erwinia carotovora. The highest the concentration of the extract, the higher the antimicrobial potency (Obais et al., 2013).
The same study evaluated the net weight of the sample potatoes after storage. All samples treated with the ethanolic sumac extracts maintained a higher weight after storage. Water extracts of sumac showed similar results as well. This shows the efficiency of Rhus coriaria against the bacteria causative of the Soft rot disease in potatoes, making it a potential alternative for preservative substances (Obais et al., 2013).
Similarly, Botrytis cinerea, which is a fungal pathogen that causes grapes table rot, was treated with sumac extracts. Extracts from the sumac fruits and leaves were applied on artificially inoculated grapes and the incidence of decays and rotted areas in the grapes was measured. After 24 h, a 92% decrease in wounds was found in the grapes inoculated with the pathogen and treated with sumac and a 72% decrease in the incidence of infection was seen. Even after 1 h only, a 70% decrease in rots area and 63% decrease in incidence was noted. The results were compared to grapes treated with citric acid and the results showed that sumac leaves were much more efficient than sumac fruits extracts and citric acid treatments (Romeo et al., 2015). Sakhr & El Khatib used different extracts of the crude Syrian Sumac to evaluate their effect as a meat tendirizer on Pectoralis Superficialis cuts. The study showed a significant decrease in shear stress and protein content with a significant increase in collagen solubility. Significant decrease in fat content has been noted promoting the effect of Sumac as a natural meat tendirizer (Sakhr and El Khatib, 2019).
4.2. Antioxidant effect of Rhus coriaria in food
Besides the therapeutic benefits of antioxidant compounds, these are also very useful in the food industry for it delays the oxidative process that deteriorates the aspect and taste of food. BHA (butylated hydroxyanisole) and BHT (butylated hydroxytoluene) are synthetic antioxidants. These have many applications in the food industry like the preventing lipids oxidation. However, BHA and BHT have been shown to have toxic and carcinogenic effects (Bursal, 2010). For this reason, more natural and safer alternatives are being requested for food application.
A study performed in Anadolu University, Turkey, evaluated the antioxidant effect of sumac extracts by calculating the Higher Induction Index of different samples of sunflower oil. The higher induction index is calculated by dividing the induction time of sunflower oil + extract over the induction time of sunflower oil without the extract. The higher the induction index, the higher the antioxidant activity is. Sumac ethyl acetate extract exhibited higher antioxidant effect at 1% concentration than BHA and BHT and the activity of sumac n-hexane was similar to that of the synthetic compounds. The induction periods were measured by Rancimat device (Bozan et al., 2002).
4.3. Sumac use in feed additive
After the use of antibiotics in feed additives was banned in Europe, alternatives are being researched on the use of herbs for that purpose. Particularly, medicinal herbs are being evaluated for its use as a safer, cheaper and healthier alternative for humans compared with synthetic chemical drugs (Salih and Gurbuz, 2015).
A study was made in Turkey to evaluate the effect of Rhus coriaria powder as feed additives on poultry with the objective of increasing the performance of laying hens and broiler. Because antibiotics have a residual effect in eggs, it should not be used in laying chicken. So, the alternative of using sumac was verified by adding 0.5 and 1% sumac powder to the feed as a supplement and check its effect on heat stress in broiler chickens. According to the study, the seeds at 0.5% could decrease negative effects of mild heat stress on broiler chickens and increase their efficiency during the first 21 days of age. However, 1% sumac fruit powder level was found to be ineffective on the performance because of its higher tannins content (Salih and Gurbuz, 2015).
A similar study was conducted in Urmia, Iran. Rhus coriaria powder was used as a supplemental feed for broiler chickens in an attempt to decrease the thiobarbituric acid reactive substances (TBARS) in the thigh meat of heat-stressed chickens. Heat stress caused a reduced efficiency of chicken and even poultry deaths. This happened because the chickens living in hot areas of tropical countries had a decrease in feed intake and weight gain. TBARS levels, which is an indicative of lipid peroxidation, increases in heat-stressed chicken because of the increase in oxidative stress and lipid oxidation in the skeletal muscles of the chicken. This affects the flavor, the texture, color and nutritional value of the meat, deteriorating its quality. To overcome this problem, different levels of Sumac were used as a feed supplement for the chicken and compared to Alpha tocopherol acetate as a control. At the end of the experiment, the pH of thigh meat was measured with a digital pH meter and TBARS was determined using trichloroacetic acid (TCA) and measuring absorbance using a spectrophotometer (Sharbati et al., 2015). The birds treated with a medium level of sumac showed the lowest thigh TBARS compared to the non-treated birds. In contrast the birds treated with a higher level showed high levels of TBARS, which worsened meat quality. Also, chicken fed medium levels of sumac showed less TBARS than those treated with Alpha tocopherol acetate (Sharbati et al., 2015).
4.4. Sumac use as colorant
Natural alternatives for additives are lately in continuous growth. The natural food colorants market in the world is expected to account for nearly 60% of the overall market by 2020. It is applied in several areas of the food industry and is a critical ingredient in many products (Dabas, 2016).
A recent study in the United States categorized some natural colorants in plants, specifically from the polyphenols present in colored plants. The anthocyanins, which are a main phenolic compound in sumac, were determined to be one of the compounds responsible for its pigmentation capacity. Moreover, the stability of these pigments was found to be increased by intermolecular pigmentation after the addition of other polyphenolics, which interact with the molecule without forming a covalent bond. Therefore, these groups would prevent the nucleophilic attack by water molecule, which makes it a more stable colorant even if mixed with water (Dabas, 2016).
Sumac was considered to be similar to wine in its colorant ability. The same compound responsible for the red-like pigmentation was found in both wine and sumac and was identified as hydroxyphenyl pyranoanthocyanins (Dabas, 2016). The study was made on staghorn sumac. Further studies should be performed on Rhus coriaria powder and its potential use as an alternative to Rhodamin B used as pickled turnips colorant.
4.5. Sumac leaf powder used as a fortifier for goat milk yogurt
The use of sumac as an antioxidant can be really broad as you could observe from this review. An Italian study was conducted using sumac leaf powder as a fortifier in goat milk yogurt (Perna et al., 2018). The goats were split into 4 groups by CSN1S1 genotype: 3 strong groups, composed of 8 goats homozygous for strong (AA) alleles, 8 goats homozygous for strong (BB) alleles, and 8 goats heterozygous for strong (AB) alleles, and 1 weak group, composed of 8 goats homozygous for weak (FF) alleles. From each animal, 3 L of individual milk were collected to manufacture 2 yogurts: 1 plain yogurt and 1 Rhus coriaria-fortified yogurt. Yogurt samples with added Rhus coriaria leaf powder and plain yogurt (without any addition) were prepared and tested for phenolic content and for antioxidant activity. Moreover, a chromatographic determination of the nature of the phenolic compounds was performed using HPLC analysis (Perna et al., 2018).
It was found that comparing different genotypes, αS1-FF plain yogurt showed lower total phenol content compared with the strong αS1-CN yogurt. Also, Rhus coriaria-fortified yogurt showed a significant increase in total phenolic compounds in comparison with plain yogurt. Moreover, the comparison with total phenol content detected in the Rhus coriaria water extract highlighted that the amount of total phenolic detectable with the Folin–Ciocalteau method in Rhus coriaria-fortified yogurt was 53.94%, whereas the remainder remains bound to milk proteins. Regardless of the considered genotype, fortified yogurt showed much higher antioxidant activity than the plain yogurt in all assays conducted. The authors hypothized that the effects of protein-polyphenol complex on antioxidant capacity are interactive and that this interaction may be causing the proteins to entrap more phenolic compounds and therefore increase the stability of polyphenols during the process. The possibility of using goat milk for the production of fortified fermented products can allow development of new nutraceutical foods (Perna et al., 2018).
5. The effect of microencapsulation on the properties of Rhus coriaria
Just like the majority of spices, sumac is used in its powdered form. The sumac berries are crushed into a powder and stored as so. However, in this form, it is more susceptible to losing its volatile compounds and consequently its flavor with time. To prolong its shelf life and the effectiveness of its compounds, researchers have suggested the use of spray drying on sumac.
Several studies have been made to evaluate the effect of spray drying conditions on the properties of sumac. Maltodextrin (MD) (Caliskan and Dirim, 2013), sodium chloride and whey (Bayram et al., 2004) were used as carrying agents. The obtained powders were analyzed for several aspects. Moisture content showed a decrease of 20% compared to the fresh berries. Water activity also was very low, around 0.18, making it more microbiologically stable (Caliskan and Dirim, 2013). Microencapsulation increased the pH of sumac powder from 3.01 to 3.2 (Caliskan and Dirim, 2013) and 4.12 (Bayram et al., 2004). However, spray drying was shown to decrease significantly the total phenolic content as well as the Radical Scavenging Activity of the powder (Caliskan and Dirim, 2013).
6. Sensory flavor profile of Rhus coriaria
In order to attain the best quality of any food product, a sensory evaluation is crucial. A single study conducted in Turkey evaluated three different extracts of Rhus coriaria for its volatile content using GC/MS technique and a flavor categorization using the Flavor Profile Analysis technique (Bahar and Altug, 2009).
Malic acid, the component that is responsible for the sour taste of sumac fruit, was detected at a significant level. It is commonly used as an acidity regulator (Bahar and Altug, 2009).
7. Other potential uses for Rhus coriaria
Sumac has proven to be a very versatile material not only used in the food industry but its components and extracts can be widely used in many distinct fields like its use as a green inhibitor of steel in water. This phenomena was due to some components found in sumac which adsorbed with metal ions forming Fe2+ organic molecule complexes (Anaee et al., 2016). Moreover, sumac can be used in dentistry as a natural anticariogenic. Rhus coriaria water extract reduced bacterial biofilm formation on orthodontic wire by Streptococcus sanguinis, Streptococcus sobrinus, Streptococcus salivarius, Streptococcus mutans and Enterococcus faecalis (Dastjerdi et al., 2014). Sumac was also shown to decrease dental biofilm formation by S. mutans via down-regulating all three GTF genes without suppressing the growth of oral bacteria (Dastjerdi et al., 2015).
8. Conclusion
Rhus coriaria, as it was previously mentioned in this review, has a wide spectrum of possible uses. The plant extracts can be used to search for bioactive natural products that help in the development of new drugs and food preservatives. It is also worthy to point out the important role of Rhus coriaria in the food industry in view of many recent findings. Although researches have been done on sumac, many possibilities could still be explored on the role that Rhus coriaria could play in the food industry. Sumac could be tested as a preservative on several products like meat and fish for example, or tested for its tenderizing effect on meat. Also, its antioxidant effect could be attempted to be applied on high fat food. Moreover, some chemical components of the Rhus coriaria plant are still unknown. Thus, there remains a significant research gap spanning the range from lead chemical discovery through process development and optimization in order to better understand the full potential of Rhus coriaria and a lot is yet to be discovered about this super food.
8.1. Future issues list
-
1
Attempts to repurpose Sumac uses are to be studied in order to explore more the potentials of this plant.
-
2
Its effect as a meat preservative could be studied, considering its low pH levels, high antioxidant activity and antimicrobial power.
-
3
What enzymes does the Sumac fruit contain? What potential benefits could it provide to the food industry?
-
4
What is the effect of different Sumac extracts on the fat, protein and physiochemical properties of different food products like meat for example?
-
5
How could the properties of Sumac be explored in the cosmetics field, considering its antioxidant properties?
-
6
Could the antifungal and antibacterial properties of Sumac be used as natural preservatives? Would it be effective and efficient?
Declarations
Author contribution statement
Khaula Sakhr, Sami El Khatib: All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We sincerely acknowledge and appreciate the support of the Lebanese International University in Lebanon, Department of Food Science and Technology.
References
- Abdelmalek A. The effect of different spices on increasing poultry meat shelf life. Assiut Environ. Res. J. 2013;38 [Google Scholar]
- AbouReidah I., Jamous R., Shtayeh M. Phytochemistry, pharmacological properties and industrial applications of Rhus coriaria L. Jordan J. Biol. Sci. 2014;7:233–244. [Google Scholar]
- AlJaber G. Antibacterial effect of two phenolic extracts from Rhus coriaria. BRJ. 2008;22:22–32. [Google Scholar]
- Almouwaly K., Alflayeh K., Ali A. Antioxidant and free radical scavenging effects of Iraqi sumac Rhus coriaria L. Baghdader Sci. J. 2013;10:921–933. [Google Scholar]
- Alwan H., Ismael S., Alwan A. Vol. 60. Mustansiriyya University; 2009. pp. 757–764. (The Suppressing Effect of Rhus Coriaria Extracts’ on Bacteria Causative of Acute Urinary Infection). [Google Scholar]
- Alwan B., Ismail S., Alwan A. Effect of Rhus coriaria crude extract on inhibition and adhesion of pseudomonas aeruginosa and E. coli clinically isolated from patients infected with acute urinary tract inflammation. Prim. Educ. College J. 2009;60:757–764. [Google Scholar]
- Anaee R., Abdullah H., Tareq M. Sumac extract as green inhibitor for steel in seawater. IJSRSET. 2016;2:353–358. [Google Scholar]
- Anweri T., Sharma M., Khan G., Iqbal M., Ali M., Alam M., Safhi M., Gupta N. Rhus coriaria ameliorates insulin resistance in non-insulin-dependent diabetes mellitus (NIDDM) rats. Acta Pol. Pharm. Drug Res. 2013;70:861–867. [PubMed] [Google Scholar]
- Ardalani H., Moghadam M., Hadipanah A., Fotovat F., Azizi A., Soltani J. Identification and characterization of chemical composition of Rhus coriaria L. fruit from Hamadan western Iran. J. Herb. Drug. 2016;6:195–198. [Google Scholar]
- Asgarpanah J., Saati S. An overview on phytochemical and pharmacological properties of Rhus coriaria L. Res. J. Pharmacogn. 2013;1:47–54. http://rjpharmacognosy.ir [Google Scholar]
- Bahar B., Altug T. Flavour characterization of sumach (rhus coriaria L.) by means of GC/MS and sensory flavour profile Analysis techniques. Int. J. Food Prop. 2009;12:379–387. [Google Scholar]
- Bayram O., Bayram M., Tekin A. Spray drying of sumac flavor using sodium chloride, sucrose, glucose and starch as carriers. J. Food Eng. 2004;69:253–260. [Google Scholar]
- Boroujeni H., Mosharraf S., Gharipour M., Samani M.A. Anti-hyperlipidemic effects of sumac (Rhus coriaria L.): can sumac strengthen anti-hyperlipidemic effect of statins. Der Pharm. Lett. 2016;8:143–147. http://scholarsresearchlibrary.com/archive.html Scholars Research Library. [Google Scholar]
- Bozan B., Kosar M., Tunalier Z., Ozturk N., Baser H. Antioxidant and free radical scavenging activities of rhus coriaria and cinnamomum cassia extracts. Acta Aliment. 2002;32:53–61. [Google Scholar]
- Bursal E. Food Research International. Erzinkan; Turkey: 2010. Evaluation of reducing power and radical scavenging activities of water and ethanol extracts from sumac (Rhus coriaria L.) [Google Scholar]
- Caliskan G., Dirim S.N. The effects of the different drying conditions and the amounts of maltodextrin addition during spray drying ofsumac extract. Food Bioprod. Process. 2013;91:539–548. [Google Scholar]
- Dabas D. Polyphenols as colorants. Adv. Food Technol. Open J. 2016;2:S1–S6. [Google Scholar]
- Dastjerdi E., Sarmast Z., Abdolazimi Z., Mahboubi A., Amdjadi P., Kamalinejad M. Effect of Rhus coriaria L. water extract on five common oral bacteria and bacterial biofilm formation on orthodontic wire. Iran. J. Microbiol. 2014;6:269–275. [PMC free article] [PubMed] [Google Scholar]
- Dastjerdi E., Monadi E., Khalighi H., Torshabi M. Down-regulation of glycosyl transferase genes in Streptococcus mutans by Punica granatum L. Flower and rhus coriaria L. Fruit water extracts. Iran. J. Pharm. Res. (IJPR) 2015;15:513–519. [PMC free article] [PubMed] [Google Scholar]
- Digrak M., Alma M., Ilcim A. Antibacterial and Antifungal Activities of Turkish Medicinal Plants. Pharm. Biol. 2001;39:346–350. [Google Scholar]
- Dogan M., Akgul A. Characteristics and fatty acid compositions of rhus coriaria cultivars from southeast Turkey. Chem. Nat. Comp. 2005;41:724–725. [Google Scholar]
- Dogan A., Celic I. Vol. 54. Yuzuncu YIL University; 2015. pp. 2092–2102. (Potential Therapeutic Properties of Rhus Coriaria on Streptozotocin-Induced Diabetic Rats). [DOI] [PubMed] [Google Scholar]
- El Khatib S., Salame A. Sumac (Rhus coriaria) extracts to enhance the microbiological safety of the red meat. Food Sci. Technol. 2019;7(4):41–52. [Google Scholar]
- Ertürk O. Antibacterial and antifungal effects of alcoholic extracts of 41 medicinal plants growing in Turkey. Czech J. Food Sci. 2010;28:53–60. [Google Scholar]
- Ferk F., Chakraborty A., Simic T., Kundi M., Knasmuller S. Antioxidant and free radical scavenging activities of sumac (Rhus coriaria) and identification of gallic acid as its active principle. BMC Pharmacol. 2007;7 abstract. [Google Scholar]
- Gulmez M., Oral N., Vatansever L. The effect of water extract of sumac (rhus coriaria L.) and lactic acid on decontamination and shelf life of raw broiler wings. Poult. Sci. 2006;85:1466–1471. doi: 10.1093/ps/85.8.1466. [DOI] [PubMed] [Google Scholar]
- Hajmohammadi Z., Shams M., Zibainejad M.J., Nimrouzi M., Fardidi P., Heydari M. Rhus coriaria in patients with hyperlipidemia: a double blind randomized clinical trial. Int. J. Med. Sci. 2016;41:10. abstract. [PMC free article] [PubMed] [Google Scholar]
- Kizil S., Turk M. Microelement contents and fatty acid composition of Rhus coiaria and pistacia terebinthus fruits spread commonly in the south eastern anatolia of Turkey. Nat. Prod. Res. 2010 doi: 10.1080/14786410903132555. (abstract) [DOI] [PubMed] [Google Scholar]
- Kossah R., Nsabimana C., Zhang H., Chen W. Optimization of extraction of polyphenols from syrian sumac Rhus coriaria and Chinese sumac rhus typhina fruits. Res. J. Phytochem. 2010;4:146–153. [Google Scholar]
- Kossah R., Nsabimana C., Zhang H., Chen W. Evaluation of antimicrobial and antioxidant activities of Syrian sumac fruit extract. J. Nat. Prod. 2013;6:96–102. [Google Scholar]
- Mirhadi K., Babazadeh D., Safarmashaei S. Orally administration effect of sumac on blood sugar in rat. Adv. Env. Biol. 2011;5:2077–2079. [Google Scholar]
- Mohammadi S., Kouhsari M., Feshani M. Antidiabetic properties of the ethanolic extract of Rhus coriaria fruits in rats. Daru. 2010;18:270–275. [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S., Zarei M., Zarei M., Salehi I. Effect of hydroalcoholic leaves extract of rhus coriaria on pain in male rats. Anesthesiol. Pain Med. 2016;6 doi: 10.5812/aapm.32128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimri L.F., Meqdam M.M., Alkofahi A. Antibacterial activity of Jordanian medicinal plants. Pharm. Biol. 1999;37:196–201. [Google Scholar]
- Obais Abed Ali Obeid, Alshouk Arkan Mahmoud, Jaafar Kholoud Abdelmajid. The antibacterial effect of sumac and myrtle extracts on the bacteria causative of soft rot disease in potatoes. Babilonia Univ. J. 2013;21:1622–1632. [Google Scholar]
- Onkar S., Mohammed A., Nida A., Ali M. New antifungal aromatic compounds from the seeds of Rhus coriaria L. Int. Res. J. Pharm. 2011;2:188–194. [Google Scholar]
- Ozcan M., Haciseferogullari H. A condiment sumac fruits: some 0physicochemical properties. Bulg. J. Plant Physiol. 2004;30:74–84. [Google Scholar]
- Ozkutlu F., Serekoglu N., Koca U., Yazici G. Selenium concentrations of selected medicinal and aromatic plants in Turkey. Nat. Prod. Commun. 2011 abstract. [PubMed] [Google Scholar]
- Perna A., Simonetti A., Grassi G., Gambacorta E. Effect of αS1-casein genotype on phenolic compounds and antioxidant activity in goat milk yogurt fortified with Rhus coriara leaf powder. J. Dairy Sci. 2018;101:7691–7701. doi: 10.3168/jds.2018-14613. [DOI] [PubMed] [Google Scholar]
- Raodah M., Alia Z., Feleeha H. The antioxidant and antimicrobial activity of Syrian sumac (rhus coriaria) fruits extract. J. Nat. Sci. Res. 2014;4:36–40. [Google Scholar]
- Reidel B. Evolution of volatile emission in Rhus coriaria organs during different stages of growth and fatty acid composition. Chem. Biodivers. 2017;14 doi: 10.1002/cbdv.201700270. (Abstract) [DOI] [PubMed] [Google Scholar]
- Romeo F., Ballistreri G., Fibroni S., Pangalo S., Nicosia M., Schena L., Rapisarda P. Chemical characterization of different Sumac and Pomegranate extracts effective against Botrytis cinerea Rots. MDPI J. 2015;20:11941–11958. doi: 10.3390/molecules200711941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhr K., El Khatib S. The use of Syrian Sumac (Rhus coriaria) as a meat tenderizer: effect on fat, protein and collagen profiles on pectoralis superficialis cut. Turk. J. Agric. - Food Sci. Technol. 2019;7(9):1203–1215. [Google Scholar]
- Salih Y.G., Gurbuz Y. Sumac (rhus coriaria L.) and ginger (zingiber officinale) as feed additive in poultry nutrition. KSU J. Nat. Sci. 2015;18:44–46. [Google Scholar]
- Shabir A. Rhus coriaria Linn, a plant of medicinal, nutritional and industrial importance: a review. J. Anim. Plant Sci. 2012;22:505–512. [Google Scholar]
- Sharbati A., Daneshyar M., Aghazadeh M., Aliakbarlu J., Hamian F. Effects of Rhus coriaria on nutrient composition, thiobarbituric acid reactive substances and color of thight meat in heat-stressed broilers. S. Afr. J. Anim. Sci. 2015;45:49–55. [Google Scholar]
- Shidfara F., Hosseinic S. The effect of sumac rhuscoriaria L. Powder on serum glycemic status, ApoB, ApoA-I and total antioxidant capacity in type 2 diabetic patients. Iran. J. Pharm. Res. (IJPR) 2014;14:1249–1255. [PMC free article] [PubMed] [Google Scholar]
- Singh S. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh O. New antifungal xanthones from Rhus coriaria seeds. Int. Res. J. Pharm. 2011;2:188–194. [PubMed] [Google Scholar]
- Zalacain A., Prodanov M., Carmona M., Alonso G.L. Optimization of extraction and identification of gallotannins from sumac leaves. J. Biosyst. Eng. 2003;84:211–216. [Google Scholar]
- Zargham H. Tannin extracted from sumac inhibits smooth cell migration. McJill J. Med. 2008;11:119–123. [PMC free article] [PubMed] [Google Scholar]